Abstract
Background
The potential for prenatal antibiotic exposure to influence asthma risk is not clear. We aimed to determine the effect of timing, dose, and spectrum of prenatal antibiotic exposure on the risk of childhood asthma.
Methods
We conducted a population-based cohort study of 84 214 mother–child dyads to examine the association of prenatal antibiotic exposure and childhood asthma using multivariable logistic regression models.
Results
Sixty-four percent of pregnant women received antibiotics. Prenatal antibiotic exposure was associated dose-dependently with increased odds of childhood asthma (adjusted odds ratio [aOR] for interquartile increase of 2 courses [interquartile range, 0–2], 1.26 [95% confidence interval {CI}, 1.20–1.33]). Among children exposed to at least 1 course in utero, the effect of timing at the first course was moderated by total maternal courses. Among pregnant women receiving a single antibiotic course, timing of exposure had no effect on childhood asthma risk. Among women receiving > 1 course, early exposure of the first course was associated with greater childhood asthma risk. Compared to narrow spectrum–only antibiotic use, broad spectrum–only antibiotic exposure was associated with increased odds of asthma (aOR, 1.14 [95% CI, 1.05–1.24]). There were effect modifications (P < .001) by maternal asthma on total courses, and on timing of the first course, significant only among those without maternal asthma.
Conclusions
Increased cumulative dose, early pregnancy first course, and broad-spectrum antibiotic exposure were associated with childhood asthma risk. Our study provides important evidence supporting judicious prenatal antibiotic use, particularly timing of use and choice of antibiotics, in preventing subsequent childhood asthma.
Keywords: prenatal antibiotic exposure, childhood asthma, genetic predisposition, dose-response relationship, antibiotic course timing
Cumulative dose, early exposure, and broad-spectrum antibiotic use in pregnancy are associated with increased risk of childhood asthma. Judicious antibiotic use during pregnancy, particularly timing of use and choice of antibiotics, may have potential to decrease childhood asthma.
Asthma is one of the most common chronic diseases in the world, resulting in not only poor health outcomes, but also substantial economic burden both to individuals and society [1, 2]. There is no cure for asthma, and the burden continues to be driven by increasing prevalence especially in westernized countries, likely related to the environment [1, 2]. The rise in the prevalence of asthma has not only been greatest in high-income countries, but has also coincided with the increasing use of antibiotics.
Antibiotics are the most commonly prescribed prenatal medications, constituting 80% of all prescription medications during pregnancy [3–5]. Previous studies examining the association between prenatal antibiotic exposure and the development of asthma have conflicting results [6–16]. Likely mechanisms include antibiotic exposure causing asthma through altered intrauterine microbiota and dysregulated immune development [12], antibiotics being merely a surrogate of the infection itself that is associated with increased asthma risk [17], or a combination of both. It is also possible that infection and antibiotic exposure merely serve as markers of familial predisposition (genetic and environmental) to asthma and/or to infection [11, 14–16]. The objective of this study was to fully understand the relationship of prenatal antibiotic exposure and childhood asthma, which then may aid in the development of clinical guidelines for optimal use of antibiotics and enhance the understanding of the early-life perturbations that predispose to asthma. We evaluated the association between the dose, timing, and spectrum of prenatal antibiotic exposure and childhood asthma by age 6 years. Furthermore, we investigated whether the association of prenatal antibiotic exposure and childhood asthma was modified by a familial predisposition to asthma, using maternal asthma status as a surrogate.
METHODS
Study Population
We conducted a large population-based cohort study of mother–child dyads of infants born between 1995 and 2003 and continuously enrolled in the Tennessee Medicaid Program (TennCare). We limited the study population to children who were singleton, term birth, non–low birth weight, otherwise healthy and whose mothers were enrolled in TennCare. The study protocol was approved by the Institutional Review Board of Vanderbilt University and the Tennessee Department of Public Health.
Prenatal Antibiotic Exposure Ascertainment
Prenatal antibiotic exposure was extracted from TennCare medical prescription fill data. The number and spectrum of antibiotic courses filled were determined for the whole pregnancy and for each trimester. We classified an antibiotic fill as narrow- or broad-spectrum based on the combination of 2 recently published classification tables [18–20]. Gestational age at the prenatal antibiotic fill was determined using date of the maternal last menstrual period from linked birth certificate (85.2%) or using a previously described and validated algorithm (14.8%) [21–24].
Childhood Asthma Ascertainment
Childhood asthma was ascertained using both pharmacy claims for asthma-specific medications and asthma-related healthcare encounters between the ages of 4.5 and 6 years. The algorithm has been reported and validated previously and is similar to that used by other United States Medicaid systems [18, 25–29].
Covariates
Covariates of maternal and child characteristics were chosen a priori based on clinical knowledge and relevance of their association with prenatal antibiotic exposure and childhood asthma.
Statistical Analysis
We performed logistic regression analyses assessing the association between prenatal antibiotic exposure and childhood asthma. The main features of antibiotic exposure were number of antibiotic courses (range, 0–10), timing (trimester-specific effect, gestational age at the first course of prenatal antibiotic exposure), and spectrum (none, narrow only, broad only, and both). The trimester-specific prenatal antibiotic exposure was defined as mutually exclusive categories including never exposed, or exposed in the first trimester only, the second trimester only, the third trimester only, or in multiple trimesters.
Among children who were exposed to at least 1 prenatal antibiotic course, we examined the association between gestational age in days at the first prenatal antibiotic course and childhood asthma. We further explored the potential interaction effect between cumulative number of courses and timing (gestational age in days) of the first course on childhood asthma outcomes.
We assessed spectrum-specific effect adjusting for covariates and total number of antibiotic courses. Potential interaction effect between number of antibiotic courses and spectrum on the risk of childhood asthma was explored. Spectrum-specific effect was further explored in the subset of children who were exposed to only 1 prenatal antibiotic course.
To examine whether genetic predisposition for asthma differentially impacts the association between prenatal antibiotic exposure and childhood asthma, we conducted interaction analyses between maternal asthma and number of antibiotic courses, and between maternal asthma and gestational age at the first course. Last, we performed sensitivity analyses by additionally adjusting for the severity of infant bronchiolitis. Additional methodological details related to study population, antibiotics and asthma ascertainment, covariates, and statistical analysis can be found in the Supplementary Materials.
RESULTS
There were in total 84 214 mother–child dyads enrolled in the study (Table 1). Fifty-one percent (n = 43 366) of children were male, and 54% (n = 45 235) of children were white, 44% (n = 37 174) were black, and 2% (n = 1805) were of other race groups. The median maternal age at delivery was 22 (interquartile range [IQR], 19–26) years. The median gestational age at delivery was 39 (IQR, 39–40) weeks, and the median birth weight was 3289 (IQR, 3005–3598) g.
Table 1.
Demographic and Health Characteristics of Mother–Child Dyads Born Between 1995 and 2003 and Continuously Enrolled in the Tennessee Medicaid Program (TennCare)
| Characteristics | Total | Prenatal Antibiotic Exposure | |
|---|---|---|---|
| No | Yes | ||
| Total | 84 214 (100) | 30 067 (36) | 54 147 (64) |
| Maternal race/ethnicity | |||
| White | 45 235 (54) | 14 304 (48) | 30 931 (57) |
| Black | 37 174 (44) | 14 977 (50) | 22 197 (41) |
| Other | 1805 (2) | 792 (2) | 1013 (2) |
| Maternal marriage status | |||
| Single | 56 647 (67) | 20 537 (68) | 36 110 (67) |
| Married | 27 558 (33) | 9531 (32) | 18 027 (33) |
| Residential classification | |||
| Major metropolitan | 27 130 (32) | 8085 (27) | 19 045 (35) |
| Standard metropolitan statistical area | 17 828 (21) | 6130 (20) | 11 698 (22) |
| Rural | 39 216 (47) | 15 842 (53) | 23 374 (43) |
| Maternal smoking status during pregnancy | |||
| No | 60 795 (72) | 22 712 (76) | 38 083 (70) |
| Yes | 23 238 (28) | 7282 (24) | 15 956 (30) |
| Maternal education level | |||
| Less than high school (< 12 y) | 38 954 (46) | 13 498 (45) | 25 456 (47) |
| High school (12 y) | 36 572 (44) | 13 312 (44) | 23 260 (43) |
| At least some college (> 12 y) | 8506 (10) | 3196 (11) | 5310 (10) |
| Maternal asthma status | |||
| No | 80 441 (96) | 29 384 (98) | 51 057 (94) |
| Yes | 3773 (4) | 683 (2) | 3090 (6) |
| Delivery method | |||
| Vaginal | 60 749 (72) | 22 107 (74) | 38 642 (71) |
| Assisted | 6247 (7) | 2157 (7) | 4090 (8) |
| Cesarean | 17 185 (20) | 5800 (19) | 11 385 (21) |
| Child sex | |||
| Male | 43 366 (51) | 15 357 (51) | 28 009 (52) |
| Female | 40 848 (49) | 14 716 (49) | 26 132 (48) |
| No. of living siblings at home | |||
| 0 | 25 363 (30) | 8746 (29) | 16 617 (31) |
| 1 | 29 688 (35) | 10 279 (34) | 19 409 (36) |
| ≥ 2 | 29 095 (35) | 11 030 (37) | 18 065 (33) |
| Gestational age, wk, median (IQR) | 39 (39–40) | 39 (39–40) | 39 (39–40) |
| Maternal age at delivery, y, median (IQR) | 22 (19–26) | 22 (19–26) | 22 (19–25) |
| Birth weight, g, median (IQR) | 3289 (3005–3598) | 3289 (3005–3600) | 3289 (3005–3584) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
A total of 54 141 of the 84 214 (64%) children were exposed to a total of 104 082 prenatal antibiotic courses (Table 2). Among them, the median number of prenatal antibiotic courses was 2 (IQR, 1–2). More than 40% of children (n = 23 362) were exposed in > 1 trimester of pregnancy, and the majority (n = 40 400 [75%]) were exposed only to narrow-spectrum antibiotics. Fourteen percent (n = 11 889) of children developed asthma by age 6 years.
Table 2.
Characteristics of Pregnant Women and Children Who Were Exposed to at Least 1 Course of Prenatal Antibiotics (n = 54 141)
| Characteristics | No. (%) |
|---|---|
| No. of prenatal antibiotic courses, median (IQR) | 2 (1–2) |
| Gestational age at the first course exposure, d, median (IQR) | 91 (40–163) |
| Trimester-specific exposure | |
| First trimester only | 9989 (18) |
| Second trimester only | 9625 (18) |
| Third trimester only | 11 163 (21) |
| Multiple trimesters | 23 362 (43) |
| Spectrum of exposure | |
| Broad-spectrum only | 5142 (9) |
| Narrow-spectrum only | 40 400 (75) |
| Both | 8599 (16) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviation: IQR, interquartile range.
Cumulative Prenatal Antibiotic Courses and Childhood Asthma
Compared with never-exposed children, exposure to prenatal antibiotics increased the odds of childhood asthma by 29% (odds ratio [OR], 1.29 [95% confidence interval {CI}, 1.24–1.35]) and 23% after adjusting for covariates (adjusted OR [aOR], 1.23 [95% CI, 1.18–1.28]).
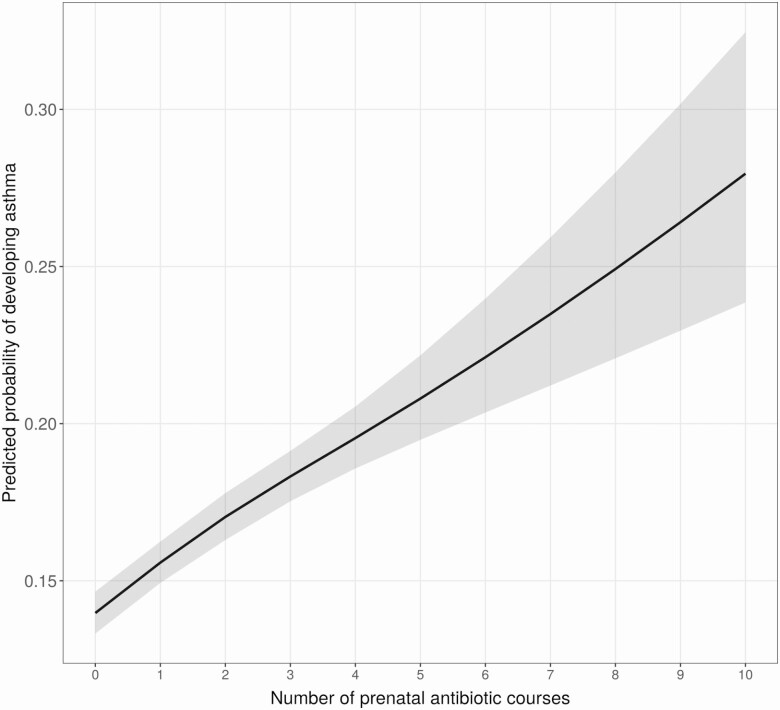
We observed a significant dose-response association between number of prenatal antibiotic courses and childhood asthma (Figure 1). With an interquartile difference of 2 antibiotic courses (IQR, 0–2), the relative odds of childhood asthma increased by 33% (OR, 1.33 [95% CI, 1.26–1.39]) and 26% (aOR, 1.26 [95% CI, 1.20–1.33]) in the univariate and adjusted analyses, respectively.
Figure 1.
Relationship between the cumulative number of prenatal antibiotic courses and childhood asthma. Multivariable logistic regression adjusted for maternal age, asthma, education, smoking, presence of group B Streptococcus infection, delivery method, and infant characteristics including birth hospitalization length of stay, gestational age, birth weight, infant race, sex, and number of living siblings at home. Gray band around the line is the 95% confidence interval.
Timing of Prenatal Antibiotic Exposure and Childhood Asthma
We classified mother–child dyads into mutually exclusive groups based on trimester of prenatal antibiotic exposure. Compared with children who were never exposed to prenatal antibiotics, the odds of childhood asthma increased by 17% (aOR, 1.17 [95% CI, 1.09–1.25]) for first trimester–only exposure, 9% (aOR, 1.09 [95% CI, 1.01–1.16]) for second trimester–only exposure, 11% (aOR, 1.11 [95% CI, 1.04–1.19]) for third trimester–only exposure, and 38% (aOR, 1.38 [95% CI, 1.32–1.45]) for multiple-trimester exposure. However, when we controlled for the total number of courses, this trimester-specific antibiotic effect persisted in children who were exposed to prenatal antibiotics in the first trimester only (aOR, 1.08 [95% CI, 1.00–1.16]) and in multiple trimesters (aOR, 1.13 [95% CI, 1.02–1.24]).
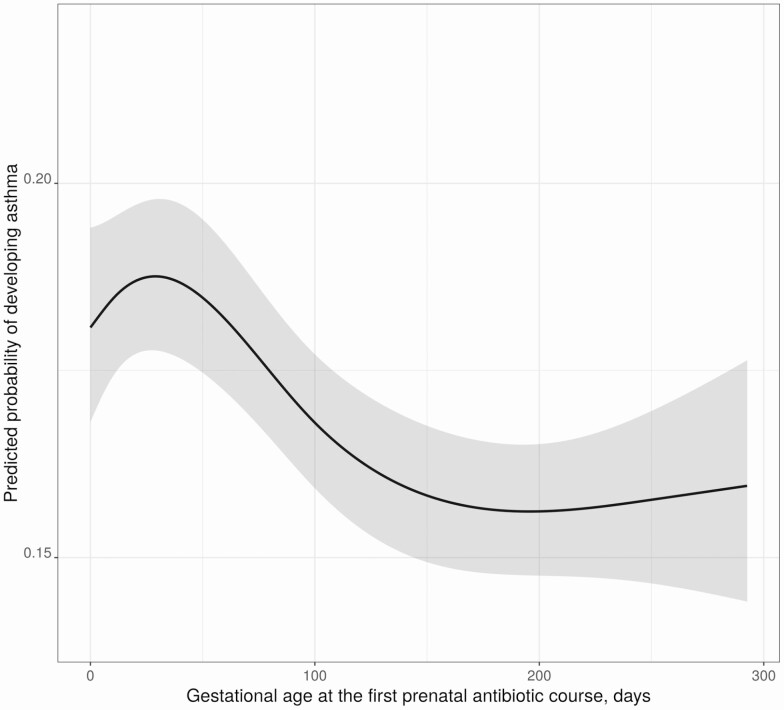
In the subset of children who were exposed to at least 1 course, we found a significant nonlinear relationship between gestational age at the first prenatal antibiotic exposure and childhood asthma after adjusting for covariates as well as total number of antibiotic courses received. We observed a protective association with advancing gestational age of first prenatal antibiotic exposure with the development of childhood asthma (Figure 2). Compared with the interquartile difference of exposure to a first prenatal antibiotic course (IQR, 40–163 days), exposure at 163 days’ gestational age was associated with 19% decreased odds of developing childhood asthma relative to an early exposure at 40 days (aOR, 0.81 [95% CI, .76–.87]).
Figure 2.
The association of timing at first prenatal antibiotic exposure with childhood asthma among children exposed to at least 1 course of prenatal antibiotics. Multivariable logistic regression adjusted for maternal age, asthma, education, smoking, presence of group B Streptococcus infection, delivery method, and infant characteristics including birth hospitalization length of stay, gestational age, birth weight, infant race, sex, and number of living siblings at home. Gray bands around the line represent the 95% confidence interval.
Interaction Effect of Timing and Cumulative Number of Prenatal Antibiotic Courses
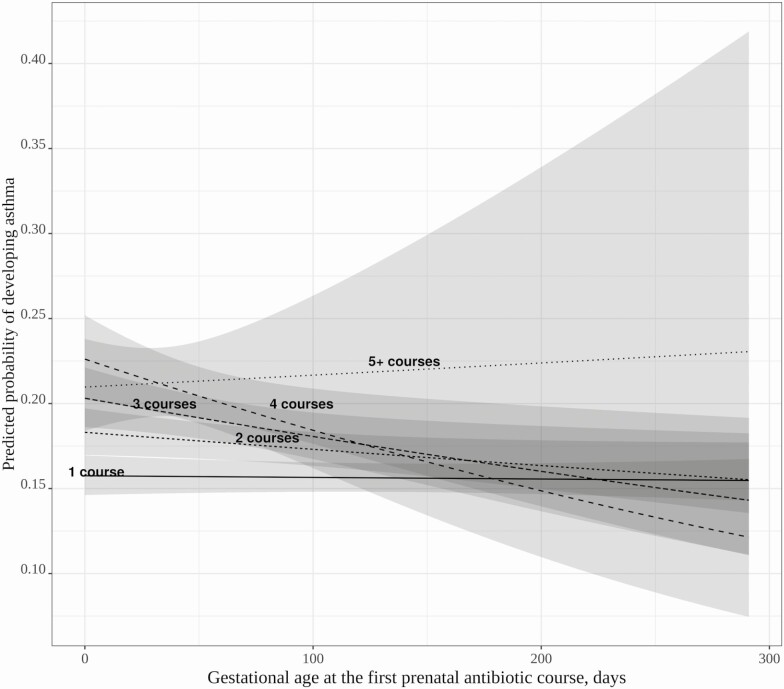
Among children who were exposed to at least 1 antibiotic course, there was a significant interaction effect between number of prenatal antibiotic courses and gestational age at the first course (P = .04; Figure 3). The increased risk of early prenatal antibiotic exposure on childhood asthma was significant only among children who were exposed to 2 or more courses, whereas timing of exposure did not affect asthma risk when children were exposed to a single prenatal antibiotic course. Subsequent subgroup analyses by specific number of courses demonstrated a consistent increased risk of childhood asthma of early timing of first course (data not shown).
Figure 3.
Effect modification of the timing of the first prenatal antibiotic exposure (gestational age in days) and the cumulative number of prenatal antibiotic courses on risk of childhood asthma. Multivariable logistic regression adjusted for maternal age, asthma, education, smoking, presence of group B Streptococcus infection, delivery method, and infant characteristics including birth hospitalization length of stay, gestational age, birth weight, infant race, sex, and number of living siblings at home. The subjects with ≥ 5 courses of antibiotics have wide confidence bands due to sparse data at later gestational ages. In the group with ≥ 5 courses of prenatal antibiotic exposure, the gestational age of first exposure tended to be earlier with a median of 25 (interquartile range [IQR], 5–51) days, whereas in those with 1–4 courses of antibiotics, median gestational age of first exposure was 95 (IQR, 43–167) days.
Prenatal Antibiotic Spectrum and Childhood Asthma
We classified the prenatal antibiotic spectrum to which pregnant women were exposed: never exposed, narrow only, broad only, or both. Compared to children who were never exposed, children who were exposed to narrow only, broad only, or both were at 1% (aOR, 1.01 [95% CI, .96–1.08]), 16% (aOR, 1.16 [95% CI, 1.06–1.27]), and 18% (aOR, 1.18 [95% CI, 1.07–1.30]) increased odds of developing childhood asthma, respectively, after adjusting for total number of antibiotic courses. There was no significant interaction effect between spectrum and number of prenatal antibiotic courses on asthma risk (P = .16). Among children who were exposed to only 1 antibiotic course, broad-spectrum antibiotics significantly increased the odds of childhood asthma by 12% (aOR, 1.12 [95% CI, 1.02–1.24]) in comparison with narrow-spectrum antibiotics.
Effect Modification of Maternal Asthma and Prenatal Antibiotic Dose and Timing
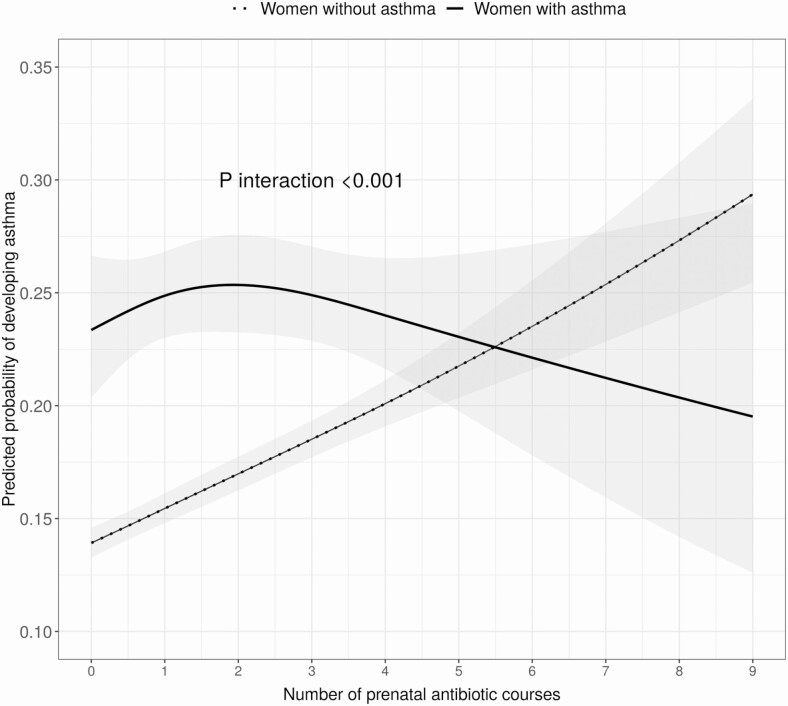
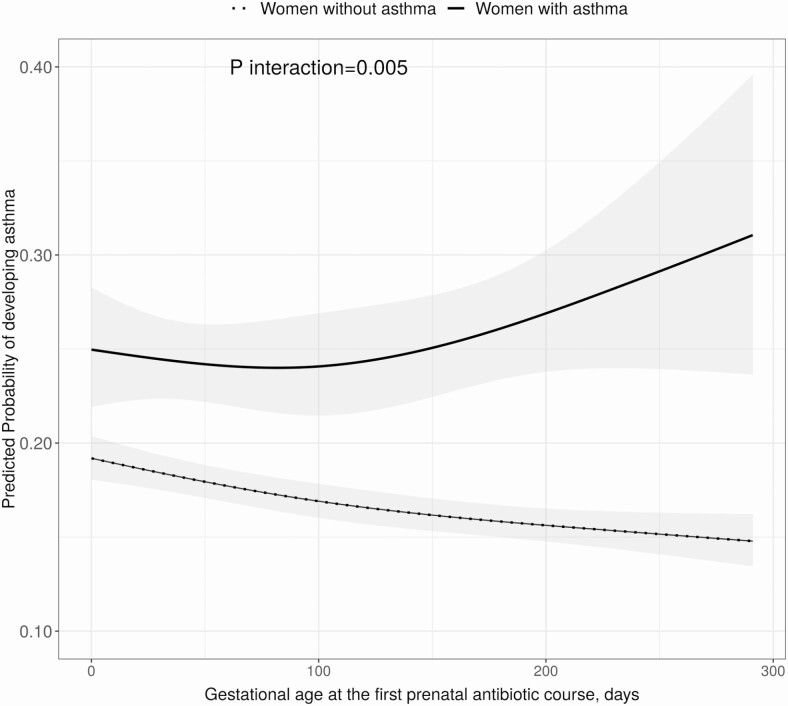
There were 3773 (4%) children whose mothers had asthma. Maternal asthma significantly modified the association between the number of prenatal antibiotic courses and asthma development (P < .001 for interaction analysis; Figure 4). The significant dose-dependent relationship between number of prenatal antibiotic courses and childhood asthma persisted only in children whose mothers did not have asthma (2 vs 0 courses: aOR,1.26 [95% CI, 1.20–1.33]) while not significant in the subset of children whose mothers had asthma (2 vs 0 courses: aOR, 1.11 [95% CI, .89–1.38]). Similarly, the significant timing effect of first prenatal antibiotic exposure on childhood asthma was observed among children whose mothers did not have asthma (Figure 5).
Figure 4.
Effect modification of maternal asthma status and the cumulative number of prenatal antibiotic courses on the risk of childhood asthma. Multivariable logistic regression adjusted for maternal age, education, smoking, presence of group B Streptococcus infection, delivery method, and infant characteristics including birth hospitalization length of stay, gestational age, birth weight, infant race, sex, and number of living siblings at home. The cumulative number of prenatal antibiotic courses was assessed with restricted cubic splines (4 knots). The gray band around the lines indicates the 95% confidence interval.
Figure 5.
Effect modification of maternal asthma status and timing of the first prenatal antibiotic course on the risk of childhood asthma among children exposed to at least 1 course of prenatal antibiotics. Multivariable logistic regression adjusted for maternal age, education, smoking, presence of group B Streptococcus infection, delivery method, and infant characteristics including birth hospitalization length of stay, gestational age, birth weight, infant race, sex, and number of living siblings at home. Gestational age in days at the first course was assessed with restricted cubic splines (3 knots). The gray band around the lines indicates the 95% confidence interval.
DISCUSSION
In this large retrospective cohort study, we report that increasing number of courses, early timing, and broad-spectrum prenatal antibiotic exposures are associated with increased asthma risk. There is a statistically significant interaction between the total number of courses and timing of the first course of prenatal antibiotics associated with subsequent childhood asthma. Moreover, the main effect of number of courses and timing of first exposure were observed among children whose mothers do not have asthma. Among children of mothers without asthma, prenatal antibiotic exposure increases the risk of childhood asthma in a dose-dependent manner, with a greater number of courses being associated with a higher risk of childhood asthma. Among children exposed to > 1 course, earlier exposure to the first course increased asthma risk compared with later first-course exposure. In contrast, although children with asthmatic mothers were more likely to be prenatally exposed to antibiotics, the number of courses and timing of the first course of prenatal exposure were not significantly associated with childhood asthma development.
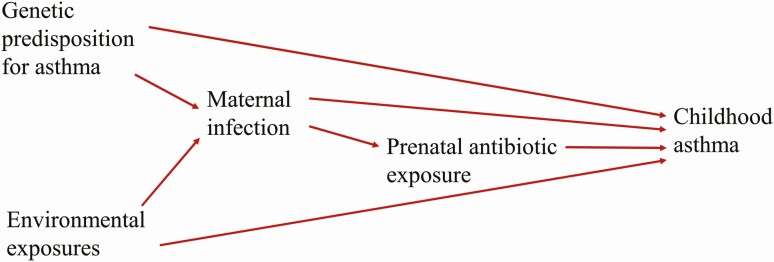
Our result of maternal asthma as an effect modifier of the association between prenatal antibiotic exposure and childhood asthma provides important evidence in understanding the relationship between prenatal antibiotic exposure and asthma development. Our finding supports the idea that multiple factors including genetic predisposition for asthma, environmental risk factors, maternal infection, and prenatal antibiotic exposure interact with each other and collectively contribute to the observed association between prenatal antibiotic exposure and childhood asthma (Figure 6). The finding that prenatal antibiotic exposure did not statistically significantly increase asthma risk in children with asthmatic mothers and there was no dose-dependent nor timing effect conforms with the findings that antibiotic exposure might be a marker of genetic predisposition and/or common environmental exposure in those with asthma [13, 15]. Örtqvist and colleagues reported that the association between prenatal antibiotics and childhood asthma vanished when compared with sibling controls in a cohort of Swedish children, suggesting that genetic predisposition to asthma and/or environmental risk factors common to the family, including shared infection, were causal factors for childhood asthma, with antibiotics being a marker of these characteristics and events [15]. In another study, Örtqvist and colleagues demonstrated that paternal antibiotic exposure during pregnancy, a negative control to prenatal antibiotic exposure and a marker of environmental exposure common to the family, was similarly associated with early-life wheeze [14].
Figure 6.
Diagram depicting the hypothesized causal mechanisms leading to the observed association between prenatal antibiotic exposure and subsequent childhood asthma development.
At the same time, our finding of a significant dose and timing effect of prenatal antibiotic exposure on childhood asthma among children whose mothers did not have asthma suggests that other explanations are plausible. Exposure to prenatal antibiotics may directly disrupt gut and intrauterine microbial diversity and function, which in turn impacts the maternal and fetal immune system and causes immune dysregulation in offspring [30]. Such disruption of the gut and intrauterine microbiome and immune system becomes pronounced when the mother and fetus are exposed to antibiotics early and repeatedly [30–32]. The altered maternal gut microbiota may then affect metabolites and substrates that are essential for fetal growth, and impact central and peripheral immune cells’ development [33].
The differential risk of broad- and narrow-spectrum antibiotic exposure observed in our study also suggests that a direct antibiotic effect on asthma is plausible. Alternatively, it is also plausible that the primary infection or factors that cause infection are the causal factors linking prenatal antibiotic exposure with asthma development. Maternal infection, through early and repetitive antigen exposure, disrupts microbial diversity and function of the developing microbiome metacommunity, and alters immunologic development, which may explain the association observed between prenatal antibiotics and childhood asthma [34]. On the other hand, it is also possible that maternal predisposition for infection, instead of infection itself, causes the observed association between prenatal antibiotic exposure and childhood asthma [11, 16]. The studies supporting this assertion showed that maternal antibiotic exposure prior to pregnancy and after delivery, markers of maternal predisposition for infection, were similarly associated with childhood asthma, as was direct prenatal antibiotic exposure [11, 16]. However, it should be noted that the effect of prepregnancy antibiotics on the maternal microbiome may persist throughout pregnancy and postdelivery maternal antibiotic exposure may disrupt mother–infant microbiome transfer.
We found that among children whose mothers did not have asthma and who were exposed to multiple courses, early pregnancy antibiotic exposure, particularly early first-course exposure, was associated with an increased risk of childhood asthma. Although early prenatal exposure often is a marker of a greater number of courses and a higher propensity for infection and asthma, our results suggest a significant timing effect even after adjusting for total courses in children whose mothers did not have asthma. This finding is consistent with our result that first trimester–only antibiotic exposure is associated with increased asthma risk. Fetal microbiome colonization has been shown to occur as early as 11 weeks of gestation [35]. Thus, early pregnancy antibiotic use may disrupt fetal microbiome colonization and affect fetal immune system development. In animal model studies, maternal antibiotic use reduces adaptive antiviral immune responses in the infant mice, suggesting an immune polarization effect of prenatal antibiotics on the offspring [31, 32]. Contrary to our finding, several studies have reported that later maternal antibiotic exposure, in the second to third trimester of pregnancy, was a significant predictor of childhood asthma [12, 36]. Other studies have reported no association between timing of prenatal antibiotic exposure and childhood asthma [9, 16]. All of these studies failed to take into consideration the total number of antibiotic courses and genetic predisposition [9, 12, 16, 36].
The strength of this study is the use of a large patient population with complete information on healthcare encounters linked with a pharmacy database that allowed us to thoroughly characterize prenatal antibiotics and the association between maternal antibiotic courses, timing, and spectrum with childhood asthma. We objectively captured prenatal antibiotic prescription fills including the exact date and antibiotic name. Similarly, we used validated asthma outcomes, which precludes recall bias.
Despite these strengths, the study has limitations that must be considered. Antibiotics are typically prescribed with an indication for infection. We could not disentangle the effect of antibiotics from the infection itself. Because treating women with infection is unavoidable, epidemiological studies like this one may not provide a thorough understanding of the independent contributions of antibiotics and infection. Second, we could not ascertain fathers’ asthma status; thus, the subsets of children whose mothers did not have asthma were a mixture of children with and without an inherited paternal genetic predisposition for asthma. However, assuming that children with such genetic predisposition would resemble the subset of children with maternal asthma, we would expect no dose and timing effect. Thus, an analysis of children without a known genetic predisposition for asthma could have an even bigger dose and timing effect of prenatal antibiotic exposure. Third, while we speculated that part of the effect of prenatal antibiotics on asthma may be through altering the microbiome and thereby immune development and phenotype, we did not actually measure the maternal microbiome and child immune phenotypes. However, the alteration of the microbiome by antibiotics has previously been shown in both human and animal studies [32, 37, 38]. Fourth, the database allows us to ascertain prescription fills of antibiotics, but not whether they were taken as prescribed. Based on the premise that prescribed antibiotics are an indication of infection during pregnancy and filling a prescription is the first step in disease management, it is likely that most fills were taken. Last, although we could include many risk factors for asthma, several risk factors were not available to include in our regression models, including the infant’s diet (breastfeeding, formula feeding, time of weaning to solids), growing up with pets, early daycare attendance, and other environmental exposures.
In this large retrospective cohort study, 64% of women received antibiotics during pregnancy. We show that both genetic predisposition for asthma as well as prenatal antibiotic exposure play a role in the development of childhood asthma. Increased cumulative dose, early prenatal exposure, and broad-spectrum antibiotics during pregnancy were associated with increased childhood asthma risk. As antibiotics are the most commonly prescribed medications during pregnancy and asthma is a lifelong chronic disease, risks and benefits of prenatal antibiotic use, timing, and choice of antibiotics should be critically evaluated prior to administration. Future studies of pregnancy antibiotic exposure should focus on the prevention of adverse effects of antibiotics, particularly their long-term impact on the newborn.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are indebted to the Tennessee Bureau of TennCare, and the Tennessee Department of Health, Office of Policy, Planning and Assessment for providing the data.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Financial support. This work was supported by the Agency for Healthcare Research and Quality (grant number R01 HS018454 to T. V. H.); the National Institutes of Health (grant numbers K24 AI77930 to T. V. H., R21 HL133742 and R21 HL123829 to P. W., K12 HD04348318 to K. N. T., and 1K12HS026395-01 to C. S.); and the National Heart, Lung, and Blood Institute.
Potential conflicts of interest. T. H. reports grants from the World Health Organization and the National Institutes of Health and personal fees from the Pfizer Vaccine Advisory Board, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Baïz N, Annesi-Maesano I. Is the asthma epidemic still ascending? Clin Chest Med 2012; 33:419–29. [DOI] [PubMed] [Google Scholar]
- 2. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355:2226–35. [DOI] [PubMed] [Google Scholar]
- 3. Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy 2015; 35:1052–62. [DOI] [PubMed] [Google Scholar]
- 4. Broe A, Pottegard A, Lamont RF, Jorgensen JS, Damkier P. Increasing use of antibiotics in pregnancy during the period 2000–2010: prevalence, timing, category, and demographics. BJOG 2014; 121:988–96. [DOI] [PubMed] [Google Scholar]
- 5. de Jonge L, Bos HJ, van Langen IM, de Jong-van den Berg LT, Bakker MK. Antibiotics prescribed before, during and after pregnancy in the Netherlands: a drug utilization study. Pharmacoepidemiol Drug Saf 2014; 23:60–8. [DOI] [PubMed] [Google Scholar]
- 6. Dom S, Droste JHJ, Sariachvili MA, et al. Pre- and post- natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin Exp Allergy 2010; 40:1378–87. [DOI] [PubMed] [Google Scholar]
- 7. Marra F, Lynd L, Coombes M, et al. Does antibiotic exposure during infancy lead to development of asthma? A systematic review and metaanalysis. Chest 2006; 129:610–8. [DOI] [PubMed] [Google Scholar]
- 8. McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands general practice database. Am J Respir Crit Care Med 2002; 166:827–32. [DOI] [PubMed] [Google Scholar]
- 9. Stensballe LG, Simonsen J, Jensen SM, Bønnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr 2013; 162:832–838.e3. [DOI] [PubMed] [Google Scholar]
- 10. Xu B, Pekkanen J, Järvelin MR, Olsen P, Hartikainen AL. Maternal infections in pregnancy and the development of asthma among offspring. Int J Epidemiol 1999; 28:723–7. [DOI] [PubMed] [Google Scholar]
- 11. Loewen K, Monchka B, Mahmud SM, ’t Jong G, Azad MB. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J 2018; 52. doi: 10.1183/13993003.02070-2017. [DOI] [PubMed] [Google Scholar]
- 12. Mulder B, Pouwels KB, Schuiling-Veninga CC, et al. Antibiotic use during pregnancy and asthma in preschool children: the influence of confounding. Clin Exp Allergy 2016; 46:1214–26. [DOI] [PubMed] [Google Scholar]
- 13. Popovic M, Rusconi F, Zugna D, et al. Prenatal exposure to antibiotics and wheezing in infancy: a birth cohort study. Eur Respir J 2016; 47:810–7. [DOI] [PubMed] [Google Scholar]
- 14. Örtqvist AK, Lundholm C, Fang F, Fall T, Almqvist C. Parental antibiotics and childhood asthma—a population-based study. J Allergy Clin Immunol Pract 2017; 5:1451–4 e4. [DOI] [PubMed] [Google Scholar]
- 15. Örtqvist AK, Lundholm C, Kieler H, et al. Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysis. BMJ 2014; 349:g6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stokholm J, Sevelsted A, Bønnelykke K, Bisgaard H. Maternal propensity for infections and risk of childhood asthma: a registry-based cohort study. Lancet Respir Med 2014; 2:631–7. [DOI] [PubMed] [Google Scholar]
- 17. de Hingh YC, van der Vossen PW, Gemen EF, et al. Intrinsic abnormalities of lymphocyte counts in children with Down syndrome. J Pediatr 2005; 147:744–7. [DOI] [PubMed] [Google Scholar]
- 18. Donovan BM, Abreo A, Ding T, et al. Dose, timing, and type of infant antibiotic use and the risk of childhood asthma [manuscript published online ahead of print 31 May 2019]. Clin Infect Dis 2019. doi: 10.1093/cid/ciz448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarpong EM, Miller GE. Narrow- and broad-spectrum antibiotic use among U.S. children. Health Serv Res 2015; 50:830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions from 2011–2016. Clin Infect Dis 2020; 70:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 2012; 207:49.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carroll KN, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, Hartert TV. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol 2005; 106:66–72. [DOI] [PubMed] [Google Scholar]
- 23. Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol 2007; 120:625–30. [DOI] [PubMed] [Google Scholar]
- 24. Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354:2443–51. [DOI] [PubMed] [Google Scholar]
- 25. Hartert TV, Speroff T, Togias A, et al. Risk factors for recurrent asthma hospital visits and death among a population of indigent older adults with asthma. Ann Allergy Asthma Immunol 2002; 89:467–73. [DOI] [PubMed] [Google Scholar]
- 26. Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc 2000; 48:651–7. [DOI] [PubMed] [Google Scholar]
- 27. Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 2005; 352:2082–90. [DOI] [PubMed] [Google Scholar]
- 28. Wakefield DB, Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr Pulmonol 2006; 41:962–71. [DOI] [PubMed] [Google Scholar]
- 29. Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med 2008; 178:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azad MB, Kozyrskyj AL. Perinatal programming of asthma: the role of gut microbiota. Clin Dev Immunol 2012; 2012:932072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol 2014; 80:250–60. [DOI] [PubMed] [Google Scholar]
- 32. Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016; 351:1296–302. [DOI] [PubMed] [Google Scholar]
- 33. Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012; 150:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirby JG, Hargreave FE, Gleich GJ, O’Byrne PM. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am Rev Respir Dis 1987; 136:379–83. [DOI] [PubMed] [Google Scholar]
- 35. Al Alam D, Danopoulos S, Grubbs B, et al. Human fetal lungs harbor a microbiome signature. Am J Respir Crit Care Med 2020. doi: 10.1164/rccm.201911-2127LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lapin B, Piorkowski J, Ownby D, et al. Relationship between prenatal antibiotic use and asthma in at-risk children. Ann Allergy Asthma Immunol 2015; 114:203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007; 1:56–66. [DOI] [PubMed] [Google Scholar]
- 38. Khan I, Azhar EI, Abbas AT, et al. Metagenomic analysis of antibiotic-induced changes in gut microbiota in a pregnant rat model. Front Pharmacol 2016; 7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.