Abstract
Declining episodic memory is common among otherwise healthy older adults, in part due to negative effects of aging on hippocampal circuits. However, there is significant variability between individuals in severity of aging effects on the hippocampus and subsequent memory decline. Importantly, variability may be influenced by modifiable protective physiological factors such as cardiorespiratory fitness (CRF). More research is needed to better understand which aspects of cognition that decline with aging benefit most from CRF. The current study evaluated the relation of CRF with learning rate on the Episodic Associative Learning (EAL) task, a task designed specifically to target hippocampal-dependent relational binding and to evaluate learning with repeated occurrences. Results show higher CRF was associated with faster learning rate. Larger hippocampal volume was also associated with faster learning rate, though hippocampal volume did not mediate the relationship between CRF and learning rate. Further, to support the distinction between learning item relations and learning higher-order sequences, which declines with aging but is largely reliant on extra-hippocampal learning systems, we found learning rate on the EAL task was not related to motor sequence learning on the alternating serial reaction time task. Motor sequence learning was also not correlated with hippocampal volume. Thus, for the first time we show that both higher CRF and larger hippocampal volume in healthy older adults are related to enhanced rate of relational memory acquisition.
INTRODUCTION
Declining episodic memory is common among cognitively normal older adults, especially after the age of 60 (Craik, 1994; Leal & Yassa, 2015; Nyberg, Lövdén, Riklund, Lindenberger, & Bäckman, 2012). Episodic memory relies on critical hippocampal processes that decline with age (Leal & Yassa, 2015), such as recollection of specific details about an experience, mnemonic discrimination (distinguishing between similar representations), and relational binding (encoding novel relationships between elements of experience) (Konkel & Cohen, 2009; Naveh-Benjamin, 2000). Evidence suggests that declining memory is in part due to negative effects of aging on hippocampal circuits critical for these processes (Driscoll et al., 2003; Geinisman, Detoledo-Morrell, Morrell, & Heller, 1995).
Significant changes in hippocampal structure and function occur in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI) (Jack et al., 2013; Jack et al., 1998; Johnson et al., 2006), yet evidence from both animals and humans demonstrates the hippocampus and associated cognitive functions are also affected during normal aging, even well before observable MCI symptoms (Gallagher & Koh, 2011). For example, older rats have been found to have, among other decrements, worse recollection (Robitsek, Fortin, Koh, Gallagher, & Eichenbaum, 2008), spatial memory (Barnes, 1979), and pattern separation (Burke et al., 2011) than young rats. Similarly, in humans, age is related to worse performance on relational memory tasks that target binding processes (Naveh-Benjamin, 2000; Naveh-Benjamin, Hussain, Guez, & Bar-On, 2003), and mnemonic discrimination tasks that target pattern separation processes (for review see Leal & Yassa, 2018; Reagh et al., 2016).
Structurally, tissue loss in the hippocampus occurs during normal aging (Raz, Rodrigue, Head, Kennedy, & Acker, 2004), and this tissue loss can be considered a proxy of lower-level degradation, such as loss of synapses and dendritic complexity. Indeed, declines in hippocampal volume relate to poorer general memory (Kramer et al., 2007; Mungas et al., 2005), episodic memory (Gorbach et al., 2017; Hedden et al., 2014; Monti et al., 2015), spatial memory (Head & Isom, 2010; Konishi & Bohbot, 2013), relational memory (Etchamendy, Konishi, Pike, Marighetto, & Bohbot, 2012), and mnemonic discrimination (for review see Yassa & Stark, 2011; Yassa et al., 2010). Thus, even otherwise cognitively normal older adults may experience declines in episodic memory processes linked to hippocampal shrinkage.
Notably, there is significant variability in the severity of age-related memory decline experienced between individuals, which may be due in part to differential effects of aging on the physiological and neurobiological processes in the hippocampus (Ash et al., 2016; Gallagher et al., 2006; Rapp & Amaral, 1992; Stark, Yassa, & Stark, 2010; Tomás Pereira, Gallagher, & Rapp, 2015). Critically, individual differences in the trajectory of age-related changes in cognition and neural systems may be influenced by modifiable protective factors such as physical activity (PA) (Hayes et al., 2015; Suwabe et al., 2018; Suwabe et al., 2017) and cardiorespiratory fitness (CRF), which is influenced largely by genetics and PA (Hayes, Forman, & Verfaellie, 2016; Hayes, Hayes, Williams, Liu, & Verfaellie, 2017). CRF is related to brain structure and function (Hayes, Hayes, Cadden, & Verfaellie, 2013) and to better cognitive function broadly (Colcombe & Kramer, 2003; Colcombe et al., 2004; Smith et al., 2010), including episodic memory (Erickson et al., 2009; Hayes et al., 2016; Szabo et al., 2011). In a study that utilized a spatial memory task, Erickson et al. (2009) found that hippocampal volume mediated the relationship between CRF and memory in older adults. Results from aerobic exercise training interventions have further shown that change in CRF relates to change in hippocampal volume (Erickson et al., 2011) and cerebral blood flow (Maass, Düzel, Goerke, Becke, Sobieray, Neumann, Lövden, et al., 2015).
While blood flow and volume are intermediate measures that are accessible in humans and may represent upstream effects of more specific structural and functional changes, other more direct measures have been evaluated in non-humans to provide insight into mechanisms. Structural changes that have been observed in response to enriched environments (of which PA is a critical aspect) include increased synaptic size and density, vascular density, rate of neurogenesis, dendritic arborization, and size and number of glial processes (Thomas, Dennis, Bandettini, & Johansen-Berg, 2012). Aerobic PA of higher intensities further creates a demand for oxygen and can result in physiological adaptations such as increased blood volume, capillary density, mitochondrial size and density, and thermoregulation (for review see Thomas et al., 2012). Human regional brain volumes thus summarize change from a combination of these micro-level mechanisms, such as increased neuropil (axonal, dendritic, and glial processes), as well as angiogenesis and neurogenesis. For instance, Pereira and colleagues (2007) showed a correlation between neurogenesis in mice and increases in regional cerebral blood volume, demonstrating cerebral blood volume could be a potential proxy for neurogenesis. They further showed increased CRF was related to increased cerebral blood volume in humans, suggesting neurogenesis may be involved in CRF’s relationship with hippocampal volume. Overall, multiple inter-dependent cellular and molecular mechanisms contribute to beneficial effects of CRF on memory, and regional brain volume provides a macro-level measure of their accumulation.
However, studies supporting relationships between CRF, hippocampal structure, and memory in older adults have primarily used spatial working memory and spatial object recall and recognition tasks. Although these tasks target some aspects of hippocampal function (e.g., spatial memory), they also emphasize one-trial learning, and while one role of the hippocampus is to acquire relations from single episodes at a time (Henke, Buck, Weber, & Wieser, 1997), the hippocampus is also involved in actively maintaining novel information over short time periods (Ranganath & D’Esposito, 2001; Watson, Voss, Warren, Tranel, & Cohen, 2013) and dynamically integrating information that connects episodes over time (Koster et al., 2018). One-trial learning does not capture this accumulation of relations over repeated occurrences with overlapping content, which requires discriminating between similar memories (e.g., seeing Bill at two coffee shops) while also accessing and strengthening the relationships between these experiences (e.g., Bill) with repeated occurrences. Because aging is known to impair relational binding (Naveh-Benjamin, 2000) and discriminating similar memories (Leal & Yassa, 2015), a task tapping into the ability to rapidly build distinct, but similar, relational memories should be maximally sensitive to hippocampal circuits affected early in aging but spared with higher CRF.
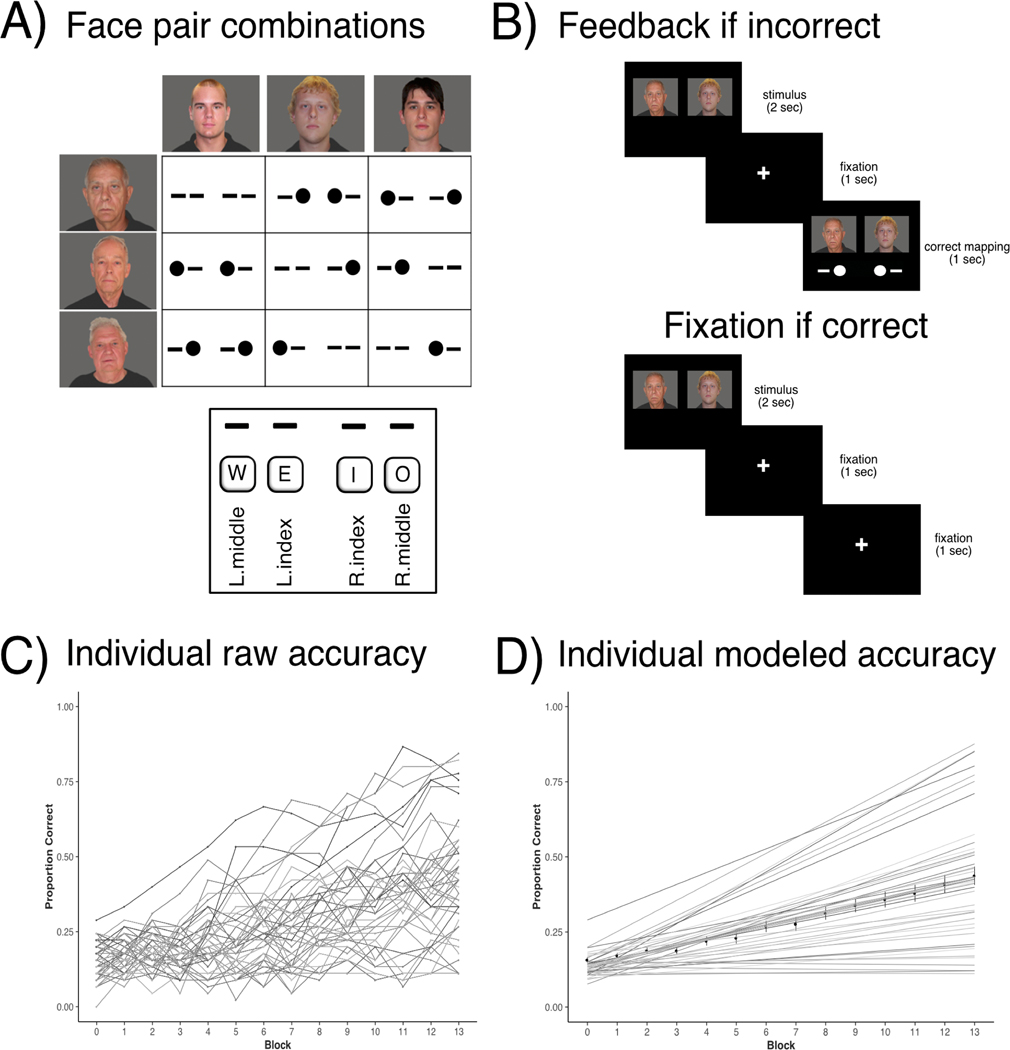
In this vein, we designed the Episodic Associative Learning (EAL) task to examine paired associates learning (Figure 1). The task measures the rate of learning item pairs with overlapping elements (e.g., A-B, A-C), for which we reliably observe strong age differences (Clark, Hazeltine, Freedberg, & Voss, 2018). Overlapping elements require each pair to be kept distinct as participants learn via trial and error. A rapid learning rate represents the ability to quickly form similar but distinct relations, which should theoretically reflect hippocampal processes of mnemonic discrimination and relational binding. However, we have not previously tested our prediction that faster EAL rate is related to hippocampal integrity in older adults, which is the first goal of this study. We also evaluate our prediction with the striatum (caudate and putamen) as control regions, as these are subcortical regions that also deteriorate with aging (Raz et al., 2005; Raz et al., 2003) and are involved in extracting regularities of experience across time (Poldrack & Packard, 2003; Seger, 2006). Second, we test the prediction that higher CRF is related to learning rate and the extent to which this is mediated by hippocampal (but not striatal) volume. Further, to distinguish between outcomes of learning item relations and higher-order sequences over time, we compare the relation of CRF with EAL rate to motor sequence learning in an alternating serial reaction time task (ASRT). ASRT performance has been shown to decrease with age (Howard & Howard, 1997), but also to depend more on the striatum than the hippocampus (for review see Howard & Howard, 2013). Notably, implicit probabilistic motor learning (such as in the ASRT) has been shown to involve the medial temporal lobe (MTL) early in the task, and then shift in later stages of the task to greater involvement of the striatum (Howard & Howard, 2013). It is worth noting that differences between young and older adults have also emerged, such that, due to age-related striatal decline, older adults may recruit the MTL more than the striatum in later stages of the task (Simon, Vaidya, Howard, & Howard, 2012). In any case, evaluating ASRT learning in relation to EAL rate, hippocampal volume, and striatal volume provides a specific test of CRF and the item-relation learning of interest in the current study.
Figure 1:
(a) Layout of face-face objects pairs and the corresponding keypress(es). (b) Incorrect and correct example trials. In the feedback, filled-in circles represent a keypress in the key that corresponds to the location of the circle, and dashes represent no keypress for that key. (c) Raw data for all participants from EAL task. (d) Data modeled by linear model for all participants, with average represented by thick black line.
Still, other cognitive processes that decline with aging, such as processing speed and working memory (Salthouse, 1994), may play a role in EAL performance. It is possible that slow processing and poor working memory could contribute to a slow learning rate on the EAL task. However, in our previous study (Clark et al., 2018), we found that while processing speed was related to EAL rate, it did not account for the age differences. Here we aim to replicate the effect for processing speed, and we further evaluate whether working memory accounts for relationships between CRF and the EAL rate.
Results from this study will provide a more comprehensive understanding of how age-sensitive learning processes correspond to individual differences in brain structure and modifiable lifestyle health characteristics.
METHODS and ANALYSES
Participants
Participants were older adults recruited from Iowa City and the surrounding communities (see Table 1). Participants were recruited using approved University email advertisements, local fliers and approved advertisements at the University of Iowa Hospitals and Clinics. Eligibility for all participants required the following criteria: 1) have no self-reported psychiatric and/or neurological condition, including depression, anxiety disorder, ADD or ADHD, epilepsy, meningitis, Parkinson’s disease, stroke, brain surgery, and head injury; 2) have no diagnoses of any of the following conditions: heart condition or other cardiovascular event, COPD, uncontrolled asthma (not on medication or inhaler for the past three months or more), cystic fibrosis, unregulated thyroid disorder (not on medication for the past 3 months or more), renal or liver disease, heart murmur, and smoking or living with someone who smokes in the past 3 months; 3) have normal color vision; 4) have corrected visual acuity of 20/40 or above; and 5) have no self-reported regular use of steroid-based medication, psychotropics, recent or current chemotherapy, or medications that indicated diagnosis of a chronic psychiatric disorder. All participants provided written informed consent approved by the University of Iowa Institutional Review Board (IRB). All study procedures were in accordance with the University of Iowa IRB’s policies. All participants were screened using the Mini-Mental State Examination (MMSE) and were excluded if they scored less than 24 points (out of 30).
Table 1.
Sample demographics
| N | Age Range | Age Mean (SD) | Years Education Mean (SD) | MMSE Mean (SD) | |
|---|---|---|---|---|---|
| Total | 45 | 60 – 76 | 66.51 (4.24) | 18.13 (2.8) | 29.18 (1.17) |
| Male | 16 | 60 – 76 | 67.12 (5.04) | 18.56 (3.39) | 28.88 (1.20) |
| Female | 29 | 60 – 74 | 66.17 (3.77) | 17.90 (2.45) | 29.34 (1.14) |
The full sample consists of 45 participants aged 60–80. Thirty-seven participants were low-active, self-reporting < 30 minutes of moderate intensity activity twice a week. Eight participants were highly-active, self-reporting performing moderate to vigorous PA for 5 or more days per week (on average) for 45 minutes per session for at least the past 2 years or longer. All participants completed at least 5 laboratory visits. Participants first attended an orientation session that included reviewing the IRB form, obtaining consent, and completing cognitive screening, health history, and self-report questionnaires, as well as a mock MRI in a simulator. The second visit included the maximal exercise test. The third and fourth visits consisted of extensive cognitive testing, with each visit lasting about 2 hours. The final visit was the MRI session, which included structural and functional scans. A subset of participants was from the pre-intervention sessions of an exercise intervention (NCT02453178).
Cardiorespiratory Fitness Testing
Maximal oxygen uptake was measured with indirect calorimetry using a maximal exercise test on a cycle ergometer with resistance increasing in two-minute intervals. Oxygen consumption was calculated from expired air samples at 15-s intervals until peak VO2 was reached. VO2max was determined and test terminated when a) respiratory exchange rate (RER) exceeded 1.10, b) participant reached 90% of age-predicted heart rate maximum, or c) heart rate and/or oxygen uptake plateaued despite an increase in resistance level. This test was also terminated if the participant showed signs of distress or if physiological signals became abnormal (blood pressure, heart rate, EKG). VO2max is the gold standard for measuring CRF. Because participants were over the age of 40, a physician was present during the testing. In all analyses we use relative VO2max (mL/kg/min) to adjust for weight.
Cognitive testing
Multiple tasks were administered to measure relational learning and memory (EAL), motor sequence learning (ASRT), working memory (Face N-back), and processing speed (Pattern and Letter Comparison).
Episodic Associative Learning.
This task has previously been described fully in Clark et al. (2018) (Experiment 2). Briefly, participants learned to respond to face pairs with either bimanual, unimanual, or no key press (Figure 1A). The correct keypress was unique for each face pair. Importantly, no single face provided information about the correct keypress.
To familiarize participants with the task, a brief practice phase (five minutes) occurred immediately before the learning phase with practice stimuli (animals and modes of transportation) and a slower pace of trials. In each trial, participants were shown a stimulus pair in the center of the screen and were to respond with their middle and index fingers on keys W, E, I, O, respectively. For each trial, if the participant responded correctly, the next trial began following a fixation screen with a centered fixation cross. If the participant responded incorrectly, however, the pair was presented again with the correct mapping shown directly below the stimuli (Figure 1B). Practice was followed by a learning phase including 14 blocks of face pairs. Each of the nine pairs appeared five times in each block in a randomized order. Face stimuli were chosen from young and older adult male neutral faces in the Center for Vital Longevity Face Database. For each participant, either young or older faces appeared on the left and the other age category of faces appeared on the right. Category (young and old faces) and position (left and right) was counterbalanced between participants. Between each block, participants received feedback regarding accuracy and speed of response of each hand. The next block began when the participant decided to continue. The task lasted for approximately 50 minutes.
EAL Analysis.
Raw accuracy data was fit to a linear mixed effects model using R (Figure 1C and 1D). Mixed effects modeling was selected over repeated-measures ANOVA to more accurately model individual differences in learning rate. Specifically, we modeled a linear slope parameter to index learning rate across blocks and a quadratic parameter to index change in learning rate. The model was fit using R’s linear mixed-effects (lme4) package (Pinheiro & Bates, 2000), which simultaneously estimates all fixed and random effects using maximum likelihood estimate. We began with the most complex model for each analysis and compared simpler models using model-comparison procedures based on the Bayesian Information Criterion (Schwarz, 1978). The primary variable of interest in our analyses was learning rate based on the linear slope parameter for each individual, which represents the speed at which the individual acquired the correct mappings for the face pairs.
ASRT.
In the ASRT, four open circles were in the center of the computer screen. On each trial, one of the four circles became filled in, and the participant was instructed to press the corresponding key (using their left and right middle and index fingers) as quickly and accurately as possible. The circle remained filled until the participant pressed the correct key, which immediately initiated the next trial in which a different circle was filled in. On alternating trials, the circles followed a specific sequence (sequences were counterbalanced across participants), while on the other trials, they were randomly ordered, with the constraint that no trial could repeat the circle from the previous trial. Participants completed 32 blocks with 90 trials each, for a total of 3,072 trials. Blocks were separated by a mandatory 30-second break. A subset of participants (36 of 45) completed this task.
ASRT Analysis.
Inaccurate trials were discarded from analysis. The difference score for each block was calculated as the difference between average RT to sequence trials and random trials, with higher difference scores representing faster responses to sequence items. For the main dependent variable, we averaged the difference scores for the blocks in the last quarter of the task (blocks 25–32), where performance seemed to plateau. In the interest of also evaluating early learning (which may be more dependent on MTL (Howard & Howard, 2013)), we averaged difference scores for the first quarter of trials (blocks 1–8).
Face N-back.
The face N-back task was administered in the scanner and consisted of participants viewing a continuous stream of neutral faces. For each face, participants were asked to determine whether each face matches the face presented n items before. This task included 1-back and 2-back conditions, with the 2-back being most demanding on working memory. Data was collected for the Face N-back task for 42 of the 45 participants. The data for one subject experienced a technical issue, and 2 subjects had discomfort because of large head size, thus the N-back task was skipped to accommodate modified scan time.
Face N-back Analysis.
Reaction time and accuracy are the primary performance outcomes. The average difference in accuracy between the 1-back and 2-back blocks represents a working memory cost. Accuracy on the 1-back blocks may vary based on non-working memory aspects such as basic facial discrimination and attention, so using accuracy on the 1-back blocks as a reference for our working memory cost allows us to more precisely evaluate differences in working memory between individuals. Since participants were encouraged to respond accurately and reaction time was less emphasized, we use accuracy cost as the main dependent variable.
Processing Speed.
Participants completed a paper-and-pencil pattern and letter comparison task (Salthouse, 1996). The pattern comparison section contained two trials, each consisting of 30 pairs of line drawings (“patterns”). The participant compared the patterns and wrote either “S” (same) or “D” (different) on a line between the items for as many items as possible within 30 seconds. The letter comparison section consisted of two trials, each consisting of one page with 15 pairs of letter strings. Again, the participant was to write S or D on a line between the letter strings for as many items as possible within 30 seconds.
Processing Speed Analysis.
We calculated the average number of correct pattern or letter string pairs completed between the two trials of each, normalized across participants within the pattern and letter sections, and then averaged the two z-score values to get a processing speed score for each person.
Magnetic Resonance Imaging
MRI Protocol.
All magnetic resonance imaging (MRI) was conducted at the Magnetic Resonance Research Facility (MRRF) at University of Iowa. Toward the beginning of data collection, in June 2016, MRRF retired a Siemens scanner and acquired a new GE scanner. Thus, MRI data were acquired with either a 3.0T MRI Siemens TIM Trio scanner using a 12-channel head coil (N = 20), or 3.0T General Electric (GE) Discovery MR750w MRI Scanner using a 32-channel head coil (N = 25). For the scans collected on the Siemens scanner, a three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) T1 scan was collected with the following parameters: echo time (TE)=3.09ms, repetition time (TR)=2530ms, inversion time (TI)=900ms, flip angle=10°, Acquisition Matrix=256 × 256×240mm, Bandwidth=219 Hz/pixel, voxel dimensions=1.00 × 1.00 × 1.00, number of slices=240. For the scans collected on the GE scanner, a three-dimensional fast spoiled gradient echo sequence (FSPGR) T1 scan was collected with the following parameters: TI=450ms, TE=3.376, TR=8.588ms, flip angle=12°, Acquisition Matrix=256×256×240, FOV=256×256×240, voxel dimensions=1.00 × 1.00 × 1.00, number of slices=240.
Analysis.
Subcortical volume estimates were calculated using Freesurfer’s automated subcortical segmentation tool (Fischl et al., 2002; Fischl et al., 2004). Subcortical volumes were adjusted based on intracranial volume (ICV) to account for individual differences in body size and gender (Raz et al., 2005). Adjustment was performed separately for each region for each hemisphere using the formula based on the analysis of covariance approach: adjusted volume = raw volume – b(ICV – mean ICV), where b is the coefficient of regression of the region volume on ICV, and ICV is the participant’s total ICV estimated via Freesurfer. For each region, the adjusted left and right hemisphere values were summed to provide an average bilateral volume. Separate hemisphere volumes and bilateral volumes for regions of interest (hippocampus, caudate, putamen) were used in the analyses.
General Analysis
Pearson correlations were computed to test relationships between our primary variables of interest. Multiple regression models were utilized to further account for covariates. For all analyses, we consider p < .05 to be statistically significant, and we compare effect sizes to previous findings. In cases where .05 < p <.10, the result is considered insignificant, though may be worthy of further investigation in more highly powered studies (Gibbs & Gibbs, 2015).
RESULTS
See Table 1 for participant demographics and Table 2 for descriptive statistics for variables of interest. Table 3 additionally reports Pearson correlation tests between covariates and additional variables of interest that will not be reported further in the results.
Table 2.
Descriptive statistics for variables of interest
| CRF Mean (SD) | Hippocampal volume Mean (SD) | Caudate volume Mean (SD) | Putamen Volume Mean (SD) | EAL Learning Rate Mean (SD) | |
|---|---|---|---|---|---|
| Total | 24.01 ml/kg/min (7.69) | 7,779.04 mm3 (666.17) | 7,090.30 mm3 (721.28) | 9,260.91 mm3 (889.64) | .02 proportion per block (.02) |
| Male | 26.73 (7.76) | 7,816.65 (665.00) | 6976.69 (683.51) | 9425.04 (853.90) | .02 (.02) |
| Female | 21.29 (7.06) | 7,758.29 (677.65) | 7152.98 (745.53) | 9170.35 (910.62) | .02 (.01) |
Table 3.
Correlations between possible covariates and additional variables of interest
| CAUDATE VOLUME | PUTAMEN VOLUME | ASRT | PROCESSING SPEED | WORKING MEMORY (NBACK ACC COST) | |
|---|---|---|---|---|---|
| AGE | r = .18 | r = .03 | r = −.16 | r = −.05 | r = .12 |
| p = .22 | p = .83 | p = .34 | p = .72 | p = .46 | |
| SEX (POINT-BISERIAL CORRELATIONS) | r = .12 | r = −.14 | r = −.02 | r = −.31 | r = −.18 |
| p = .44 | p = .36 | p = .88 | p = .04* | p = .28 | |
| YEARS EDUCATION | r = .08 | r = .06 | r = .30 | r = .15 | r = −.11 |
| p = .57 | p = .71 | p = .08 | p = .33 | p = .50 |
Statistical significance at p<.05
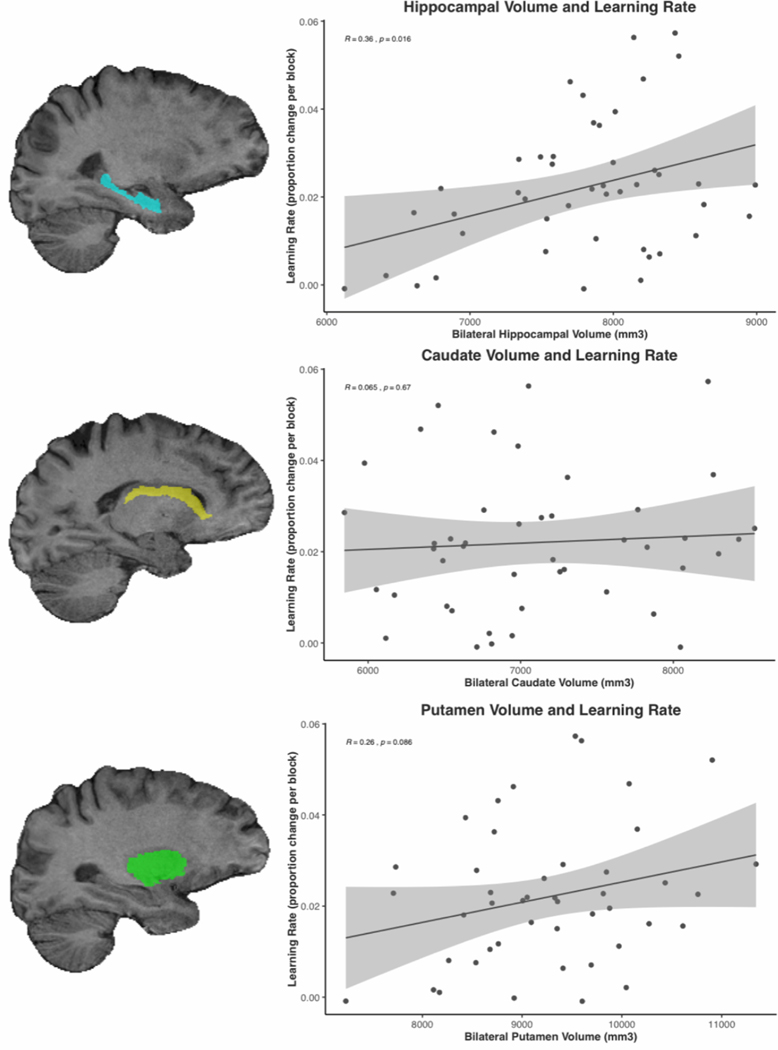
Hippocampal volume is associated with faster learning on EAL task.
We first tested whether hippocampal volume predicts the rate at which individuals learned the face pairs and their associated responses. Consistent with predictions, individuals with larger hippocampal volume more quickly acquired the correct responses (r = .36, p = .02) (Figure 2). This was observed for both hemispheres (right: r = .35, p = .02; left: r = .35, p = .02). Neither age, sex, nor years of education were related to hippocampal volume or EAL rate, so no covariates were included in this analysis.
Figure 2:
Example subject-specific masks for right hippocampus, right caudate, right putamen from coronal slices. Scatterplots showing relationships between bilateral hippocampal volume, bilateral caudate volume, bilateral putamen volume, and EAL learning rate.
Unlike hippocampal volume, volume of neither the cauduate (r = .06, p = .67) nor putamen (r = .26, p = .09) significantly correlated with EAL rate (see Figure 2). For both structures, the relationship of bilateral volume with learning rate did not differ from the relationship of left and right hemisphere volume with learning rate (right caudate: r = .02, p = .91; left caudate: r = .10, p = .49; right putamen: r = .25, p = .10; left putamen: r = .26 p = .09). These findings support that EAL rate is uniquely related to hippocampal volume rather than being related to other subcortical regions involved in motor learning. The results suggest EAL rate involves processes that extend beyond statistical regularities over time.
To evaluate the relationship between different types of learning, we also examined learning rate on the ASRT. Participants demonstrated sequence learning as, on average, responses became faster over time to the sequenced relative to random items (comparison of average reaction times for sequence vs random trials for the last 8 blocks of task, t(36) = 3.1, p = .002). To support their distinction, EAL rate was not correlated with ASRT learning (r = .11, p = .51). Further, ASRT learning was not correlated with hippocampal volume (bilteral: r = .002, p = .1; right: r = .02, p = .90; left: r = −.01, p = .90). Surprisingly, though, ASRT learning was also not correlated with caudate (bilateral: r = −.28, p = .10; right: r = −.26, p = .12; left: r = −.27, p = .11), or putamen (bilateral: r = −.09, p = .60; right: r = −.14, p = .42; left: −.03, p = .84) volume. As exploratory analyses, we also evaluated early learning on the ASRT. Performance early in the task (calculated as average difference in RT to random and sequence trials across the first 8 blocks) was not correlated with bilateral hippocampal volume (r = .15, p = .39), bilateral caudate volume (r = .01, p = .93), bilateral putamen volume (r = −.08, r = .64), or with EAL slope (r = −.26, p = .12). It should be noted, though, that this stage of the task did not show evidence of learning, as reaction times were not significantly different between sequence and random trials for any of the first 8 blocks (block 8: t = −1.5, p = .93). Overall, results provide additional evidence that EAL performance is uniquely tied more to hippocampal than striatal integrity.
To evaluate potential contributions of processing speed and working memory, we tested whether the average processing speed measure from the Pattern and Letter Comparison tasks and the cost measure from the Face N-back task were related to EAL rate and hippocampal volume. We found that EAL rate was not related to average processing speed (r = .23, p = .13), but was related to working memory cost (r = −.34, p = .03), such that individuals who learned faster on the EAL also had less cost on the face N-back task. However, bilateral hippocampal volume was not correlated with processing speed (r = .08, p = .60), or with working memory cost (r = .11, p = .51). Further, the relationship between hippocampal volume and EAL rate remained significant when including processing speed (β(42) = .34, (SE = .14), t = 2.4, p = .02) and working memory cost (β(36) = .36, (SE = .15), t = 2.5, p = .02) as covariates in a regression model. Thus, neither processing speed nor working memory accounted for the relationship between hippocampal volume and EAL rate.
Cardiorespiratory fitness positively predicts learning rate on EAL task.
Next, we tested whether CRF predicted EAL rate. Although CRF was related to sex (point-biserial correlation: r = −.35, p = .02), since learning rate was not related to sex (r = −.03, p = .85), we did not include sex as a covariate. Similarly, although age was related to CRF (r = −.37, p = .01), age was not related to learning rate (r = −.20, p = .20), so age was not included as a covariate. Lastly, years of education was related to CRF (r = .34, p = .02), but not to learning rate (r = .16, p = .29). Thus, no covariates were used in this analysis. As predicted, individuals with higher CRF had a faster EAL rate (r = .45, p = .002). CRF also had a positive but non-statistically significant relationship with processing speed (r = .28, p = .06). However, since processing speed was not related to learning rate on the EAL task, processing speed could not account for the relationship between CRF and EAL rate. Additionally, CRF was not related to working memory cost (r = −.03, p = .88), which also discounts the possibility that working memory is a confound in the association between CRF and EAL rate.
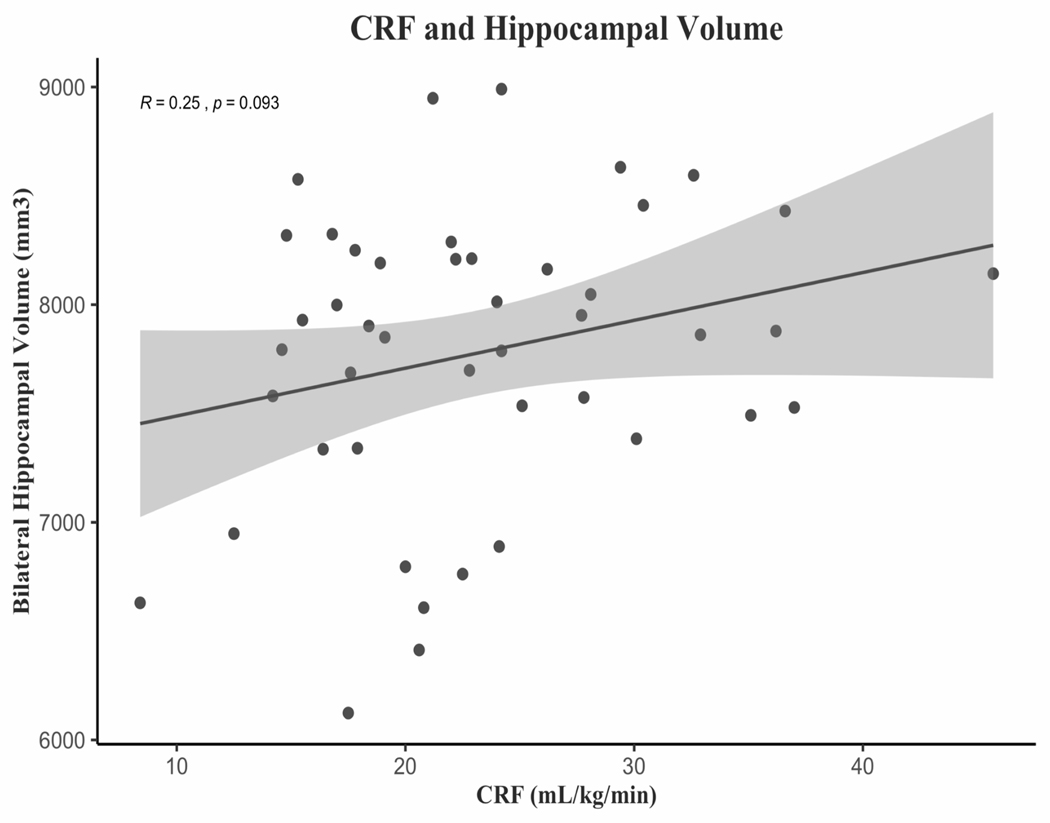
Relation of cardiorespiratory fitness with hippocampal volume.
CRF was positively related to hippocampal volume, but surprisingly, this relationship was not significant (r = .25, p = .09, Figure 3). Though the relationship was not statistically significant, the effect size of r = .25 is similar to what has been found in different samples of older adults (Erickson et al., 2009). This relationship was slightly stronger for the left hippocampus than the right, though neither were significant (left: r = .28, p = .07; right: r = .21, p = .15). As comparison, the correlations of CRF with bilateral caudate volume (r = .03, p = .8) and bilateral putamen volume (r = .01, p = .96) were both non-significant with near zero effect sizes.
Figure 3:
Relationship between CRF and bilateral hippocampal volume
Hippocampal volume does not mediate the relationship between CRF and EAL rate.
Because CRF positively predicted learning rate and hippocampal volume was positively associated with EAL rate, we ran a mediation model to test whether hippocampal volume acts as a mediator in this relationship. We do acknowledge, however, that since CRF was not statistically predictive of hippocampal volume, it is unlikely hippocampal volume would be a strong mediator. Based on the lack of contribution of age, sex, and education to the relationships between variables of interest, no covariates were included in the mediation model. The steps outlined by Baron and Kenny (1986) were completed in R using the mediate package.
Results indicated hippocampal volume does not fully mediate the relationship between CRF and learning rate. The first step of setting up the mediation is to test for the direct effect of the independent variable on the dependent variable. As previously shown, the total effect of CRF on learning rate was significant (ß(43) = .45, (SE = .14), t = 3.3, p = .002). The direct effect of CRF on EAL rate after taking hippocampal volume into account as a mediator was reduced but was still significant (ß(42) = .39, (SE = .14), t = 2.8, p =.007). By itself, CRF explained 18% of the variance in EAL rate (adjusted R-squared: 0.18). When adding hippocampal volume into the model, the model explained 23% of the variance (adjusted R-squared = 0.23). This can indicate some degree of mediation, but the final and critical step of mediation is to determine whether the indirect effect (i.e., the amount that hippocampal volume mediates the relationship) is significant. We tested the indirect effect using a bootstrap estimation approach with 5000 simulations, which revealed that the indirect effect was nearly, but not statistically, significant (b = .07, 95% CI [−.002, .18], p = .06). This finding reveals that hippocampal volume accounts for some variability but does not significantly mediate the relationship between CRF and learning rate in our sample.
DISCUSSION
In the current study, we found CRF positively predicted learning rate on the EAL task, which we designed to heavily draw on hippocampal processes that decline with age. We found a specific relationship of EAL performance with hippocampal volume, such that learning rate was related to hippocampal volume but not volume of other subcortical structures involved in motor learning (e.g., caudate and putamen). Critically, hippocampal volume was related to learning rate on the EAL task, but not to CRF, and hippocampal volume did not mediate the relationship between CRF and learning rate. These findings provide evidence that CRF, which has been shown in previous studies to be a modifiable physiological characteristic associated with hippocampal structure and function, is related to better episodic memory acquisition. Future research will be needed to further delineate structural and functional mechanisms beyond hippocampal volume.
Our finding of a relationship between CRF and episodic learning rate aligns with previous findings of positive relationships between CRF and cognitive outcomes (Erickson et al., 2009; Hayes et al., 2016; Hayes et al., 2013; Hayes et al., 2017; Szabo et al., 2011), and is supported by a large body of work in animals and humans that has shown beneficial effects of PA on the brain, specifically the hippocampus, and hippocampal-dependent learning and pattern separation (Creer, Romberg, Saksida, van Praag, & Bussey, 2010; Hayes et al., 2015; Suwabe et al., 2017; van Praag, Shubert, Zhao, & Gage, 2005). Previous studies have also demonstrated a relationship between CRF and hippocampal volume (for review see Voss et al., 2019). Thus, it is surprising CRF was not related to hippocampal volume in our sample. It is worth noting, though, that our sample size (45 participants) was smaller than other studies that have focused on detecting a relationship between CRF and hippocampal volume (e.g., Erickson et al., 2009 included N = 165). Another potential difference is that we utilized a stationary bicycle ergometer for our graded maximal exercise test, whereas Erickson and colleagues (2009) used a treadmill. Bicycle ergometers were specifically chosen for our population of older adults for safety and comfort, and because the associated intervention trial was a cycling intervention. There is evidence that CRF derived from treadmill versus cycling ergometer differs systematically, with the treadmill producing higher CRF values (Buchfuhrer et al., 1983). Although systematic scaling should not affect a correlation, the effects of maximal exercise test modality on brain and cognitive outcomes is unknown and deserves future study for verification.
Critically, previous work linking CRF to hippocampal volume and cognition in older adults has not examined performance on tasks that require acquisition of object-based relations over time, a process that may be more sensitive to subtle aging processes in the hippocampus (Reagh et al., 2016). The EAL task uniquely addresses this gap by providing a measure of acquisition rate across repeated co-occurrences of paired elements. We have previously shown robust age differences in performance on this task (Clark et al., 2018), suggesting it is sensitive to processes changing during cognitively normal aging. The task draws on relational binding and mnemonic discrimination for the acquisition of overlapping but distinct relations over repeated co-occurrences. To target mnemonic discrimination processes, the relations used in the EAL task have overlapping elements across pairs but also require distinct representations in order to learn the unique responses. For these reasons, the EAL task is a useful tool for examining subtle age-related differences, and the current study revealed a novel relationship between CRF and learning rate. This study also provides validation that the EAL task is a useful tool for targeting associative bindings (see Supplementary materials), and the addition of multiple data points throughout the task allows researchers to model acquisition curves and more thoroughly probe binding processes over repeated occurrences of item pairs.
We also found that learning on the EAL task was distinct from other aspects of cognition. EAL rate was not related to sequence learning, which has been shown to be less affected by age than the learning processes involved in the EAL task. Sequence learning was also not related to hippocampal volume. We further tested whether working memory and processing speed could account for the relationships between CRF, hippocampal volume, and learning rate. As working memory and processing speed are known to decline with age, it is possible that these age-related changes could influence performance on the EAL task. In our previous study (Clark et al., 2018), we found that individual differences in processing speed did not account for differences in EAL rate. We did not, however, measure working memory in the previous study. In the current study, the relationship between CRF and processing speed was not significant, but was close to our cut-off of p = .05 (CRF and processing speed was p = .06). However, because processing speed was not related to EAL rate, nor was it related to hippocampal volume, it is unlikely that processing speed accounts for the relationship between hippocampal volume and EAL rate. We also found that although working memory was related to EAL rate, it was not related to hippocampal volume or CRF. Thus, the results suggest working memory cannot account for the relationships between CRF, hippocampal volume, and EAL rate. This finding of specificity is consistent with, and extends upon, our previous findings by showing that learning rate on the EAL task is related to hippocampal volume independent of processing speed, higher-level motor learning, and working memory with faces.
The EAL paradigm involves learning unique responses to overlapping pairs of stimuli, thus relying on hippocampal circuits to create and continuously strengthen distinct bindings. The multiple, interleaved presentations of each pair require rapid binding, and continuous maintenance, updating, and integration of relations, processes supported by the hippocampus (Henke et al., 1997; Ranganath & D’Esposito, 2001). Koster and colleagues (2018) recently provided evidence of a big-loop recurrence process within the hippocampus that allows it to both store representations of distinct episodes and integrate information across related episodes, which together would support richly constructive yet precise episodic memories. Koster et al. (2018) found that big-loop recurrence could account for successful inference, which consists of binding information that does not exist simultaneously but does have common relations across episodes. While our task does not rely on inference in this same sense, a rapid EAL rate requires managing overlapping yet distinct relations across trials. Future research could use the EAL task and ultra-high-resolution imaging to test whether the activity of hippocampal circuits during rapid learning is similar to the recirculation of hippocampal inputs that supports inference.
Hippocampal volume was used in the current study as a biomarker of hippocampal integrity. As such, measuring hippocampal volume has many strengths. Automated segmentation of the hippocampus is quite robust (Fischl et al., 2002), has been widely used, and does not require manual corrections or decisions. Hippocampal volume has also been shown to be a sensitive biomarker to other health factors (Erickson et al., 2011; Kleemeyer et al., 2016; for review see Ott, Johnson, Macoveanu, & Miskowiak, 2019). Nevertheless, volumetric measurements do involve some limitations. As the hippocampus is far from a homogenous region, structurally or functionally, examination of bilateral hippocampal volume as measured from the entire hippocampus may be too broad. Findings within the last few decades have supported the differential roles of distinct regions within the human hippocampus (for review see Poppenk, Evensmoen, Moscovitch, & Nadel, 2013), specifically specialization along the longitudinal-axis. The hippocampus can be generally divided into an anterior (ventral in rodents) and posterior (dorsal in rodents) portion. Of relevance for the current study, in humans, the anterior hippocampus has been found to be involved in encoding of novel stimuli, whereas the posterior region is known for its involvement in spatial processing (Poppenk et al., 2013; Ryan, Lin, Ketcham, & Nadel, 2010; Woollett & Maguire, 2011). Regarding PA and CRF in relation to anterior and posterior sections of the hippocampus, the results are mixed, but generally favor the anterior hippocampus. Low-intensity walking has been found to be more strongly correlated with anterior compared to posterior hippocampus (Varma, Tang, & Carlson, 2016), and a 1-year aerobic exercise intervention selectively increased the volume of the anterior, but not the posterior, hippocampus (Erickson et al., 2011). In the same study by Erickson et al. (2011), change in CRF was related to increases in both anterior and posterior hippocampus. Both Maass, Düzel, Goerke, Becke, Sobieray, Neumann, Lövdén, et al. (2015) and Thomas et al. (2016) found a specific relation between increase in CRF and increase in anterior hippocampal volume, suggesting greater sensitivity to CRF in the anterior hippocampus. However, sub-regional measurements do have methodological challenges. As automation is not as robust for sub-regions in 3T, decisions must be made as to how to segment anterior and posterior for each specific sample, and segmentation may then require significant manual input.
In addition to the possibility that more specific hippocampal volumetric measures may reveal stronger relationships with CRF and learning than the volume of the entire hippocampus, there is also evidence that other measures of hippocampal structure and function may serve as important contributors to the CRF-cognition relationship. For instance, Schwarb and colleagues (2017) found hippocampal viscoelasticity mediated the relationship between CRF and relational memory performance in young adults. Viscoelasticity measures microstructural integrity of the hippocampus, which may be more sensitive to individual differences than volume measurements. Further, tissue density, measured via diffusivity, has also been found to be sensitive to changes in CRF in older adults (Kleemeyer et al., 2016). Importantly, changes in density were related to changes in hippocampal volume, which suggests both measures are sensitive to CRF changes. For our purposes of extending from other studies examining hippocampal volume and understanding the role of the hippocampus in the relationship between CRF and EAL rate, volume measurements of the whole hippocampus were sufficient. Our findings do support future research extending into sub-regions or MTL systems as well as research that evaluates other measures of hippocampal microstructure, such as viscoelasticity and diffusivity.
Another limitation of this study is the cross-sectional nature. The strongest evidence for a relationship between CRF, hippocampal volume, and memory would come from a randomized controlled trial involving a structured exercise intervention known to increase CRF. Though multiple cross-sectional studies have shown evidence of a relationship between aerobic exercise and cognition (for review see Kramer & Colcombe, 2018; Voss et al., 2019), none have utilized a task like the EAL, which specifically targets aspects of hippocampal function that robustly decline with aging and are important for episodic memory acquisition. The cross-sectional design allowed us to establish a relationship between CRF and EAL rate. Based on this, current results support using the EAL task as an outcome in intervention studies to evaluate effects of aerobic PA and changes in CRF on EAL rate.
Importantly, while CRF is determined in part by genetics, it is also influenced by frequency and intensity of PA. Thus, if CRF is modifiable, which can in turn influence the severity of age-related effects on the brain, individuals may be able to influence their personal trajectory by increasing PA, which would increase the possibility of preventing age-related decline. As PA remains difficult to objectively quantify, CRF serves as an important marker for physiological processes that may be induced by PA. Further, CRF has been shown to have a positive relationship with brain function independent of PA. Specifically, CRF is related to higher functional connectivity in networks that are diminished by age (Voss et al., 2016). Mechanistically, research supports that the health benefits of PA come from a variety of pathways, including increases in heart rate and repetitive muscle contraction and usage, all of which trigger a beneficial slew of neurotrophic pathways, decreased inflammation, improved body composition, and improved metabolic processes (for review see Warburton, Nicol, & Bredin, 2006). These pathways may also be systemically affected by CRF, such that individuals with higher CRF have elevated baseline activity of certain pathways and processes. It is also possible that differing levels of CRF, or other physiological characteristics, influence the extent to which PA acutely and chronically impacts the body and brain.
In sum, our findings support a relationship between CRF, hippocampal volume, and learning rate on a task that was specifically designed to engage hippocampal processes that decline with aging. We have shown that higher CRF predicts faster associative learning and that hippocampal volume, while not related to CRF in our sample, was related to faster associative learning. The EAL task can be used in the future as a rich source of data that represents the acquisition speed of associative bindings, which provides insight about the integrity and function of hippocampal circuitry. The current study moves this field forward by combining strong measures of CRF and brain structure with a novel task that is sensitive to age and targets hippocampal-based learning.
Supplementary Material
Acknowledgments
Grant sponsor: NIH/NIA
Grant number: 5R21AG048170
REFERENCES
- Ash JA, Lu H, Taxier LR, Long JM, Yang Y, Stein EA, & Rapp PR (2016). Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats. Proceedings of the National Academy of Sciences, 113(43), 12286–12291. doi: 10.1073/pnas.1525309113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA (1979). Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. Journal of Comparative and Physiological Psychology, 93(1), 74–104. doi: 10.1037/h0077579 [DOI] [PubMed] [Google Scholar]
- Baron R, & Kenny D. (1986). The moderator-mediator variable distinction in social psychological research. Journal of personality and social psychology, 51(6), 1173–1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, & Whipp BJ (1983). Optimizing the exercise protocol for cardiopulmonary assessment. Journal of Applied Physiology, 55(5), 1558–1564. doi: 10.1152/jappl.1983.55.5.1558 [DOI] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, & Barnes CA (2011). Age-associated deficits in pattern separation functions of the perirhinal cortex: A cross-species consensus. Behavioral neuroscience, 125(6), 836–847. doi: 10.1037/a0026238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Hazeltine E, Freedberg M, & Voss MW (2018). Age differences in episodic associative learning. Psychology and Aging, 33(1), 144–157. doi: 10.1037/pag0000234 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, & Kramer AF (2003). Fitness Effects on the Cognitive Function of Older Adults. Psychological Science, 14(2), 125–130. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, . . . Elavsky S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, 101(9), 3316–3321. doi: 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM (1994). Memory Changes in Normal Aging. Current Directions in Psychological Science, 3(5), 155–158. doi: 10.1111/1467-8721.ep10770653 [DOI] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, & Bussey TJ (2010). Running enhances spatial pattern separation in mice. Pnas, 107(5), 2367–2372. doi: 10.1073/pnas.0911725107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, & Sutherland RJ (2003). The Aging Hippocampus: Cognitive, Biochemical and Structural Findings. Cerebral Cortex, 13(12), 1344–1351. doi: 10.1093/cercor/bhg081 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, . . . Kramer (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus, 19(10), 1030–1039. doi: 10.1002/hipo.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, . . . Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 3017–3022. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchamendy N, Konishi K, Pike GB, Marighetto A, & Bohbot VD (2012). Evidence for a virtual human analog of a rodent relational memory task: a study of aging and fMRI in young adults. Hippocampus, 22(4), 869–880. doi: 10.1002/hipo.20948 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert MS, Dieterich M, Haselgrove C, . . . Dale AM (2002). Whole Brain Segmentation: Neurotechnique Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron, 33(3), 341–355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, . . . Dale AM (2004). Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex, 14(1), 11–22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, & Wilson IA (2006). Individual differences in neurocognitive aging of the medial temporal lobe. Age, 28(3), 221–233. doi: 10.1007/s11357-006-9017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, & Koh MT (2011). Episodic memory on the path to Alzheimer’s disease. Current Opinion in Neurobiology, 21(6), 929–934. doi: 10.1016/j.conb.2011.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, & Heller RE (1995). Hippocampal markers of age-related memory dysfunction: Behavioral, electrophysiological and morphological perspectives. Progress in Neurobiology, 45(3). [DOI] [PubMed] [Google Scholar]
- Gibbs NM, & Gibbs SV (2015). Misuse of ‘ trend ‘ to describe ‘ almost significant ‘ differences in anaesthesia research. 115(May), 337–339. doi: 10.1093/bja/aev149 [DOI] [PubMed] [Google Scholar]
- Gorbach T, Pudas S, Lundquist A, Orädd G, Josefsson M, Salami A, . . . Nyberg L. (2017). Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiology of Aging, 51, 167–176. doi: 10.1016/j.neurobiolaging.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML, Hayes JP, Cadden M, Peterson KM, Allsup K, . . . Verfaellie M. (2015). Physical Activity Is Positively Associated with Episodic Memory in Aging. Journal of the International Neuropsychological Society, 21(10), 780–790. doi: 10.1017/S1355617715000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Forman DE, & Verfaellie M. (2016). Cardiorespiratory Fitness is Associated with Cognitive Performance in Older but Not Younger Adults. Journals of Gerontology - Series B Psychological Sciences and Social Sciences, 71(3), 474–482. doi: 10.1093/geronb/gbu167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, & Verfaellie M. (2013). A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Frontiers in Aging Neuroscience, 5(JUL), 1–16. doi: 10.3389/fnagi.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Williams VJ, Liu H, & Verfaellie M. (2017). FMRI activity during associative encoding is correlated with cardiorespiratory fitness and source memory performance in older adults. Cortex, 91, 208–220. doi: 10.1016/j.cortex.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, & Isom M. (2010). Age effects on wayfinding and route learning skills. Behavioural Brain Research, 209(1), 49–58. doi: 10.1016/j.bbr.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, & Buckner RL (2014). Multiple Brain Markers are Linked to Age-Related Variation in Cognition. Cerebral Cortex, 1–13. doi: 10.1093/cercor/bhu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, & Wieser HG (1997). Human hippocampus establishes associations in memory. Hippocampus, 7(3), 249–256. doi: [DOI] [PubMed] [Google Scholar]
- Howard JH, & Howard DV (1997). Age differences in implicit learning of higher order dependencies in serial patterns. Psychology and Aging, 12(4), 634–656. [DOI] [PubMed] [Google Scholar]
- Howard JH, & Howard DV (2013). Aging mind and brain: Is implicit learning spared in healthy aging? Frontiers in Psychology, 4(November), 1–6. doi: 10.3389/fpsyg.2013.00817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, . . . Trojanowski JQ (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12(2), 207–216. doi: 10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, . . . Kokmen E. (1998). Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology, 51(4), 993–999. doi: 10.1212/WNL.51.4.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, . . . Alexander GE (2006). Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiology of Aging, 27(11), 16041612. doi: 10.1016/j.neurobiolaging.2005.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemeyer MM, Kühn S, Prindle J, Bodammer NC, Brechtel L, Garthe A, . . . Lindenberger U. (2016). Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. NeuroImage, 131, 155–161. doi: 10.1016/j.neuroimage.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Konishi K, & Bohbot VD (2013). Spatial navigational strategies correlate with gray matter in the hippocampus of healthy older adults tested in a virtual maze. Frontiers in Aging Neuroscience, 5(February), 1–8. doi: 10.3389/fnagi.2013.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, & Cohen NJ (2009). Relational memory and the hippocampus: representations and methods. Frontiers in neuroscience, 3(2), 166–174. doi: 10.3389/neuro.01.023.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R, Chadwick MJ, Chen Y, Berron D, Banino A, Düzel E, . . . Kumaran D. (2018). Big-Loop Recurrence within the Hippocampal System Supports Integration of Information across Episodes. Neuron, 99(6), 1342–1354.e1346. doi: 10.1016/j.neuron.2018.08.009 [DOI] [PubMed] [Google Scholar]
- Kramer AF, & Colcombe SJ (2018). Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study - Revisited. Psychological Science, 13(2), 213–217. doi: 10.1177/1745691617707316 [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, . . . Chui HC (2007). Longitudinal MRI and Cognitive Change in Healthy Elderly. Neuropsychology, 21(4), 412–418. doi: 10.1037/0894-4105.21.4.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2015). Neurocognitive Aging and the Hippocampus across Species. Trends in Neurosciences, 38(12), 800–812. doi: 10.1016/j.tins.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, & Yassa MA (2018). Integrating new findings and examining clinical applications of pattern separation. Nature Neuroscience, 21(2), 163–173. doi: 10.1038/s41593-0170065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, . . . Düzel E. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585–593. doi: 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, . . . Düzel E. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry(August), 1–9. doi: 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Monti JM, Cooke GE, Watson PD, Voss MW, Kramer AF, & Cohen NJ (2015). Relating Hippocampus to Relational Memory Processing across Domains and Delays. Journal of Cognitive Neuroscience, 27(2), 1–10. doi: 10.1162/jocn_a_00717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, . . . Chui HC (2005). Longitudinal volumetric MRI change and rate of cognitive decline. Neurology, 65(4), 565–571. doi: 10.1212/01.wnl.0000172913.88973.0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. (2000). Adult age differences in memory performance: tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. doi: 10.1037/0278-7393.26.5.1170 [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, & Bar-On M. (2003). Adult age differences in episodic memory: further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(5), 826–837. doi: 10.1037/0278-7393.29.5.826 [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, & Bäckman L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. doi: 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Ott CV, Johnson CB, Macoveanu J, & Miskowiak K. (2019). Structural changes in the hippocampus as a biomarker for cognitive improvements in neuropsychiatric disorders: A systematic review. European Neuropsychopharmacology, 1–11. doi: 10.1016/j.euroneuro.2019.01.105 [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, . . . Small (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5638–5643. doi: 10.1073/pnas.0611721104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, & Bates DM (2000). Linear mixed-effects models: basic concepts and examples. Mixed-Effects Models in S and S-PLUS, 3–56. doi: 10.1007/978-1-4419-03181_1 [DOI] [Google Scholar]
- Poldrack RA, & Packard MG (2003). Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia.Special Issue: Functional neuroimaging of memory, 41, 245–251. doi: 10.1016/S00283932(02)00157-4 [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L. (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. doi: 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Ranganath C, & D’Esposito M. (2001). Medial temporal lobe activity associated with active maintenance of novel information. Neuron, 31(5), 865–873. doi: 10.1016/S08966273(01)00411-1 [DOI] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1992). Individual differences in the cognitive and neurobiological consequences of normal aging. Trends in Neurosciences, 15(9), 340–345. doi: 10.1016/0166-2236(92)90051-9 [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, . . . Acker JD (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, & Acker JD (2004). Differential aging of the medial temporal lobe: a study of a five-year change. Neurology, 62(3), 433–438. doi: 10.1212/01.WNL.0000106466.09835.46 [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, & Acker JD (2003). Differential aging of the human striatum: longitudinal evidence. AJNR. American journal of neuroradiology, 24(9), 1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, & Yassa MA (2016). Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus, 26(4), 417–422. doi: 10.1002/hipo.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, & Eichenbaum H. (2008). Cognitive Aging: A Common Decline of Episodic Recollection and Spatial Memory in Rats. Journal of Neuroscience, 28(36), 8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Lin C, Ketcham K, & Nadel L. (2010). The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus, 20(1), 11–18. doi: 10.1002/hipo.20607 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1994). The aging of working memory. Neuropsychology, 8(4), 535–543. doi: 10.1037/0894-4105.8.4.535 [DOI] [Google Scholar]
- Salthouse TA (1996). The processing-speed theory of adult age differences in cognition. Psychological review, 103(3), 403–428. [DOI] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Daugherty AM, & Hillman CH (2017). Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. NeuroImage, 153(December 2016), 179–188. doi: 10.1016/j.neuroimage.2017.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464. doi: 10.1214/aos/1176344136 [DOI] [Google Scholar]
- Seger CA (2006). The basal ganglia in human learning. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry, 12(4), 285–290. doi: 10.1177/1073858405285632 [DOI] [PubMed] [Google Scholar]
- Simon JR, Vaidya CJ, Howard JH, & Howard DV (2012). The effects of aging on the neural basis of implicit associative learning in a probabilistic triplets learning task. Journal of Cognitive Neuroscience, 24(2), 451–463. doi: 10.1162/jocn_a_00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Strauman TA, Welsh-Bohmer K, Jeffrey N, & Sherwood A. (2010). Aerobic Exercise and Neurocognitive Performance: a Meta-Analytic Review of Randomized Controlled Trials. Psychosom Med, 72(3), 239–252. doi: 10.1097/PSY.0b013e3181d14633.Aerobic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, & Stark CEL (2010). Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learning & memory, 17(6), 284–288. doi: 10.1101/lm.1768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe K, Byun K, Hyodo K, Reagh ZM, Roberts JM, Matsushita A, . . . Soya H. (2018). Rapid stimulation of human dentate gyrus function with acute mild exercise. Proceedings of the National Academy of Sciences, 201805668–201805668. doi: 10.1073/pnas.1805668115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwabe K, Hyodo K, Byun K, Ochi G, Yassa MA, & Soya H. (2017). Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus, 27(3), 229–234. doi: 10.1002/hipo.22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo AN, McAuley E, Erickson KI, Voss MW, Prakash RS, Mailey EL, . . . Kramer AF (2011). Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology, 25(5), 545–553. doi: 10.1037/a0022733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Bandettini PA, & Johansen-Berg H. (2012). The effects of aerobic activity on brain structure. Frontiers in Psychology, 3(MAR), 1–9. doi: 10.3389/fpsyg.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Rawlings NB, Stagg CJ, Matthews L, Morris M, . . . Johansen-Berg H. (2016). Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage, 131, 162–170. doi: 10.1016/j.neuroimage.2015.10.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás Pereira I, Gallagher M, & Rapp PR (2015). Head west or left, east or right: Interactions between memory systems in neurocognitive aging. Neurobiology of Aging, 36(11), 3067–3078. doi: 10.1016/j.neurobiolaging.2015.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, & Gage FH (2005). Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience: the official journal of the Society for Neuroscience, 25(38), 8680–8685. doi: 10.1523/JNEUROSCI.173105.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Tang X, & Carlson MC (2016). Hippocampal sub-regional shape and physical activity in older adults. Hippocampus, 26(8), 1051–1060. doi: 10.1002/hipo.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, & van Praag H. (2019). Exercise and Hippocampal Memory Systems. Trends in Cognitive Sciences, 23(4), 318–333. doi: 10.1016/j.tics.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, . . . Kramer AF (2016). Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage. doi: 10.1016/j.neuroimage.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DER, Nicol CW, & Bredin SSD (2006). Health benefits of physical activity: The evidence. Cmaj, 174(6), 801–809. doi: 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, & Cohen NJ (2013). Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus, 23(7), 570–580. doi: 10.1002/hipo.22115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K, & Maguire EA (2011). Acquiring “the knowledge” of London’s layout drives structural brain changes. Current Biology, 21(24), 2109–2114. doi: 10.1016/j.cub.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, & Stark CEL (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34(10), 515–525. doi: 10.1016/j.tins.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, & Stark CEL (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage, 51(3), 1242–1252. doi: 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.