Abstract
Objective
To identify novel serum proteins involved in the pathogenesis of PsA as compared with healthy controls, psoriasis (Pso) and AS, and to explore which proteins best correlated to major clinical features of the disease.
Methods
A high-throughput serum biomarker platform (Olink) was used to assess the level of 951 unique proteins in serum of patients with PsA (n = 20), Pso (n = 18) and AS (n = 19), as well as healthy controls (HC, n = 20). Pso and PsA were matched for Psoriasis Area and Severity Index (PASI) and other clinical parameters.
Results
We found 68 differentially expressed proteins (DEPs) in PsA as compared with HC. Of those DEPs, 48 proteins (71%) were also dysregulated in Pso and/or AS. Strikingly, there were no DEPs when comparing PsA with Pso directly. On the contrary, hierarchical cluster analysis and multidimensional scaling revealed that HC clustered distinctly from all patients, and that PsA and Pso grouped together. The number of swollen joints had the strongest positive correlation to ICAM-1 (r = 0.81, P < 0.001) and CCL18 (0.76, P < 0.001). PASI score was best correlated to PI3 (r = 0.54, P < 0.001) and IL-17 receptor A (r = –0.51, P < 0.01). There were more proteins correlated to PASI score when analysing Pso and PsA patients separately, as compared with analysing Pso and PsA patients pooled together.
Conclusion
PsA and Pso patients share a serum proteomic signature, which supports the concept of a single psoriatic spectrum of disease. Future studies should target skin and synovial tissues to uncover differences in local factors driving arthritis development in Pso.
Keywords: psoriasis, psoriatic arthritis, biomarker, spondyloarthritis, ankylosing spondylitis, proximity extension assay
Rheumatology key messages
PsA and psoriasis have a shared serum proteomic signature.
Expression of ICAM-1 and CCL18 had the most significant correlation to joint disease activity.
Expression of PI3 and IL-17 receptor A had the most significant correlation to skin disease activity.
Introduction
Psoriasis (Pso) is a common autoimmune disease that causes excessive scaling, redness and itchiness of skin at prototypical sites of the body. Approximately 20% of patients with Pso will at some point in their life develop PsA [1]. A clinical diagnosis of PsA is typically made in a patient with Pso or psoriatic nail disease with concomitant arthritis. PsA is clinically heterogeneous and other manifestations include those of the SpA spectrum, such as enthesitis, dactylitis and SpA. Adding to this heterogeneity is that in ∼15% of the cases of PsA, arthritis manifests prior to Pso [1]. Both cutaneous and rheumatic manifestations of Pso negatively impact quality of life and should be treated appropriately [2].
Tremendous advances have been made in the treatment options available for Pso. The current and emerging therapeutics can almost completely reverse skin inflammation in a majority of patients, but their capacity to halt arthritis is less impressive [3]. This discrepancy is well-illustrated by examining the current gold standard of trial outcome measures: a 90% improvement for Pso disease severity (Psoriasis Area and Severity Index, PASI90), compared with a 20% improvement for arthritis severity (ACR20). Numerous factors could explain the trailing treatment response in arthritis, including drug bioavailability, the cellular target and cellular turnover at the target tissue, as well as (still unidentified) differences in tissue-specific drivers of pathogenesis [4–6].
It is unknown whether the immunologic drivers in Pso vs PsA patients are different [7, 8]. This raises the question of whether these diseases are part of the same spectrum or distinct entities [8, 9]. Pso is one of the strongest known clinical risk factors for the development of arthritis, thus providing a unique opportunity to better understand arthritis development and improve treatment. It has historically been difficult to identify early PsA in Pso patients in daily clinical practice and there are currently no serum diagnostic biomarkers used in care. This impedes clarification of the presence or absence of a window of opportunity for treating early PsA. To overcome these important open questions, Pso and PsA should be studied head-to-head to uncover potential differences in pathogenesis that could serve as therapeutic targets, as well as to identify possible biomarkers to be used in early diagnosis.
Genetic studies reveal vast overlap between Pso and PsA, in which the few differences found were variants related to chromatin marks on a subset of T lymphocytes and CD8 T cells, and to variants in the IL-23 receptor [10, 11]. In comparative studies from peripheral blood mononuclear cells, Pso patients with PsA have higher expression of genes associated with the IFN signature in their monocytes [12, 13], and their T cells more readily produce IL-2 and IL-22 upon re-stimulation [14, 15]. Recent work has also shown that patients with PsA have higher levels of auto-antibodies directed against two previously identified putative auto-antigens of Pso, namely (carbamylated) LL37 and ADAMTSL5 [16, 17]. So far, serum-based biomarker studies have revealed elevated levels of high-sensitivity CRP, pro-inflammatory cytokines (e.g. IL-6, IL-33, TNF-α), adipokines and changes in markers of bone/cartilage damage in the Pso patients with PsA [18–27].
Overall, there is a scarcity of head-to-head serum biomarker comparisons in well-defined cohorts of Pso and PsA. The current study measured serum biomarkers in the early stage of PsA as compared with Pso matched for skin disease severity. We used a novel high-throughput proteomic platform capable of screening over 950 proteins in a small volume of serum. Previously, this technology proved valuable in providing new mechanistic insights into the pathogenesis of immune-mediated diseases of skin [28, 29], but results have not yet been reported in patients with rheumatic disease. The goal was to determine whether this biomarker platform could identify novel serum protein disturbances in PsA as compared with HC, Pso and AS (non-psoriatic reference group), and to specify which proteins best reflected major skin and joint manifestations.
Methods
Study design
This study was performed at the University Medical Centre Utrecht and conducted in compliance with the Helsinki principles. Ethical approval was obtained from the institutional review board and all patients signed written informed consent before participation. Clinical parameters and serum samples were collected from a cohort of patients with Pso, PsA and AS as part of larger prospective observational study performed at the outpatient clinic of the Department of Rheumatology and Clinical Immunology.
For this study 79 patients were recruited. The Pso cohort (Pso, n = 20) included patients with a dermatologist-confirmed diagnosis of Pso in whom concomitant PsA was clinically excluded by a rheumatologist (in training). Patients with PsA (n = 20) fulfilled ClASsification of Psoriatic ARthritis (CASPAR) criteria [30]. Patients with a clinical diagnosis of AS (n = 19), all without a history of Pso, were included as a non-psoriatic reference group. Serum samples were collected from healthy controls (HC, n = 20) from the University Medical Centre Utrecht.
Serum proteomic analysis
Serum samples were collected, centrifuged at 1700g for 10 min at 4°C and stored directly at −80°C. Frozen serum aliquots were shipped on dry ice to the Olink Facility (Uppsala, Sweden) without prior thawing and measured according to manufacturer’s instructions as previously published [31]. The Olink high-throughput proteomic platform employs a proximity extension-assay technology, in which oligonucleotide-labelled antibody pairs bind to a protein target. DNA reporter molecules bind to these antibodies, and are amplified to provide relative protein concentrations. One serum aliquot of 250 μl was used to run 11 different Olink platform ‘panels’ encompassing 1012 proteins, some of which were run in more than one panel (panels: CARDIOMETABOLIC, CARDIOVASCULAR II, CARDIOVASCULAR III, CELL REGULATION, DEVELOP-MENT, IMMUNE RESPONSE, INFLAMMATION, METABOLISM, NEUROLOGY, ONCOLOGY II and ORGAN DAMAGE). Only data that passed Olink internal quality control were used for analysis. We removed samples entirely if they did not pass Olink internal quality control in >80% of the data. We removed proteins entirely if they were below the limit of assay detection in >40% of the samples. Some proteins were measured in multiple panels, in which case the protein data with the fewest missing values after quality control was used for analysis.
Statistical approach
For analysis of clinical characteristics, contingency analysis of two groups were performed using Chi-squared tests for categorical variables, and independent samples T-tests or Mann–Whitney U tests for continuous variables. Contingency analysis of more than two groups were conducted with one-way independent analysis of variance or Kruskal–Wallis for continuous variables, and with χ2 test for categorical variables. Spearman’s rank correlation was used to correlate disease activity parameters to protein levels. Unless otherwise stated, a P-value of <0.05 was considered statistically significant.
The statistical analysis of proteomic data was performed on protein data received by Olink without further normalization (quantile normalization did not impact the overall results, data not shown). Olink protein data are expressed as an arbitrary unit (Normalized Protein eXpression, ‘NPX’) representing the relative protein concentration based on a log2 scale (i.e. absolute protein quantity cannot be compared across different proteins). Protein levels were compared between groups based on the likelihood ratio test and considered statistically significant at a false discovery rate (FDR)-corrected P-value of <0.05, referred to as differentially expressed proteins (DEPs). Analysis was performed to compare two groups (e.g. HC vs PsA) or to compare multiple groups (HC, Pso, PsA, AS), as specified in the text. Hierarchical cluster analysis was based on Ward’s method to create heatmaps (R pheatmap package, version 1.0.12). Classical multidimensional scaling was performed with the R built-in ‘stats’ package (cmdscale function), using the Euclidean distance matrix between samples based on protein data. The hierarchical cluster analysis and multidimensional scaling were performed using DEPs between groups based on a nominal P-value <0.05. The protein data shown in figures of hierarchical cluster analysis underwent Z-score normalization for the sake of visualization in heatmaps. Venn diagrams were modified from web-based BioVenn tool [32]. Reactome pathway and Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway enrichment analysis for DEPs was performed based on hypergeometric test using ReactomePA package (version 1.28.0) and clusterProfiler package (version 3.12.0), respectively. Statistical analysis was performed in R (version 3.6) and SPSS (version 25, SPSS Inc., Chicago IL, USA).
Results
Cohort description
Clinical characteristics of the study participants are shown in Table 1. The Pso and PsA groups were matched for age, gender and PASI score. The PsA cohort was recruited early after disease onset, typically with <1 year of disease duration. Except for two patients with PsA, none of the study participants was being treated with DMARDs. Following quality control (see Methods), a total of 951 unique proteins and 77 samples (18 Pso, 20 PsA, 19 AS, 20 HC) were retained for further analysis.
Table 1.
Baseline characteristics
| HC (N = 20) | Pso (N = 18) | PsA (N = 20) | AS (N = 19) | |
|---|---|---|---|---|
| Age (years) | 43 (13) | 37 (15) | 41 (9) | 40 (12) |
| Female, n (%) | 7 (35) | 7 (39) | 7 (35) | 5 (26) |
| BMI (kg/m2) | – | 29.9 (7.9) | 27.7 (4.5) | 24.2 (3.4)* |
| Smoker, n (%) | – | 6 (40) | 7 (35) | 2 (11) |
| Disease duration (years) | ||||
| Pso | – | 12.4 (6.1–18.6) | 20.0 (7.2–31.4) | – |
| PsA | – | – | 0.7 (0.1–8.3) | – |
| AS | – | – | – | 5.6 (0.4–13.4) |
| NSAID use, n (%) | – | 1 (6)* | 11 (55) | 11 (58) |
| DMARD use, n (%) | – | 0 (0) | 2 (10) | 0 (0) |
| CRP (mg/l) | – | 2.8 (1–6) | 2.8 (2–4) | 3.2 (1–7) |
| ESR (mm/h) | – | 5 (2–8) | 5 (2–13) | 5 (3–14) |
| Pso indices | ||||
| PASI | – | 2.7 (2–7) | 3.0 (1–6) | – |
| Nail involvement, n (%) | – | 9 (56.3) | 13 (72) | – |
| Vulgaris type only, n (%) | – | 12 (67) | 14 (78) | – |
| SpA manifestations | ||||
| Swollen joint count, of 76 | – | – | 3 (1–9)* | 0 (0–0) |
| Tender joint count, of 78 | – | – | 3 (1–10)* | 0 (0–0) |
| Dactylitis ever, n (%) | – | – | 7 (35)* | 0 (0) |
| Enthesitis, n (%) | – | – | 9 (47)* | 3 (16) |
| Inflammatory back pain, n (%) | – | – | 3 (15)* | 19 (100) |
| BASDAI (range 0–10) | – | – | – | 4.2 (2–5) |
Significant (P-value <0.05). Presented data are from time of baseline visit, unless otherwise indicated. Categorical data are presented with frequencies (%) and continuous data are shown as mean (s.d.) (normally distributed variables) or median (interquartile range) (non-normally distributed variables). Pso: psoriasis; HC: healthy control; DMARD use: in past 3 months; NSAID use: daily on stable dose; PASI: Psoriasis Area and Severity Index (range 0–72).
Major proteins changes in PsA serum compared with HC serum
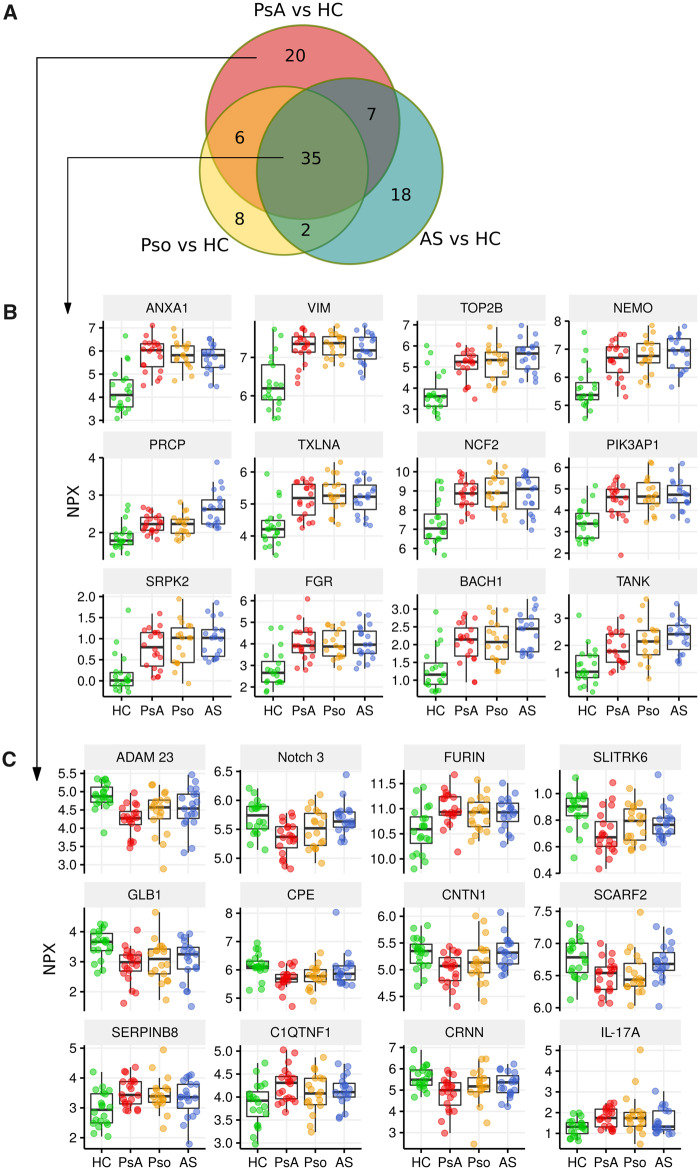
We first set out to specifically compare the serum of PsA to HC and found 68 differentially expressed proteins (DEPs) (FDR-corrected P < 0.05) (supplementary Table S1, available at Rheumatology online). Most of the top DEPs between PsA and HC have not previously been implicated in the pathogenesis of PsA, which included proteins such as ANXA1, ADAM23 and VIM (supplementary Fig. S1A, available at Rheumatology online). Hierarchical cluster analysis revealed that the serum proteomic profile of PsA patients could be clearly distinguished from the serum proteomic profile of HC (supplementary Fig. S1B, available at Rheumatology online).
Common and unique protein disturbances in serum of PsA
We first examined whether those serum proteins changes were unique to PsA, or if they were also dysregulated in Pso and/or AS. Of the 68 DEPs between PsA and HC, 48 proteins (71%) were also dysregulated in Pso and/or AS (Fig. 1A). The most significant DEPs between the groups were proteins that all had higher serum levels in patient groups as compared with HC (Fig. 1B). This list again included the proteins ANXA1, VIM and TOP2B. In total, 20 proteins (29%) were dysregulated in PsA as compared with HC, which were not dysregulated in AS or Pso as compared with HC (Fig. 1C). This list included proteins ADAM23, Neurogenic locus notch homologue protein 3 (Notch 3) and SLITRK6. Interestingly, many of the proteins in this list were lower in the serum of PsA as compared with that from HC.
Fig. 1.
Common and unique protein disturbances in serum of PsA
(A) Overlap in DEPs between patient groups vs HC. This Venn diagram shows the number of DEPs between each patient group as compared with HC. For example, there were 68 DEPs when comparing PsA with HC, of which 35 proteins were also differentially expressed when comparing Pso with HC or comparing AS with HC. Results are based on FDR-corrected P-value <0.05. (B) Common DEPs in all patient groups. Thirty-five proteins were differentially expressed in all patient groups as compared with HC. The 12 most significant proteins are displayed as boxplots (DEPs with the lowest FDR-corrected P-value). (C) DEPs only found in PsA vs HC. Twenty proteins were differentially expressed in PsA compared with HC, but not dysregulated in other patient groups compared with HC. The 12 most significant proteins are displayed in boxplots (DEPs with the lowest FDR-corrected P-value). HC: healthy control; Pso: psoriasis; NPX: Normalized Protein eXpression; DEPs: differentially expressed proteins; FDR: false discovery rate.
We next compared patient groups directly. Importantly, there were no DEPs when directly comparing PsA with Pso based on FDR-corrected P < 0.05. An exploratory analysis (based on nominal P-value) comparing PsA with Pso can be found in supplementary Fig. S2A and B, available at Rheumatology online. We found that CLEC4A and SOD1 were the only proteins significantly different between patient groups, being elevated in AS (supplementary Fig. S3, available at Rheumatology online). Some specific proteins that have previously been implicated in the pathogenesis of these disease are displayed in supplementary Fig. S4, available at Rheumatology online. The list of DEPs can be found in supplementary Tables S1–S4, available at Rheumatology online. Taken together, we identified 20 proteins uniquely dysregulated in PsA, while the majority of protein disturbances were also dysregulated in Pso and/or AS.
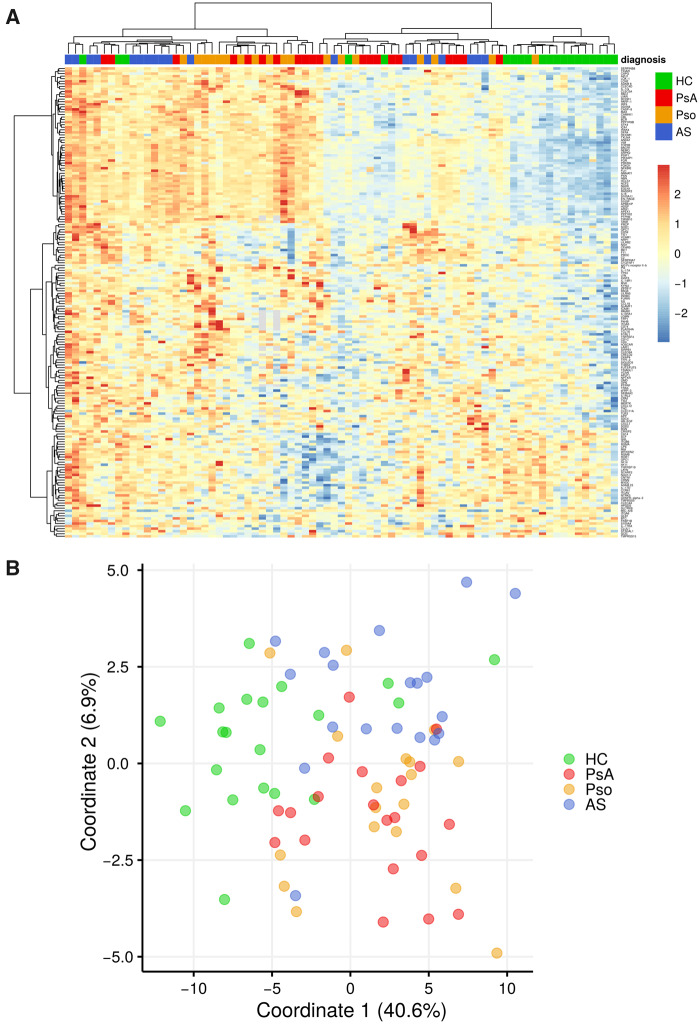
Overall serum proteomic signature is similar in PsA and Pso
Hierarchical cluster analysis showed that most patients, regardless of diagnosis, clustered separately from HC. The serum proteomic profile of PsA patients grouped closer to the Pso patients than to the AS patients (Fig. 2A). Using an alternative method of analysing the data, namely multidimensional scaling analysis, we also found that HC grouped separately from patients, and that PsA and Pso grouped close together (Fig. 2B). Finally, pathway enrichment analysis on the sets of DEPs between patient groups vs HC similarly revealed that very similar pathways were enriched in PsA and Pso (supplementary Fig. S5, available at Rheumatology online).
Fig. 2.
Overall serum proteomic signature is similar in PsA and Pso
(A) Serum in Pso and PsA overlap based on hierarchical clustering analysis. Hierarchical clustering shown in the heatmap reveals HC cluster separately from the different patient groups. The clustering also reveals that most Pso and PsA patients cluster separately from AS patients. This analysis is based on DEPs with nominal P-value <0.05 when comparing all groups. (B) Serum in Pso and PsA overlap based on MDS. The MDS plot reveals that HC cluster separately from the different patient groups. Similar to the hierarchical clustering (3A), Pso and PsA tend to cluster separately from AS. This analysis is based on DEPs with nominal P-value <0.05 when comparing all groups. HC: healthy control; Pso: psoriasis; DEPs: differentially expressed proteins; MDS: multidimensional scaling.
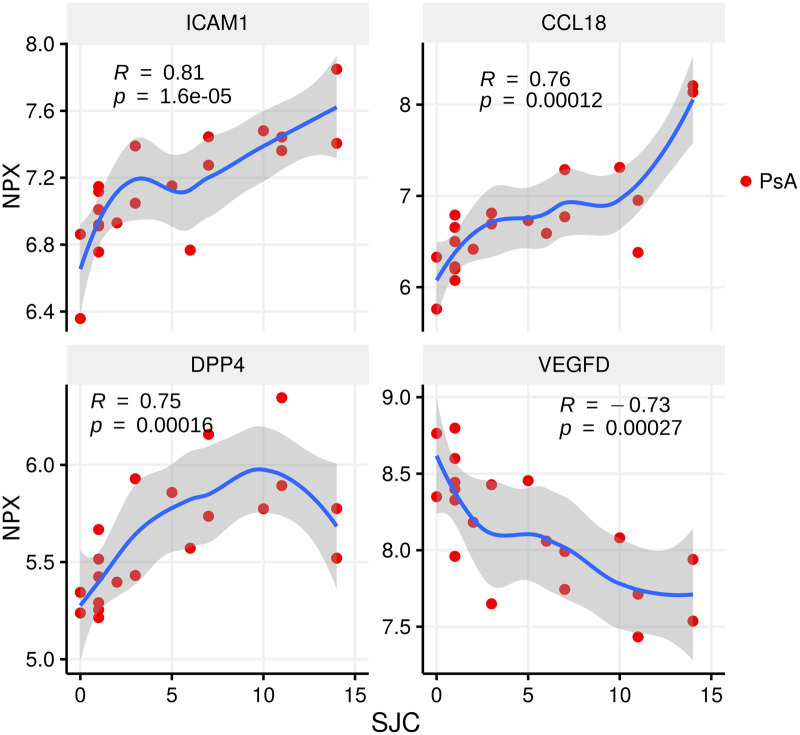
Proteins reflecting joint and skin disease activity
We next examined which serum proteomic changes best reflected the major disease manifestations with respect to joint and skin disease activity in patients with PsA and Pso. The number of swollen joints had the strongest positive correlation to Intracellular adhesion molecule 1 (ICAM-1; r = 0.81, P < 0.001), C-C motif chemokine 18 (CCL18; r = 0.76, P < 0.001) and dipeptidyl peptidase 4 (DPP4) (r = 0.75, P < 0.001), whereas swollen joint count had the strongest negative correlation to VEGFD (r = −0.73, P < 0.001) (Fig. 3).
Fig. 3.
Top proteins related to arthritis activity
The swollen joint count (SJC) vs relative protein levels of ICAM-1, CCL18, DPP4 and VEGFD. Spearman’s rank correlation (R) and P-value in PsA are displayed in the figure. Locally estimated scatterplot smoothing (loess) curve is shown. NPX: Normalized Protein eXpression; ICAM-1: Intracellular adhesion molecule 1; CCL18: C-C motif chemokine 18; DPP4: Dipeptidyl peptidase 4.
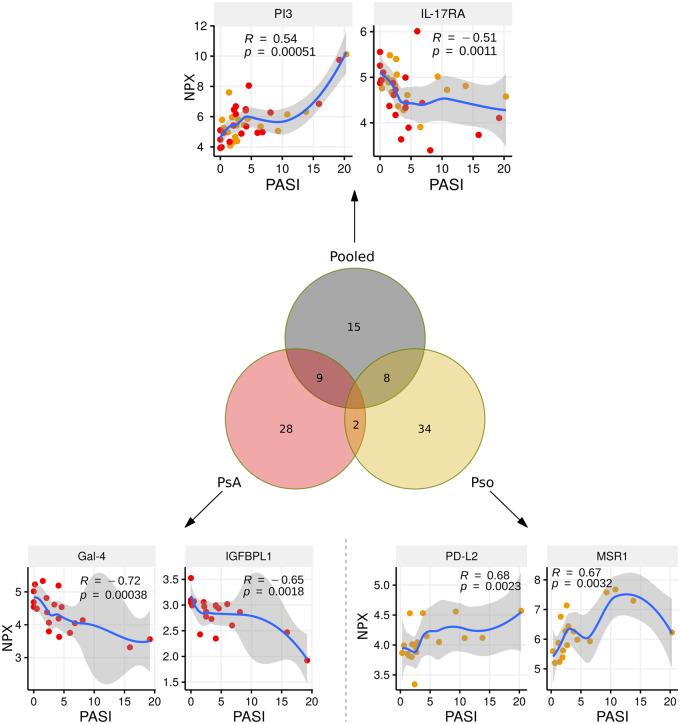
When PsA and Pso patients were considered as one group (data pooled together), PASI scores had the strongest correlation to the proteins PI3 (r = 0.54, P < 0.001), IL-17 receptor A (r = −0.51, P < 0.01), MMP-1 (r = 0.47, P = 0.01) and SERPINB8 (r = 0.46, P < 0.01). Surprisingly, there were more proteins that correlated to PASI score when analysing the Pso and PsA cohorts separately as compared with analysing the Pso and PsA patients pooled together (Fig. 4). PASI score was correlated to Gal-4 (r = −0.72, P < 0.001) and IGFBPL1 (r = −0.65, P < 0.01), but only in patients with PsA. PASI score was correlated to PD-L2 (r = 0.68, P < 0.01) and MSR1 (r = 0.67, P < 0.01), but only in patients with Pso (Fig. 4).
Fig. 4.
Top proteins related to Pso activity
The Venn diagram shows the total number of proteins that significantly correlate to PASI score (nominal P-value <0.05). The analysis was performed when pooling PsA and Pso patients (grey circle), taking PsA patients only (red circle) or taking Pso patients only (orange circle). The PASI was correlated to relative protein levels. Spearman’s rank correlation (R) and P-value are shown in the figure. Locally estimated scatterplot smoothing (loess) curve is shown. Pso: psoriasis; PASI: Psoriasis Area and Severity Index; NPX: Normalized Protein eXpression; PI3: Elafin; IL-17RA: IL-17 receptor A; Gal-4: Galectin-4; IGFBPL1: Insulin-like growth factor-binding protein-like 1; PD-L2: Programmed cell death 1 ligand 2; MSR1: Macrophage scavenger receptor types I and II.
Discussion
This study found large proteomic disturbances in the serum of patients with PsA and revealed that the strongest proteomic changes occurred in novel proteins not yet linked to the pathogenesis of PsA. Importantly, the majority of protein changes in serum of patients with PsA were similarly disturbed in patients with Pso in whom PsA was excluded. From over 950 proteins screened, we were able to narrow down specific proteins of interest correlating to the major clinical manifestations of these diseases.
This is one of few head-to-head serum proteomic comparisons in a well-characterized cohort of patients with PsA and Pso. Our PsA cohort consisted of patients with early disease onset and was carefully matched to have similar clinical characteristics (including PASI score) to the Pso patients. From a clinical perspective, our results indicate that none of the evaluated serum proteins (singularly) is a likely candidate for a simple diagnostic biomarker capable of discriminating early PsA from Pso. In other words, a simple blood test to differentiate PsA from Pso may not be a feasible goal for daily clinical practice, at least not based on the proteins we evaluated. Instead, our results primarily contribute to the understanding of the pathogenesis of PsA, which includes specifying potential drug targets. From a pathophysiological perspective, our data support the ‘two phenotypes of one disease’ hypothesis [8, 9].
Our study adds important insight into the question as to which type of tissue sample is best suited to unravel the pathogenesis of PsA. PsA and Pso fall within a spectrum of diseases with shared genetic background and presumably shared immunologic drivers. From a clinician point of view, however, they are distinct: some patients develop (poly)arthritis, which requires specific clinical intervention. Therefore, there must be specific drivers (local and/or systemic) within this overlapping psoriatic spectrum that enable the development of overt arthritis manifestations. Our broad analysis reveals that PsA and Pso are extremely difficult to discriminate based on serum proteomic changes, underscoring that other sites of the body, such as synovial tissue, should be an important target of future research. It will still be important to find methods of incorporating appropriate control groups, ideally Pso patients in whom PsA is excluded by a rheumatologist, even when studying tissue sites such as synovial tissue. Surprisingly, we found that many serum proteins were related to PASI score when dichotomizing the analysis for Pso only and PsA only. This may indicate there are different primary drivers of cutaneous inflammation and/or secondary systemic responses upon inflammation occurring in PsA compared with Pso. A comparison of the skin in PsA compared with Pso as tissue site has only been addressed in a small number of studies and therefore warrants specific tissue comparisons [33, 34].
We here identified specific proteins strongly associated with joint disease activity. ICAM-1 is a molecule important for trans-endothelial migration of leucocytes via interaction with LFA-1. ICAM-1 has previously been identified in the pathogenesis of Pso and PsA [35, 36]. In RA synovial tissue it was shown that ICAM-1 expression marked a specific myeloid synovial tissue phenotype [37]. Interestingly, previous attempts to target LFA-1 with mAbs for the treatment of Pso lead to the new-onset arthritis in many patients enrolling in the trials [38], supporting the notion that the balance of leucocyte extravasation mediated by ICAM-1 could be important in arthritis development. VEGFD is one of the members of the endothelial growth factors involved in angiogenesis and lymphangiogenesis in cancer, and while this specific family member has not been described in rheumatic disease [39], VEGF has been implicated in the pathogenesis of arthritis [40]. Considering that we performed a broad, unbiased serologic screening, our data again highlight the importance of angiogenesis in PsA, which is in agreement with existing histologic data in PsA showing increased angiogenesis to be an important feature of PsA synovial tissue [7, 35, 41]. Two additional proteins were strongly correlated to arthritis activity: CCL18 and DPP4. DPP4 is currently a target for type 2 diabetes mellitus, and the role of DPP4 in development of arthritis is still unclear [42]. CCL18 is expressed by endothelial cells in the synovial tissue of RA and has been identified as a disease activity marker in RA and other diseases [43].
A strength of our study is the broad set of protein panels we have measured. We hence observed that the strongest protein disturbances were not well-known cytokines and chemokines, but rather proteins not previously implicated in the pathogenesis of rheumatic disease, including ADAM23 and Notch 3. ADAM23 is a non-proteolytic member of the ‘A disintegrin and metalloproteases’ (ADAM) family known for high expression in brain and roles in neuronal differentiation, but also shown to inhibit cell adhesion and cell migration in cancer cells, possibly via interaction with integrin αvβ3 [44, 45]. Notch 3 has very broad functions and is aberrantly expression in psoriatic skin, and was shown to modulate Th cell phenotypes function [46, 47]. Our patient cohorts have an expected overlapping pathogenic spectrum (Pso, PsA, AS). Future studies should consider including other rheumatic diseases with more distinct clinical features and pathogenesis (e.g. gout and OA) in order to further address the specificity of the protein changes. While the protein disturbances were not specific to PsA, this per se does not preclude their importance in pathogenesis or their role as potential therapeutic target: many of the current therapeutics (e.g. TNF-α inhibitors) are effective across a range of distinct clinical entities considered to be driven by different pathways.
Some of the more familiar proteins changes included IL-6 and IL-17A, which are known drug targets for rheumatologic diseases. Studies in RA highlight that serum levels of cytokines are unlikely to predict clinical response to mAbs targeting that respective cytokine [48, 49]. Nevertheless, we detected elevated levels of IL-6 in PsA and also found a positive correlation between IL-6 levels and joint disease activity measures, which supports current efforts examining IL-6 as a potential therapeutic target for patients with PsA.
Our study was designed to recruit PsA patients without DMARDs use and early after disease onset, resulting in PsA patients with mostly oligoarthritis. The serum proteomic results best represent the oligoarthritis pattern in PsA, but our cohort does not represent the entire spectrum of PsA patients, i.e. those with very severe polyarticular disease. Our choice to avoid patients with DMARDs is underscored by recent data using the same proteomic platform in Pso patients confirming that most proteins undergo vast changes upon initiation of immunomodulatory drugs [29].
A limitation of the current study is the relatively small cohort size, which means that we may have underestimated the number of proteins that are different between Pso and PsA groups due to stringent FDR-correction. Realistically, it is challenging to include large numbers of patients in basic science studies with very severe disease that are not (yet) treated with immunomodulatory drugs. Clearly, it will be necessary to (i) replicate the major protein disturbances identified by our screening and (ii) determine whether the proteins are downstream biomarkers of the disease or directly involved in the pathogenesis. Functional validation will be necessary to determine which of these specific factors or combination of factors contribute to the pathogenesis of PsA.
To overcome some of the aforementioned challenges we recommend that, similar to sharing gene expression data, these proteomic datasets can be publicly shared (e.g. repositories). Firstly, this provides additional scientific transparency of the results. Secondly, by sharing datasets the proteins can be compared across diseases (determine specificity) and allow for rapid validation and identification of those proteins worth pursuing for in vitro experiments. These collaborative efforts should maximize the yield of costly scientific endeavours, whilst ensuring acknowledgement of data in a competitive scientific landscape.
In summary, we have identified novel serum protein disturbances in PsA and furthermore establish that both Pso patients and PsA patients with oligoarthritis have an overall shared serum proteomic signature.
Supplementary Material
Acknowledgements
We would like to thank the patients for participating in the study. We would like to thank the clinical study team (Nienke Kleinrensink, Nanette Vincken, Anne Karien Marijnissen, Anneloes van Loo, Karin Schrijvers and Joke Nijdeken). W.T. was supported by the China Scholarship Council (CSC) No. 201606300050.
Funding: This study was in part funded by Janssen. This study was in part funded by Health∼Holland, Top Sector Life Sciences & Health.
Disclosure statement: T.R. received consultancy fees from Jansen in 2016 and 2017 on topics that were unrelated to the content of this manuscript. T.R. is currently an employee of AbbVie, with no conflicts of interest regarding the work of this manuscript. D.B. received consultancy fees from Janssen in 2018 and 2019 on topics that were unrelated to the content of this manuscript. Ernesto Munoz-Elias and Samuel DePrimo are Janssen R&D LLC employees. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Kavanaugh A, Gottlieb A, Morita A. et al. The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies. Ann Rheum Dis 2019;78:1215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furue K, Ito T, Furue M.. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine 2018;111:182–8. [DOI] [PubMed] [Google Scholar]
- 4. Belasco J, Louie JS, Gulati N. et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol 2015;67:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krueger JG, Kirkham B, Ritchlin CT.. Basic and translational science: a report from the GRAPPA 2016 Annual Meeting. J Rheumatol 2017;44:679–83. [DOI] [PubMed] [Google Scholar]
- 6. Ospelt C, Frank-Bertoncelj M.. Why location matters—site-specific factors in rheumatic diseases. Nat Rev Rheumatol 2017;13:433–42. [DOI] [PubMed] [Google Scholar]
- 7. Veale DJ, Fearon U.. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. [DOI] [PubMed] [Google Scholar]
- 8. Boehncke W-H. Psoriasis and psoriatic arthritis: flip sides of the coin? Acta Derm Venereol 2016;96:436–41. [DOI] [PubMed] [Google Scholar]
- 9. Sakkas LI, Bogdanos DP.. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 2017;16:10–5. [DOI] [PubMed] [Google Scholar]
- 10. Bowes J, Budu-Aggrey A, Huffmeier U. et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun 2015;6:6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stuart PE, Nair RP, Tsoi LC. et al. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet 2015;97:816–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollock RA, Abji F, Liang K. et al. Gene expression differences between psoriasis patients with and without inflammatory arthritis. J Invest Dermatol 2015;135:620–3. [DOI] [PubMed] [Google Scholar]
- 13. Abji F, Pollock RA, Liang K, Chandran V, Gladman DD.. Brief report: CXCL10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol 2016;68:2911–6. [DOI] [PubMed] [Google Scholar]
- 14. Capsoni F, Molteni S, Raeli L. et al. Differential expression of interleukin-2 by anti-CD3-stimulated peripheral blood mononuclear cells in patients with psoriatic arthritis and patients with cutaneous psoriasis. Clin Exp Dermatol 2014;39:385–90. [DOI] [PubMed] [Google Scholar]
- 15. Benham H, Norris P, Goodall J. et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther 2013;15:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan Y, Qiu J, Lin Z-T. et al. Identification of novel autoantibodies associated with psoriatic arthritis. Arthritis Rheumatol 2019;71:941–51. [DOI] [PubMed] [Google Scholar]
- 17. Frasca L, Palazzo R, Chimenti MS. et al. Anti-LL37 antibodies are present in psoriatic arthritis (PsA) patients: new biomarkers in PsA. Front Immunol 2018 2018;9:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandran V, Cook RJ, Edwin J. et al. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (Oxford) 2010;49:1399–405. [DOI] [PubMed] [Google Scholar]
- 19. Alenius G-M, Eriksson C, Dahlqvist R. S. Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin Exp Rheumatol 2009;27:120–3. [PubMed] [Google Scholar]
- 20. Xue Y, Jiang L, Cheng Q. et al. Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLoS One 2012;7:e46740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Liu L, Rui W. et al. New interleukins in psoriasis and psoriatic arthritis patients: the possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology 2017;233:37–46. [DOI] [PubMed] [Google Scholar]
- 22. Dalbeth N, Pool B, Smith T. et al. Circulating mediators of bone remodeling in psoriatic arthritis: implications for disordered osteoclastogenesis and bone erosion. Arthritis Res Ther 2010;12:R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartosińska J, Michalak-Stoma A, Juszkiewicz-Borowiec M, Kowal M, Chodorowska G.. The assessment of selected bone and cartilage biomarkers in psoriatic patients from Poland. Mediators Inflamm 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cubillos S, Krieg N, Norgauer J.. Effect of vitamin D on peripheral blood mononuclear cells from patients with psoriasis vulgaris and psoriatic arthritis. PLoS One 2016;11:e0153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amin TE, ElFar NN, Ghaly NR. et al. Serum level of receptor activator of nuclear factor kappa-B ligand in patients with psoriasis. Int J Dermatol 2016;55:e227–33. [DOI] [PubMed] [Google Scholar]
- 26. Kim DS, Shin D, Lee MS. et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol 2016;43:305–10. [DOI] [PubMed] [Google Scholar]
- 27. Mitsui A, Tada Y, Takahashi T. et al. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol 2016;41:183–9. [DOI] [PubMed] [Google Scholar]
- 28. Brunner PM, Suárez-Fariñas M, He H. et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J, Tomalin L, Lee J. et al. Reduction of inflammatory and cardiovascular proteins in the blood of patients with psoriasis: differential responses between tofacitinib and etanercept after 4 weeks of treatment. J Invest Dermatol 2018;138:273–81. [DOI] [PubMed] [Google Scholar]
- 30. Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 31. Assarsson E, Lundberg M, Holmquist G. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hulsen T, Vlieg J, de, Alkema W.. BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics 2008;9:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veale DJ, Barnes L, Rogers S, FitzGerald O.. Immunohistochemical markers for arthritis in psoriasis. Ann Rheum Dis 1994;53:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Šahmatova L, Sügis E, Šunina M. et al. Signs of innate immune activation and premature immunosenescence in psoriasis patients. Sci Rep 2017;7:7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veale D, Yanni G, Rogers S. et al. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum 1993;36:893–900. [DOI] [PubMed] [Google Scholar]
- 36. Veale D, Rogers S, Fitzgerald O.. Immunolocalization of adhesion molecules in psoriatic arthritis, psoriatic and normal skin. Br J Dermatol 1995;132:32–8. [DOI] [PubMed] [Google Scholar]
- 37. Dennis G, Holweg CTJ, Kummerfeld SK. et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 2014;16:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viguier M, Richette P, Aubin F. et al. Onset of psoriatic arthritis in patients treated with efalizumab for moderate to severe psoriasis. Arthritis Rheum 2008;58:1796–802. [DOI] [PubMed] [Google Scholar]
- 39. Stacker SA, Achen MG.. Emerging roles for VEGF-D in human disease. Biomolecules 2018;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Balogh E, Biniecka M, Fearon U, Veale DJ, Szekanecz Z.. Angiogenesis in inflammatory arthritis. Isr Med Assoc J 2019;5:345–52. [PubMed] [Google Scholar]
- 41. Kruithof E, Baeten D, Rycke L, De. et al. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther 2005;7:R569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim SC, Schneeweiss S, Glynn RJ. et al. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis 2015;74:1968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lieshout AWT, van Fransen J, Flendrie M. et al. Circulating levels of the chemokine CCL18 but not CXCL16 are elevated and correlate with disease activity in rheumatoid arthritis. Ann Rheum Dis 2007;66:1334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsia H-E, Tüshaus J, Brummer T. et al. Functions of “A disintegrin and metalloproteases (ADAMs)” in the mammalian nervous system. Cell Mol Life Sci 2019;76:3055–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ota M, Mochizuki S, Shimoda M. et al. ADAM23 is downregulated in side population and suppresses lung metastasis of lung carcinoma cells. Cancer Sci 2016;107:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ota T, Takekoshi S, Takagi T. et al. Notch signaling may be involved in the abnormal differentiation of epidermal keratinocytes in psoriasis. Acta Histochem Cytochem 2014;47:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao Z, Wang W, Xu H. et al. Engagement of activated Notch signalling in collagen II-specific T helper type 1 (Th1)- and Th17-type expansion involving Notch3 and Delta-like1. Clin Exp Immunol 2011;164:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cuppen BVJ, Welsing PMJ, Sprengers JJ. et al. Personalized biological treatment for rheumatoid arthritis: a systematic review with a focus on clinical applicability. Rheumatology (Oxford) 2016;55:826–39. [DOI] [PubMed] [Google Scholar]
- 49. Romão VC, Fonseca JE.. Major challenges in rheumatology: will we ever treat smarter, instead of just harder? Front Med 2019;6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.