Abstract
Background
Sporadic late-onset nemaline myopathy (SLONM) is a rare, acquired, adult-onset myopathy, characterized by proximal muscle weakness and the pathognomonic feature of nemaline rods in muscle fibres. Sporadic late-onset nemaline myopathy is associated with cardiac pathology in case reports and small case series, but the severity of cardiac disease is generally mild and rarely requires specific treatment. This case report describes severe heart failure as an early feature of SLONM, which responded to specific treatments, and highlights SLONM as a potentially reversible cause of heart failure.
Case summary
A 65-year-old woman presented with progressive muscle weakness and a dramatic loss of muscle bulk in her thighs, followed by progressive effort breathlessness over an 18-month period. She required a wheelchair to ambulate. A diagnosis of SLONM was made on histopathological assessment of a muscle biopsy along with electron microscopy. An echocardiogram showed a severely dilated and impaired left ventricle. She was treated with standard heart failure medications and autologous stem cell transplantation, which resulted in improvement of both her cardiac and muscle function, and allowed her to walk again and resume near-normal functional performance status.
Discussion
Cardiomyopathy can be a relatively early and life-threatening feature of SLONM and even in severe cases can be effectively treated with standard heart failure medications and autologous stem cell transplantation.
Keywords: Cardiac failure, Autologous stem cell transplantation, SLONM, Nemaline rods, Case report
Learning points
Sporadic late-onset nemaline myopathy (SLONM) is a rare myopathy that can present with severe cardiomyopathy as a prominent feature.
Sporadic late-onset nemaline myopathy and the associated cardiomyopathy can be treated successfully with heart failure medications and autologous stem cell transplantation.
In cases of cardiomyopathy and proximal muscle weakness, a diagnosis of SLONM should be considered.
Introduction
Sporadic late-onset nemaline myopathy (SLONM) is a rare acquired adult-onset myopathy characterized by muscle atrophy. It presents as proximal muscle weakness and has the characteristic histopathological finding of nemaline rods in muscle fibres. It varies in terms of clinical presentation and progression of the disease. In a significant proportion of cases, SLONM is associated with monoclonal gammopathy of unknown significance (MGUS), which is associated with an unfavourable outcome due to respiratory failure. Cardiac involvement has been reported in SLONM-MGUS in a few case reports and one cohort study,1–3 which demonstrates it can be life-threatening.4 There is a paucity of data on management of cardiac dysfunction in SLONM. Recently, autologous stem cell transplantation following high-dose melphalan has shown to reverse features of SLONM if started early.5–8 Therefore, while the disease can be lethal, it is now considered treatable if recognized early.
Timeline
| September 2014 | Progressive muscle weakness, initially affecting neck muscles and then gait, dysphagia and speaking difficulties. Mild dyspnoea with limited exercise tolerance |
| March 2016 |
Neurology review—proximal myopathy and muscle wasting Diagnosis of MGUS and AF |
| May 2017 | Gradual worsening dyspnoea and reduced exercise tolerance |
| 21 June 2017 | 1st muscle biopsy—inconclusive |
| 16 August 2017 | 2nd muscle biopsy—nemaline rods identified |
| 13 November 2017 | Severe left ventricular (LV) systolic dysfunction on echocardiogram. Commenced heart failure medications |
| 18 January 2018 | Autologous stem cell transplantation |
| 30 April 2018 | Resolution of symptoms with preserved LV systolic function and improvement in neuromuscular status |
Case presentation
A 65-year-old retired geography teacher presented in March 2016 with a 2-year history of progressive widespread global muscle weakness, dysphagia, and a dramatic loss of muscle bulk in her thighs. Approximately 1 year later, she developed gradual worsening exertional dyspnoea and reduced exercise tolerance, having previously been able to play 36 holes of golf. She did not experience any palpitations, chest pains, orthopnoea, or paroxysmal nocturnal dyspnoea. Her only medical history was a monoclonal gammopathy of uncertain significance (MGUS) and an incidental finding of atrial fibrillation for which she was taking Bisoprolol 5 mg once a day and warfarin with a target INR of 2–3.
On initial physical examination, she had mild to moderate weakness of neck flexion, shoulder abduction, elbow flexion, and extension. Distal strength in the upper limbs was normal. In the lower limbs, she had profound weakness of hip flexion and extension with striking muscle atrophy of the quadriceps bilaterally. She had moderate weakness of knee flexion and extension with minimal weakness of ankle dorsiflexion. Her reflexes were brisk and normal. Cardiac examination was unremarkable apart from an irregularly irregular pulse at a rate of 112 b.p.m. A year later, she remained in atrial fibrillation but was now noted to have a pan-systolic murmur, radiating to her axilla, and bilateral ankle swelling. Her Jugular venous pulse (JVP) was raised at 8 cm. She had scattered bibasal crepitations consistent with mild pulmonary oedema. There were no ascites.
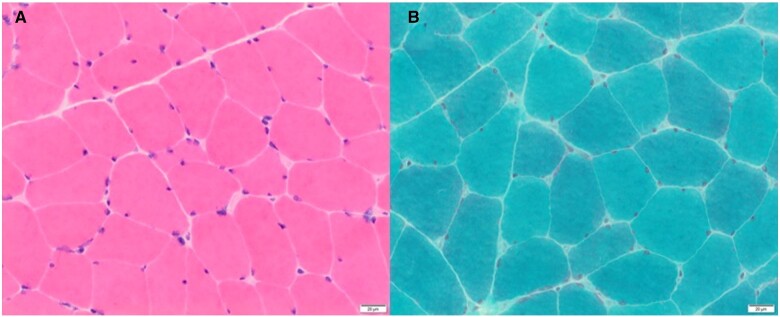
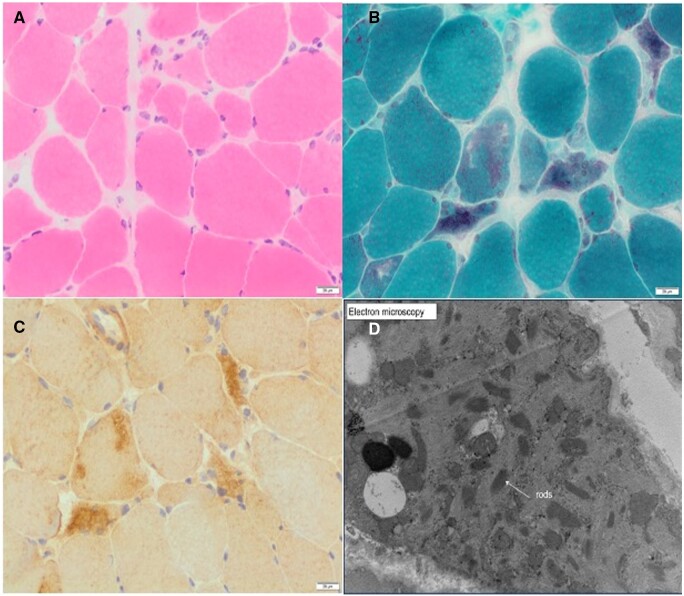
Her baseline blood tests were unremarkable with a normal full blood count, renal, liver, and thyroid function tests. She had a normal creatine kinase and was negative for the antibodies tested, including anti-nuclear antibody level (Hep-2), Mi-2, Ku, PM/Scl-100, PM/Scl-75, Jo-1, SRP, PL-7, PL-12, EJ, OJ, HMGCoAR. Neurophysiology testing was inconclusive and unable to differentiate between a neuropathic or myopathic process. Therefore, she underwent a deltoid muscle biopsy (Figure 1) and despite extensive analysis, the underlying aetiology remained unclear. Two months later, after worsening weakness, she had a second biopsy of her left vastus lateralis (Figure 2). This showed more pronounced changes including increased variability of muscle fibre diameter, frequent atrophic fibres, and rare necrotic fibres (Figure 2A). Importantly, the second biopsy also demonstrated fibres with rod-like structures on modified Gormori trichrome staining (Figure 2B), which were not apparent in the previous biopsy (Figure 1B). The presence of presumed nemaline rods and the clinical picture was suggestive of SLONM. Myotilin immunostaining (Figure 2C) and electron microscopy were performed (Figure 2D) to confirm the presence of nemaline rods filling atrophic fibres. The typical ‘lattice’ structure of nemaline rods was confirmed on electron microscopy.6
Figure 1.
Histopathological findings from deltoid biopsy. (A) Haemotoxylin and Eosin (H&E) staining of skeletal muscle with a moderate increase in the variability of muscle fibre diameter. Scattered moderately atrophic fibres. Few rare necrotic fibres are present (not shown) without obvious inflammation. Blood vessels appear normal. (B) Modified Gomori trichrome stain shows no vacuoles or ragged red fibres. There are no clear-cut rods.
Figure 2.
Histopathological findings from left vastus lateralis biopsy. (A) H&E staining showing skeletal muscle with significant variability of muscle fibre diameters. Scattered atrophic fibres including round, polygonal, and angulated forms. There is no significant increase in endomysial connective tissue. A few rare pale necrotic fibres may be present. No obvious inflammation is present. (B) Modified Gomori trichrome highlights the presence of relatively frequently lobulated fibres and scattered fibres, which appear to contain aggregates of rod-like structures. (C) Myotilin staining showing myofibril aggregates, which co-localize with rods. (D) Electron microscopy confirms the presence of nemaline rods, which have a high electron density and an internal lattice structure.
Her ECG showed atrial fibrillation with a rate of 112 b.p.m., incomplete left bundle branch block with QRS duration of 98 ms. Her initial echocardiogram showed a severely dilated left and right ventricle with severely impaired function (ejection fraction 25–30%) (Supplementary material online, VideosS1 and S2). She had a severely dilated left atrium. There was a moderate functional mitral and tricuspid regurgitation, but no other significant valvular disease. She had a normal right heart and no signs of pulmonary hypertension. Common and reversible causes of heart failure were considered and addressed, such as ischaemic heart disease, toxic damage, inflammatory, infiltrative, metabolic and genetic, arrhythmias, and high output states. She had no risk factors for coronary artery disease such as hypertension and had not been exposed to any cardiotoxic drugs or radiation. Her routine blood tests were normal.
On receiving the results of the initial echocardiogram, heart failure therapy was commenced. This included ramipril (titrated to the maximal dose over a 4-week period), an increase in bisoprolol to the maximum tolerated dose of 10 mg and bumetanide 1 mg twice daily. She was switched from warfarin to apixaban 5 mg twice daily in view of her atrial fibrillation. Two months later, spironolactone 25 mg once daily was added and amiodarone 200 mg once daily. Her butmetanide was reduced to 1 mg once daily.
She also had a successful direct current (DC) cardioversion to optimize her cardiac status in preparation for an autologous stem cell transplantation, which was performed in January 2018 following high-dose melphalan. She had stem cell return in early February 2018.
On commencing standard heart failure medications, her left ventricular systolic function improved from 30% to 40% over a 2-month period. Following the DC cardioversion and autologous stem cell transplant, her ejection fraction improved further to 56% measured by Simpson’s biplane method (Supplementary material online, Videos S3 and S4). She reverted back to atrial fibrillation a month later and digoxin 125 μg once daily was added to improve rate control. She had a substantial improvement in her neuromuscular performance and she was able to resume playing golf without experiencing significant dyspnoea. A repeat echocardiogram in January 2019 showed that she maintained a normal ejection fraction of 56% and remains on her standard heart failure medication. This includes apixaban 5 mg twice daily, digoxin 125 μg once daily, Ramipril 2.5 mg once daily, Bisoprolol 5 mg once daily, and spironolactone 12.5 mg once daily.
Discussion
In a review of 76 cases, SLONM had a mean age of onset at 52 years and was associated with MGUS in just over half of these cases.3 Cardiomyopathy has been reported in SLONM in a few case studies and one cohort study which detected an incidence of 11%.1–3 It is associated with a worse prognosis9; however, large studies and statistical data are lacking. This case highlights unusual features of cardiomyopathy in SLONM including a relatively early and severe phenotype. Previous studies show variability in terms of the severity and type of cardiomyopathy (hypertrophic or dilated) with the majority of studies showing mild cardiomyopathy. Monforte and colleagues performed a single-centre cohort study of six patients with SLONM all of whom had cardiac involvement. While conduction abnormalities or arrhythmias were the most common pathology, the type of cardiomyopathy varied significantly. Nevertheless, a mild reduction in ejection fraction was present in the majority of patients. Additionally, in previous case studies, cardiomyopathy was a late event in SLONM presenting 2–5 years after the initial presentation,1,7,10 while in this case, it was both a relatively early and severe feature of the disease. Significantly, this case highlights that life-threatening cardiomyopathy can be a feature of SLONM and can be reversible with standard heart failure medications alongside chemotherapy with an autologous stem cell transplantation. Previous studies have also demonstrated that chemotherapy alone can improve left ventricular function in SLONM.7
While the underlying mechanism of cardiac failure in SLONM remains unclear, our patient as well as others have demonstrated improvement of cardiomyopathy with standard heart failure medication and chemotherapy followed by an autologous stem cell transplantation.7 This finding supports the notion that cardiomyopathy is disease-related. It is hypothesized that striated cardiac muscle is susceptible to the same damaging mechanism that affects the skeletal muscle, although histopathological data from heart muscle biopsy has yet to be reported.
The limitations of this study are the lack of cardiac biopsy and cardiac MRI as the patient was too ill to perform these investigations. However, the course of her cardiomyopathy is well documented with sequential echocardiograms. This case demonstrates clear histopathological features of SLONM on peripheral muscle biopsy and evidence of reversal of cardiac failure after commencing routine heart failure medications, chemotherapy, and autologous stem cell transplantation, which may help guide clinical management of patients presenting with cardiac failure and muscle weakness.
Conclusion
In conclusion, cardiologists should be aware of this rare but reversible cause of cardiac failure. Sporadic late-onset nemaline myopathy should be considered in patients presenting with muscle weakness and cardiomyopathy as early detection and treatment with conventional heart failure therapy, chemotherapy, and autologous stem cell transplantation can lead to significant improvement and prevent further progression.
Lead author biography

Dr Casmir Turnquist received her DPhil from the University of Oxford in 2015 with funding from the National Institutes of Health -Oxford Biomedical Research Fellowship. Her research in the laboratories of Professor Xin Lu and Dr Curtis Harris explores the role of tumour suppressor genes, p53 and ASPP2, in neurodegenerative disease and neuroinflammation. She was the recipient of the Foulkes Fellowship, Nuffield Department of Medicine Graduate Prize, and the National Cancer Institute Director’s Innovation Award. She is currently a Graduate-Entry medical student at the University of Oxford.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to thank the patient and her family for allowing us to write this case.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in accordance with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Contributor Information
Casmir Turnquist, University of Oxford Medical School, John Radcliffe Hospital, Oxford, UK.
Joanna C Grogono, Department of Cardiology, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, UK.
Monika Hofer, Department of Neuropathology, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Alex Pitcher, Department of Cardiology, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, UK.
References
- 1. Belkhribchia MR, Tazi I, Louhab N, Kissani N, Mahmal L, Pereon Y.. Autologous stem cell transplantation in a patient with sporadic late-onset nemaline myopathy and monoclonal gammopathy: first Moroccan experience. Presse Med 2017;46:122–125. [DOI] [PubMed] [Google Scholar]
- 2. Monforte M, Primiano G, Silvestri G, Mirabella M, Luigetti M, Cuccagna C. et al. Sporadic late-onset nemaline myopathy: clinical, pathology and imaging findings in a single center cohort. J Neurol 2018;265:542–551. [DOI] [PubMed] [Google Scholar]
- 3. Schnitzler LJ, Schreckenbach T, Nadaj-Pakleza A, Stenzel W, Rushing EJ, Van Damme P. et al. Sporadic late-onset nemaline myopathy: clinico-pathological characteristics and review of 76 cases. Orphanet J Rare Dis 2017;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarullo FM, Vitale G, Di Franco A, Sarullo S, Salerno Y, Vassallo L. et al. Nemaline myopathy and heart failure: role of ivabradine; a case report. BMC Cardiovasc Disord 2015;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voermans NC, Benveniste O, Minnema MC, Lokhorst H, Lammens M, Meersseman W. et al. Sporadic late-onset nemaline myopathy with MGUS: long-term follow-up after melphalan and SCT. Neurology 2014;83:2133–2139. [DOI] [PubMed] [Google Scholar]
- 6. Benveniste O, Laforet P, Dubourg O, Solly S, Musset L, Choquet S. et al. Stem cell transplantation in a patient with late-onset nemaline myopathy and gammopathy. Neurology 2008;71:531–532. [DOI] [PubMed] [Google Scholar]
- 7. Belhomme N, Maamar A, Le Gallou T, Minot-Myhié M-C, Larralde A, Champtiaux N. et al. Rare myopathy associated to MGUS, causing heart failure and responding to chemotherapy. Ann Hematol 2017;96:695–696. [DOI] [PubMed] [Google Scholar]
- 8. Voermans NC, Minnema M, Lammens M, Schelhaas HJ, Kooi AVD, Lokhorst HM. et al. Sporadic late-onset nemaline myopathy effectively treated by melphalan and stem cell transplant. Neurology 2008;71:532–534. [DOI] [PubMed] [Google Scholar]
- 9. Palmucci L, Doriguzzi C, Mongini T, Chiadò-Piat L.. Adult onset nemaline myopathy: a distinct nosologic entity? Clin Neuropathol 1993;12:153–155. [PubMed] [Google Scholar]
- 10. Kotchetkov R, Dyszkiewicz-Korpanty A, Kukreti V.. Chemotherapy with stem cell transplantation is more effective than immunotherapy in sporadic late onset nemaline myopathy with monoclonal gammopathy. Bone Marrow Transplant 2018;53:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.