Abstract
Background
Pulmonary hypertension (PH) is one of the significant complications of sarcoidosis. In the clinical classification of PH proposed in the recent world symposium of PH 2018, sarcoidosis-associated PH is classified in Group 5. The mechanisms of sarcoidosis-associated PH are very heterogeneous. There is no evidence of effective treatment for this condition.
Case summary
We report a case of a 46-year-old female who developed severe pulmonary hypertension due to sarcoidosis. Her haemodynamics, exercise capacity, and functional class remarkably improved after the treatment with pulmonary arterial hypertension (PAH) targeted drugs including macitentan and tadalafil and secondary immunosuppressive therapy with corticosteroids.
Discussion
This case emphasizes the importance of considering the underlying mechanisms of sarcoidosis-associated PH in order to choose appropriate treatment.
Keywords: Sarcoidosis, Pulmonary hypertension, Pulmonary arterial hypertension targeted drug, Immunosuppression, Case report
Learning points
Pulmonary hypertension is one of the significant complications of sarcoidosis. The mechanisms of sarcoidosis-associated PH are very heterogeneous.
Pulmonary-targeted drugs could improve the patient’s haemodynamic status without causing clinically significant acute pulmonary oedema and hypoxia.
Immunosuppressive therapy may be useful to treat active sarcoidosis that could be evaluated with the 18F-fluorodeoxyglucose positron emission tomography.
Introduction
Sarcoidosis is a multisystem granulomatous disorder characterized by the formation of non-necrotizing granulomas in various organs. Pulmonary hypertension (PH) is one of the significant complications of sarcoidosis, occurring in 6–74% of patients.1,2 Sarcoidosis-associated PH is categorized in Group 5: PH with unclear and/or multifactorial mechanisms, in the updated clinical classification of PH.3 Several pathophysiological mechanisms, including parenchymal lung disease, extrinsic compression of pulmonary vessels, direct myocardial involvement, or granulomatous arteriopathy, are involved in developing sarcoidosis-associated PH.4 The presence of PH markedly increases mortality among these patients. However, no treatment of proven benefit has been established for this complex disease.5 Only one prospective randomized controlled trial supported the efficacy on haemodynamics of pulmonary vasodilator (bosentan) in the short term;6 however, the results of small retrospective studies on pulmonary vasodilators or immunosuppressive therapy were variable.7–9 Here, we report a 46-year-old female patient who developed severe PH due to sarcoidosis. She was treated with sequential combination therapy of pulmonary arterial hypertension (PAH)-targeted drugs and immunosuppressive therapy. She had an improvement in both pulmonary haemodynamics and exercise capacity.
Timeline
| Time | Events |
|---|---|
| 15 years prior to admission |
|
| Initial presentation |
|
| Day 1 |
|
| Day 15 |
|
| Day 20 |
|
| 2 months after admission |
|
| 7 months after admission |
|
Case presentation
A 46-year-old female was diagnosed with sarcoidosis approximately 15 years ago based on bilateral hilar lymphadenopathy but without any involvement of other organs. The patient was asymptomatic and did not receive any medications. However, she reported progressive exertional dyspnoea and leg oedema in the past few months and was admitted to our hospital, presenting with World Health Organization (WHO) functional Class IV. She had no heart murmur and fine crackles in the lower lung fields. The six-minute walk distance (6MWD) was 300 m, with desaturation from 85% to 61%. Laboratory tests showed serum brain natriuretic peptide (BNP) level of 32.9 pg/mL, KL-6 of 1140 U/mL, and normal serum concentrations of angiotensin-converting enzyme. Chest radiography showed bilateral hilar lymphadenopathy with bilateral interstitial lung opacities in the lower lung fields. Pulmonary function testing revealed a normal pattern but severe impairment of the diffusing capacity of the lung for carbon monoxide (DLCO), 16.2% of the predicted value. Transthoracic echocardiography revealed right atrial and ventricular dilatation with dislocation of the interventricular septum toward the left ventricle and trace tricuspid valve regurgitation with an elevated trans-tricuspid pressure gradient (TR-PG) of 42 mmHg, but normal left ventricle ejection fraction of 71%. Chest computed tomography (CT) showed mediastinal and hilar lymphadenopathy (Figure 1A), lung fibrosis, bilateral interstitial opacities, and thickened interlobular septa (Figure 2A). First, diuretics and oxygen therapy were initiated for 2 weeks. Leg oedema and dyspnoea improved gradually; however, echocardiography results were almost similar to those on admission, and TR-PG of 40 mmHg. Trans-bronchoscopic lung biopsy could have promoted a better understanding of the underlying pathophysiological mechanisms driving the PH. However, because of her respiratory condition, we decided not to perform it. Instead, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) of the enlarged mediastinal lymph nodes revealed non-caseating epithelioid cell granuloma, confirming the diagnosis of sarcoidosis (Figure 3). Right heart catheterization (RHC) revealed precapillary PH with a mean pulmonary artery pressure (mPAP) of 43 mmHg and pulmonary vascular resistance (PVR) of 9.59 WU (Table 1). Based on these findings, the definitive diagnosis of sarcoidosis-associated PH was made. Oral PAH-targeted therapy with an endothelin-receptor antagonist (macitentan 10 mg) was initiated, and, after excluding a deterioration of oxygenation, a phosphodiesterase type-5 inhibitor (tadalafil 20 mg) was added 5 days later. After 2 months, we observed improvements in the patient’s functional status from WHO functional Class IV to II. The 6MWD without supplemental oxygen improved from 300 m to 370 m, with desaturation from 93% to 72%. The second RHC demonstrated that mPAP decreased from 43 mmHg to 35 mmHg and PVR from 9.59 to 6.23 WU (Table 1). The 18F-fluorodeoxyglucose positron emission tomography (F-18 FDG-PET) highlighted bilateral uptake in hilar and mediastinal lymph nodes but not in the myocardium (Figure 4). As the patient still suffered from dyspnoea and the mPAP was elevated, we decided to start immunosuppressive therapy with prednisone 30 mg per day for 4 weeks tapering the dose by 10 mg every 4 weeks. Five months after starting prednisone clinical symptoms improved. In 6MWD without oxygen, she covered a walking distance of 400 m with saturation decreasing from 99% to 59%. The third RHC showed improvement of mPAP (23 mmHg) and PVR (5.03 WU) (Table 1). As exertional desaturation was still evident, domiciliary oxygen treatment was continued.
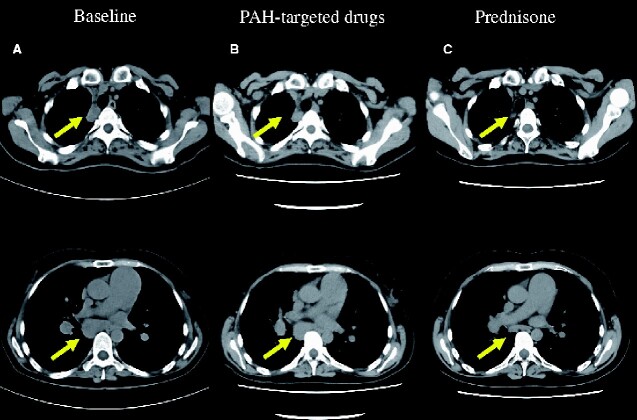
Figure 1.
The comparison of chest computed tomography (mediastinal window). (A): Computed tomography on admission showed mediastinal and hilar lymphadenopathy (yellow arrows). (B): Computed tomography after initiation of macitentan 10 mg and tadalafil 20 mg showed mediastinal and hilar lymphadenopathy same as that on admission. (C) Computed tomography after administration of prednisone revealed diminution in size of mediastinal and hilar lymph nodes.
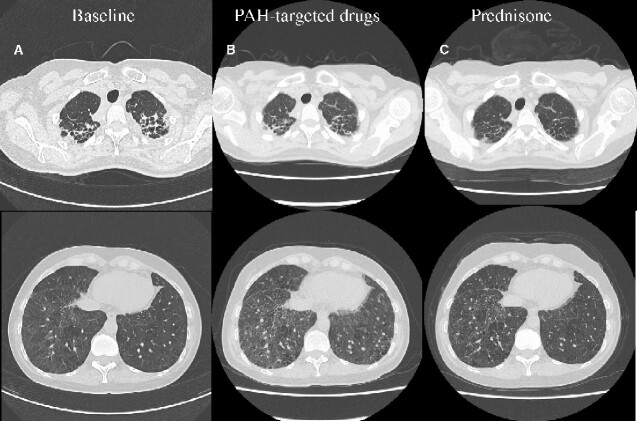
Figure 2.
The comparison of chest computed tomography (lung window). (A) Computed tomography on admission showed lung fibrosis, bilateral interstitial opacities, and thickened interlobular septa. (B) Computed tomography after initiation of macitentan 10 mg and tadalafil 20 mg showed lung fibrosis, bilateral interstitial opacities, and thickened interlobular septa same as that on admission. It newly revealed slight pulmonary oedema. (C) Computed tomography after administration of prednisone revealed some improvement in lung fibrosis, bilateral interstitial opacities, and thickened interlobular septa.
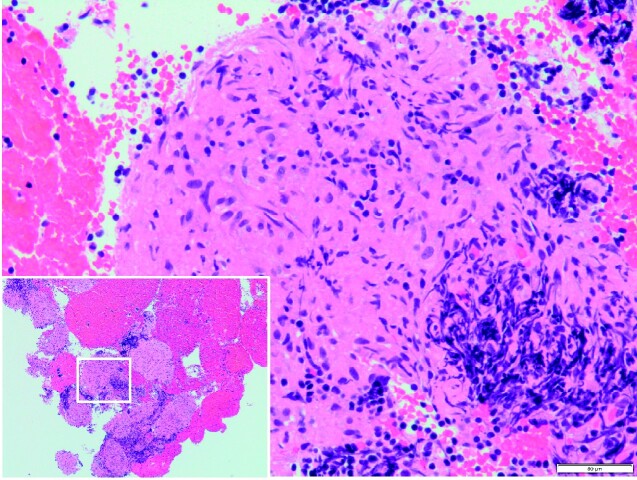
Figure 3.
Endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS-TBNA) of the enlarged mediastinal lymph nodes revealed non-caseating epithelioid cell granuloma, leading to the diagnosis of biopsy-proven sarcoidosis.
Table 1.
Clinical course and haemodynamics
| Baseline | 2 weeks after | 2 months after | 7 months after | |
|---|---|---|---|---|
| Therapies | Oxygen | Oxygen Macitentan 10 mg Tadalafil 20 mg | Oxygen Macitentan 10 mg Tadalafil 20 mg Prednisone | |
| Characteristics | ||||
| 6MWD (min) | 300 | 370 | 400 | |
| DLCO (% of predicted value) | 16.2 | 17.6 | 23.3 | |
| Haemodynamics | ||||
| Mean PAP (mmHg) | 43 | 35 | 27 | |
| Mean RAP (mmHg) | 1 | 3 | 1 | |
| PAWP (mmHg) | 15 | 15 | 7 | |
| Cardiac index (L/min/m2) | 1.96 | 3.21 | 2.56 | |
| PVR (WU) | 9.59 | 6.23 | 5.03 |
6MWD, 6-minute walk distance; DLCO, the diffusing capacity of the lungs for carbon monoxide; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrium pressure.
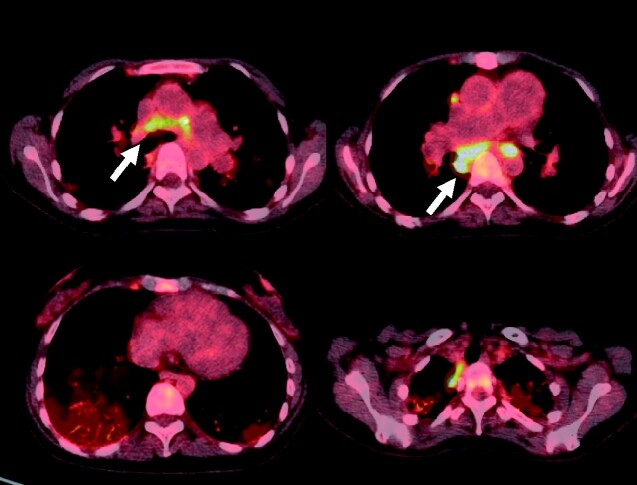
Figure 4.
The 18F-fluorodeoxyglucose positron emission tomography (F-18 FDG-PET) revealed bilateral uptake in hilar and mediastinal lymphadenopathy areas (white arrows) but not in the heart.
Discussion
Sarcoidosis-associated PH is classified in Group 5, subgroup 5.2: systemic disorders, of the clinical classification of PH.3 The mechanisms of PH are very heterogeneous and poorly understood.10 Sarcoidosis-associated PH can result from lung parenchymal disease of pulmonary fibrosis and hypoxaemia, granulomatous involvement of arterioles or pulmonary veins, compression of the proximal pulmonary arteries by hilar lymph nodes, and cardiac sarcoidosis.11,12 Therefore, each sarcoidosis patient needs an individual assessment of the pathophysiological mechanisms driving the PH, which is crucial for treatment selection. The use of PAH-targeted drugs is controversial. PAH-targeted drugs may improve short-term haemodynamics, however, may have the potential risk of worsening oxygenation by reversing physiologic hypoxaemic vasoconstriction and increasing blood flow to areas with poor ventilation, causing increased shunt or ventilation/perfusion (V/Q) inequality.13 In addition, they may also cause acute pulmonary oedema and hypoxaemia through preferential vasodilation of the pulmonary arteries in the setting of downstream venular obliteration by granulomas.2,14 Furthermore, in the largest cohort study of sarcoidosis-associated PH patients, only 4 out of 11 patients treated with immunosuppression alone showed haemodynamic improvements.13 Due to multiple pathophysiologic mechanisms, there is no gold standard strategy for treating sarcoidosis-associated PH patients so far.
In the present case report, as the patient suffered from the hypoxia even at the rest, oxygen was administered. Right heart catheterization performed 2 weeks after administration showed precapillary PH with slightly high pulmonary artery wedge pressure (PAWP) which may be due to low cardiac output caused by an enlarged right ventricle and compression of the left ventricle. Considering the results of her investigations, she was diagnosed with sarcoidosis-associated PH. As the left ventricle ejection fraction was normal and no arrhythmias were identified, cardiac sarcoidosis was deemed less likely. Mortality of sarcoidosis-associated PH is high, with no treatment of proven benefit. Therefore, we decided to trial available oral medical therapy and prepared the patient for a lung transplant in case this therapy was not effective. The PAH-targeted drugs macitentan 10 mg and tadalafil 20 mg were initiated to improve vasoreactivity. Haemodynamic status improved without causing clinically significant acute pulmonary oedema and hypoxia, although CT after initiation of PAH-targeted drugs showed slight pulmonary oedema. The possible reasons for the improvement in exercise capacity without clinically significant hypoxia are as follows: firstly, combination therapy of PAH-targeted drugs improved cardiac output drastically. Secondly, circulating plasma volume was well-controlled by diuretics. Because of persistent dyspnoea, F-18 FDG-PET was performed to evaluate the activity of sarcoidosis. Bilateral uptake was observed in hilar and mediastinal lymph nodes compressing the proximal pulmonary arteries that might be one of the causes of PH, immunosuppressive therapy was administrated. Follow-up CT revealed the diminution in the size of mediastinal and hilar lymph nodes. We observed further functional improvement.
Even though the treatment of sarcoidosis-associated PH remains controversial due to the limited data available mostly from case reports and small non-randomized series, considering the underlying mechanisms of PH will allow effective management of these patients. We would recommend long-term careful follow-up for this patient using WHO functional class, 6MWD, serum BNP, echocardiography, and RHC if needed according to PH risk stratification.15 To the best of our knowledge, no study has demonstrated the long-term efficacy of PAH-targeted drugs for the treatment of sarcoidosis-associated PH, which remains a life-threatening disease. More studies are necessary to investigate the treatment of sarcoidosis-associated PH.
Lead author biography

After graduating from university in 2013, Keiko Sumimoto went into the path of cardiology after 2 years of residency. After working as a general cardiologist for 3 years, she is studying echocardiography at Kobe University Graduate School.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors are grateful to Dr Yuichiro Matsuoka, Dr Hiroyuki Onishi, and PT PhD Yasunori Tsuboi for helpful discussion.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: None declared.
References
- 1. Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M. et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006;129:1246–1252. [DOI] [PubMed] [Google Scholar]
- 2. Shino M, Lynch J, Fishbein M, McGraw C, Oyama J, Belperio J. et al. Sarcoidosis-associated pulmonary hypertension and lung transplantation for sarcoidosis. Semin Respir Crit Care Med 2014;35:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shlobin OA, Baughman RP.. Sarcoidosis-associated pulmonary hypertension. Semin Respir Crit Care Med 2017;38:450–462. [DOI] [PubMed] [Google Scholar]
- 5. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 6. Baughman RP, Culver DA, Cordova FC, Padilla M, Gibson KF, Lower EE. et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014;145:810–817. [DOI] [PubMed] [Google Scholar]
- 7. Hostettler K, Baty F, Kleiner R, Junker L, Tamm M, Brutsche M.. Bosentan for patients with steroid-resistant pulmonary sarcoidosis: a randomised controlled trial. Swiss Med Wkly 2018;148:w14677.. [DOI] [PubMed] [Google Scholar]
- 8. Judson MA, Highland KB, Kwon S, Donohue JF, Aris R, Craft N. et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2011;28:139–145. [PubMed] [Google Scholar]
- 9. Milman N, Burton CM, Iversen M, Videbæk R, Jensen CV, Carlsen J.. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant 2008;27:329–334. [DOI] [PubMed] [Google Scholar]
- 10. Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML.. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005;128:1483–1489. [DOI] [PubMed] [Google Scholar]
- 11. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A. et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41. [DOI] [PubMed] [Google Scholar]
- 12. Duong H, Bonham CA.. Sarcoidosis-associated pulmonary hypertension: pathophysiology, diagnosis, and treatment. Clin Pulm Med 2018;25:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boucly A, Cottin V, Nunes H, Jaïs X, Tazi A, Prévôt G. et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017;50:1700465. [DOI] [PubMed] [Google Scholar]
- 14. Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW.. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest 2006;130:1481–1488. [DOI] [PubMed] [Google Scholar]
- 15. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O. et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53:1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.