Abstract
Background
Takotsubo syndrome (TTS) is a disease characterized by an acute and reversible myocardial injury typically precipitated by stressful and/or emotional triggers. Despite extensive research, its pathogenesis remains incompletely understood. Spasm of epicardial coronary arteries has been proposed as a potential pathogenic factor in TTS.
Case summary
Herein, we report the case of a 68-year-old female admitted to the emergency department after developing chest pain in concomitance with an intense emotional stress. A diagnosis of non-ST-segment elevation myocardial infarction (NSTEMI) was made. Coronary angiography disclosed normal coronary arteries, and left ventriculography showed an inferior focal akinesia with basal and apical hyperkinesis, so that a diagnosis of ‘focal TTS’ was made. Two months later, the patient was re-admitted with NSTEMI, and repeat coronary angiography showed an irregular subocclusive stenosis of a well-developed first obtuse marginal branch. After intracoronary nitroglycerine infusion, a complete recover of the vessel patency was noted, and a diagnosis of epicardial spasm was made. Intracoronary optical coherence tomography was performed to assess a residual ‘hazy’ region, which confirmed a normal vessel morphology and a residual focal area of spasm without signs of instability.
Discussion
Whether TTS and coronary artery spasm are two expressions of the same disease, or rather two separate entities with overlapping mechanisms remains unknown, and further research is warranted to solve this issue. Meanwhile, the opportunity of performing provocative tests for coronary spasm in patients with suspected TTS might be considered to gain more insights into this hypothesis.
Keywords: Takotsubo syndrome, Coronary spasm, MINOCA, Case report
Learning points
Takotsubo syndrome (TTS) and coronary artery spasm have similar predisposing factors and are often present in the same patients.
In TTS patients, it might be useful to perform a provocative test in order to rule out the presence of epicardial spasm and initiation of β-blocker therapy should be considered more carefully in that specific subgroup of patients with evidence of spasm.
Introduction
Takotsubo syndrome (TTS) is a disease characterized by an acute and reversible myocardial injury which typically recovers within days or weeks. Takotsubo syndrome frequently occurs in post-menopausal women and is often preceded by stressful and/or emotional events.1
In contrast with acute coronary syndrome (ACS), TTS’s ischaemia is not related to the thrombotic occlusion of an epicardial coronary artery, but most likely to a microvascular dysfunction associated with a sympathetic activation with release of catecholamines and endothelin.2
The first diagnosis and treatment of TTS patients remain a challenge since they usually present symptoms resembling those of ACS. Furthermore, the rate of TTS recurrence per-patient year is not low and it is often associated to newly stressors.3
Coronary artery spasm (CAS), which is frequently associated with an adrenergic surge, has been proposed as a potential pathogenic factor in TTS. One of the most intriguing theories in this field is that an abnormal coronary vasomotion may lead to a diffuse, transient spastic subocclusion/occlusion of epicardial vessels and to a critical ischaemia, which can be reproduced by provocative testing with acetylcholine early after a TTS episode.4,5
Whether TTS and CAS are two expressions of the same disease, or rather two separate entities with overlapping mechanisms remain unknown.6
Timeline
| Initial presentation | A 68-year-old woman was admitted to the emergency room for an acute chest pain referring a recent emotional stress |
| 28 February 2020 | Cardiac catheterization was performed and diagnosed a myocardial bridge and a focal takotsubo syndrome |
| 2 months later | The patient came back to our department for similar symptoms |
| 28 April 2020 | Cardiac catheterization was performed and diagnosed a focal coronary artery spasm, with a residual intimal hyperplasia evaluated at the optical coherence tomography |
| Discharge therapy | Calcium-channel blockers were added |
Case presentation
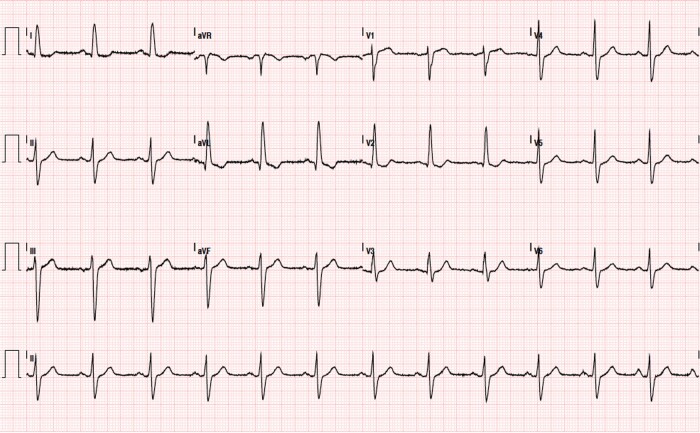
A 68-year-old woman was referred to our emergency department for the onset of an acute severe chest pain irradiated to the interscapular regions and to both arms lasting about 2 h. She had diabetes mellitus, hypertension, and former smoking as cardiovascular risk factors. Another relevant comorbidity was a mixed anxiety-depressed disorder under pharmacological treatment. She also reported a recent intense emotional stress. The patient appeared comfortable, with unlaboured breathing. The temperature was normal, the pulse 100 b.p.m., the blood pressure 140/90 mmHg, and the oxygen saturation 97% while she was breathing ambient air. No clinical signs of heart failure were present. High-sensitivity Troponin I levels were increased (i.e. 0.198 and 3.896 ng/mL in two consecutive samples, reference range <0.04 ng/mL). Electrocardiogram (ECG) showed sinus rhythm, signs of left ventricular hypertrophy and aspecific ST-T-wave abnormalities (Figure 1). After excluding an acute aortic syndrome by emergent chest computed tomography angiography a diagnosis of ACS was made, and was admitted to the Cardiology unit.
Figure 1.
Electrocardiogram at the time of takotsubo syndrome showing sinus rhythm, signs of left ventricular hypertrophy and aspecific ST-T wave abnormalities.
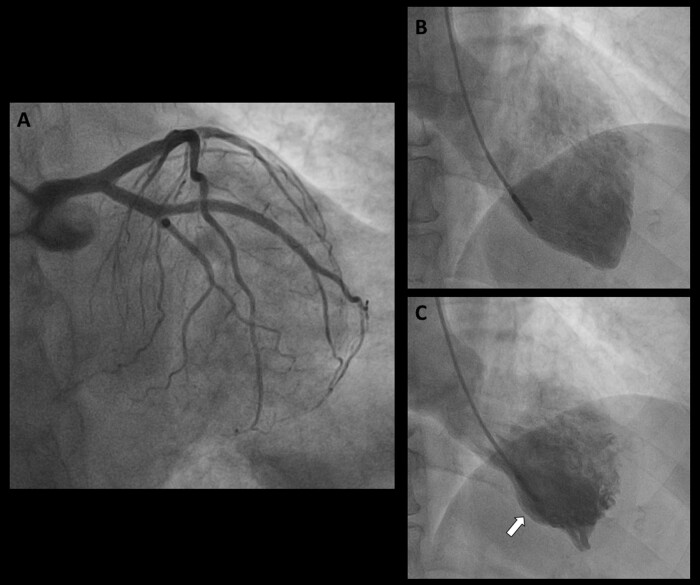
Baseline coronary angiography revealed non-obstructive coronary artery disease, and an intramyocardial bridge of the mid-left anterior descending (LAD) coronary artery (Figure 1A). Left ventriculogram showed a preserved global left ventricle function and an inferior focal akinesia with basal and apical hyperkinesis (Figure2B and C, Supplementary material online, Video S1). Based on the clinical and angiographic findings, a diagnosis of ‘focal’ TTS was made. Trans-thoracic echocardiography revealed a regional hypokinesia of the inferior mid-basal wall and septum. A cardiac magnetic resonance imaging was attempted to better define the cardiomyopathy findings, which the patient was not able to perform due to severe claustrophobia. Eventually, the patient was discharged with Bisoprolol 5 mg daily, Ramipril 2.5 mg daily, and Aspirin 100 mg daily planning a clinical follow-up at 3 months.
Figure 2.
Baseline coronary angiography showing non-obstructive coronary artery disease (A). Left ventriculogram showed a preserved global left ventricle function and a severe inferior focal akinesia with basal and apical hyperkinesis (B, C, white arrow).
However, 2 months later, the patient came back to the emergency department of our institution with recurrent chest pain and dyspnoea arising 3 h before. During the medical interview, she referred a newly emotional stress has been occurred. Physical examination and ECG were unchanged compared with the previous episode. N-terminal pro b-type natriuretic peptide was 244 pg/mL (normal values <150 pg/mL) and hs-TnI levels were abnormal with a rise-and-fall pattern. InterTAK score, which takes into account both clinical and diagnostic findings, was 60 reflecting a probability of a recurrence of TTS of 54%. Echocardiographic evidence of hypokinesis of the inferior septum, basal inferior wall, and mid-apical infero-lateral walls was reported.
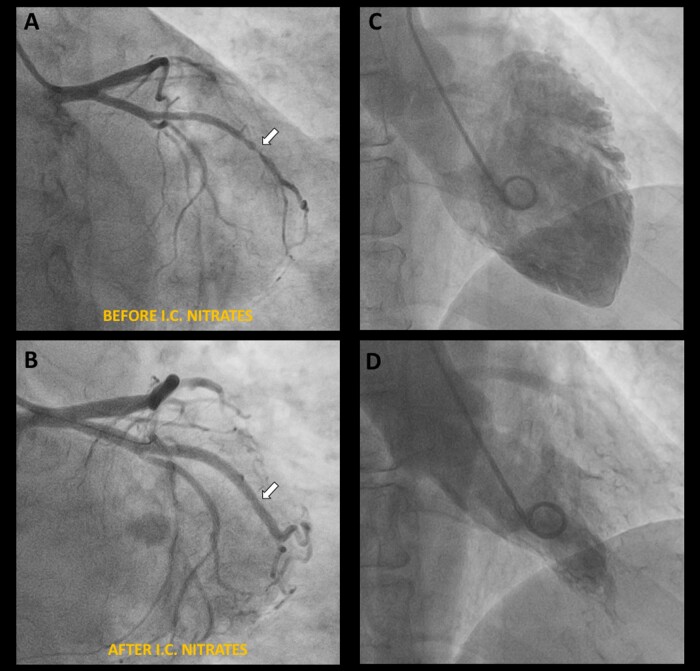
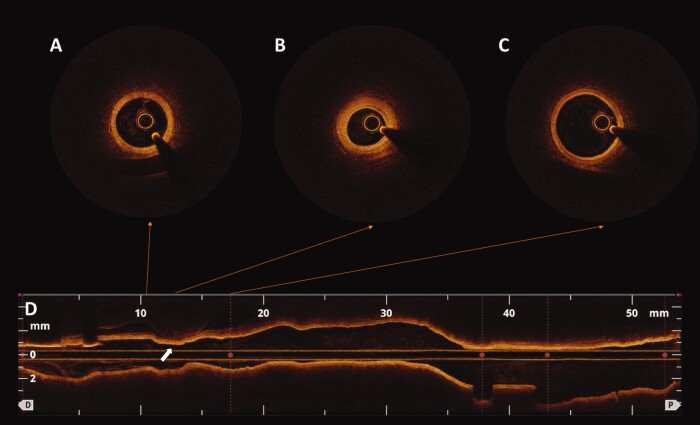
Coronary angiography showed an irregular subocclusive stenosis of a well-developed first obtuse marginal branch (Figure 3A, Supplementary material online, Video S2). However, after administration of intracoronary nitroglycerine, a complete recover of the vessel patency was noted, and a diagnosis of epicardial spasm was made (Figure 3B, Supplementary material online, Video S3). Due to the persistence of a mild residual stenosis with focal area of angiographic ‘haziness’, intracoronary optical coherence tomography (OCT) imaging was performed which confirmed a normal vessel morphology (Figure4A and C) and a residual focal area of spasm without any signs of instability (Figure 4B). Left ventriculogram showed an improved left ventricular function with only a mild residual hypokinesia of the inferior mid-ventricular wall, significantly improved compared with the previous exam (Figure3C and D, Supplementary material online, Video S4). Pre-discharge echocardiography revealed a full recovery of the regional wall motion abnormalities observed during both episodes (Supplementary material online, Video S5).
Figure 3.
Follow-up coronary angiography showing an irregular subocclusive stenosis of a well-developed first obtuse marginal branch (A, white arrow) and no other significant stenoses (A). After administration of intracoronary nitrates, a complete recover of the vessel patency was noted, and a diagnosis of epicardial spasm was made (B, white arrow). Left ventriculogram showed an improved left ventricular function without frank areas of akinesia (C, D).
Figure 4.
Intracoronary optical coherence tomography imaging showing a normal coronary vessel (A, C) with a focal area of intimal hyperplasia (B) corresponding to the region of residual spasm (D, white arrow), in the absence of any signs of instability.
Based on these new findings, medical therapy was optimized by adding Amlodipine 5 mg daily, and the patient was discharged. No further events occurred after 1 month of follow-up.
Discussion
Takotsubo syndrome and CAS share some predisposing factors and pathogenic mechanism, such as emotional triggers and adrenergic hyper-activation, and some evidence suggests a potential pathogenic link between these two conditions.
Takotsubo syndrome consists of a sustained, significant but self‐limiting event leading to a reversible impairment of a limited area of myocardium, typically defined as stunned myocardium. It is likely that critical transient spastic obstruction of an epicardial coronary vessel lasts long enough to determine myocardial stunning but not long enough to cause an irreversible damage to the involved area of myocardium. A number of mechanisms might favour this, such as: (i) an increased autonomic tone or critical spasmogenic stimuli, which result in increased circulating levels of catecholamines and/or stress-related neuroreceptors; (ii) an abnormal sensitivity of the endocrine and neuroendocrine myocardial receptors; and (iii) microvascular spasm.1,7
Dote et al.8 firstly proposed a causal relationship between TTS and multivessel CAS after observing that most patients of a TTS series had spontaneous or induced CAS at coronary angiography; similarly, Tsuchihashi et al.9 reported a rate of CAS of about 20% in a cohort of 48 patients with TTS.
More recently, Angelini10 proposed a fascinating theory on the pathogenesis of TTS, according to which a significantly increased coronary spasticity may cause a diffuse, transient spastic obliteration of coronary arteries and to a critical ischaemia, eventually leading to TTS.
Although intriguing, this hypothesis remains speculative and needs to be confirmed in future studies. Yet, seeking CAS in patients with TTS may provide significant advantage both for a better therapeutic management and for a more precise prognostic stratification.
In fact, while β‐blockers may provide some protection against recurrence of TTS attenuating the catecholamine surge, they may also increase tendency to spasm in these patients, probably due to the blockade of coronary β2-receptors, leaving α-adrenergic receptors unopposed.4
When epicardial coronary spasm is observed in patients with a previous episode of TTS, therapy with β-blocker should be considered with caution, and calcium-channel blocker therapy should be initiated.
Takotsubo syndrome is likely determined by the interplay of multiple, diverse pathophysiological mechanisms. In specific subsets of patients, some determinants of TTS may have a greater burden: this may be the case of CAS. Such speculation may be even more interesting if one considers that some coronary anatomic variants have been associated with TTS, such as wrap-LAD11—might patients with such variants be at greater risk of TTS if having also a CAS?
In conclusion, our report supports the notion of a potential interrelation between CAS and TTS. Whether TTS and CAS are two expressions of the same disease, or rather two separate entities with overlapping mechanisms remain unknown. Meanwhile, seeking CAS in patients with TTS by means of invasive provocative tests using acetylcholine or ergonovine might help to gain more insights into this issue.
Lead author biography

Marco Lombardi obtained his medical degree in 2018 at the University Campus Bio-Medico of Rome, Italy. Currently, he is completing the Cardiology residency at the Catholic University of the Sacred Heart of Rome, Italy.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
References
- 1. Pelliccia F, Kaski JC, Crea F, Camici PG.. Pathophysiology of takotsubo syndrome. Circulation 2017;135:2426–2441. [DOI] [PubMed] [Google Scholar]
- 2. Lüscher TF, Templin C.. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur Heart J 2016;37:2816–2820. [DOI] [PubMed] [Google Scholar]
- 3. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Lüscher TF. et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 4. Lanza GA, Careri G, Crea F.. Mechanisms of coronary artery spasm. Circulation 2011;124:1774–1782. [DOI] [PubMed] [Google Scholar]
- 5. Angelini P, Uribe C.. Is transient takotsubo syndrome associated with cancer? Why, and with what implications for oncocardiology? J Am Heart Assoc 2019;8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D. et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G. et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J 2010;31:1319–1327. [DOI] [PubMed] [Google Scholar]
- 8. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M.. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol 1991;21:203–214. [PubMed] [Google Scholar]
- 9. Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Owa M. et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol 2001;38:11–18. [DOI] [PubMed] [Google Scholar]
- 10. Angelini P. Transient left ventricular apical ballooning: a unifying pathophysiologic theory at the edge of Prinzmental angina. Cathet Cardiovasc Intervent 2008;71:342–352. [DOI] [PubMed] [Google Scholar]
- 11. Arcari L, Limite LR, Cacciotti L, Alonzo A, Musumeci MB, Passaseo I. et al. Tortuosity, recurrent segments, and bridging of the epicardial coronary arteries in patients with the takotsubo syndrome. Am J Cardiol 2017;119:243–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.