Abstract
Background:
In recent years, a number of clinical trials for antibody drugs targeting programmed cell death protein 1 (PD1)/programmed cell death 1 ligand 1 (PD-L1) have been carried out on recurrent or metastatic head and neck squamous cell carcinoma (R/M SCCHN) and reported promising prospects. To further evaluate and understand the effects and risk of anti-PD1/PD-L1 monotherapy vs standard of care (SoC) in R/M SCCHN, we conducted this meta-analysis of published randomized controlled trials.
Method:
The potential eligible trials were searched from Cochrane library and Pubmed and Embase databases. Randomized controlled trials evaluating the effects and risk of anti-PD1/PD-L1 monotherapy vs SoC in platinum refractory R/M SCCHN were selected. The outcomes, including objective response rate, disease control rate, progression-free survival, overall survival, and treatment-related adverse events, were extracted and pooled.
Results:
1345 patients with R/M SCCHN from three randomized controlled trials were enrolled in this analysis. Compared with SoC, anti-PD1/PD-L1 monotherapy could provide statistically significant overall survival benefit, hazard ratio (95% confidence interval ) = 0.79 [0.70–0.90]. However, we observed no significant difference between 2 treatments in progression-free survival (hazard ratio [95% confidence interval] = 0.96 [0.85–1.09]). Furthermore, anti-PD1/PD-L1 monotherapy caused less treatment-related adverse events than standard of care.
Conclusion:
Anti-PD1/PD-L1 monotherapy could indeed reduce the risk of death in R/M SCCHN patients, and provide higher safety vs SoC. Expression level of PD-L1 may be a useful biomarker for selecting patients with better response to anti-PD1/PD-L1 monotherapy.
Keywords: checkpoint inhibitor, head and neck squamous cell carcinoma, meta-analysis, PD1, PD-L1
1. Introduction
Head and neck carcinomas represent a class of biologically diverse solid tumors, including tumors in larynx, oropharynx, oral cavity, hypopharynx, and nasopharynx.[1,2] In the world, it is the sixth most prevalent malignant tumor, with approximately 830,000 patients diagnosed annually.[3] Squamous cell carcinoma constitutes 90% to 95% of all head and neck carcinomas.[4] At present, radiotherapy or surgery is effective for patients in early stage of SCCHN. However, most diagnosed patients are at advanced stage. Despite aggressive multidisciplinary treatments consisting of surgery, radiation, and chemotherapy, more than half of these patients have disease recurrence or suffer distant metastases within three years of treatment.[5,6] For these recurrent or metastatic patients, the first-line treatment had been the EXTREME regimen (cetuximab and platinum-based chemotherapy plus 5-fluorouracil), with limited efficacy and significant toxicity.[7,8]
After tumor progression, second-line treatment options were generally limited to a single-agent chemotherapy or cetuximab, and delivered limited survival benefits.[9] Therefore, there is an urgent need for a novel therapy that can provide better options. Immunotherapies have achieved great success in treating a variety of tumors by regulating immune checkpoints in T cells, such as cytotoxic T-lymphocyte protein 4 and programmed cell death protein 1 (PD1)/programmed cell death 1 ligand 1 (PD-L1).[10,11] It has been confirmed that immune escape and abnormal immune regulation play a key role in the occurrence and progression of head and neck cancer.[12] Over recent years, more and more clinical trials for antibody drugs targeting PD1/PD-L1 have been carried out on recurrent or metastatic head and neck squamous cell carcinoma (R/M SCCHN) and reported promising prospects.[13–17] Based on the findings of these trials, 2 anti-PD1 agents (pembrolizumab and nivolumab) obtained approval of the US Food and Drug Administration for the therapy of platinum-refractory R/M SCCHN in 2016.[5] Furthermore, several completed and ongoing phase III randomized controlled trials (RCTs) have provided new data. Here, we conducted this meta-analysis of RCTs to evaluate the efficacy and risk of anti-PD1/PD-L1 therapy in R/M SCCHN patients.
2. Methods
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.[18] The data used in this study were based on the published clinical researches, no ethical approval and patient consent are required.
2.1. Literature search
The relevant literatures published before May 30, 2020 were retrieved from the following databases: Cochrane Library, EMBASE, and PubMed. The main keywords used were: avelumab, atezolizumab, durvalumab, pembrolizumab, nivolumab, cemiplimab, checkpoint inhibitors, PD-L1, PD1, cancer, carcinoma, head and neck neoplasms, head and neck, tumor. The search strategy is combined with [MeSH Terms] and [Text Word]. We also did a manual search, using the reference lists of identified studies, to include other potentially eligible literatures.
2.2. Inclusion criteria
Literatures were selected according to the following criteria:
-
1.
the literature was about a randomized controlled trial on SCCHN patients who had recurrence or progression after a treatment containing platinum;
-
2.
the experimental group received anti-PD1/PD-L1 monotherapy;
-
3.
the control group received SoC therapy (single-agent chemotherapy or cetuximab);
-
4.
outcomes data, such as overall survival (OS), progression-free survival (PFS), treatment-related adverse events (trAEs), should be included.
Literatures should be excluded if they are:
-
1.
conference abstracts, reviews, letters, case reports, trial protocols, or comments;
-
2.
studies in which necessary data could not be extracted.
If there are literatures reporting on the same clinical trial, the literature that provide more and newer data will be selected.
2.3. Data extraction
Two authors (ZP, WYW) independently reviewed the selected literatures and extracted data as needed. Any disagreements were settled by discussion or consensus with another author (LX). The following data from each selected study were extracted: the trial name, publication year, patients number, PD-L1 status, antibody drug used, and multiple outcomes. Hazard ratio with 95% confidence interval (CI) was extracted for the statistical analysis of PFS and OS. Number of patients with certain events, such as complete response, partial response, stable disease, and adverse reactions, were also extracted for the evaluation of objective response rate (ORR), disease control rate (DCR), and trAEs.
2.4. Quality assessment
Two authors (ZP, WYW) independently assessed the methodological quality of selected literatures using the Cochrane Collaboration's tool.[19] Disagreements were also settled down by discussion among authors or consensus with a third investigator (LX).
2.5. Statistical analysis
We performed the meta-analysis for the extracted data using Review Manager software (RevMan version 5.3) provided by the Cochrane Collaboration. Time to event data OS and PFS were pooled as hazard ratio (HR) with 95% CI. DCR, ORR, and trAEs data were pooled as risk ratio (RR) with 95% CI. The Cochran Q test and I2 test were used to assess heterogeneity between studies. We used a random-effects model for meta-analysis when the heterogeneity test is statistically significant (I2 ≥ 50%, P < .1). Otherwise, a fixed-effect model was used. P < .05 is thought to have statistical significance for all outcomes data. Stata software (version 14.0) was used to assess the publication bias of the analysis results.
3. Results
3.1. Search results
We identified 1262 literatures, of which three RCT studies (with data for 1345 participants) were selected according to the inclusion criteria for this analysis (Figure 1).[20–22] All trials included SCCHN patients who had recurrence or progression after a treatment containing platinum. The antibody drugs used in the experimental arm of these RCT studies included one PD-L1 inhibitor and 2 PD1 inhibitors. The control arm in three RCT studies was the same, receiving a SoC therapy (single-agent chemotherapy or cetuximab). The characteristics of the 3 selected trials were presented in Table 1. The quality evaluation result of the included trials was shown in Figure 2. The result indicated that the included trials were with high quality.
Figure 1.
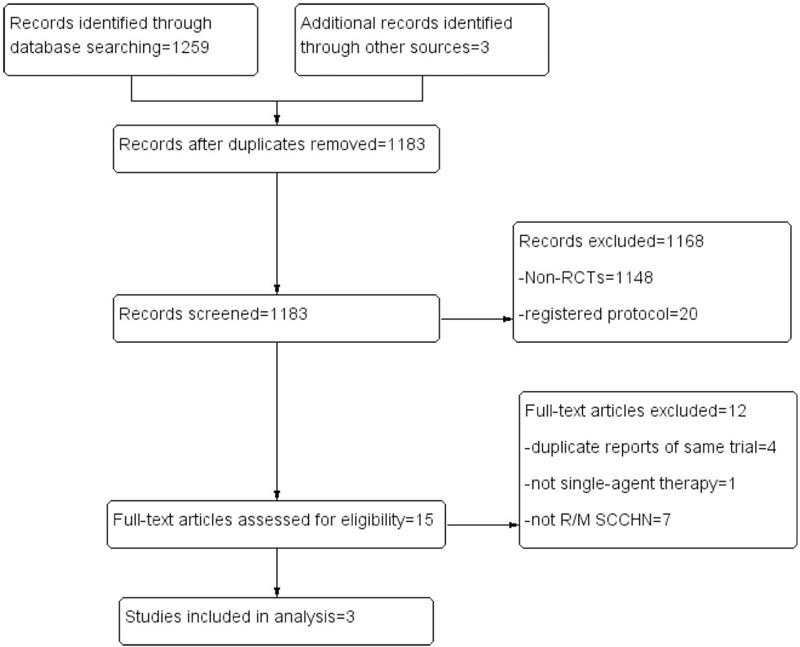
Flow diagram of study search and selection.
Table 1.
Characteristics of included trials.
| Median OS (mo) | |||||||||
| Trial | Year | Study design | Arms and interventions | N | Total | PD-L1+ | PD-L1− | 12- month OS rate | Median PFS |
| CheckMate141(XXXXX) | 2018 | A phase III randomized, open-label, multi-center trial | Nivolumab:3 mg/kg Q2W | 240 | 7.5 | TC≥1%: 8.2 | TC<1%: 6.5 | 36.0% | 2.0 mo |
| Standard of Care:Investigator's choice of methotrexate, docetaxel, or cetuximab | 121 | 5.1 | TC≥1%: 4.7 | TC<1%: 5.5 | 16.6% | 2.3 mo | |||
| KEYNOTE-040(XXXXX) | 2019 | A phase III randomized, open-label, multi-center trial | Pembrolizumab:200 mg Q3W | 247 | 8.4 | CPS≥1: 8.7TC≥50%: 11.6 | CPS<1: 6.3TC<50%: 6.5 | 37.0% | 2.1 mo |
| Standard of care:Investigator's choice of methotrexate, docetaxel, or cetuximab | 248 | 6.9 | CPS≥1: 7.1TC≥50%: 6.6 | CPS<1: 7.0TC<50%: 7.1 | 26.5% | 2.3 mo | |||
| EAGLE(XXXXX) | 2020 | A phase III randomized, open-label, multi-center trial | Durvalumab:10 mg/kg Q2W | 240 | 7.6 | TC≥25%: 9.8 | TC<25%: 7.6 | 37.0% | 2.1 mo |
| Standard of Care:Investigator's choice of cetuximab, taxane, methotrexate, or fluoropyrimidine | 249 | 8.3 | TC≥25%: 9.0 | TC<25%: 8.0 | 30.5% | 3.7mo | |||
N = number of patients, mo = months, OS = overall survival, PFS = progression-free survival, TC = tumour cell proportion score, CPS = combined positive score.
Figure 2.
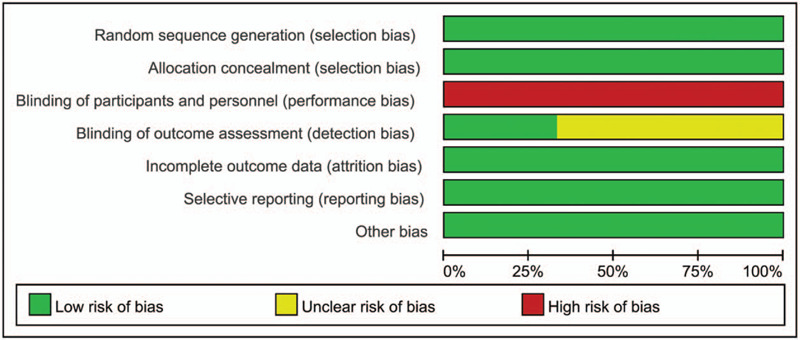
Quality evaluation of the included trials.
3.2. Efficacy data
Pooled analysis of all three RCTs indicated that anti-PD1/PD-L1 monotherapy led to an improvement in OS vs SoC with statistical significance (HR [95% CI] = 0.79 [0.70–0.90]). Due to the low heterogeneity (I2 = 26%), we used a fixed-effect model. The result was shown in Figure 3A. There were no publication bias on Egger test (P = .204) and Begg test (P = .296) in this analysis. However, the pooled analysis of PFS showed no statistical difference between 2 arms (HR [95% CI] = 0.96 [0.85–1.09]; Fig. 3 B).
Figure 3.
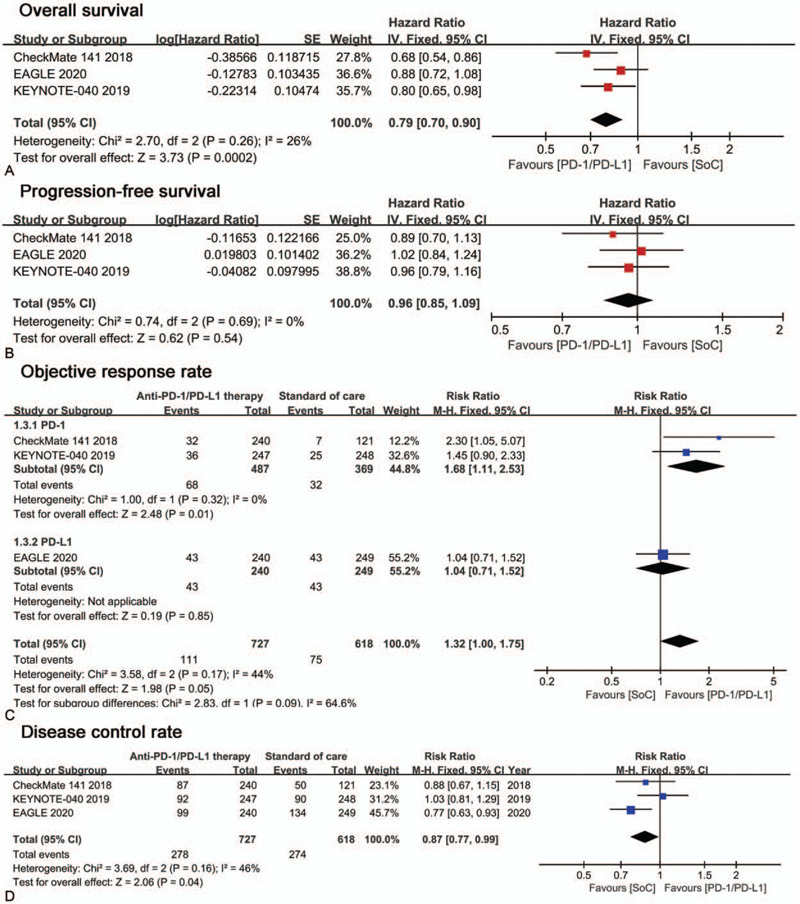
Forest plots of efficacy outcomes comparison: anti-PD1/PD-L1 monotherapy versus standard of care. Outcomes assessed are (A) Overall survival, (B) Progression-free survival, (C) Objective response rate, (D) Disease control rate.
Pooled analysis of ORR showed a risk ratio (RR) of 1.32 (95% CI: 1.00–1.75; Fig. 3C), which suggested a better effect of anti-PD1/PD-L1 therapy, but without statistical significance. ORR subgroup analysis showed that patients seems benefit more from anti-PD1 monotherapy (RR [95%CI] = 1.68 [1.11–2.53]) than from anti-PD-L1 monotherapy (RR [95%CI] = 1.04 [0.71–1.52]). Pooled analysis for DCR showed a risk ratio of 0.87 (95%CI=0.77–0.99; Fig. 3D), which suggested a better response to standard of care therapy with statistical significance.
3.3. Safety data
The safety of the treatments was evaluated by the incidence of trAEs of any grade and grade 3 to 4. The pooled analysis showed a risk ratio of 0.74 (95%CI = 0.69–0.79; Fig. 4A) for any grade trAEs and 0.38 (95%CI = 0.30–0.48; Fig. 4B) for grade 3 to 4 trAEs, which suggested a higher safety of anti-PD1/PD-L1 monotherapy.
Figure 4.
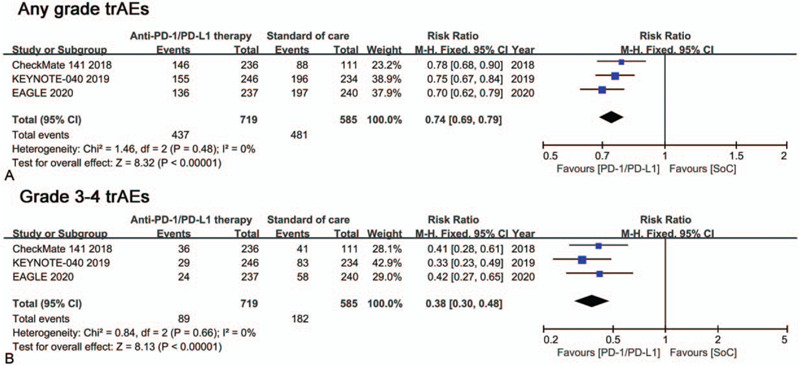
Forest plots of trAEs comparison: anti-PD1/PD-L1 monotherapy versus SoC. (A) Any grade trAEs, (B) Grade 3-4 trAEs.
4. Discussion
Over the last decade, SCCHN patients who recurred or metastasized after platinum chemotherapy have limited effective therapy options and a poor prognosis. The advent of the era of immune checkpoint therapy brings new hope for the therapy of R/M SCCHN, which is characterized as immunosuppression and high tumor mutation burden. The PD1 and its ligand PD-L1 axis is a key immune checkpoint inhibiting the excessive activation of T cells and can be targeted to reactivate anti-tumor immune response of T cells.[23] Recent years, there are more and more clinical researches of PD1/PD-L1 antibodies on R/M SCCHN. In a previously published meta-analysis, only 2 RCT studies on PD1 inhibitors were included.[24] In addition, for time-to-event outcomes OS and PFS, that study used odds ratio as effect measures, which is commonly used in case-control studies, resulting in an unreliable estimate and wrong interpretation.
Recently, the results of randomized controlled trial EAGLE have just been published, evaluating PD-L1 inhibitor durvalumab in patients with R/M SCCHN. Here, we conducted an updated meta-analysis of RCTs including EAGLE to further understand the effects and risk of anti-PD1/PD-L1 monotherapy vs SoC in R/M SCCHN. The standard of care means the investigator's choice of a single-agent, standard doses of cetuximab, docetaxel, paclitaxel, or methotrexate. Our results show that, compared with SoC, anti-PD1/PD-L1 therapies offer a great improvement on OS, with a 21% lower risk of death. But the progression-free survival (PFS) is quite similar in 2 groups, probably because some patients might not benefit from anti-PD1/PD-L1 monotherapy. In KEYNOTE 040, the PFS and OS were longer with anti-PD1 agent (pembrolizumab) in patients with a PD-L1 tumor proportion score (TPS; the percentage of tumor cells with membranous PD-L1 staining) of 50% or higher (Table 1). Similarly, in CheckMate141, patients with a PD-L1 tumor cell (TC) expression level of 1% or higher were associated with prolongation of OS compared to SoC. In EAGLE, the OS was also improved in PD-L1 positive (TC ≥ 25%) patients compared to SoC. In a word, PD-L1 expression status may be a predictive biomarker for the efficacy of anti-PD1/PD-L1 monotherapy in R/M SCCHN patients. Because of different testing method and score criteria for PD-L1 expression status, we did not conduct a sub-group meta-analysis on OS for the included 3 RCTs.
Furthermore, the results of sub-group meta-analysis on ORR showed that patients seem to benefit more from anti-PD1 therapy than from anti-PD-L1 therapy. Compared with SoC, anti-PD1 therapy has a 68% higher of ORR, yet anti-PD-L1 therapy has no difference.
SoC has a 13% higher of disease control rate vs anti-PD1/PD-L1 monotherapy. However, the 12-month OS rate is higher for anti-PD1/PD-L1 monotherapy compared with SoC in the included three studies (Table 1). Regarding safety, trAEs of any grade occurred in 60.8% in the anti-PD1/PD-L1 arm, 82.2% in the SoC arm. Grade 3 to 4 trAEs occurred in 12.9% patients receiving anti-PD1/PD-L1 monotherapy, 31.5% patients receiving SoC.
A limitation of this analysis is that only 3 RCTs are included in this study. Second, the included three RCTs are of open-label design and supported by pharmaceutical industry funding. Third, only 1 anti-PD-L1 therapy is included.
On the whole, these data lend support to anti-PD1/PD-L1 monotherapy as a better clinical choice compared with SoC for R/M SCCHN patients, who experienced tumor progression after platinum-based therapy. The expression status of PD-L1 may be a biomarker in patients’ selection for better response to anti-PD1/PD-L1 agents. Unified evaluation criteria for PD-L1 expression status are needed in further clinical trials.
Author contributions
Conceptualization: Peng Zhu, Xin Liu.
Data curation: Wendi Zhang.
Formal analysis: Peng Zhu.
Software: Peng Zhu.
Supervision: Xin Liu.
Validation: Peng Zhu, Yanwei Wang.
Visualization: Xin Liu.
Writing – original draft: Yanwei Wang.
Writing – review & editing: Peng Zhu, Wendi Zhang.
Glossary
Abbreviations: CI = confidence interval, DCR = disease control rate, HR = hazard ratio, ORR = objective response rate, OS = overall survival, PD1 = programmed cell death protein 1, PD-L1 = programmed cell death 1 ligand 1, PFS = progression-free survival, R/M SCCHN = recurrent or metastatic head and neck squamous cell carcinoma, RCTs = randomized controlled trials, trAEs = treatment-related adverse events.
References
- [1].Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw 2017;15:761–70. [DOI] [PubMed] [Google Scholar]
- [2].Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 2016;91:386–96. [DOI] [PubMed] [Google Scholar]
- [3].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [4].Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology 2016;91:13–23. [DOI] [PubMed] [Google Scholar]
- [5].Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008;83:489–501. [DOI] [PubMed] [Google Scholar]
- [7].Steinbichler TB, Lichtenecker M, Anegg M, et al. Persistent head and neck cancer following first-line treatment. Cancers (Basel) 2018;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- [9].Guardiola E, Peyrade F, Chaigneau L, et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer 2004;40:2071–6. [DOI] [PubMed] [Google Scholar]
- [10].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tong CC, Kao J, Sikora AG. Recognizing and reversing the immunosuppressive tumor microenvironment of head and neck cancer. Immunol Res 2012;54:266–74. [DOI] [PubMed] [Google Scholar]
- [13].Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with >/=25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019;107:142–52. [DOI] [PubMed] [Google Scholar]
- [14].Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- [16].Segal NH, Ou SI, Balmanoukian A, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer 2019;109:154–61. [DOI] [PubMed] [Google Scholar]
- [17].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- [21].Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. [DOI] [PubMed] [Google Scholar]
- [23].Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol 2015;33:3293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang BC, Cao RB, Li PD, et al. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: a systematic review and meta-analysis. Cancer Med 2019;8:5969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


