Abstract
Background:
To evaluate the effectiveness and safety of thermal mineral waters therapy for pain relief, and functional improvement, and quality of life (QoL) in patients with osteoarthritis (OA).
Methods:
Cochrane Library, Web of science, EMBASE, ClinicalTrials.gov and PubMed were systematically searched for randomized controlled trials. Study inclusion criteria included assessment of the visual analog scale and Western Ontario and McMaster Universities scores and the lequesne index to evaluate the effects of thermal mineral waters on pain relief and functional improvement. Also, studies that used the European quality of life 5-dimension scale and health assessment questionnaire to assess the impact of thermal mineral waters therapy on improving QoL were included.
Results:
Sixteen studies were included. A meta-analysis showed that thermal mineral waters therapy could significantly reduce pain as measured visual analog scale and Western Ontario and McMaster Universities assessments (P < .001). Thermal mineral waters significantly reduced the lequesne index (P < .001) and improved joint function. Finally, compared with a control group, European quality of life 5-dimension scale and health assessment questionnaire improved significantly in patients with OA receiving thermal mineral waters therapy (P < .05). There is no evidence that thermal mineral waters is unsafe for treating OA.
Conclusion:
Thermal mineral waters therapy is a safe way to relieve pain, improve physical functions, and QoL in patients with OA.
Keywords: meta-analysis, osteoarthritis, physical therapy, thermal mineral waters
1. Introduction
Osteoarthritis (OA) is a chronic disease that affects joints such as the hands, knees, and hips, causing considerable pain, increased disability, and progressive cartilage degeneration.[1] As the most common type of arthritis, OA is the main cause of decreased function and reduced quality of life (QoL).[2] It often occurs in adults over the age of 50 and is one of the main causes of disability worldwide.[3,4] Due to the increasing rate of obesity and the aging population, the prevalence of OA may increase in the next 20 years.[5]
At present, there is no specific medicine for OA, and clinical treatment methods for OA include nonpharmacologic and pharmacologic modalities.[6] Pharmacologic therapy is mainly limited to analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) or selective cyclooxygenase 2 (COX-2) inhibitors. However, the use of NSAIDs can cause damage to the gastrointestinal tract,[7] and the use of COX-2 inhibitors is complicated by side effects in the cardiovascular system.[8] Compared with NSAIDs and COX-2 inhibitors, acetaminophen is better tolerated, but does not always provide adequate pain relief.[9] Pharmacologic therapy is limited to the relief of symptoms such as pain, stiffness, and impaired mobility.[10,11] It is worth noting that complementary and alternative medicinal therapies have been widely promoted, and there are a growing number of reports about the use of these therapies by patients with OA.[12]
The term balneotherapy comes from the Latin term balneum, meaning bath. The term is usually used for bathing in thermal or mineral water, and although it is different from hydrotherapy, both terms have been accepted for all forms of water-based treatments.[13] The most important uses of mineral water are bathing, drinking and inhalation. The physical properties and chemical effects of thermal mineral waters therapy may play an important role in the treatment of diseases. The environment, density, buoyancy, specific gravity, viscosity, hydrostatic pressure, temperature, and dissolved substances of mineral water will affect balneotherapy.[14] In vitro tests and clinical studies have shown that certain water-soluble minerals can be absorbed through the skin. Immersion during thermal mineral waters therapy has many physiological effects and causes changes in the neuro-immuno-endocrine system.[15,16] The 2013 International Osteoarthritis Research Society International guidelines first recommended balneotherapy combined with biomechanical intervention, intra-articular corticosteroids, oral COX-2 inhibitors and antidepressants for the treatment of multi-joint OA with comorbidities.[17]
Since prehistoric times, thermal mineral waters therapy has been used to treat rheumatic diseases.[18] The practice and research of rheumatism began in European spas, such as the Aix-les-Bains in France, the Strathpeffer in Scotland, and Bath in England.[13,19] In recent years, thermal mineral waters therapy has become a popular form of treatment, suitable for all forms of arthritis. Reports on the treatment of OA with thermal mineral waters therapy have shown positive results.[20–35] Previous systematic reviews and meta-analyses were limited to studying the efficacy of balneotherapy on pain and function in patients with knee OA. In contrast, the efficacy of balneotherapy on hip or hand OA has received less attention.[36–39] Harzy et al first reviewed the short-term and long-term therapeutic effects of thermal mineral waters therapy for OA in the knee. They included 493 patients in the study, and all interventions were able to reduce pain and improve functional ability.[36] A review by Tenti et al concluded that spa therapy seems to play a role in the treatment of knee OA and that it may complement traditional therapies, but it should not replace traditional therapies entirely.[37] Matsumoto et al conducted a meta-analysis on the effects of balneotherapy on pain relief, stiffness, and physical function in patients with knee OA. The Western Ontario and McMaster Universities (WOMAC) scores were used as the outcome measure. The results of the meta-analysis showed that, compared with a control group, the WOMAC score showed that balneotherapy could effectively relieve pain and stiffness and improve function for people with OA. However, there was high heterogeneity (88% – 93%) in the data.[38] Antonelli et al conducted a meta-analysis on the impact of balneotherapy and spa therapy on the QoL of patients by including 17 studies and concluded that balneotherapy and spa therapy could significantly improve the QoL of patients with knee OA.[39]
In this study, recent and updated evidence was collected to evaluate the analgesic effects and improvements in joint function attributed to thermal mineral waters therapy in the treatment of OA. In addition, this study evaluated the effects of thermal mineral waters therapy on reducing pain, stiffness, joint function, and QoL in patients with OA.
2. Methods
2.1. Search strategy
This study does not need the approval of the ethics committee because it is based on literature research. Five electronic databases were searched for studies in which mineral water hydrotherapy was used to treat OA, including the Cochrane Library, Web of Science, EMBASE, ClinicalTrials.gov, and PubMed. The keywords"“thermal mineral waters,” “spa therapy,” “balneotherapy,” “osteoarthritis,” and “randomized controlled trial,” and corresponding terms of medical subject headings (MeSH) were used to conduct the search. Each keyword was combined with the corresponding MeSH word with the “OR” operator, and then the 5 keywords were combined with the “AND” operator. The search was limited to randomized controlled clinical trials (RCTs), but there were no restrictions on the release date. Articles were screened by 2 independent reviewers (TM and YM) to remove ineligible articles. If required, disagreements were resolved by a third reviewer (LG). The systematic review was further evaluated to ensure that all appropriate studies were included. The search was conducted in July 2020.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows:
-
1)
The study was an RCT with either parallel or case-crossover design;
-
2)
Sufficient information on the visual analog scale (VAS), Lequesne index (LI), or WOMAC was provided at baseline and at the end of follow-up, or the study presented the net change values in each study group;
-
3)
The length of follow-up was not limited;
-
4)
Patients in the experimental group received hydrotherapy or hydrotherapy adjuvant therapy; or
-
5)
The intervention for the experimental group contained thermal mineral waters therapy, while interventions for the control group were not limited, with the exception of thermal mineral waters therapy.
Studies were excluded if:
-
1)
they were published in a language other than English; or
-
2)
there was insufficient or unextractable data. Articles were screened by 2 independent reviewers to remove ineligible articles. If required, disagreements were resolved by a third reviewer.
2.3. Data extraction and outcome measures
Data was extracted from qualifying studies by 2 reviewers (XS and YL) using standardized forms. The study database recorded basic information of the qualified studies (first author, country, and published date), characteristics of the study participants (sex, age, and location of OA), characteristics of the mineral water (mineral composition and average temperature), the study interventions, duration of the intervention, and follow-up periods. The outcome measures comprised pain outcomes (VAS, WOMAC),[40,41] physical function outcomes (LI, functional index for hand osteoarthritis score, minimal clinically important improvement, range of movement, knee injury and osteoarthritis outcome score,[42–45] and QoL evaluation (the Arthritis Impact Measurement Scales, the European Quality of life 5-Dimension Scale (EQ-5D), the Nottingham Health Profile, the Short Form 36-item Health Survey (SF-36), or the Health Assessment Questionnaire (HAQ).[46–48]
2.4. Quality assessment
Two investigators (XS and HH) independently assessed the risk of bias in each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.[49] Differences were resolved through discussion or consultation with a third reviewer (HB). Studies were evaluated in terms of allocation sequence generation (selection bias), concealment of allocation (selection bias), outcome assessment (performance bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting data), and other bias categories. The risk of bias was graded for each potential source of bias as either high, low, or unclear, and the justification for these judgments is presented in the “risk of bias” table (Figs. 1 and 2).
Figure 1.
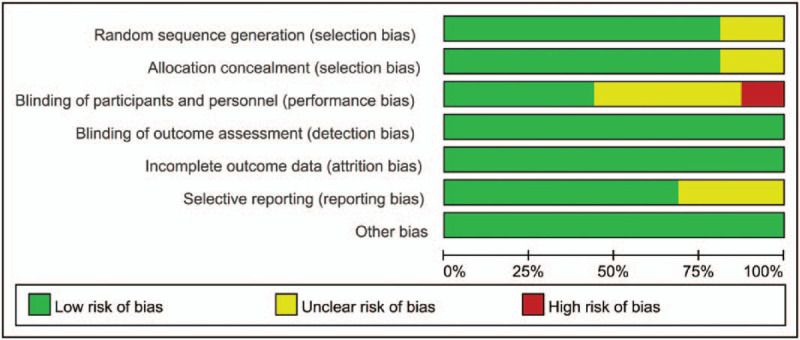
Risk of bias. author judgments about each risk of bias item, presented as percentages across all included studies.
Figure 2.
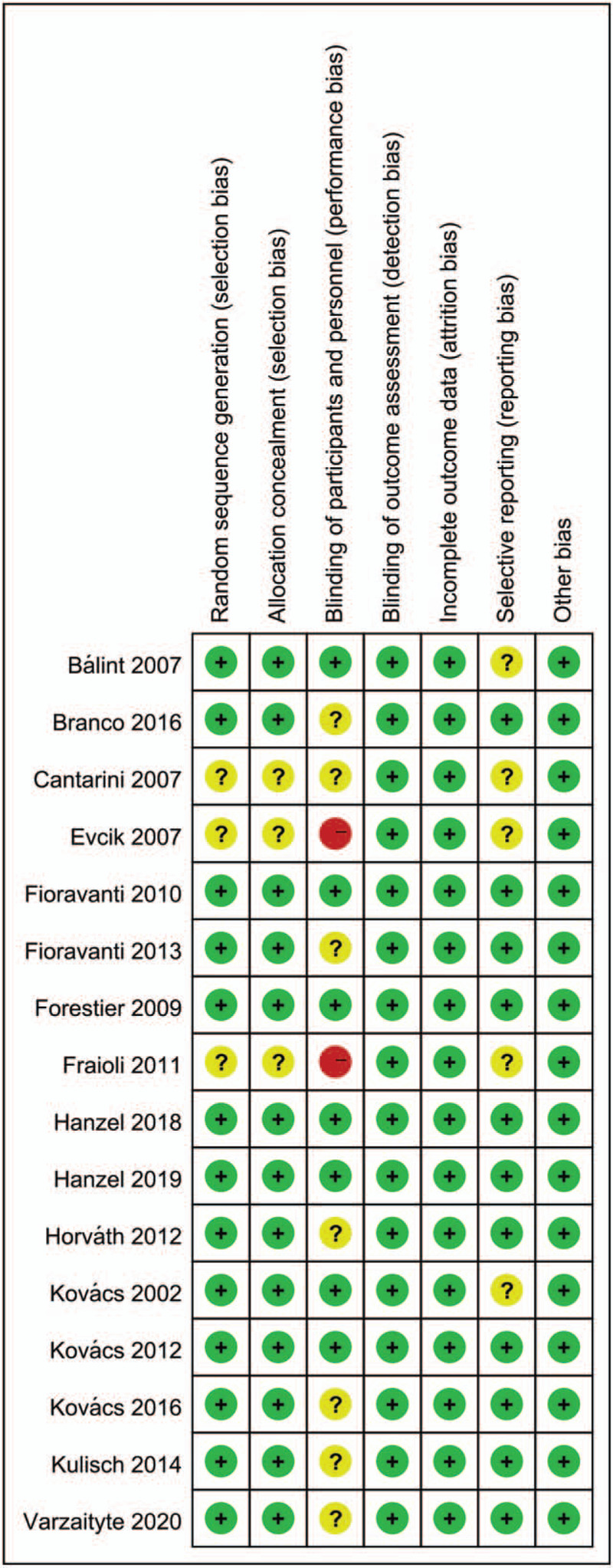
Risk of bias summary. author judgments about each risk of bias item for each included study.
2.5. Statistical analysis
The standardized mean difference (SMD) and 95% confidence interval (CI) were calculated for continuous data. An inverse variance fixed or random-effects model was applied in calculating the weighted mean difference (WMD). Statistical heterogeneity was assessed with the I2 test. An I2 > 50% indicates medium or high heterogeneity, in which case the random-effects model was used. For outcome indicators such as VAS and WOMAC, a subgroup analysis was performed. In order to observe the impact of any study on the aggregate WMD, the WMD and the corresponding 95% CIs were recalculated by eliminating individual studies one by one and performing sensitivity analysis. A P-value <.05 indicated a significant difference. All analyses were conducted using Stata 15.1 (Stata Corporation, College Station, TX).
3. Results
3.1. Study selection
A total of 534 related studies were obtained from the four databases. A total of 309 duplicate articles were excluded. After screening the title and abstract, 191 articles were excluded. After reading the full text and careful review of the remaining 34 studies, 18 articles were excluded for several reasons, including that they were not RCTs (n = 6), did not include people with OA (n = 10), or were not written in English (n = 2). Finally, 16 RCT studies met the inclusion criteria and were included in the meta-analysis. The study selection process (Fig. 3) followed the PRISMA guidelines.[50]
Figure 3.
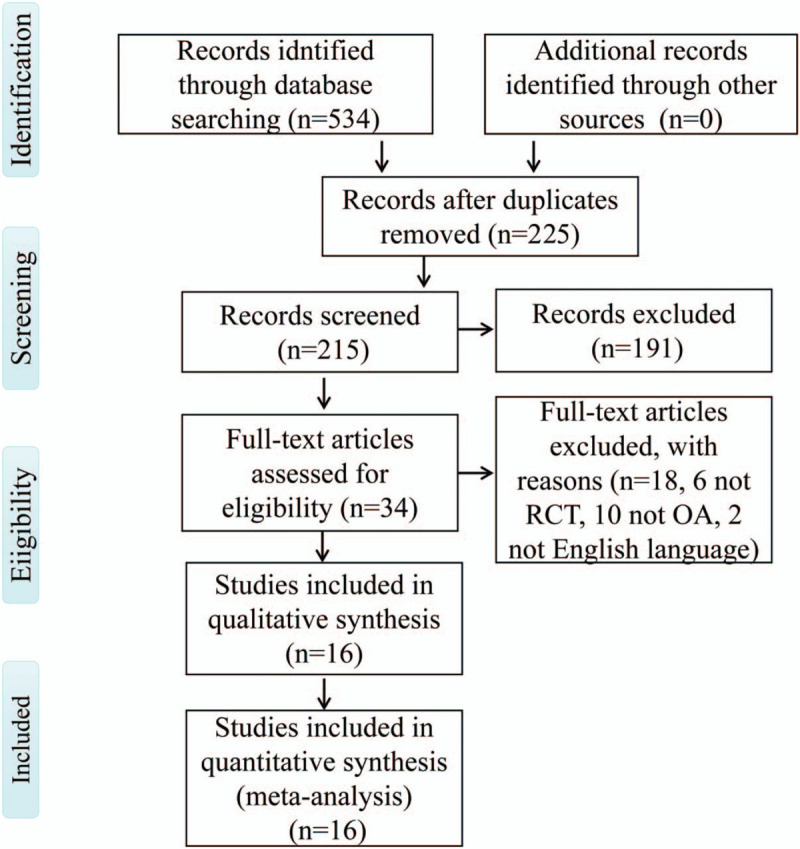
Flowchart for the selection of included studies.
3.2. Study characteristics
Table 1 summarizes the data from the trials selected for this meta-analysis. The 16 RCTs that qualified for inclusion had a total of 1273 participants. The intervention groups included 649 patients (50.98%), while the control groups included 624 (49.02%). Five of the study intervention groups used a combination of mud-bath therapy and mineral waters immersion therapy,[20,25,29,31,34] and the remaining 11 studies used only mineral waters immersion therapy to treat OA. Due to the high incidence of OA in females, more females participated in the studies. The included studies were published between 2002 and 2020. The duration of each treatment session was between 15 to 30 minutes in duration at a temperature of 34° to 39°C. The treatment cycle was between 2 to 10 weeks. The length of follow-up was 1 month to 1 year. Selected studies enrolled subjects with a diagnosis of OA in the hand, knee, or hip.
Table 1.
Characteristics of the included randomized controlled trials.
| Study | Design | Characteristics of subjects | Mineral composition | Intervention group | Comparison group | Intervention and treatment duration | QoL Scales | Location of OA | Outcome measurements | Follow-up |
| Fraioli et al (2011) | RCT 3 parallel groups | Italy age: > 50 yr M/F:unclear | HCO3-, SO42–, Mg2+ | Group I, Mud-bath therapy + mineral water immersion, N = 17 | Group II, Standard treatment, N = 44 | 47°C,15 min; 3 full cycles | ND | Knee | VAS, LI | 1 yr |
| Branco et al (2016) | RCT 3 parallel groups | Brazil Age: 64.8 ± 8.9 yr M/F:25/115 | The sulfurous water: H2S, SO42–,HCO3, F−, Na+; non-sulfurous water: CI−, Fe3+, Mn2+,F− | Group I, mineral water immersion, N = 47 | Group II, non-sulfurous water immersion, N = 50; Group III, standard treatment, N = 43 | 37°C–39°C, 20 min; 3 times a wk for 10 wks | HAQ | Knee | VAS, LI | 2 mo |
| Hanzel et al (2018) | RCT 2 parallel groups | Hungary Age: 66.7 ± 4.79 yr M/F: 17/33 | Na+, Ca2+, Mg2+, NH4+, K+, et al | Group I, mineral water immersion, N = 26 | Group II, tap water immersion, N = 24 | 34°C, 30 min; 3–5 d of treatment per wk, a total of 15 treatments | SF-36 | Hip or knee | VAS, WOMAC ROM | 3 mo |
| Kulisch et al (2014) | RCT 2 parallel groups | Hungary Age: 45–75 yr M/F:17/60 | Na+, Cl−, K+, I−, Mg2+, F−, Ca2+, HCO3-, Fe3+, et al | Group I, mineral water immersion, N = 38 | Group II, tap water immersion, N = 39 | 34°C, 30 min; 5 d of treatment per wk, a total of 15 treatments | EQ-5D | Knee | VAS, WOMAC | 15 wk |
| Hanzel et al (2019) | RCT 3 parallel groups | Hungary Age: 67.3 ± 4.48 yr M/F: 24/50 | Na+, Ca2+, Mg2+, NH4+, K+, Fe2+, Li+, Cl−, Br− | Group I, mineral water immersion, N = 26 | Group II, tap water immersion, N = 24; group III, organic fraction, N = 24 | 34°C, 30 min; 5 times a wk for 3 wk | SF-36 | Hip or knee | VAS, WOMAC, ROM | 3 mo |
| Fioravanti et al (2010) | RCT 2 parallel groups | Italy age: 54–81 yr M/F: 20/60 | HS−, CO2, HCO3−, SO42−, Ca2+, Mg2+, et al | Group I, mud-bath therapy + mineral water immersion, N = 40 | Group II, standard treatment, N = 40 | 38°C, 15 min; 12 times in 2 wk | AIMS1 | Knee | VAS, LI | 9 mo |
| Varzaityte et al (2020) | RCT 3 parallel groups | Lithuania age: 64.6 ± 11.4 yr M/F:13/87 | Na+, Cl−, et al | Group I, mineral water immersion, N = 30; | Group II, Mud-bath therapy, N = 32; Group III, standard treatment, N = 30 | 36°C–42°C, 20 min; 10 procedures every other d | SF-36 | Knee | VAS, KOOS | 1 mo |
| Bálint et al (2007) | RCT 2 parallel groups | Hungary age: 50–75 yr M/F: 33/19 | Na+, K+, NH4+, Ca2+, Mg2+, et al | Group I, mineral water immersion, N = 27 | Group II, Short wave therapy, N = 25 | 34°C, 30 min; 5 d for 4 wk | ND | Knee | WOMAC | 3 mo |
| Kovács et al (2002) | RCT 2 parallel groups | Hungary M/F: 41/17 | NaHCO3, H2SiO3,et al | Group I, mineral water immersion, N = 31 | Group II, tap water immersion, N = 27 | 36°C, 30 min; 15 d | ND | Knee | VAS, Symptom scores | 3 mo |
| Cantarini et al (2007) | RCT 3 parallel groups | Italy age: 48–86 yr M/F: 47/27 | H2AsO3, Fe2+, et al | Group I, mud-bath therapy + mineral water immersion, N = 30 | Group II, tap water immersion, N = 24; group III, standard Treatment, N = 20 | Mud-bath therapy: 45°C, 20 min; mineral water immersion: 38°C, 15 min; 15 times in 3 wk | AIMS1 | Knee | VAS | 3 mo |
| Kovács et al (2012) | RCT 2 parallel groups | Hungary Age: 47–73 yr M/F: 3/42 | Na+, K+, NH4+, Ca2+, Mg2+, Fe3+, Mn2+, et al | Group I, mineral water immersion, N = 24 | Group II, Standard treatment, N = 21 | 37°C, 20 min; 15 times in 3 wk | HAQ, EQ-5D | Hand | VAS | 6 mo |
| Fioravanti et al (2014) | RCT 2 parallel groups | Italy Age: 50–75 yr M/F: 8/52 | Ca2+, Mg2+, Na+, K+, NH4+, F−, HCO3−, et al | Group I, mud-bath therapy + mineral water immersion, N = 30 | Group II, standard treatment, N = 30 | Mud-bath therapy: 43°C, 20 min; mineral water immersion: 38°C, 15 min; 2 wk | HAQ, SF-36 | Hand | VAS, FIHOA | 6 mo |
| Horváth et al (2012) | RCT 2 parallel groups | Hungary Age: 50–70 yr M/F: 12/51 | K+, Na+, Ca2+, NH4+, Mg2+, Fe3+, et al | Group I, II mineral water immersion N = 42 | Group II, standard treatment, N = 21 | 36°C and 38°C, 20 min; 15 times in 3 wk | HAQ, SF-36 | Hand | VAS | 13 wk |
| Kovács et al (2016) | RCT 3 parallel groups | Hungary Age: 40–75 yr M/F: unclear | Na+, K+, NH4+, Ca2+, Mg2+, Fe3+, et al | Group I, mineral water immersion, N = 21 | Group III, standard treatment, N = 20 | 36°C, 20 min; 15 times in 3 wk | EQ-5D | Hip | VAS, WOMAC, MCII | 12 wk |
| Forestier et al (2009) | RCT 2 parallel groups | France Age: 63.0 ± 9.1 (group I); 64.3 ± 10.4 (group II) M/F: 237/145 | ND | Group I, mud-bath therapy + mineral water immersion + massage, N = 195 | Group II, standard Treatment, N = 187 | Mud-bath therapy: 45°C, 15 min; massage: 38°C, 10 min; mineral water immersion: 37°C, 15 min; 3 wk | SF-36 | Knee | VAS, WOMAC, MCII | 9 mo |
| Evcik et al (2007) | RCT 3 parallel groups | Turkey Age: 39–78 M/F: 6/74 | Na+, HCO3−, SO42−, Ca2+, Mg2+, et al | Group I, mineral water immersion, N = 25 | Group II, Mud-bath therapy, N = 29; group III, hot-pack therapy, N = 26 | Mineral water immersion: 36°C, 20 min; 10 times in 5 wk | NHP | Knee | VAS, WOMAC, | 3 mo |
AIMS1 = arthritis impact measurement scale, EQ-5D = European quality of life 5-dimension scale, FIHOA = functional index for hand osteoarthritis score, HAQ = health assessment questionnaire, KOOS = knee injury and osteoarthritis outcome score, LI = Lequesne index, M/F = male/female., MCII = minimal clinically important improvement, ND = no data, NHP = Nottingham health profile, RCT = randomized controlled clinical trials, ROM = range of movement, SF-36 = short form 36-item health survey, VAS = visual analogue scale, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
3.3. Risk of Bias of the Eligible Studies
3.3.1. Allocation
Fraioli et al and Cantarini et al stated that allocation was “randomized” without mentioning the specific allocation method.[20,29] Evcik et al stated that the patients were allocated to the groups in order of their admittance to the study.[35] Varzaityte et al randomly allocated participants according to the allocation ratio of 1:1:1.[26] Other investigators used methods such as a computer-generated randomization chart, a computer-generated table”, a random number generator, or by tossing a coin. Therefore, the studies were assessed to have a low risk of bias.
3.3.2. Blinding
Five studies by Bálint et al, Hanzel et al, and Kovács et al, had a double-blind design, in which the surgeons and assessors were unaware of participant allocation.[22,24,27,28,32] Thus, detection and performance bias were considered to be of low risk in these studies. For the single-blind design and the studies in which blindness was not mentioned, performance bias was treated as unclear or high risk, respectively. In addition, detection bias was treated as low risk.
3.3.3. Incomplete outcome data
In Branco et al, Evcik et al Fioravanti et al, Forestier et al, and Kulisch et al, more than 90% (139/140, 75/80, 76/80, 349/382, 75/77) of participants completed follow-up assessments.[21,23,31,34,35] In Fraioli et al, for personal reasons, 3 of the patients in group A were unable to complete the second cycle of treatment scheduled 6 months after the first, but all 3 completed the third cycle, which began 1 year after the first.[20] We assessed these studies as having low risk. Patients in other studies completed the study without withdrawal, and they were categorized as low risk.
3.3.4. Selective reporting
Bálint et al, Cantarini et al, Evcik et al, Fraioli et al, and Kovács et al did not provide their trial register number and were assessed as having an unclear risk of bias.[20,27,28,29,35] Other studies were considered low risk.
3.4. Effect of thermal mineral waters therapy on pain relief and functional improvement
3.4.1. Pain relief
Figure 4 demonstrates the effect of using 2 different pain assessment methods on the pain relief from thermal mineral waters therapy. Figure 4A shows a significant difference on the VAS scale, but the heterogeneity was high [SMD: –1.02; 95% CI: –1.57, –0.46; P < .00001; I2 = 93.9%]. In Figure 4B, statistical significance was observed on the WOMAC score, but the heterogeneity was high [SMD: –0.54; 95% CI: –0.68, –0.39; P < .00001; I2 = 92.5%]. In addition, the study of Forestier et al was sensitive to both VAS and WOMAC (Fig. 5A,B).[34] According to the type of OA, a subgroup analysis showed that the VAS scale and WOMAC score of knee OA were significantly reduced following thermal mineral waters therapy [SMD: –1.50; 95% CI: –2.34, -0.65; P = .001; I2 = 96.1%; SMD: –0.52; 95% CI: –0.68, –0.36; P < .00001; I2 = 94.5%]. The WOMAC score of hip OA and the VAS scale of hand OA were significantly reduced [SMD: –0.64; 95% CI: –1.05, –0.24; P = .002; I2 = 35.1%; SMD: –0.53; 95% CI: –1.02, –0.04; P = .035; I2 = 58.2%] but the VAS scale in hip OA did not change significantly [SMD: 0.1; 95% CI: –0.54, –0.75; P = .753; I2 = 58.7%].
Figure 4.
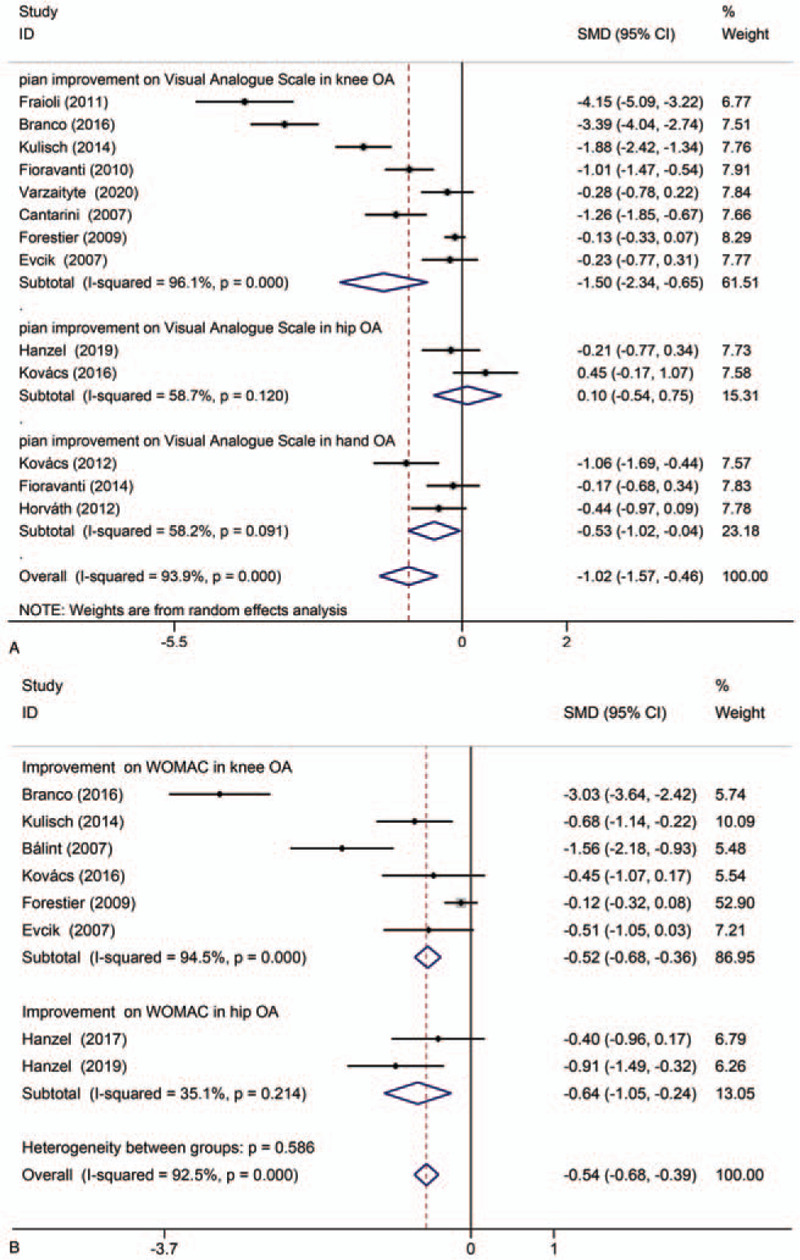
Meta-analyses of the effects of thermal mineral waters therapy on pain relief: (A) Forest plot displaying the weighted mean difference and 95% confidence intervals for the effect of thermal mineral waters therapy on VAS; (B) Forest plot displaying the weighted mean difference and 95% confidence intervals for the effect of thermal mineral waters therapy on WOMAC. CI = confidence interval, SMD = standardized mean difference.
Figure 5.
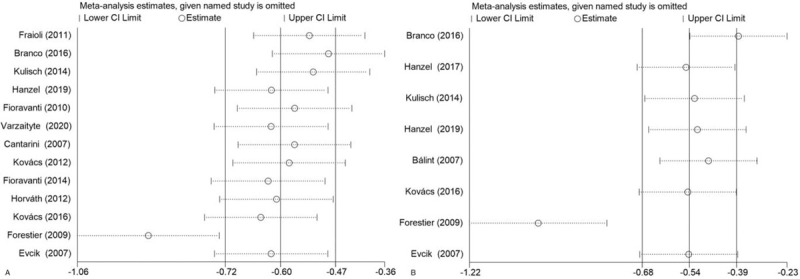
Sensitivity analysis: (A) VAS score; (B) WOMAC score. CI = confidence interval.
3.4.2. Functional improvement
As shown in Figure 6, the 3 studies were suitable for inclusion in the meta-analysis of LI function scores. Compared with the control group, the treatment group showed a significantly reduced LI (SMD: –2.48; 95% CI: –4.40, –0.56; P = .011; I2 = 96.5%).
Figure 6.
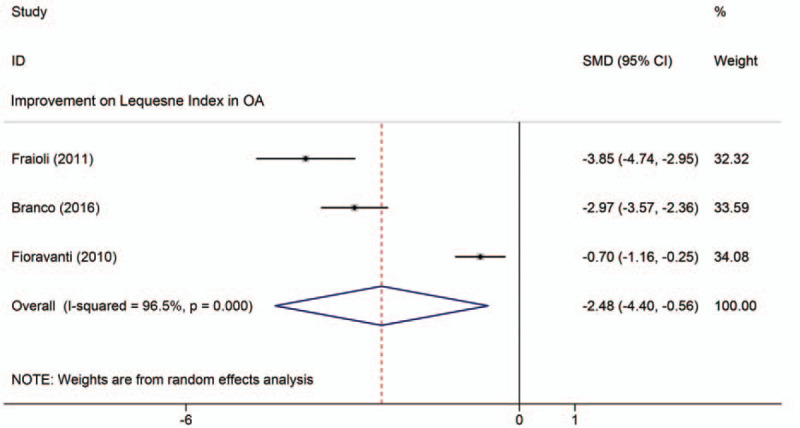
Meta-analyses of the effects of thermal mineral waters therapy on functional improvement: Forest plot displaying the weighted mean difference and 95% confidence intervals for the effect of thermal mineral waters therapy on LI. CI = confidence interval, SMD = standardized mean difference.
3.5. Effect of thermal mineral waters on quality of life improvement
The QoL was significantly improved in 3 studies that used the SF-36 questionnaire at the end of the treatment process and during the 3-month follow-up period.[22,24,31] Varzaityte et al reported that 1 month after mineral waters treatment, SF-36 was found to be positive, indicating that the intervention group's physical activity increased and pain was reduced. Horváth et al reported that when mineral water temperature is controlled at 38°C or 36°C, the SF-36 shows that the physical component summary (PCS) and the mental component summary (MCS) scores after treatment and during follow-up were substantially different and indicated a permanent improvement.[32] As shown in Figure 7A, the utility index of the EQ-5D questionnaire showed permanent and significant improvement of general health-related quality of life in the group receiving thermal water therapy compared to the control group during the follow-up period [SMD: 0.51; 95% CI: 0.20, 0.83; P = .001; I2 = 0.0%].[23,30,33] Meta-analysis of the 4 studies found that the HAQ score were significantly improved after thermal mineral waters therapy [SMD: –1.13; 95% CI: –2.22, –0.04; 0 = 0.042; I2 = 93.5%] (Fig. 7B).[21,30–32]
Figure 7.
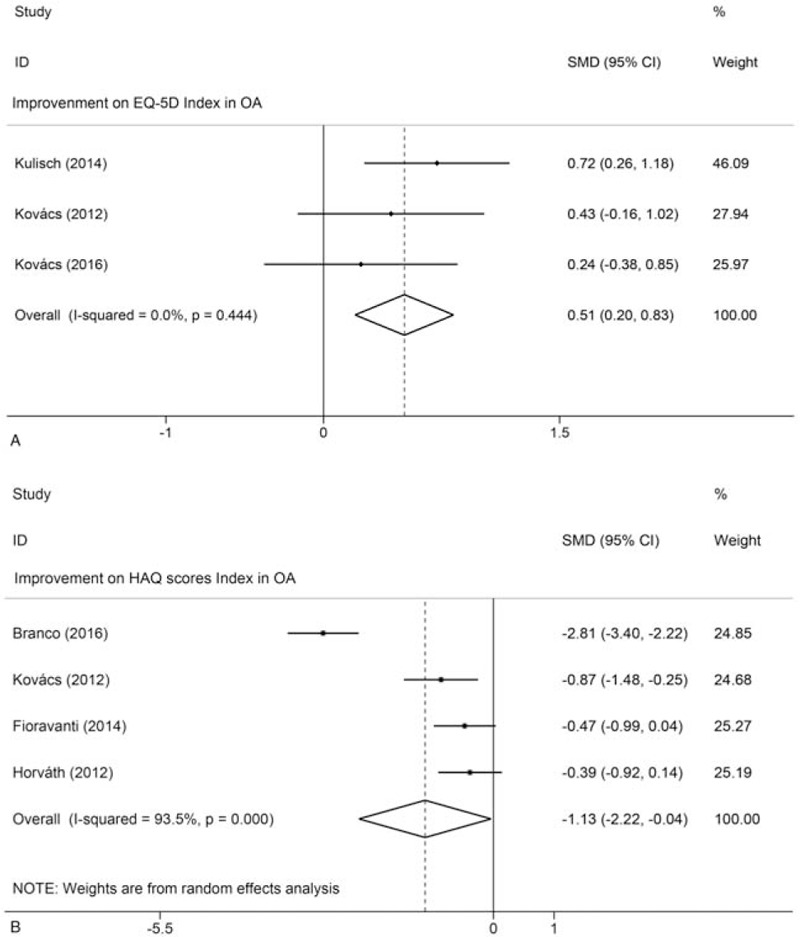
Meta-analyses of the effects of thermal mineral waters therapy on QoL: (A) Forest plot displaying the weighted mean difference and 95% confidence intervals for the effect of thermal mineral waters therapy on EQ-5D; (B) Forest plot displaying the weighted mean difference and 95% confidence intervals for the effect of thermal mineral waters therapy on HAQ. CI = confidence interval, SMD = standardized mean difference.
3.6. Safety evaluation of thermal mineral waters therapy
Only 4 trials reported adverse effects, but none of the participants withdrew from the study due to adverse events.[25,27,29,31] Fioravanti et al reported that 11% of patients in the treatment group had side effects after treatment, but the satisfaction in the clinical efficacy reached 89.9%.[25] Bálint et al reported that 2 patients in the thermal mineral waters group and 1 patient in the tap water group complained of itching after 2 to 3 treatments, which lasted only a few days.[27] Cantarini et al and Fioravanti et al reported, respectively, that in the treatment group, 9 patients and 5% of patients had side effects due to treatment, but these were of light intensity, and they did not interrupt the therapy.[29,31]
4. Discussion
OA is a chronic degenerative disease with high morbidity and prevalence. It requires multidisciplinary therapeutic intervention combined with primary and secondary prevention strategies, involving medication, physical therapy, hydrotherapy, and climate therapy.[51] In recent years, thermal mineral waters therapy has been widely used to treat various joint diseases and has been reported in clinical trials of OA in the knee, hip, and hand.[52–54] Systematic review and meta-analysis of the effects of thermal mineral waters therapy on OA pain relief, joint function, quality of life, and safety implications was conducted in the present study. The results found that thermal mineral waters therapy can effectively reduce the pain experienced by patients with OA, and improves their physical functions as indicated by reducing their VAS score, WOMAC index, and LI index. In addition, thermal mineral waters therapy can improve the QoL of patients with OA, evidenced by significant changes in EQ-5D and HAQ. Generally, thermal mineral waters therapy is a safe way to relieve pain and knee stiffness and improve joint function in patients with OA.
The minerals in mineral water can be absorbed into the systemic circulation through the skin. However, the mechanisms of this process have been largely unexplored. Although the experimental methods, treatment techniques, and cell culture procedures used have differed between studies, a growing number of studies have confirmed the effects of mineral water in anti-inflammatory processes, antioxidant levels, cartilage protection, and immunosuppression.[55] In psoriasis, H2S donors (NaHS and GYY4137) have been shown to have anti-inflammatory and anti-angiogenic effects, confirming the beneficial effects of sulfur in mineral water.[56,57] In 2013, Prandelli et al used human primary monocytes to test the beneficial effects of Sirmione thermal water (Lombardia, Italy), rich in sodium chloride, bromide, and iodide, and NaHS at a concentration of 2.5 mM,[58] and found evidence that saline affected the Langerhans cells of the skin. However, the data obtained in vitro experiments are usually inconsistent with the observed clinical effects.[59] In a recent double-blind, randomized controlled trial of patients with knee and hip OA, the organic part separated from the entire Szigetvár medicinal water and redissolved in tap water, showing that a significant improvement in the clinical outcomes for patients compared to those observed in tap water alone.[24] Interestingly, all the RCTs that were included in the present study detailed trace elements of sulfur in the mineral water. From the available evidence, it can be hypothesized that sulfur plays an important role in hot mineral water. The penetration of trace elements may be affected by soaking time, water temperature, the composition of its solute content, and many other factors.[60] The specific mechanisms still require further clinical and in vitro experimental studies.
Verhagen et al published a Cochrane systematic review in 2007, which included seven RCTs (498 patients), showing that thermal mineral waters therapy can reduce pain and improve quality of life when compared with no treatment.[19] Harzy et al used 9 RCTs (493 patients) to reach a similar conclusion and proposed that using thermal mineral waters therapy can relieve pain and improve function in patients with OA for up to 24 weeks.[36] Tenti et al evaluated 14 RCTs, and 1198 patients believed that thermal mineral waters therapy had a positive effect in the treatment of knee OA. These 3 reviews indicate that immersion in thermal mineral water can effectively relieve pain and improve physical function.[37] The latest meta-analysis published in 2017 compared eight RCTs (359 cases and 375 controls), which is consistent with the present study, found that the improvements in WOMAC score indicated that balneotherapy can effectively relieve pain and stiffness in the clinic for a prolonged time and that it improves body function.[38]
Xiang et al performed a meta-analysis of mud-bath therapy for knee OA patients and showed that mud-bath therapy, whether used alone or in combination with hydrotherapy, was not an important factor of high heterogeneity. The meta-analysis also emphasized the need to include a combination of therapies in the evaluation of such treatments.[61] In contrast, the most advanced meta-analysis to evaluate the effectiveness of mud-bath therapy for OA treatment showed that mud-bath can also effectively relieve knee pain.[62] Similar to the study of Xiang et al, the present study did not support the effectiveness of mud-bath therapy but separately showed the positive effects of thermal mineral waters therapy on pain relief and recovery of function. The present meta-analysis included higher quality studies and obtained enough data through various evaluation indicators to analyze the effects of thermal mineral waters therapy on pain and functional recovery. Therefore, compared with isolated mud-bath therapy, thermal mineral waters therapy may have a greater positive effect.
The main outcome measures were the VAS and WOMAC physical function scores to measure the overall pain score. Pain and joint limitation are not only the main manifestations of OA but also the primary clinical symptoms observed by doctors. The pain of OA is mainly due to inflammation of the synovial membrane or degradation of articular cartilage.[63] Similar to previous studies, the present results confirmed that thermal mineral waters therapy could significantly relieve pain, VAS [P < .00001; I2 = 93.9%] and WOMAC [P < .00001; I2 = 92.5%] scores are significantly reduced. The WOMAC pain score includes multiple aspects, such as exercise, rest, and standing,[41] which may be more meaningful for measuring the effect of thermal mineral waters therapy on pain relief. It is worth noting that, as far as the authors know, this is the first meta-analysis of the effects of mineral water on different diseased parts of the body. The findings show that mineral water can effectively relieve the pain of hand and knee OA. The VAS scale did not change significantly in hip OA [P = .753; I2 = 58.7%], but thermal mineral waters therapy significantly reduced the WOMAC score [P = .002; I2 = 35.1%]. This outcome may be related to the research that was included, which has not been reported in previous reviews. A previous meta-analysis showed that balneotherapy with Hungarian thermal spring mineral water effectively treated back pain and OA of the knees and hands.[64] At the same time, using LI to evaluate the effect of functional recovery, functional outcomes were significantly improved [P = .011; I2 = 96.5%]. The mechanism by which thermal mineral waters reduce joint pain is not well understood. It is reasonable to assume that pain relief is related to mechanical, thermal, and chemical factors. The heat and buoyancy provided by thermal mineral waters help to reduce muscle tension, which may increase the pain threshold of nerve endings. The absorption of salt through the skin seems limited, but there is little research on this problem. Therefore, the therapeutic effect of balneotherapy appears to be related to the local interaction between mineral water and skin surface structure.
Chronic pain caused by OA and difficulty performing activities of daily living can cause a significant decrease in QoL. The EQ-5D global health-related quality of life questionnaire and HAQ index values showed that, compared with the control group, the thermal mineral waters therapy group had a greater and longer-lasting improvement. As further evidence of the beneficial effect of mineral water treatment, compared with tap water treatment, the SF-36 questionnaire showed that the results of mineral water treatment were significantly better. The RCTs included 3 studies of hand OA and all reported improved quality of life.[30–32] Although there are 4 reports of adverse reactions in the literature, none of the participants withdrew from the study.[25,27,29,31] In conclusion, the present results are consistent with other studies in the literature, confirming that the beneficial effects of thermal mineral waters therapy on patients with OA will continue over time and significantly improve QoL. Occasionally, minor and short-term side effects may occur after mineral waters treatment of OA, but there is minimal risk to patient safety.
This meta-analysis has multiple advantages over previously reported analyses. First of all, only high-quality articles were included, which provided sufficient data. Second, the analysis included not only knee OA patients but also hip and hand OA patients, which enables subgroup analysis to evaluate the pain relief effect of thermal mineral waters therapy compared between body locations. This led to a comprehensive evaluation of the effects of thermal mineral waters therapy in reducing pain and improving QoL. Third, the reliability of the results are improved by applying the VAS scale and WOMAC score for pain assessment in the meta-analysis. Finally, a rigorous search strategy and data extraction techniques, performed by 2 researchers independently, contribute to improving the credibility of the results.
The study also has some limitations. Firstly, this meta-analysis includes 16 RCTs with high heterogeneity (92.5%–96.5%). Through sensitivity analysis, it was found that articles by Forestier et al were sensitive to both the research team's VAS and WOMAC, which may be causing the heterogeneity.[34] Secondly, there is clinical heterogeneity due to different interventions, methods, controls, and differences during follow-up between the studies. In addition, a difference in the composition of mineral water may be present, as 1 study did not clarify the composition of the water.[34] The proportion of female participants in the study is relatively high. Due to the differences in publication and research design quality, the research also has heterogeneity in methodology. These factors may be related to the differences between the groups and affect the overall effect and heterogeneity in this analysis. Although there is no existing evidence that the pathophysiological mechanism of OA pain is different in each joint, the location of OA may interfere with the results and increase the heterogeneity of certain outcome indicators. Secondly, the severity of knee OA in the population included in the meta-analysis is not consistent, so it may overestimate the impact on pain relief and the improvement of thermal mineral waters therapy. Third, trials were conducted in different countries and regions. Although the questionnaire was translated into the participant's native language, social and cultural differences may have played a role in determining the patient's perceived and reported QoL. Fourth, due to the different intervention periods, water temperature, and treatment frequencies, the optimal parameters need to be determined in future studies. Therefore, more research should be conducted to further evaluate the long-term efficacy of mineral water in the treatment of OA. Similarly, multiple treatment groups need to be studied to find the optimal water temperature and treatment period. In addition, further RCTs are required to study the effect of mineral waters therapy on hip OA.
5. Conclusions
In conclusion, thermal mineral waters therapy is a safe way to relieve pain and improve physical function and improve the QoL in patients with OA. It may be more effective for knee and hand OA in reducing pain. Current evidence provides comprehensive guidance for the long-term clinical application of thermal mineral waters therapy in OA patients.
Author contributions
Conceptualization: Tianwen Ma, Yuanqiang Ma.
Data curation: Xiaopeng Song, Yue Li.
Formal analysis: Xiaopeng Song, Hailong Hu, Hui Bai.
Funding acquisition: Li Gao.
Investigation: Li Gao.
Methodology: Tianwen Ma, Li Gao.
Resources: Xiaopeng Song, Yuanqiang Ma.
Software: Tianwen Ma.
Supervision: Li Gao.
Writing – original draft: Tianwen Ma.
Writing – review & editing: Hailong Hu, Hui Bai, Yue Li.
Glossary
Abbreviations: COX-2 = cyclooxygenase 2, EQ-5D = European Quality of life 5-Dimension Scale, HAQ = Health Assessment Questionnaire, LI = Lequesne index, NSAIDs = nonsteroidal anti-inflammatory drugs, OA = osteoarthritis, QoL = quality of life, RCT = randomized controlled trial, SF-36 = Short Form 36-item Health Survey, VAS = visual analog scale, WOMAC = Western Ontario and McMaster Universities.
References
- [1].Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- [2].Wu Y, Zhu S, Lv Z, et al. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabi 2019;33:1863–75. [DOI] [PubMed] [Google Scholar]
- [3].Pereira D, Peleteiro B, Araújo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270–85. [DOI] [PubMed] [Google Scholar]
- [4].Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010;26:355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: An evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ofman JJ, Maclean CH, Straus WL, et al. A meta analysis of severe upper gastrointestinal complications of nonsteroidal anti-inflflammatory drugs. J Rheumatol 2002;29:804–12. [PubMed] [Google Scholar]
- [8].Bresalier RS, Sandler RS, Quan H, et al. Trial investigations: cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092–102. [DOI] [PubMed] [Google Scholar]
- [9].Pincus T, Wang X, Chung C, et al. Patient preference in a crossover clinical trial of patients with osteoarthritis of the knee or hip: face validity of self-report questionnaire ratings. J Rheumatol 2005;32:533–9. [PubMed] [Google Scholar]
- [10].Skou St, Roos EM. Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol 2019;120:112–7. [PubMed] [Google Scholar]
- [11].Jotanovic Z, Mihelic R, Sestan B, et al. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets 2014;15:635–61. [DOI] [PubMed] [Google Scholar]
- [12].Ng JY, Azizudin AM. Rheumatoid arthritis and osteoarthritis clinical practice guidelines provide few complementary and alternative medicine therapy recommendations: a systematic review. Clin Rheumatol 2020;39:2861–73. [DOI] [PubMed] [Google Scholar]
- [13].Johnson RH. Arthur Stanley Wohlmann, the first government balneologist in New Zealand. Med Hist Suppl 1990;10:114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vaidya B, Nakarmi S. A qualitative study of patients’ beliefs and perception on medicinal properties of natural hot spring bath for musculoskeletal problems. J Environ Public Health 2020;2020:3694627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fioravanti A, Cantarini L, Guidelli GM, et al. Mechanisms of action of spa therapies in rheumatic diseases: what scientific evidence is there? Rheumatol Int 2011;31:1–8. [DOI] [PubMed] [Google Scholar]
- [16].Gutenbrunner C, Bender T, Cantista P, et al. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol 2010;54:495–507. [DOI] [PubMed] [Google Scholar]
- [17].McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- [18].Cozzi F, Ciprian L, Carrara M, et al. Balneotherapy in chronic inflammatory rheumatic diseases-a narrative review. Int J Biometeorol 2018;62:2065–71. [DOI] [PubMed] [Google Scholar]
- [19].Verhagen AP, Bierma-Zeinstra SM, Boers M, et al. Balneotherapy for osteoarthritis. Cochrane Database Syst Rev 2007;17:CD006864. [DOI] [PubMed] [Google Scholar]
- [20].Fraioli A, Serio A, Mennuni G, et al. A study on the efficacy of treatment with mud packs and baths with Sillene mineral water (Chianciano Spa Italy) in patients suffering from knee osteoarthritis. Rheumatol Int 2011;31:1333–40. [DOI] [PubMed] [Google Scholar]
- [21].Branco M, Rêgo NN, Silva PH, et al. Bath thermal waters in the treatment of knee osteoarthritis: a randomized controlled clinical trial. Eur J Phys Rehabil Med 2016;52:422–30. [PubMed] [Google Scholar]
- [22].Hanzel A, Horvát K, Molics B, et al. Clinical improvement of patients with osteoarthritis using thermal mineral water at Szigetvár Spa-results of a randomised double-blind controlled study. Int J Biometeorol 2018;62:253–9. [DOI] [PubMed] [Google Scholar]
- [23].Kulisch Á, Benkö Á, Bergmann A, et al. Evaluation of the effect of Lake Hévíz thermal mineral water in patients with osteoarthritis of the knee: a randomized, controlled, single-blind, follow-up study. Eur J Phys Rehabil Med 2014;50:373–81. [PubMed] [Google Scholar]
- [24].Hanzel A, Berényi K, Horváth K, et al. Evidence for the therapeutic effect of the organic content in Szigetvár thermal water on osteoarthritis: a double-blind, randomized, controlled clinical trial. Int J Biometeorol 2019;63:449–58. [DOI] [PubMed] [Google Scholar]
- [25].Fioravanti A, Iacoponi F, Bellisai B, et al. Short- and long-term effects of spa therapy in knee osteoarthritis. Am J Phys Med Rehabil 2010;89:125–32. [DOI] [PubMed] [Google Scholar]
- [26].Varzaityte L, Kubilius R, Rapoliene L, et al. The effect of balneotherapy and peloid therapy on changes in the functional state of patients with knee joint osteoarthritis: a randomized, controlled, single-blind pilot study. Int J Biometeorol 2020;64:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bálint GP, Buchanan WW, Adam A, et al. The effect of the thermal mineral water of Nagybaracska on patients with knee joint osteoarthritis-a double blind study. Clin Rheumatol 2007;26:890–4. [DOI] [PubMed] [Google Scholar]
- [28].Kovács I, Bender T. The therapeutic effects of Cserkeszölö thermal water in osteoarthritis of the knee: a double blind, controlled, follow-up study. Rheumatol Int 2002;21:218–21. [DOI] [PubMed] [Google Scholar]
- [29].Cantarini L, Leo G, Giannitti C, et al. Therapeutic effect of spa therapy and short wave therapy in knee osteoarthritis: a randomized, single blind, controlled trial. Rheumatol Int 2007;27:523–9. [DOI] [PubMed] [Google Scholar]
- [30].Kovács C, Pecze M, Tihanyi Á, et al. The effect of sulphurous water in patients with osteoarthritis of hand. Double-blind, randomized, controlled follow-up study. Clin Rheumatol 2012;31:1437–42. [DOI] [PubMed] [Google Scholar]
- [31].Fioravanti A, Tenti S, Giannitti C, et al. Short- and long-term effects of mud-bath treatment on hand osteoarthritis: a randomized clinical trial. Int J Biometeorol 2014;58:79–86. [DOI] [PubMed] [Google Scholar]
- [32].Horváth K, Kulisch Á, Németh A, et al. Evaluation of the effect of balneotherapy in patients with osteoarthritis of the hands: a randomized controlled single-blind follow-up study. Clin Rehabil 2012;26:431–41. [DOI] [PubMed] [Google Scholar]
- [33].Kovács C, Bozsik Á, Pecze M, et al. Effects of sulfur bath on hip osteoarthritis: a randomized, controlled, single-blind, follow-up trial: a pilot study. Int J Biometeorol 2016;60:1675–80. [DOI] [PubMed] [Google Scholar]
- [34].Forestier R, Desfour H, Tessier JM, et al. Spa therapy in the treatment of knee osteoarthritis: a large randomised multicentre trial. Ann Rheum Dis 2010;69:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Evcik D, Kavuncu V, Yeter A, et al. The efficacy of balneotherapy and mud-pack therapy in patients with knee osteoarthritis. Joint Bone Spine 2007;74:60–5. [DOI] [PubMed] [Google Scholar]
- [36].Harzy T, Ghani N, Akasbi N, et al. Short- and long-term therapeutic effects of thermal mineral waters in knee osteoarthritis: a systematic review of randomized controlled trials. Clin Rheumatol 2009;28:501–7. [DOI] [PubMed] [Google Scholar]
- [37].Tenti S, Cheleschi S, Galeazzi M, et al. Spa therapy: can be a valid option for treating knee osteoarthritis? Int J Biometeorol 2015;59:1133–43. [DOI] [PubMed] [Google Scholar]
- [38].Matsumoto H, Hagino H, Hayashi K, et al. The effect of balneotherapy on pain relief, stiffness, and physical function in patients with osteoarthritis of the knee: a meta-analysis. Clin Rheumatol 2017;36:1839–47. [DOI] [PubMed] [Google Scholar]
- [39].Antonelli M, Donelli D, Fioravanti A. Effects of balneotherapy and spa therapy on quality of life of patients with knee osteoarthritis: a systematic review and meta-analysis. Rheumatol Int 2018;38:1807–24. [DOI] [PubMed] [Google Scholar]
- [40].Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983;16:87–101. [DOI] [PubMed] [Google Scholar]
- [41].Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [42].Lequesne MG, Mery C, Samson M, et al. Indexes of severity for osteoarthritis of the hip and knee. Validation–value in comparison with other assessment tests. Scand J Rheumatol Suppl 1987;65:85–9. [DOI] [PubMed] [Google Scholar]
- [43].Dreiser RL, Maheu E, Guillou GB, et al. Validation of an algofunctional index for osteoarthritis of the hand. Rev Rhum Engl Ed 1995;62:43S–53S. [PubMed] [Google Scholar]
- [44].Tubach F, Ravaud P, Beaton D, et al. Minimal clinically important improvement and patient acceptable symptom state for subjective outcome measures in rheumatic disorders. J Rheumatol 2007;34:1188–93. [PMC free article] [PubMed] [Google Scholar]
- [45].Soucie JM, Wang C, Forsyth A, et al. Range of motion measurements: reference values and a database for comparison studies. Haemophilia 2011;17:500–7. [DOI] [PubMed] [Google Scholar]
- [46].Gignac MA, Cao X, Mcalpine J, et al. Measures of disability: Arthritis Impact Measurement Scales 2 (AIMS2), Arthritis Impact Measurement Scales 2-Short Form (AIMS2-SF), The Organization for Economic Cooperation and Development (OECD) Long-Term Disability (LTD) Questionnaire, EQ-5D, World Health Organization Disability Assessment Schedule II (WHODASII), Late-Life Function and Disability Instrument (LLFDI), and Late-Life Function and Disability Instrument-Abbreviated Version (LLFDI-Abbreviated). Arthritis Care Res (Hoboken) 2011;63: (Suppl 11): S308–24. [DOI] [PubMed] [Google Scholar]
- [47].Bruce B, Fries JF. The Stanford Health Assessment. Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Salaffi F, Di Carlo M, Carotti M, et al. The impact of different rheumatic diseases on health-related quality of life: a comparison with a selected sample of healthy individuals using SF-36 questionnaire, EQ-5D and SF-6D utility values. Acta Biomed 2019;89:541–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].J. P. T. Higgin, S. Green, “Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 March 2011,” The Cochrane Collaboration 2011; https://www.cochranehandbook.org. [Google Scholar]
- [50].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am 2020;104:293–311. [DOI] [PubMed] [Google Scholar]
- [52].Küçükdeveci AA. Nonpharmacological treatment in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2019;33:101482. [DOI] [PubMed] [Google Scholar]
- [53].Bai R, Li C, Xiao Y, et al. Effectiveness of spa therapy for patients with chronic low back pain: an updated systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bernetti A, Mangone M, Alviti F, et al. Spa therapy and rehabilitation of musculoskeletal pathologies: a proposal for best practice in Italy. Int J Biometeorol 2020;64:905–14. [DOI] [PubMed] [Google Scholar]
- [55].Cheleschi S, Gallo I, Tenti S. A comprehensive analysis to understand the mechanism of action of balneotherapy: why, how, and where they can be used? Evidence from in vitro studies performed on human and animal samples. Int J Biometeorol 2020;64:1247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gobbi G, Ricci F, Malinverno C, et al. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Lab Invest 2009;89:994–1006. [DOI] [PubMed] [Google Scholar]
- [57].Mirandola P, Gobbi G, Micheloni C, et al. Hydrogen sulfide inhibits IL-8 expression in human keratinocytes via MAP kinase signaling. Lab Invest 2011;91:1188–94. [DOI] [PubMed] [Google Scholar]
- [58].Prandelli C, Parola C, Buizza L, et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int J Immunopathol Pharmacol 2013;26:633–46. [DOI] [PubMed] [Google Scholar]
- [59].Gruner S, Zwirner A, Boonen H, et al. Der Einfluss einer Behandlung mit Salz des Toten Meeres (Tomesa-Therapie) auf epidermale Langerhanszellen-Eine klinische Studie [effect of treatment with salt from the Dead Sea (Tomesa therapy) on epidermal Langerhans cells--a clinical study]. Z Hautkr 1990;65:1146–51. [PubMed] [Google Scholar]
- [60].Kosińska B, Grabowski ML. Sulfurous balneotherapy in Poland: a vignette on history and contemporary use. Adv Exp Med Biol 2019;1211:51–9. [DOI] [PubMed] [Google Scholar]
- [61].Xiang J, Wu D, Li J. Clinical efficacy of mudpack therapy in treating knee osteoarthritis: a meta-analysis of randomized controlled studies. Am J Phys Med Rehabil 2016;95:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hou C, Liang L, Chu X, et al. The short-term efficacy of mud therapy for knee osteoarthritis: a meta-analysis. Medicine (Madr) 2020;99:e19761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sherwood J. Osteoarthritis year in review 2018: biology. Osteoarthritis Cartilage 2019;27:365–70. [DOI] [PubMed] [Google Scholar]
- [64].Bender T, Bálint G, Prohászka Z, et al. Evidence-based hydro- and balneotherapy in Hungary-a systematic review and meta-analysis. Int J Biometeorol 2014;58:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


