Abstract
Background:
In the present study, we aimed to detect the expression of CXCL2 in epithelial ovarian cancer (OC) and explore its clinical significance.
Methods:
TCGA (The Cancer Genome Atlas) database was adopted to assess the significance of CXCL2. Tissue microarray and immunohistochemical staining were used to detect the expression of CXCL2 in epithelial OC, and its correlation with clinicopathological features and prognosis was statistically analyzed.
Results:
CXCL2 was highly expressed in epithelial OC tissues compared with the adjacent tissues. Such up-regulation of CXCL2 was significantly correlated with tumor differentiation (P = .001), tumor stage (P = .01), tumor location (unilateral or bilateral) (P = .003), and metastasis (P = .003). Kaplan-Meier and Cox proportional hazards regression analyses showed that high expression of CXCL2 was not an independent predictor of poor prognosis in epithelial OC.
Conclusions:
Collectively, the high expression of CXCL2 might be related to the invasion and metastasis of epithelial OC.
Keywords: CXCL2, epithelial ovarian cancer, immunohistochemistry, prognosis, tissue chips
1. Introduction
Ovarian cancer (OC) is the fifth leading cause of cancer-related death among women worldwide.[1,2] As the early symptoms of OC are not obvious, most patients have reached a more advanced stage of OC at the time of diagnosis. Therefore, the survival time of OC is relatively short. Although OC is sensitive to chemical reactions and responds well to platinum drugs/paclitaxel at the initial stage of treatment, the 5-year recurrence rate is still 60% to 80%. It has been shown that the occurrence and progression of tumors can induce an adaptive immune response, and anti-tumor immunity is significantly correlated with the prognosis of those patients.[3] More and more evidence shows that cell-mediated tumor immune response is a part of tumor immune monitoring.
Chemokines are a group of small proinflammatory cytokines that attract, activate, and regulate leukocytes in inflammatory tissues and play an important role in immune defense, anti-infection, and inflammatory response. Recent studies have shown that they play a critical role in tumorigenesis and development.[4] CXCL2 is a chemokine produced by neutrophils, which plays a role in recruiting neutrophils, releasing granzyme, and expressing adhesion molecules, thus enhancing the inflammatory effect. CXCL2 shares receptors with interleukin-8 (IL-8) and IL-8RB/CXCR2. CXCR2 can be activated by a variety of CXC chemokines, such as CXCL1, CXCL2, and CXCL5. The combination of CXCL2 and CXCR2 can promote the chemotaxis of neutrophils and endothelial cells and accelerate the angiogenesis, drug resistance, transformation, and growth of the tumor. Some studies have shown that the over-expression of CXCL2 is involved in the progression and invasion of tumors, such as esophageal squamous cell carcinoma (ESCC).[5] The expression of CXCL2 is found in different tumors, including brain cancer, breast cancer, colorectal cancer, and OC.[6–8] It has also been found that CXCL2 negatively regulates the cell cycle of hepatocellular carcinoma (HCC) through the ERK1/2 signaling pathway.[9] CXCL2 plays a role as a carcinogenic gene in breast and colorectal cancer but functions as a tumor suppressor gene in HCC, which may be attributed to different molecular mechanisms of tumor development in tissues. In colorectal cancer, the CXCL2-CXCR2 axis promotes tumorigenesis by regulating Gαi-2 and Gαq/11, which is related to irinotecan R (CPT-11R) LoVo cells as cancer stem cell properties.[10] Wang et al[11] have found that CXCL2/CXCR2 forms an autocrine loop by activating the ERK1/2 pathway, which contributes to the spread of primary ESCC. On the contrary, the expression of CXCL2 decreases the ability to inhibit the proliferation and colonization of esophageal cancer cells.[12] However, the effect of CXCL2 over-expression on clinical outcomes in patients with epithelial OC remains largely unexplored.
In the present study, the expression of CXCL2 in epithelial OC tissues and its adjacent tissues was assessed using the cDNA microarray database and tissue microarray (TMA). Moreover, the potential correlation between the expression of CXCL2 and the clinicopathological features of epithelial OC was explored. Besides, we also evaluated the relationship between the expression of CXCL2 and the prognosis of OC patients.
2. Materials and methods
2.1. Data extraction
The expression data of CXCL2 at the mRNA level were extracted from the TCGA (The Cancer Genome Atlas) database, and the expression of CXCL2 at the mRNA level in epithelial OC tissues and normal ovarian tissues was compared.
2.2. Patients and tissue samples
This study was approved by the institutional review board/ethics committee. The written informed consent was obtained from every participant.
A total of 121 OC specimens were obtained from the Affiliated Hospital of Xuzhou Medical College from 2005 to 2009. In patients with nest cancer, tumor tissue and its adjacent tissue were fixed by formalin and then embedded by paraffin. Inclusion criteria were set as follows: the patients did not receive any treatment before the operation, and the postoperative follow-up data were complete. Exclusion criteria were set as follows: patients with other parts of the primary focus, and those suffering from severe immune diseases. The clinical data included sex, age, degree of differentiation, tumor location, pathological classification, differentiation, FIGO pathological stage, metastasis, lymph node metastasis, carbohydrate antigen 125 (CA125) level, and follow-up data (including 5-year survival rate). The survival time was calculated from the date of operation to the date of death or the last follow-up time. The tumor stage was determined according to the FIGO system, and the degree of tumor differentiation was determined by the criteria of the World Health Organization (WHO).
2.3. Immunohistochemistry
Tissue microarray system (Quick-Ray, UT06,) was used for 2.0-mm tissue in each patient in the TMA analysis. TMA sections were dewaxed with a 100% xylene solution and rehydrated with ethanol gradient solution. Antigen retrieval was carried out by boiling the samples in citrate buffer solution (pH 6.0) for 5 minutes, followed by a 15-minute incubation with PBS containing 5% goat serum to block non-specific binding. TMA sections were incubated with the primary antibody against CXCL2 (PeproTech, Rocky Hill, CT), followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (sc-2004, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The staining of CXCL2 was independently evaluated by 2 pathologists under double-blind conditions. The positive rate of staining was 0% to 100%. The grade of staining intensity was set as follows: 0=no staining; 1 = mild; 2 = moderate; and 3 = high. The final staining score was obtained by multiplying the intensity grade with the proportion of positive cells. The cutoff value expressed by CXCL2 was calculated by the X-tile software program. The degree of staining was quantified by 2 grades, and the final CXCL2 staining score was defined as follows: 0 to 150, low expression; 150 to 300, high expression.
2.4. Statistical analysis
The experimental data were analyzed by SPSS20.0 statistical software and expressed by the expression of CXCL2 at the mRNA level. The experimental variables were compared by the U test or t test. The relationship between the CXCL2 expression and clinicopathological features was analyzed by Pearson correlation analysis, the survival rate was calculated by the Kaplan-Meier method and Cox proportional risk regression model, and the difference was analyzed by logarithmic rank-sum test.
3. Results
The over-expression of CXCL2 at the mRNA level in OC was statistically analyzed by the TCGA database, and the expression of CXCL2 at the mRNA level in OC tissue (n = 426) was higher compared with the normal tissue (num = 88). The average expression of CXCL2 was significantly different between the tumor tissues and normal tissues. The survival analysis showed that the overall survival (OS) rate of the patients with higher CXCL2 expression was significantly poorer compared with the patients with lower CXCL2 expression (HR = 0.7, P = .015)(Fig. 1).
Figure 1.
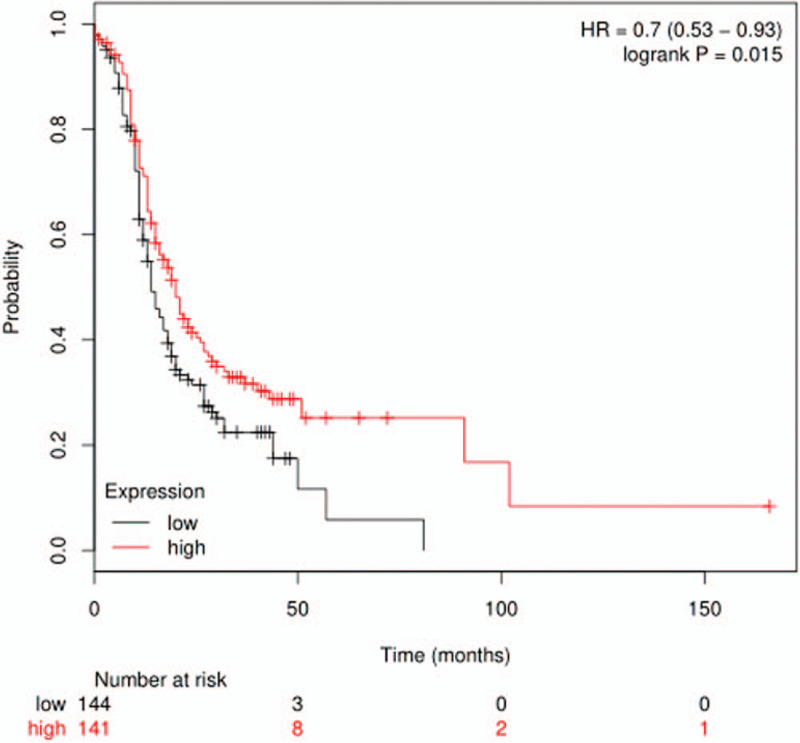
The overall survival rate of the patients with higher CXCL2 expression was significantly poorer compared with those showing lower CXCL2 expression (HR = 0.7, P = .015).
3.1. The expression of CXCL2 protein in OC tissues and its adjacent tissues by immunohistochemical staining
To further study the expression of CXCL2 at the protein level in OC tissues and its adjacent tissues, 110 cases of primary epithelial carcinoma and 110 cases of paracancerous tissues were analyzed by immunohistochemical staining. Figure 2A shows the H&E staining in OC tissue, Figure 2B indicates that weak or negative staining of CXCL2 could be found in ovarian cyst tissues, and Figure 2C reveals that positive staining of CXCL2 could be found in the cytoplasm of the cancer cells. The CXCL2 staining score of cancer tissues was higher compared with the ovarian cyst tissues. The high expression of cytoplasmic protein CXCL2 was detected in 43.6% (48/110) of epithelial OC tissues. Therefore, combined with the data on CXCL2 expression at the mRNA level from the TCGA database, this study confirmed the over-expression of CXCL2 at the protein level in epithelial OC.
Figure 2.
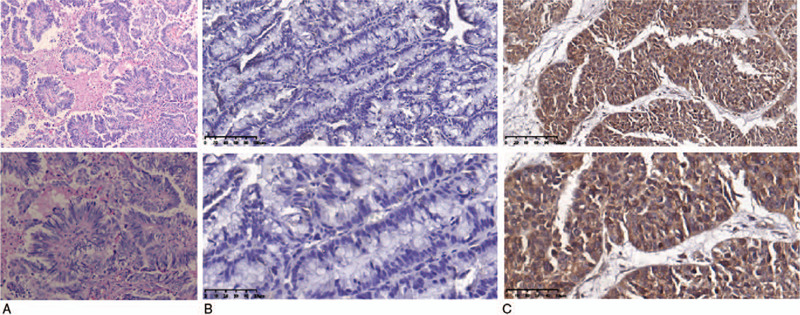
A shows HE staining in ovarian cancer, B shows negative staining of CXCL2 in ovarian cyst tissues, and C shows positive staining of CXCL2 in ovarian cancer tissues.
In the present study, we studied the expression of CXCL2 at the protein level in the cytoplasm of 110 patients with epithelial OC and found that the expression of CXCL2 was significantly correlated with the differentiation of OC (P = .001), FIGO tumor stage (P = .001), unilateral or bilateral tumor location (P = .0003) and metastasis (P = .0003) (Table 1). Further analysis showed that the expression of CXCL2 at the protein level was low in 62 cases (56.4%) and high in 48 cases (43.6%). The results implied that the high expression of CXCL2 in the cytoplasm of primary epithelial OC was significantly correlated with the degree of differentiation (P = .022), FIGO tumor stage (P = .001), unilateral or bilateral tumor location (P = .022), and metastasis (P = .005) (Table 2). However, there was no significant correlation between the CXCL2 expression and other clinicopathological features, including age, lymph node metastasis, and CA125 level.
Table 1.
Correlation between CXCL2 expression in ovarian cancer tissues and clinicopathological features of epithelial ovarian cancer patients.
| Clinicopathological feature | Groups | Cases | CXCL2 (x ± s) | CXCL2 expression | χ2/z | P |
| Age | <55 | 75 | 132.13 ± 78.6 | 120 (0–290) | 1.151 | .250 |
| ≥55 | 28 | 145.71 ± 76.34 | 170 (0–290) | |||
| Differentiation | High | 11 | 60.00 ± 55.54 | 40 (0–190) | 14.738 | .001 |
| middle | 39 | 126.92 ± 83.03 | 100 (0–290) | |||
| low | 58 | 151.98 ± 67.09 | 160 (0–290) | |||
| FIGO stage | I∼II | 32 | 97.19 ± 72.98 | 100 (0–290) | 3.023 | .003 |
| IV∼III | 76 | 148.88 ± 73.49 | 160 (0–290) | |||
| Location (single or double) | single | 45 | 109.89 ± 81.8 | 90 (0–290) | 2.962 | .003 |
| double | 63 | 150.48 ± 68.71 | 160 (0–290) | |||
| Distant metastasis | nought | 26 | 92.69 ± 79.64 | 95 (0–290) | 2.987 | .003 |
| have | 81 | 145.74 ± 71.63 | 140 (0–290) | |||
| Lymph node metastasis | nought | 47 | 125.85 ± 88.75 | 120 (0–290) | 1.372 | .170 |
| have | 52 | 149.52 ± 66.25 | 160 (30–290) |
Table 2.
Correlation between different expression levels of CXCL2 in ovarian cancer tissues and clinicopathological features of epithelial ovarian cancer patients.
| CXCL2 expression | ||||||
| Low | High | |||||
| Clinicopathological feature | Cases | % | Cases | % | χ2 | P |
| Age | ||||||
| <55 | 51 | 68.00 | 24 | 32.00 | 1.844 | .174 |
| ≥55 | 15 | 53.57 | 13 | 46.43 | ||
| Differentiation | ||||||
| High | 10 | 90.91 | 1 | 9.09 | 7.604 | .022 |
| middle | 29 | 74.36 | 10 | 25.64 | ||
| l ow | 31 | 54.39 | 26 | 45.61 | ||
| FIGO stage | ||||||
| I∼II | 28 | 90.32 | 3 | 9.68 | 11.964 | .001 |
| IV∼III | 42 | 55.26 | 34 | 44.74 | ||
| Location (single or double) | ||||||
| single | 35 | 77.78 | 10 | 22.22 | 5.242 | .022 |
| double | 35 | 56.45 | 27 | 43.55 | ||
| Distant metastasis | ||||||
| nought | 23 | 88.46 | 3 | 11.54 | 7.724 | .005 |
| have | 47 | 58.75 | 33 | 41.25 | ||
| Lymph node metastasis | ||||||
| nought | 34 | 72.34 | 13 | 27.66 | 3.608 | .058 |
| have | 28 | 53.85 | 24 | 46.15 | ||
In the present study, we aimed to assess the correlation between the survival time of patients with epithelial OC and the factors, such as high expression of CXCL2, degree of differentiation, FIGO stage, location of the tumor, lymph node metastasis and metastasis, and the prognostic value of each factor was assessed by Kaplan-Meier analysis (Table 3). Our data showed that the survival time of patients with poorly differentiated epithelial OC was significantly shorter than that of patients with high and moderate differentiation (P = .002). The survival time of patients with FIGO stage IV ∼ III was significantly shorter than that of patients with stage I ∼ II (P = .0001). The survival time of patients with lymph node metastasis was significantly shorter than that of patients without lymph node metastasis (P = .0001). The survival curve suggested that the OS rate of the group with high expression of CXCL2 was decreased, while there was no statistical significance (P = .184) (Fig. 3).
Table 3.
Kaplan-Meier univariate analysis of survival time of patients with epithelial ovarian cancer after surgery (months).
| Clinicopathological feature | Survival (x ± s) | Survival 95%CI | P |
| Age | |||
| <55 | 51 | 24 | .174 |
| ≥55 | 15 | 13 | |
| CXCL2 | |||
| Low expression | 45.424 ± 2.337 | 40.843∼50.005 | .723 |
| hyperexpression | 43.958 ± 2.655 | 38.755∼49.161 | |
| Differentiation | |||
| High | 51.579 ± 4.415 | 42.926∼60.233 | .002 |
| middle | 51.109 ± 2.387 | 46.432∼55.787 | |
| low | 39.445 ± 3.439 | 34.664∼44.225 | |
| FIGO stage | |||
| I∼II | 54.817 ± 2.259 | 50.390∼59.244 | .0001 |
| IV∼III | 40.796 ± 2.083 | 36.714∼44.878 | |
| Location (single or double) | |||
| single | 47.299 ± 2.544 | 42.313∼52.284 | .272 |
| double | 43.250 ± 2.305 | 38.732∼47.768 | |
| Distant metastasis | |||
| nought | 56.686 ± 1.796 | 53.166∼60.207 | .0001 |
| have | 41.400 ± 2.050 | 37.382∼45.418 | |
| Lymph node metastasis | |||
| nought | 56.761 ± 1.209 | 54.392∼59.131 | .0001 |
| have | 34.810 ± 2.471 | 29.967∼39.653 | |
Figure 3.
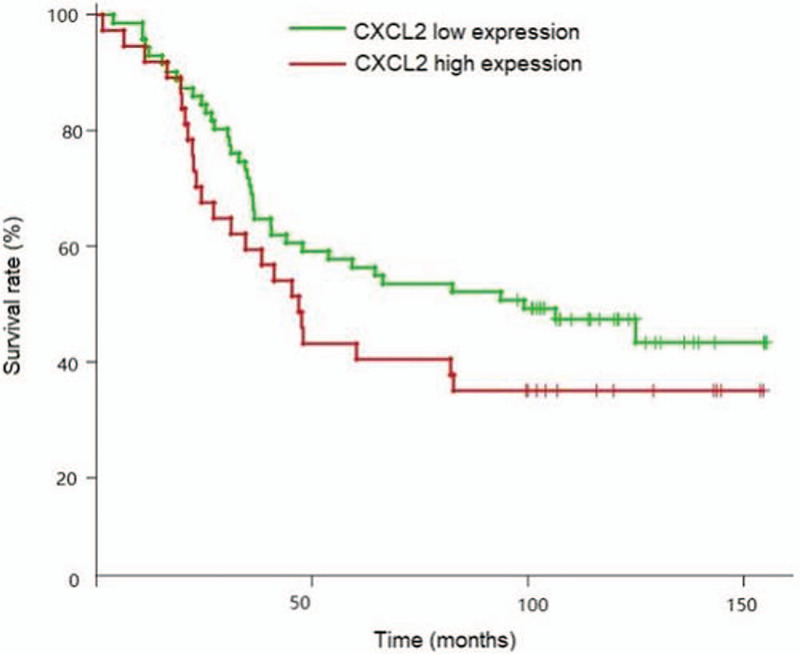
Kaplan-Meier survival curve for ovarian cancer patients.
4. Discussion
As a potential carcinogen, CXCL2 has been used to detect several types of human cancers, such as ESCC,[5] melanoma, and so on. CXCL2-CXCR2 is considered to be the receptor of IL-8, coupled with G α I to promote neutrophil chemotaxis and endothelial cells, leading to angiogenesis, tumor drug resistance, growth, and conversion.[7] However, the role of CXCL2 in OC remains largely unexplored. Some studies have discussed the potential role of CXCL2 in OC. The expression of CXCL2 at the mRNA level in OC tissues is significantly higher than that in normal ovarian tissues.[6] Previous studies have reported that the down-regulation of CXCL2 can significantly inhibit the expressions of metastatic genes in OC.[13] The up-regulation of CXCL2 can promote the progression of OC.[6] In this study, the statistical analysis of the TCGA database revealed that the expression of CXCL2 at the mRNA level in OC tissues (n = 426) was higher than that in normal tissues (n = 88). According to the average expression value of CXCL2 mRNA, there was a significant difference between the tumor tissues and normal tissues (P < .01).
In the current work, we detected the expression of CXCL2 at the protein level in OC tissues and their adjacent tissues. Moreover, the potential correlation between its expression and clinicopathological features of OC was explored. Besides, we also investigated the relationship between the expression of CXCL2 and the prognosis of OC patients. Immunohistochemical staining showed that the expression of CXCL2 at the protein level in 110 epithelial OC tissues (including each stage of OC) was higher than that in paracancerous tissues. Such over-expression was related to tumor epithelial cells, but not to inflammatory cells or non-epithelial stroma. The expression of CXCL2 was significantly correlated with the degree of invasion and metastasis of the primary tumor. The high expression of CXCL2 was associated with tumor stage and more prone to metastasis. These results suggested that the over-expression of CXCL2 in the cytoplasm of OC could promote the invasion and metastasis of OC cells. The data of this study showed that the high expression of CXCL2 in the cytoplasm was associated with a very poor OS, while there was no statistical significance. Multivariate analysis of variance showed that CXCL2 could not be used as an independent prognostic factor in patients with epithelial OC. This finding was similar to the relevant research results.[14] However, the over-expression of CXCL2 might still be an important prognostic factor for the prognosis of human epithelial OC. The expression of CXCL2 had potential value in determining the treatment regimen of OC patients. The pathophysiological role of CXCL2 in the carcinogenesis and progression of epithelial OC remains unclear. Chronic inflammation may increase the risk of cancer.[15,16] CXCL2 is over-expressed and chemotactic to neutrophils to bind to its receptor CXCR2 in inflammation. In addition to its role in the inflammatory response, chemokines also play an important role in tumorigenesis. Chemokines can regulate tumor-related angiogenesis, induce the release of proteolytic enzymes and stimulate the proliferation of tumor cells via the degradation of extracellular matrix and basement membrane, autocrine or paracrine, to enhance the ability of metastasis and invasion of tumor cells. The over-expression of A-kinase associated protein 1 (AKIP1) in cervical cancer cells increases the expressions of CXCL1, CXCL2, and CXCL8. These 3 chemokines are involved not only in endothelial angiogenesis by binding to endothelial receptor CXCR2, but also in the proliferation and cloning of cervical cancer cells induced by AKIP1. Therefore, AKIP1 plays a key role in the pathogenesis and disease progression of cervical cancer depending on CXCL1, CXCL2, and CXCL8.[17] It has been previously reported that NF- kappa B pathway activates the transcription of CXCL1 and CXCL2 genes.[18] The study has reported that in patients with breast cancer, gastric cancer, colorectal cancer, liver cancer, and bladder cancer, the expressions of CXCL1 and CXCL2 are associated with reduced tumor size, metastasis, and OS rate.[7,19–22]
The acquisition of cancer invasiveness is accompanied by the loss of epithelial characteristics and the increase of the mesenchymal phenotype. Such a process is called epithelial-mesenchymal transformation (EMT). It has been found that the number of CD8+ tumor-infiltrating lymphocytes (CD8+ TILs) in mesenchymal subtypes is decreased.[23] The number of CD8 + TILs and OS rate were negatively correlated with the invasion of MDSCs (myeloid-derived suppressor cells, bone marrow-derived suppressive cells) in advanced OC. The Snail can induce cancer progression by up-regulating CXCR2 ligands and recruiting MDSCs. The blockade of CXCR2 is an immunotherapeutic approach to inhibit the progression of spiral high tumor EMT.[14]
Although the multivariate analysis did not prove that the expressions of these chemokines were an independent predictor of poor prognosis in OC, the increased levels of chemokines were associated with Snail and MDSC infiltration. To overcome the possible limitation of small sample size, it is necessary to conduct clinical studies in the future to evaluate the values of serum CXCL1 and CXCL2 as biomarkers. It has been found that in OC patients, Snail up-regulates the expression of CXCL1/2 through CXCR2 and enhances the tumor invasion of MDSCs. MDSCs can inhibit tumor immunity and promote tumor progression. Therefore, CXCR2 and its ligands may become therapeutic targets for tumors with high Snail expression. These findings provide new insights into the mechanism of EMT and tumor immunity.[14] Our results showed that CXCL2 was over-expressed in the cytoplasm of epithelial OC cells rather than inflammatory cells. However, the relationship between CXCL2 protein expression and clinicopathological features is limited by tumor TMA. The autocrine or paracrine mechanism and signaling pathway of CXCL2 in OC remain to be clarified. Further in vitro and in vivo experimental studies are greatly required to explore the biological functions of CXCL2 and its receptors in epithelial OC.
Author contributions
Conceptualization: Jingting Jiang, Bin Xu.
Data curation: Fenghua Zhang, Bin Xu.
Formal analysis: Bin Xu.
Funding acquisition: Jingting Jiang, Changping Wu.
Investigation: Changping Wu.
Methodology: Fenghua Zhang, Changping Wu.
Project administration: Changping Wu.
Resources: Fenghua Zhang, Yun Xu.
Software: Bin Xu.
Supervision: Yun Xu, Changping Wu.
Validation: Changping Wu.
Visualization: Changping Wu.
Writing – original draft: Fenghua Zhang, Yun Xu.
Writing – review & editing: Fenghua Zhang, Changping Wu.
Glossary
Abbreviations: AKIP1 = A-kinase associated protein 1, CXCL2 = CXC chemokine ligand-2, CXCR2 = CXC chemokine receptor-2, EMT = epithelial-mesenchymal transformation, MDSC = myeloid-derived suppressor cells, OS = overall survival, TMA = tissue microarray.
References
- [1].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [3].Wang X, Zhao X, Feng C, et al. IL-36gamma transforms the tumor microenvironment and promotes type 1 lymphocyte-mediated antitumor immune responses. Cancer Cell 2015;28:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khan S, Cameron S, Blaschke M, et al. Differential gene expression of chemokines in KRAS and BRAF mutated colorectal cell lines: role of cytokines. World J Gastroenterol 2014;20:2979–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dong QM, Zhang JQ, Li Q, et al. Clinical significance of serum expression of GRObeta in esophageal squamous cell carcinoma. World J Gastroenterol 2011;17:2658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dong YL, Kabir SM, Lee ES, et al. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-(B activation via EGFR-transactivated Akt signaling. PLoS One 2013;8:e83789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012;150:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Doll D, Keller L, Maak M, et al. Differential expression of the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and their impact on metastatic disease and survival. Int J Colorectal Dis 2010;25:573–81. [DOI] [PubMed] [Google Scholar]
- [9].Jun Ding, Kangdi Xu, Jie Zhang, et al. Overexpression of CXCL2 inhibits cell proliferation and promotes apoptosis in hepatocellular carcinoma. BMB Rep 2018;51:630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen MC, Baskaran R, Lee NH, et al. CXCL2/CXCR2 axis induces cancer stem cell characteristics in CPT-11-resistant LoVo colon cancer cells via Gαi-2 and Gαq/11. J Cellular Physiol 2019;234:11822–34. [DOI] [PubMed] [Google Scholar]
- [11].Wang B, Khachigian LM, Esau L, et al. A key role for early growth response-1 and nuclear factor-kappaB in mediating and maintaining GRO/CXCR2 proliferative signaling in esophageal cancer. Mol Cancer Res 2009;7:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bruyere C, Lonez C, Duray A, et al. Considering temozolomide as a novel potential treatment for esophageal cancer. Cancer 2011;117:2004–16. [DOI] [PubMed] [Google Scholar]
- [13].Kavandi L, Collier MA, Nguyen H, et al. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell Biochem 2012;113:3143–52. [DOI] [PubMed] [Google Scholar]
- [14].Taki M, Abiko K, Baba T, et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun 2018;9:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Inoue Y, Iwata T, Okugawa Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology 2013;84:100–7. [DOI] [PubMed] [Google Scholar]
- [16].Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic system predicts survival after surgery for stage IV colorectal cancer. Am J Surg 2013;205:22–8. [DOI] [PubMed] [Google Scholar]
- [17].Zhang W, Wu Q, Wang C, et al. AKIP1 promotes angiogenesis and tumor growth by upregulating CXC-chemokines in cervical cancer cells. Mol Cell Biochem 2018;448:311–20. [DOI] [PubMed] [Google Scholar]
- [18].Burke SJ, Lu D, Sparer TE, et al. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metabol 2014;306:E131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheng WL, Wang CS, Huang YH, et al. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol 2011;22:2267–76. [DOI] [PubMed] [Google Scholar]
- [20].le Rolle AF, Chiu TK, Fara M, et al. The prognostic significance of CXCL1 hypersecretion by human colorectal cancer epithelia and myofibroblasts. J Transl Med 2015;13:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li L, Xu L, Yan J, et al. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res 2015;34:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang H, Ye YL, Li MX, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017;36:2095–104. [DOI] [PubMed] [Google Scholar]
- [23].Murakami R, Matsumura N, Mandai M, et al. Establishment of a novel histopathological classification of high-grade serous ovarian carcinoma correlated with prognostically distinct gene expression subtypes. Am J Pathol 2016;186:1103–13. [DOI] [PubMed] [Google Scholar]


