Abstract
Nosocomial infections (NI) are common complications after cardiac surgery. To date, there have been few manuscripts investigating NI in the intensive care unit after cardiac surgery. Our study was designed to investigate the characteristics of the distribution of pathogenic bacteria, antibiotic resistance and risk factors for NI.
A total of 1360 patients received standard postoperative care, including antibiotic prophylaxis. Microbiological examinations of sputum, blood, catheter tips and excrement were performed as clinically indicated to isolate pathogens. Thirty potential associated variables were collected and compared between the 2 different groups according to the development of NI using univariate and multivariate analyses.
Eighty-nine patients (6.54%) acquired a microbiologically documented NI. There was a significant difference in mortality between the 2 groups with or without postoperative NI (23.60% vs 2.28%, P < .00). A total of 98 pathogens (73.13%) were isolated from sputum, 32 pathogens (23.88%) from blood and only 1 (0.75%) from urine. Three (2.24%) surgical site infections were detected, including 2 superficial surgical site infections and 1 mediastinitis. The most common pathogens were Gram-negative bacteria (78.36%), followed by Gram-positive bacteria (14.93%) and fungi (6.71%). The major pathogenic species had different levels of drug resistance, and most of them exhibited multidrug resistance. Six out of thirty variables were identified as independent risk factors for the development of NI, namely, duration of surgery, low cardiac output syndrome, continuous veno-venous hemofiltration, mechanical ventilation time, reintubation and tracheostomy.
We analyzed the characteristics of the distribution of pathogens, antibiotic resistance and risk factors for NI in our center and provided some suggestions for clinical practice. In addition to antibiotic treatment, avoidance of risk factors and aggressive infection control measures may be crucial to stop or prevent outbreaks.
Keywords: fungi, G- bacilli, G+ cocci, multiple drug resistance, nosocomial infections, risk factors
1. Introduction
Nosocomial infections (NI) include all infections acquired between 48 hour after hospital admission and 2 days after hospital discharge. The cardiac surgical intensive care unit (ICU) is a special ward with a higher incidence of NI and usage rate of antibiotics, owing to the severity of illnesses, complexity of surgeries and common use of invasive devices (endotracheal tubes, central venous catheters, peripheral arterial catheters and urinary catheters).[1] NI are associated with increased morbidity and mortality, as well as increased length of ICU stay and healthcare costs.[2,3] Furthermore, the burden of antimicrobial resistance in the ICU is increasing, which has been attributed to the increased difficulty of clinical treatment and hospital control of NI. Therefore, initial antibiotic treatments should be updated to pathogen-specific treatments as soon as possible. The aims of our study were to investigate the characteristics of distribution of pathogenic bacteria, antibiotic resistance and risk factors for NI.
2. Materials and methods
2.1. Study population and design
From January 2018 to December 2018, 1381 patients (≥18 years old) undergoing open heart surgery were transferred to our cardiac surgical ICU. All patients were eligible for the investigation, except for those who died within 24 hour postoperatively (n = 21). Since it was a retrospective observational study, ethical approval was not necessary. All patients received standard postoperative care that complied with published guidelines.[4,5] Antibiotic prophylaxis was a single administration of second-generation cephalosporin. Body temperature was recorded every 6 hour routinely and anytime when necessary. Hematologic tests and chest radiographs were performed regularly. Microbiological examinations of the sputum, blood, catheter tips and excrement of these patients were performed as clinically indicated to isolate pathogens according to the criteria of the Clinical and Laboratory Standards Institute. One pathogen cultured positively multiple times in the same patient within 1 week was regarded as a single infection. All enrolled patients were divided into 2 different groups: the NI group and non-NI group, according to the development of NI or not. Thirty potential related perioperative parameters, including age, gender, BMI, and smoking index, were collected retrospectively and compared between the 2 groups. Postoperative acute kidney injury refers to 1 of the following situations:
-
(1)
serum creatinine increased by at least 0.3 mg/dL compared with the baseline within 48 hour;
-
(2)
serum creatinine at least 1.5-fold higher than the baseline within 7 days; or
-
(3)
urine volume ≦ 0.5 mL/kg/h lasting 6 hour.
2.2. Definitions
All definitions were based on criteria established by the Centers for Disease Control and Prevention. The most common types of NI are hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), bloodstream infection (BSI), catheter-related bloodstream infection (CRBSI), catheter-associated urinary tract infection and surgical site infection (SSI).[6–8] VAP is a subset of HAP that occurs more than 48 h after endotracheal intubation. In our study, both HAP and VAP were included into the type of pneumonia. CRBSI is a subset of BSI characterized by the presence of central venous catheters and signs of catheter insertion site infection. SSI can be divided into three types: superficial SSI, deep SSI and mediastinitis.
Antibiotic resistance means acquired resistance, except for natural resistance. Multidrug-resistant bacteria means that 1 bacteria was resistant to three or more classes of antibiotics, and bacteria sensitive only to colistin and/or tigecycline were defined as extensively drug resistant bacteria.[9] Smoking index refers to the product of number of cigarettes per day and years of smoking. VIS score can be utilized to evaluate the circulation situation and usage of intropes, it equals dopamine (ug/kg·min) + dobutamine (ug/kg·min) + 10 × milrinone (ug/kg·min) + 100 × epinephrine (ug/kg·min) +100 × norepinephrine (ug/kg·min) + 10000 × pituitrin (ug/kg·min). Low cardiac output syndrome (LCOS) refers to the syndrome of reduced cardiac output (CO < 2.0L/min/m2) and peripheral malperfusion.
2.3. Data analysis
Continuous variables are shown as means plus SD; categorical data are presented as proportions. For comparisons of continuous variables, the t-test or Wilcoxon test was used, depending on the distribution of data. The categorical data were compared using the chi-square or Fisher exact test. Variables associated with the development of NI in the univariate analysis (p < 0.05) were included into a forward multivariable logistic regression model. All statistical analyses were performed using SPSS 21. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Source of nosocomial infection and associated mortality
Out of 1360 patients enrolled into our study, 89 patients (6.54%) acquired a microbiologically documented NI. Among these 89 patients, 21 patients died, resulting in a mortality rate of 23.60%. For the other 1271 patients without NI, the mortality rate was only 2.28% (29/1271, p < 0.00). A total of 56 patients (62.92%) developed a single infection, while 33 patients (37.08%) experienced 2 or more different nosocomial infections. A total of 134 strains of pathogenic bacteria were detected, of which 98 (73.13%) were isolated from sputum and 32 (23.88%) were isolated from blood. Three (2.24%) SSIs were detected in our study (2 superficial SSIs and 1 mediastinitis). Only 1 (0.75%) bacterial strain was isolated from urine. The details of the sources of NI and associated mortality are shown in Figure 1. The distribution of pathogens for each type of NI were different. Microorganisms causing pneumonia were Gram-negative bacilli, while the main pathogens of BSI were Gram-positive cocci (Fig. 2).
Figure 1.
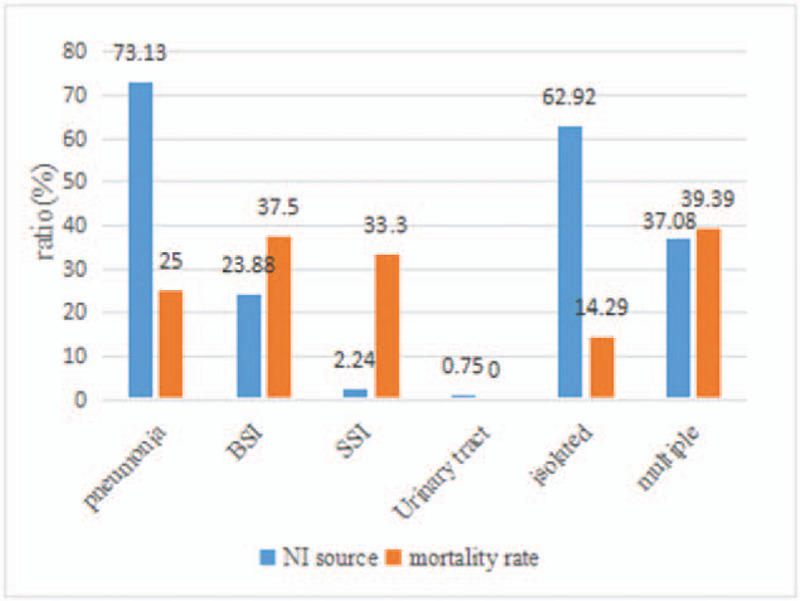
Source of NI and associated mortality.
Figure 2.
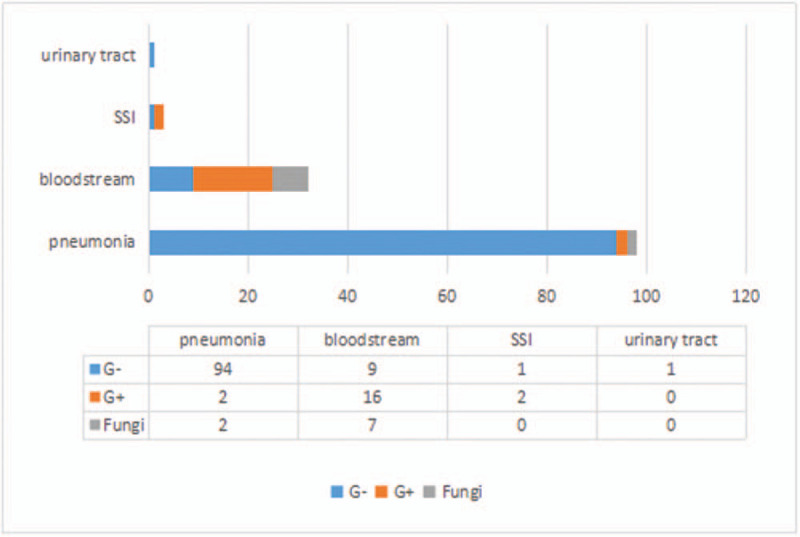
Distribution of pathogens in clinical specimens.
3.2. Constituent ratio of pathogenic bacteria
A total of 134 strains of pathogenic bacteria were isolated in our study. The most common pathogens were Gram-negative bacteria (78.36%), followed by Gram-positive bacteria (14.93%). Table 1 presents the constituent ratio of predominant infectious pathogens.
Table 1.
Constituent ratio of pathogenic bacteria.
| Pathogenic Bacteria | Strains | Ratio (%) |
| Gram-negative | 105 | 78.36 |
| Acinetobacter baumannii | 50 | 37.31 |
| Klebsiella pneumoniae | 19 | 14.18 |
| Pseudomonas aeruginosa | 17 | 12.69 |
| Enterobacter cloacae | 8 | 5.97 |
| Escherichia coli | 6 | 4.48 |
| others | 5 | 3.73 |
| Gram-positive | 20 | 14.93 |
| Staphylococcus epidermidis | 9 | 6.72 |
| Enterococcus faecium | 6 | 4.48 |
| Staphylococcus aureus | 3 | 2.24 |
| others | 2 | 1.49 |
| Fungi | 9 | 6.72 |
| candida | 8 | 5.97 |
| aspergillus | 1 | 0.75 |
3.3. Antibiotic resistance of main pathogens of NI in clinical practice
Along with the development and inappropriate use of antibiotics, especially wide-spectrum ones, drug resistance in bacteria changes constantly. Timely and continuous determination of antibiotic susceptibility is required. The details of antibiotic resistance for common pathogens are as follows (Tables 2 and 3).
Table 2.
Resistant rates of common Gram-positive bacteria.
| Acinetobacter baumannii | Klebsiella pneumoniae | Pseudomonas aeruginosa | Enterobacter cloacae | Escherichia coli | |||||||||||
| Antibiotics | tested strains | resistant strains | resistant rate (%) | tested strains | resistant strains | resistant rate (%) | tested strains | resistant strains | resistant rate (%) | tested strains | resistant strains | resistant rate (%) | tested strains | resistant strains | resistant rate (%) |
| Ampicillin | – | – | – | 13 | 12 | 92.31 | – | – | – | – | – | – | 6 | 5 | 83.33 |
| Piperacillin/Tazobactam | – | – | – | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 0 | 0 | 6 | 0 | 0 |
| Ceftazidime | 44 | 44 | 100.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 3 | 37.50 | 6 | 3 | 50.00 |
| Ceftriaxone | 50 | 45 | 90.00 | 19 | 5 | 26.32 | – | – | – | 8 | 3 | 37.50 | 6 | 3 | 50.00 |
| Cefperazone-Sulbactam | 50 | 17 | 34.00 | – | – | – | 17 | 1 | 5.88 | - | – | – | – | – | – |
| Cefepime | 50 | 44 | 88.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 3 | 37.50 | 6 | 2 | 33.33 |
| Aztreonam | – | – | – | 19 | 2 | 10.53 | 17 | 3 | 17.65 | 8 | 4 | 50.00 | 6 | 3 | 50.00 |
| Gentamicin | 50 | 44 | 88.00 | 19 | 2 | 10.53 | 17 | 2 | 11.76 | 8 | 0 | 0 | 6 | 3 | 50.00 |
| Amikacin | 7 | 7 | 100.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 0 | 0 | 6 | 0 | 0 |
| Imipenem | 48 | 44 | 91.67 | 19 | 2 | 10.53 | 17 | 5 | 29.41 | 8 | 0 | 0 | 6 | 0 | 0 |
| Meropenem | 47 | 42 | 89.36 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 0 | 0 | 6 | 0 | 0 |
| Levofloxacin | 50 | 21 | 42.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 1 | 12.50 | 6 | 5 | 83.33 |
| Ciprofloxacin | 50 | 45 | 90.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 1 | 12.50 | 6 | 5 | 83.33 |
| Sulfamethoxazole | 50 | 41 | 82.00 | 19 | 5 | 26.32 | – | – | – | 8 | 6 | 75.00 | 6 | 2 | 33.33 |
| Minocycline | 42 | 16 | 38.10 | – | – | – | – | – | – | – | – | – | – | – | – |
| Tobramycin | 50 | 42 | 84.00 | 19 | 2 | 10.53 | 17 | 1 | 5.88 | 8 | 0 | 0 | 6 | 0 | 0 |
| Tigecycline | 49 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 100 | – | – | – | – | – | – |
Table 3.
Resistant rates of common Gram-positive bacteria.
| Staphylococcus epidermidis | Enterococcus faecium | |||||
| Antibiotics | tested strains | resistant strains | resistant rate (%) | tested strains | resistant strains | resistant rate (%) |
| Penicillin G | 9 | 9 | 100 | 6 | 6 | 100 |
| Oxacillin | 9 | 8 | 88.89 | 6 | 6 | 100 |
| High concentration of Gentamicin | 9 | 7 | 77.78 | 6 | 6 | 100 |
| Levofloxacin | 9 | 6 | 66.67 | 6 | 6 | 100 |
| Ciprofloxacin | 9 | 6 | 66.67 | 6 | 6 | 100 |
| Clindamycin | 9 | 5 | 55.56 | - | - | - |
| Rifampicin | 9 | 2 | 22.22 | 6 | 3 | 50 |
| Sulfamethoxazole | 9 | 4 | 44.44 | - | - | - |
| Vancomycin | 9 | 0 | 0 | 6 | 1 | 16.67 |
| Linezolid | 9 | 0 | 0 | 6 | 0 | 0 |
| Daptomycin | 9 | 0 | 0 | 6 | 0 | 0 |
A total of 9 strains of fungi were isolated in our study, 8 of which were Candida species, 7 C albicans and 1 C tropicalis. All of the 7 C albicans isolates were susceptible to azoles, 5-fluorocytosine and amphotericin B. Aspergillosis was naturally resistant to fluconazole but sensitive to voriconazole and echinocandins.
3.4. Risk factors for NI
Table 4 shows the results of all potential variables associated with the development of NI. Eighteen of the 30 original variables, which had significant differences, were studied further using multivariate stepwise logistic regression analysis. The following six independent risk factors for the development of NI were identified in our study: duration of surgery, LCOS, continuous veno-venous hemofiltration, mechanical ventilation time, reintubation and tracheostomy (Table 5).
Table 4.
Univariate analysis of perioperative risk factors of NI in patients after open heart surgery.
| Variable | NI (n = 89) | non-NI (n = 1271) | P |
| Age (yr) | 67.90 ± 11.76 | 61.83 ± 9.65 | .002 |
| Male | 56 (62.9%) | 740 (58.2%) | .425 |
| BMI | 27.35 ± 2.15 | 25.2 ± 3.56 | .023 |
| Smoking index | 170.35 ± 101.48 | 152.00 ± 87.96 | .760 |
| Preoperative LVEF (%) | 43 ± 11.32 | 47 ± 9.01 | .001 |
| Preoperative creatinine (umol/L) | 78.50 ± 24.08 | 62.56 ± 26.50 | .223 |
| Preoperative albumine (g/L) | 40.75 ± 3.86 | 41.01 ± 5.44 | .923 |
| History of COPD | 9 (10.1%) | 97 (7.6%) | .511 |
| Diabetes mellitus | 29 (32.6%) | 365 (28.7%) | .595 |
| Prior cancer treatment | 2 (2.3%) | 26 (2.1%) | .896 |
| History of cerebrovascular disease | 6 (6.7%) | 77 (6.1%) | .698 |
| History of immunosuppression | 2 (2.2%) | 11 (0.9%) | <.001 |
| endocarditis | 3 (3.4%) | 40 (3.1%) | .899 |
| Urgent Operation | 10 (11.2%) | 89 (7.0%) | .022 |
| Type of operation | |||
| CABG | 18 | 479 | .079 |
| Valve | 20 | 450 | |
| Combination of CABG and valve | 12 | 130 | |
| Aortic root replacement | 8 | 48 | |
| Aortic arch surgery | 31 | 165 | |
| Duration of Surgery (min) | 616.00 ± 88.47 | 525.83 ± 77.57 | .019 |
| CPB time (min) | 277.50 ± 33.93 | 239.08 ± 45.61 | .040 |
| Aortic crossclamp time (min) | 169.70 ± 15.13 | 151.25 ± 25.85 | .061 |
| Blood transfusion volume within 24h (mL) | 654.37 ± 126.81 | 472.15 ± 154.93 | .018 |
| reoperation | 3 (3.37%) | 35 (2.75%) | .768 |
| VIS score of intropes | 347 ± 189.70 | 178 ± 202.15 | .013 |
| LCOS | 29 (32.58%) | 57 (4.48%) | <.001 |
| Postoperative AKI | 23 (29.2%) | 76 (5.98%) | .002 |
| CVVH | 14 (15.73%) | 25 (1.97%) | .001 |
| Urgent insertion of IABP | 8 (8.98%) | 40 (3.15%) | .008 |
| Mechanical ventilation time (h) | 32.30 ± 22.1 | 8.67 ± 18.49 | <.001 |
| Reintubation | 20 (22.47%) | 71 (5.59%) | .001 |
| Tracheostomy | 18 (52.9%) | 3 (4.5%) | <.001 |
| ICU stay (d) | 21 ± 17.69 | 4.68 ± 8.18 | .012 |
| Postoperative stroke | 13 (14.61%) | 50 (3.93%) | .006 |
AKI = acute kidney injury, COPD = chronic obstructive pulmonary disease, CPB = cardiopulmonary bypass, CVVH = continuous veno-venous hemofiltration, IABP = intra-aortic balloon pump, ICU = intensive care unit, LCOS = low cardiac output syndrome, LVEF = left ventricular ejection fraction.
Table 5.
Multivariate stepwise logistic regression analysis of risk factors for the development of NI.
| Variable | OR (95% CI) | P |
| duration of surgery | 3.94 (1.07–10.04) | .04 |
| LCOS | 4.76 (1.43–15.72) | .01 |
| CVVH | 5.89 (1.27–16.12) | .008 |
| mechanical ventilation time | 15.24 (4.07–36.72) | .001 |
| reintubation | 21.09 (11.15–44.21) | .001 |
| tracheostomy | 12.38 (3.56–24.21) | .002 |
CI = confidence interval, CVVH = continuous veno-venous hemofiltration, LCOS = low cardiac output syndrome.
4. Discussion
In our retrospective study, HAP was the main cause of postoperative NI, accounting for 73.13% of cases. It was also a leading cause of mortality, as high as 17.34%. In accordance with our results, mechanical ventilation time and reintubation were independent risk factors for the development of NI. The development of HAP was associated with the use of endotracheal intubation and mechanical ventilation. Intubation destroys the normal barrier of the epiglottis and decreases the cough reflex and movement of cilia, which lead to impaired clearance of the infectious pathogen from airway secretions. Sputum was a good culture medium for bacteria. Mechanical ventilation also contributed to the development of VAP and the risk peaked within the first week. The initial step of VAP is the colonization of the upper respiratory tract by potentially pathogenic bacteria. Aspiration of these microorganisms either through the endotracheal tube or a leak around the cuff allowed them to enter the lower respiratory tract. This event, along with decreased host immunity, can result in the development of NI. The key to control the development of VAP is to reduce the duration of intubation, including the use of a semi-recumbent position, sedation holds, protocolized weaning and daily assessment for the possibility of extubation.
The predominant bacteria for HAP was Acinetobacter baumannii. A baumannii is a conditional pathogen that may cause NI in critically ill patients. A baumannii has simple growth requirements and may survive in a desiccated environment for prolonged periods.[10] Contaminated environmental sources and transmission via medical personnel may cause outbreaks of NI.[11,12]A baumannii has been associated with high morbidity and mortality.[13,14] Vincent JL and colleagues reported that infection with A baumannii was independently associated with a greater risk for hospital death among 14414 ICU patients.[15] In recent years, the incidence and antibiotic resistance of A baumannii infection have increased rapidly. Treatment of A baumannii is difficult, owing to its resistance to various antibiotics and remarkable ability to acquire new resistance via different mechanisms, such as plasmids, transposons, integrons and resistance islands. Antimicrobial resistance poses a serious threat worldwide. In early 1990, carbapenem-resistant (CPR) strains of A baumannii were first discovered. CPR-A baumannii is often resistant to all classes of antibiotics, except for colistin and tigecycline.[16] Furthermore, a large dose of sulbactam, fluoroquinolones, aminoglycosides and tetracyclines may also have antibacterial activity against CPR-A baumannii.[17–20] In our study, we discovered that the carbapenem resistance rate of A baumannii had reached up to >50%. As much as 90% of A baumannii were sensitive to colistin and tigecycline, while the resistance rates to cefoperazone-sulbactam, levofloxacin and minocycline were 34.00%, 42.00% and 38.10%, respectively. We routinely analyzed the drug sensitivity of gentamicin, instead of amikacin, as a representative of aminoglycosides, and the resistance rate exceeded 80%.
The principles of treatment for A baumannii were as follows:  antimicrobial susceptibility results;
antimicrobial susceptibility results;  combination therapy;
combination therapy;  adequate dose;
adequate dose;  sufficient treatment period; and
sufficient treatment period; and  personal administration. Optimal therapy was established according to antimicrobial susceptibility results. However, for multidrug resistant or extensively drug resistant A baumannii, the recommended therapy was colistin/tigecycline combined with other agents (ie, carbapenem, sulbactams, fluoroquinolones or minocycline).
personal administration. Optimal therapy was established according to antimicrobial susceptibility results. However, for multidrug resistant or extensively drug resistant A baumannii, the recommended therapy was colistin/tigecycline combined with other agents (ie, carbapenem, sulbactams, fluoroquinolones or minocycline).
Except for A baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and other Gram-negative bacilli were also common pathogens for HAP. As proved in our study, all of them were clinically sensitive to carbapenem, extended-spectrum cephalosporins and fluoroquinolones.
BSI is also a serious and common type of NI. It is more likely to occur in patients immunosuppressed and malnourished patients and those using various invasive devices. CRBSI is a subset of BSI with the presence of central venous catheters. Immediately after insertion, the catheter becomes coated with plasma protein. Bacteria could migrate from the skin along the surface of catheter. This may happen a few hours or more than 1 week after insertion. Femoral venous catheters have the highest rate of infection, followed by internal jugular and subclavian catheters.[21] For CRBSI, once infection is suspected, the central catheter should be removed as soon as possible. In our research, the most common pathogens for BSI were Gram-positive cocci (50%), for example, Staphylococcus and Enterococcus. Within the past few decades, the resistance rate among Gram-positive cocci have obviously increased. We reported that 78.13% of Gram-positive bacteria were resistant to methicillin and only 6.25% were resistant to vancomycin. However, all of them were sensitive to linezolid.
Fungal infection is not unusual after cardiac surgery. Risk factors for fungal infection include immunosuppression, malnutrition, diabetes mellitus and long-term use of extended-spectrum antibiotics.[22,23] In our research, Candida was the most common agent of fungal infection (88.89%; 8/9), which was consistent with previous studies.[24–26] Among 8 strains of Candida, 6 were C albicans and 2 were C. tropicalis, with 7 strains isolated from the bloodstream. Other than Candida, Aspergillosis was also a common agent of fungal infection. Lung was the most frequent site of aspergillosis infection.[27] Consistent with our results, most Candida infections, except for C glabrata, were susceptible to fluconazole and voriconazole. Aspergillosis was naturally resistant to fluconazole. Echinocandins were the best choice for definite and severe fungal infection, because they remained close to 100% effective.
As discovered in our research, the development of NI was associated with longer surgery duration, postoperative LCOS and utilization of continuous veno-venous hemofiltration. This finding suggested that NI may occur in patients with more severe diseases or more complex surgical procedures. For these populations, more attention should be paid to preoperative assessment and preparation and the choice of optimal surgical procedures and efforts should be made to minimize the duration of the surgery. Once postoperative LCOS or acute kidney injury has developed, sterile practices should be strictly followed for invasive operations, and a timely adjustment of the prescribed antibiotics according to the condition of the patient is crucial.
The distribution of pathogenic bacteria and antibiotic resistance of NI after cardiac surgery varies distinctly worldwide, and while our research data only provide the epidemiological profiles and trends of our institution, our results have significant implications for the prevention and treatment of NI.
5. Conclusion
NI is a common postoperative complication of open-heart surgery and is associated with increased morbidity and mortality. Currently, antimicrobial resistance, which differs in specific regions and/or populations, has become a great challenge for the treatment of NI. Therefore, we analyzed the characteristics of the distribution of pathogens, antibiotic resistance and independent risk factors for NI in patients after open-heart surgery and provided some suggestions for clinical practice. In addition to antibiotic treatment, avoidance of risk factors and aggressive infection control measures, including identifying the source of infection, environmental cleaning, contact precautions, isolation of infected patients and hand hygiene, may be crucial to stop or prevent outbreaks.
Acknowledgments
In writing this paper, I have benefited from the input of my teachers and colleagues. They generously helped me collect materials I needed and made many invaluable suggestions. I hereby extend my gratitude for their kind help. Particularly, I am deeply indebted to Professor Qian Zhai, my supervisor, who guided me throughout my writing of this thesis. She carefully read the whole draft and offered painstaking and valuable feedback. Her standards of academic excellence have made my revision an exciting and gratifying experience. I also wish to sincerely thank my director, whose brilliant ideas and perceptive observations have proved immensely constructive. Furthermore, none of this would have been possible without the help of the following individuals and organizations: our department and its staff and the Department of Cardiovascular Surgery of Qilu Hospital of Shandong University and its staff.
Author contributions
Conceptualization: zhengqin liu, xiquan zhang, Qian Zhai.
Data curation: zhengqin liu.
Formal analysis: zhengqin liu.
Investigation: zhengqin liu, Qian Zhai.
Methodology: zhengqin liu, xiquan zhang, Qian Zhai.
Project administration: zhengqin liu, xiquan zhang, Qian Zhai.
Resources: zhengqin liu.
Software: zhengqin liu.
Supervision: xiquan zhang, Qian Zhai.
Validation: zhengqin liu, Qian Zhai.
Writing – original draft: zhengqin liu.
Writing – review & editing: zhengqin liu, xiquan zhang, Qian Zhai.
Glossary
Abbreviations: BSI = blood stream infection, CPR = carbapenem resistant, CRBSI = catheter-related blood stream infection, HAP = hospital-acquired pneumonia, ICU = intensive care unit, LCOS = low cardiac output syndrome, LVEF = left ventricular ejection fraction, NI = nosocomial infections, SSI = surgical site infection, VAP = ventilator-associated pneumonia.
References
- [1].Welsby IJ, Bennett-Guerrero E, Atwell D, et al. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg 2002;94:1072–8. [DOI] [PubMed] [Google Scholar]
- [2].Bouza E, Hortal J, Munoz P, et al. European Study Group on Nosocomial Infections, European Workgroup of Cardiothoracic Intensivists. Postoperativeinfections after major heart surgery and prevention of ventilator-associated neumonia: a one-day European prevalence study (ESGNI-008). J Hosp Infect 2006;64:224–30. [DOI] [PubMed] [Google Scholar]
- [3].Michalopulos A, Geroulanos S, Rosmarakis ES, et al. Frequency, characteristics, and redictors of microbiologically documented nosocomial infections after cardiac surgery. Eur J Cardiothorac Surg 2006;2:456–60. [DOI] [PubMed] [Google Scholar]
- [4].De Santo LS, Romano G, Amarelli C, et al. Surgical repair of acute type A aortic dissection: continuous pulmonary perfusion during retrograde cerebral perfusion prevents lung injury in a pilot study. J Thorac Cardiovasc Surg 2003;126:826–31. [DOI] [PubMed] [Google Scholar]
- [5].Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care-associated pneumonia 2003:recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1–36. [PubMed] [Google Scholar]
- [6].Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128–40. [DOI] [PubMed] [Google Scholar]
- [7].Horan TC, Gaynes RP. Mayahall CG. Surveillance of nosocomial infections. Hospital epidemiology and infection control. Philadelphia: Lippincot Williams & Wilkins; 2004. 1659–702. [Google Scholar]
- [8].ATS Board of Directors. In: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- [9].Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [10].Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med 2015;36:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tal-Jasper R, Katz DE, Amrami N, et al. Clinical and epidemiological significance of Carbapenem resistance in Acinetobacter baumannii infections. Antimicrob Agents Chemother 2016;60:3127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gavaldà L, Soriano AM, Cámara J, et al. Control of endemic extensively drug-resistant Acinetobacter baumannii with a cohorting policy and cleaning procedures based on the 1 room, 1wipe approach. Am J Infect Control 2016;44:520–4. [DOI] [PubMed] [Google Scholar]
- [13].Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: evolution of antimicrobial esistance-treatment options. Semin Respir Crit Care Med 2015;36:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wisplinghoff H, Paulus T, Lugenheim M, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect 2012;64:282–90. [DOI] [PubMed] [Google Scholar]
- [15].Vincent JL, Rello J, Marshall J, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–9. [DOI] [PubMed] [Google Scholar]
- [16].Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents 2012;39:105–14. [DOI] [PubMed] [Google Scholar]
- [17].Oliveira MS, Prado GV, Costa SF, et al. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother 2008;61:1369–75. [DOI] [PubMed] [Google Scholar]
- [18].Jaruratanasirikul S, Wongpoowarak W, Aeinlang N, et al. Pharmacodynamics modeling to optimize dosage regimens of sulbactam. Antimicrob Agents Chemother 2013;57:3441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lob SH, Hoban DJ, Sahm DF, et al. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents 2016;47:317–23. [DOI] [PubMed] [Google Scholar]
- [20].Buisson Y, TranVanNhieu G, Ginot L, et al. Nosocomial outbreaks due to amikacin-resistant tobramycin-sensitive Acinetobacter species: correlation with amikacin usage. J Hosp Infect 1990;15:83–93. [DOI] [PubMed] [Google Scholar]
- [21].Vincent, Jean-Louis. Nosocomial infection in the ICU. Microbes Infection 2004;6:1003–7. [Google Scholar]
- [22].Ma CF, Li FQ, Shi LN, et al. Surveillance study of species distribution, antifungal susceptibility and mortality of nosocomial candidemia in a tertiary care hospital in China. BMC Infect Dis 2013;13:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Poikonen E, Lyytikäinen O, Anttila VJ, et al. Secular trend in candidemia and the use of fluconazole in Finland, 2004-2007. BMC Infect Dis 2010;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miceli MH, Díaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis 2011;11:142–51. [DOI] [PubMed] [Google Scholar]
- [25].Gavaldà J, Meije Y, Fortún J, et al. ESCMID Study Group for Infections in Compromised Hosts. Invasive fungal infections in solid organ transplant recipients. Clin Microbiol Infect 2014;20: (Suppl 7): 27–48. [DOI] [PubMed] [Google Scholar]
- [26].Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012;4:163–5. [DOI] [PubMed] [Google Scholar]
- [27].Gao LY, Yu J, Li RY. Epidemiology of aspergillosis in mainland China. Chin J Mycol (Zhongguo Zhen Jun Xue Za Zhi) 2010;5:247–51. [Google Scholar]


