Abstract
To evaluate the antiviral effect and safety of arbidol and Lianhuaqingwen Capsule (LH) in treating patients with Coronavirus disease 2019 (COVID-19).
108 patients with COVID-19 were divided into 2 groups, including 40 patients in the arbidol group and 68 patients in the arbidol + LH group. Patients in the arbidol + LH group received 200 mg of arbidol and 1400 mg of LH per 8 hour, and the arbidol group was given 200 mg arbidol per 8 hour. Blood routine examination, blood biochemistry detection, SARS-CoV-2 nucleic acid detection, and chest CT scans were performed to evaluate the clinical effects between the 2 groups.
No statistically significant differences were observed between the 2 groups in terms of preoperative characteristics including the baseline characteristics, laboratory indicators, and chest CT. On day 7 after admission, patients in the arbidol + LH group showed a higher level of Lymphocytes count, and a lower level of serum amyloid A and C-reactive protein levels (P < .05). Moreover, the median time from admission to the first negative result of the SARS-CoV-2 nucleic acid detection was shorter in the arbidol + LH group (P < .05). Analysis based on CT scan results showed a better extinction of lung inflammation in the arbidol + LH group. No apparent side effects were found in both groups. No patients were transferred to the intensive care unit (ICU) treatment.
Arbidol combined with LH treatment may be more effective in improving the prognosis and accelerating the SARS-CoV-2 clearance in patients with COVID-19.
Keywords: arbidol, coronavirus disease 2019, Lianhuaqingwen capsule
1. Introduction
Coronaviruses are a group of enveloped viruses named for their coronary appearance with positive single-stranded RNA genomes.[1] In December 2019, a kind of novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was detected in Wuhan. And more than 80,000 cases of Coronavirus disease 2019 (COVID-19) have been reported in China.[2–5] So far, the infection keeps spreading in the world, and more and more cases were confirmed in other countries.[6,7]
It has been hypothesized that the immunopathological response was triggered by the viral antigen; the most strategic treatment was to stop the viral replication at the beginning so that the peak viral load and the subsequent immunopathological damage will be minimized.[8] So far, the number of diagnoses of this disease is still multiplying, and effective antiviral treatment is therefore required urgently.[6,7]
Since no specific antiviral targeted drugs for COVID-19 is currently available, supportive care remains the most critical management of patients with COVID-19. Other therapeutic regimens, including antimicrobial therapy for any associated bacterial, viral infections, and strict implementation of appropriate infection control precautions are also important.[2–5] At present, some drugs are effective in eliminating SRS-CoV-2 and improving symptoms. Traditional Chinese medicines have received broad adoption in treating COVID-19.[9] Lianhuaqingwen (LH) capsule, a Chinese patent medicine, has played a positive role in the treatment of SARS-CoV-2. Moreover, LH exerted broad-spectrum effects on a series of influenza viruses by inhibiting viral propagation and regulating immune function.[10,11]
Recently, clinical evidence on arbidol monotherapy in patients with COVID-19 was reported in some studies. However, the clinical evidence on LH in COVID-19 therapy is limited. Thus, we reported the antiviral effects and safety in patients with COVID-19 that compare a combination of arbidol and LH against arbidol only.
2. Methods
2.1. Patients
The single-center, retrospective cohort study included individuals who were diagnosed with laboratory-confirmed COVID-19 between February 1, 2020, and March 8, 2020, at the Hubei No.3 People's Hospital of Jianghan University. All experimental protocols were conducted according to the Declaration of Helsinki and approved by the Human Research Ethics Committees of Hubei No.3 People's Hospital of Jianghan University (Wuhan, China) and all participants in the present study signed the informed consent for retrospective access to patients’ records and files. Patients aged 18 years or older with pneumonia were eligible, and chest CT imaging showed patchy shadows and interstitial changes. COVID-19 was diagnosed by real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay for throat swab specimens.[8] All patients with COVID-19 enrolled in this study were diagnosed according to World Health Organization interim guidance.[12]
Exclusion criteria:
-
1)
Patients with respiratory failure and requiring noninvasive or invasive mechanical ventilation;
-
2)
Patients with chest imaging that showed obvious lesion progression within 24 to 48 hours >50%;
-
3)
Patients with shock or other severe systemic diseases (i.e., malignancy, autoimmune diseases, liver, or renal diseases) that affected the assessment of efficacy in treatment;
-
4)
pregnancy, lactation and women who are pregnant; patients who participated in clinical trials of other drugs within the past month;
-
5)
patients who were allergic to the components of the 2 drugs.
2.2. Precedures
Patients eligible were divided into 2 groups: including arbidol combined with LH (arbidol + H) group and arbidol alone group. Oral drugs were given once the patients were diagnosed with COVID-19 and hospitalized in our hospital. Arbidol + LH group received 200 mg of arbidol (CSPC Ouyi Pharmaceutical Co Ltd., Hebei, China) and 1400 mg LH (SHIJIAZHUANG YILING PHARMACEUTICAL CO LTD, Hebei, China) per 8 hour, while the arbidol group was given 200 mg arbidol per 8 hour. The administration period is about 5 to 21 days until coronavirus is detected negative by RT-PCR for 3 times.
All patients received appropriate supportive care and regular clinical and laboratory monitoring. Renal and liver function and blood routine examination were assessed on the first day of admission and every 7 days throughout the treatment course. Patients’ throat swab was collected for RT-PCR tests every other day after admission to the hospital. The chest CT scans were performed on days 1, 5, 10, 14, 18, and 21, patients’ lung CT scans were assessed for degree of involvement and classified as none, unilateral pneumonia, and bilateral pneumonia. None corresponded to lung CT without inflammation, unilateral pneumonia to lung CT with one side inflammation, bilateral pneumonia to lung CT with 3 side inflammation. All patients were monitored for any clinical signs of depression or acute confusion.
2.3. Statistical analysis
Data were expressed as the mean ± standard deviation for normally distributed continuous numerical variables or median and interquartile range for non-normally distributed numerical variables and the frequency (percentage) for categorical variables. In comparisons of unmatched groups, Student t test was applied to continuous variables, and χ2 or Fisher exact test was used for categorical variables. In the comparisons of matched groups, means were assessed by Student t test for paired samples, and frequencies were subjected to McNemar test. Differences were considered statistically significant when the P value <.05. All data were analyzed by SPSS software, version 26.0 (IBM Corp, Armonk, NY).
3. Results
3.1. Basal characteristics of patients with COVID-19
A total of 108 patients were included in this study, the mean age of all patients was 54.8 years, including 47 males and 61 females. Patients were divided into 2 groups, including arbidol + LH (68 cases) and arbidol (40 cases) group. As shown in Table 1, baseline characteristics were generally similar between the 2 groups. Patients’ baseline characteristics including age, sex, smoking history had no significant differences between the 2 groups. Moreover, there were no statistical differences in the clinical symptoms and primary diseases of the 2 groups of patients (Table 1). Fever was the most common symptom at the onset of illness, other symptoms were present in some of the patients.
Table 1.
Baseline characteristics of patients on first day of adimission.
| Arbidoln = 40 | Arbidol+LHn = 68 | P value | |
| Age (yr) | 54.8 ± 19.1 | 59.5 ± 15.6 | .168 |
| Male (n) | 15 (37.5%) | 32 (47.1%) | .333 |
| Smoking history (n) | 12 (30.0%) | 27 (39.7%) | .311 |
| Chronic obstructive pulmonary disease | 0 (0.0%) | 1 (1.5%) | 1.000 |
| Drinking history (n) | 2 (5.0%) | 7 (10.3%) | .548 |
| Hypertension (n) | 16 (40.0%) | 28 (41.2%) | .904 |
| Diabetes | 2 (5.0%) | 9 (13.2%) | .300 |
| Coronary disease | 4 (10.0%) | 8 (11.8%) | .972 |
| Symptoms | |||
| Fever | 37 (92.5%) | 62 (91.2%) | .904 |
| Cough | 24 (60.0%) | 44 (64.7%) | .625 |
| Expectoration | 12 (30.0%) | 19 (27.9%) | .819 |
| Diarrhea | 6 (15.0%) | 12 (17.6%) | .722 |
| Nausea | 1 (2.5%) | 4 (5.9%) | .739 |
Data were expressed as n (%), and mean ± standard deviation.
Overall, no statistically significant differences in support measures were observed between the 2 groups (Table 2). We found that 33 (30.56%) patients needed a double nasal catheter for oxygen, 28 (25.92%) patients received immunoglobulin therapy, and 67 (62.03%) patients received broad-spectrum antibacterial therapy, 14 (12.96%) patients received corticosteroid therapy, no patients received invasive ventilation or vasopressor therapy (Table 2).
Table 2.
Support measures offered during the course of coronavirus disease 2019, by patient group.
| Arbidoln = 40 | Arbidol+LHn = 68 | P value | |
| Double nasal catheter for oxygen | 13 (32.5%) | 20 (29.4%) | .737 |
| Immunoglobulin therapy | 9 (22.5%) | 19 (27.9%) | .533 |
| Corticosteroid therapy | 4 (10.0%) | 10 (14.7%) | .482 |
| Number of antibacterial therapy agents | 24 (60.0%) | 43 (63.2%) | .738 |
| Invasive ventilation | 0 (0.0%) | 0 (0.0%) | |
| Vasopressor therapy | 0 (0.0%) | 0 (0.0%) |
Data were expressed as n (%).
3.2. Laboratory monitoring indicators of patients in arbidol + LH and arbidol alone groups
After hospitalization, patients were performed laboratory monitoring every 7 days, including renal and liver function and blood count. As shown in Table 3, on the first day of admission, we found a markedly decrease in Lymphocytes (LY) count and Eosinophil (EOS) count of patients with COVID-19, while patients’ erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and serum amyloid A (SAA) levels were increased, respectively. There were no significant differences in white blood cell (WBC) count, neutrophils (NEUT) count, LY count, EOS count, ES, CRP, and SAA.
Table 3.
Laboratory monitoring indicators in coronavirus disease 2019 patients on 1, 7, 14, 21 days of admission, by patient group.
| First day of admission | Seventh day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| WBC (×109 /L) | 5.49 ± 1.46 | 6.08 ± 2.54 | .182 | 6.22 ± 1.60 | 7.43 ± 3.71 | .053 |
| NEUT (×109 /L) | 4.34 ± 1.30 | 4.48 ± 1.96 | .693 | 6.94 ± 0.75 | 7.14 ± 2.63 | .640 |
| LY (×109 /L) | 0.70 ± 0.23 | 0.78 ± 0.21 | .068 | 1.13 ± 0.29 | 1.28 ± 0.40 | .041 |
| EOS (×109 /L) | 0.14 ± 0.03 | 0.16 ± 0.07 | .089 | 0.25 ± 0.06 | 0.27 ± 0.14 | .393 |
| ESR (mm/h) | 63.81 ± 42.65 | 69.39 ± 38.17 | .484 | 41.34 ± 24.87 | 46.78 ± 28.47 | .318 |
| CRP (mg/L) | 55.93 ± 25.34 | 65.17 ± 26.07 | .075 | 26.32 ± 18.45 | 16.87 ± 14.61 | .004 |
| SAA (mg/L) | 171.45 ± 97.04 | 195.71 ± 89.53 | .190 | 75.26 ± 45.39 | 27.87 ± 19.85 | <.001 |
| Fourteenth day of admission | Twenty-first day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| WBC (×109 /L) | 5.48 ± 0.78 | 6.63 ± 3.88 | .067 | 4.97 ± 0.52 | 5.28 ± 1.65 | .251 |
| NEUT (×109 /L) | 4.48 ± 1.62 | 4.90 ± 3.62 | .490 | 3.53 ± 0.92 | 3.46 ± 1.71 | .812 |
| LY (×109 /L) | 1.46 ± 0.40 | 1.74 ± 0.43 | .001 | 1.85 ± 0.47 | 2.01 ± 0.51 | .108 |
| EOS (×109 /L) | 0.44 ± 0.21 | 0.56 ± 0.31 | .032 | 1.32 ± 0.43 | 1.42 ± 0.76 | .447 |
| ESR (mm/h) | 36.92 ± 11.25 | 21.78 ± 7.04 | <.001 | 15.89 ± 9.30 | 17.42 ± 8.04 | .370 |
| CRP (mg/L) | 19.54 ± 17.32 | 15.40 ± 13.48 | .169 | 2.91 ± 1.65 | 2.56 ± 1.82 | .320 |
| SAA (mg/L) | 8.76 ± 6.56 | 7.19 ± 5.31 | .177 | 3.78 ± 2.67 | 4.65 ± 3.19 | .216 |
Data were expressed as mean ± standard deviation.
CRP = C-reactive protein, EOS = Eosinophil, ESR = erythrocyte sedimentation rate, LH = Lianhuaqingwen, LY = Lymphocytes, NEUT = neutrophils, SAA = serum amyloid A, WBC = white blood cell.
As shown in Table 3, after 7 days of admission, patients in the arbidol + LH group showed a higher level of LY count, compared with that in the arbidol group (1.28 ± 0.40 vs 1.13 ± 0.29, P < .05). Moreover, compared with the arbidol group, patients in the arbidol + LH group showed a lower level of CRP (39.87 ± 14.61 vs 46.32 ± 18.45, P < .05) and SAA (27.87 ± 18.85 vs 35.26 ± 15.39, P < .05). The WBC count, NEUT count, EOS count, and serum ESR level were similar in the 2 groups.
After 14 days of admission, patients in the arbidol + LH group showed a higher level of LY count and EOS count, compared with that in the arbidol group (1.74 ± 0.43 vs 1.46 ± 0.40, P < .05; 0.56 ± 0.31 vs 0.44 ± 0.21, P < .05). Moreover, compared with the arbidol group, patients in the arbidol + LH group showed a lower level of ESR (21.78 ± 7.04 vs 36.92 ± 11.25, P < .05) No significant differences were observed in WBC count, NEUT count, CRP, and SAA between the 2 groups. After 21 days of admission, the WBC count, LY count, NEUT count, EOS count, ESR, CRP, and SAA were similar in the 2 groups (Table 3).
3.3. Efficacy of arbidol + LH and arbidol alone in treating COVID-19
As shown in Table 4, the lung CT examination results were similar in the 2 groups on the first day of hospitalization, overall, 28 (25.93%) of 108 patients had unilateral pneumonia, and 80 (74.07%) of 108 patients had bilateral pneumonia. After 7 days of therapy, the chest CT scans were improving in both the arbidol + LH group and the arbidol group, there was no difference between the 2 groups. However, 47 (69.1%) of 68 patients’ throat swab specimens were positive for SARS-CoV-2's test by RT-PCR in the arbidol + LH group on the 7th day of admission, compared with 36 (90%) of 36 in the arbidol group (P < .05, Fig. 1).
Table 4.
The CT examination results of patients in two groups, data were expressed as n (%).
| First day of admission | Seventh day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| CT findings | (n = 40) | (n = 68) | (n = 40) | (n = 68) | ||
| None | 0 (0.0%) | 0 (0.0%) | .533 | 0 (0.0%) | 0 (0.0%) | .324 |
| Unilateral pneumonia | 9 (22.5%) | 19 (27.9%) | 11 (27.5%) | 25 (36.8%) | ||
| Bilateral pneumonia | 31 (77.5%) | 49 (72.1%) | 29 (72.5%) | 43 (63.2%) | ||
| Fourteenth day of admission | Twenty-first day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| CT findings | (n = 40) | (n = 68) | (n = 40) | (n = 68) | ||
| None | 6 (15.0%) | 19 (27.9%) | .037 | 16 (40.0%) | 44 (64.7%) | .041 |
| Unilateral pneumonia | 21 (52.5%) | 40 (58.8%) | 20 (50.0%) | 21 (30.9%) | ||
| Bilateral pneumonia | 13 (32.5%) | 9 (13.3%) | 4 (10.0%) | 3 (4.4%) | ||
LH = Lianhuaqingwen.
Figure 1.
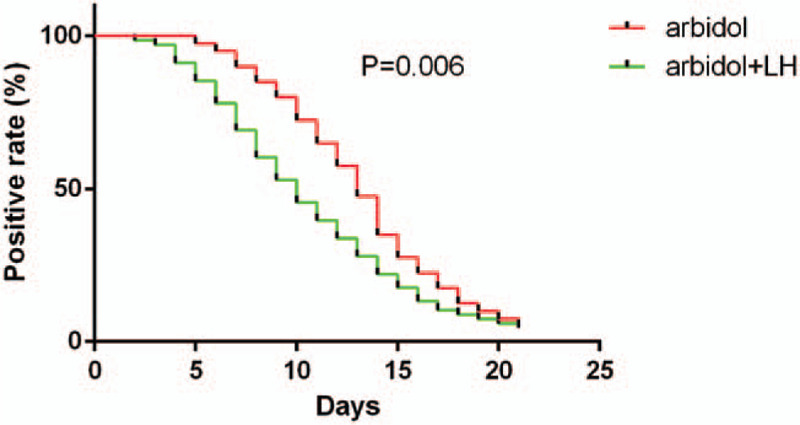
The positive rate of severe acute respiratory syndrome coronavirus 2 nucleic acid throat swab tests by RT-PCR between the 2 groups. The median time from admission to first negative result of the detection of severe acute respiratory syndrome coronavirus 2 nucleic acid was shorter in arbidol + Lianhuaqingwen group (P < .05).
After 14 days of admission, patients in the arbidol + LH group showed better improvement in CT scans (P < .05). In the arbidol + LH group, 19 (27.9%) patients’ pulmonary inflammation was completely absorbed, 40 (58.8%) patients had unilateral pneumonia, and 9 patients (13.3%) still had bilateral pneumonia. While in the arbidol group, only 6 (15.0%) patients’ pulmonary inflammation was completely absorbed, 21 (52.5%) patients had unilateral pneumonia, and 13 (32.5%) of 40 patients still had bilateral pneumonia. For SARS-CoV-2's tests, 19 (27.9%) of 68 patients were positive in the arbidol + LH group, and in arbidol group, 19 (47.5%) of 40 patients nasopharyngeal specimens were still positive (P < .05) (Table 5 and Fig. 1).
Table 5.
severe acute respiratory syndrome coronavirus 2 test in arbidol + Lianhuaqingwen group and arbidol alone group, data were expressed as n (%).
| First day of admission | Seventh day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| Positive | 40 (100.0%) | 68 (100.0%) | 36 (90.0%) | 47 (69.1%) | .013 | |
| Fourteenth day of admission | twenty-first day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| Positive | 19 (47.5%) | 19 (27.9%) | .040 | 2 (5.0%) | 3 (4.4%) | .739 |
LH = Lianhuaqingwen.
After 21 days of admission, 44 (64.7%) of 68 patients’ pulmonary inflammation was completely absorbed in the arbidol + LH group, compared with 16 (40%) of 40 in the arbidol group. Moreover, 21 (30.9%) and 3 (4.4%) patients in the arbidol + LH group had unilateral pneumonia and bilateral pneumonia, respectively, compared with 20 (50.0%) and 4 (10.0%) patients had unilateral pneumonia and bilateral pneumonia in the arbidol group (P < .05). After 21 days of admission, only 3 of 68 (4.4%) patients in the arbidol + LH group and 2 (5%) of 40 patients in the arbidol group showed positive for SARS-CoV-2's test, respectively.
6 (8.82%) of 68 patients in the arbidol + LH group and 4 (10%) of 40 patients in the arbidol group had an elevation of alanine transaminase (ALT) on admission. 7 patients in the arbidol group and 3 patients in the arbidol + LH group showed an elevated level of ALT on 7th day of admission. All patients’ ALT levels become normal before they were discharged. 2 patients in the arbidol group showed an elevation in creatinine (Cr) levels, and the Cr levels were normal before they were discharged. Overall, the level of ALT, aspartate aminotransferase, albumin, Cr, hemoglobin were similar in the 2 groups during the hospitalization of these patients (Table 6). No side effects such as nausea, vomiting, dizziness, abdominal pain, abdominal distension, and diarrhea were observed in this study.
Table 6.
Safety of arbidol + Lianhuaqingwen and arbidol alone in treating coronavirus disease 2019.
| First day of admission | Seventh day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| ALT (mmol/L) | 49.51 ± 28.36 | 45.93 ± 21.68 | .462 | 50.83 ± 35.80 | 41.38 ± 20.60 | .084 |
| AST (mmol/L) | 35.56 ± 23.81 | 41.65 ± 29.76 | .273 | 35.61 ± 29.31 | 41.43 ± 21.60 | .240 |
| ALB (g /L) | 38.38 ± 6.37 | 36.41 ± 5.25 | .085 | 36.81 ± 3.24 | 35.1 ± 6.39 | .118 |
| Cre (mmol/L) | 65.37 ± 31.46 | 76.53 ± 59.76 | .276 | 53.53 ± 32.14 | 62.43 ± 43.84 | .266 |
| Hb (g/h) | 126.54 ± 23.42 | 130.82 ± 12.13 | .214 | 125.27 ± 21.98 | 123.88 ± 23.77 | .764 |
| Fourteenth day of admission | Twenty-first day of admission | |||||
| arbidol | Arbidol+LH | P value | arbidol | Arbidol+LH | P value | |
| (n = 40) | (n = 68) | (n = 40) | (n = 68) | |||
| ALT (mmol/L) | 56.64 ± 23.76 | 48.93 ± 26.16 | .129 | 31.25 ± 15.3 | 29.88 ± 16.10 | .665 |
| AST (mmol/L) | 36.83 ± 22.42 | 39.43 ± 19.43 | .527 | 31.32 ± 13.56 | 30.15 ± 9.70 | .604 |
| ALB (g /L) | 38.93 ± 6.35 | 37.07 ± 6.32 | .260 | 36.85 ± 3.82 | 37.93 ± 4.83 | .341 |
| Cre (mmol/L) | 53.54 ± 26.65 | 62.85 ± 22.78 | .056 | 56.91 ± 12.54 | 61.76 ± 15.63 | .098 |
| Hb (g/h) | 127.84 ± 19.65 | 119.18 ± 19.08 | .089 | 128.65 ± 20.93 | 121.88 ± 18.72 | .192 |
Data were expressed as mean ± standard deviation.
ALB = albumin, ALT = alanine transaminase, AST = aspartate aminotransferase, Cre = creatinine, HB = hemoglobin, LH = Lianhuaqingwen.
4. Discussion
The SARS-CoV-2 infection causes a spectrum of respiratory illness, from asymptomatic to fatal pneumonia, and virus replication has an essential role in the inflammatory process.[13,14] Arbidol was reported to have a direct antiviral effect in early viral replication in vitro for SARS-CoV.[15,16] In our study, we analyzed the efficacy and safety of arbidol and LH in patients with COVID-19. We demonstrated the antiviral effect of arbidol in patients with COVID-19. Moreover, we demonstrated the antiviral effect of LH for SARS-CoV-2, and the combination therapy of arbidol with LH was more effective than arbidol alone for treating SARS-CoV-2. No severe side effects were found in the 2 groups.
Patients with COVID-19 has a similar feature with other respiratory virus infection, SARS-CoV-2 could trigger a strong innate inflammatory immune response, and cause depletion of LYs after infection.[20–22] In our study, we found the absolute value of LY was reduced in most COVID-19 patients on the first day of admission. However, after treatment, the LY count was increased in the arbidol + LH group and arbidol group. Currently, the vaccine for COVID-19 treatment is being tested, and no licensed vaccines or antiviral treatments are available for COVID-19. It is supposed that effective antiviral agents would decrease the peak viral load and delay the progression of immunopathological damage. Arbidol had been recommended by the National Health Commission and National Administration of Traditional Chinese Medicine for the treatment of COVID-19, and the antiviral effect of arbidol was reported in recent studies.[17–19] In our study, we also demonstrated the positive antiviral effect of arbidol in treating patients with COVID-19.
It is supposed that the cytokine storm plays a crucial role in causing fatal pneumonia.[23] Cytokine storm was also confirmed in COVID-19 patients, excessive amounts of proinflammatory cytokines were found in the serum of COVID 19 patients.[7,24] In the prevention and treatment of COVID-19, Traditional Chinese medicines have also received broad adoption, especially in treating cases of mild symptoms.[9] Li found LH could effectively suppress the release of cytokine factors such as TNF-α, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10 in host cells infected with SARS-COV-2, in a dose-dependent manner.[24] In this study, we found patients in the combination group showed a more significant increase in LY count and EOS count. Moreover, the median time from admission to the first negative result of throat swab for SARS-CoV-2 in the arbidol + LH group was shorter than that in the arbidol alone group. These results suggest that arbidol with LH therapy could accelerate the viral clearance of patients with COVID-19.
CT examination of the chest also provides great help in the diagnosis and evaluation of the curative effect.[21] Analysis based on chest CT scan results showed a better improvement of lung inflammation in the arbidol + LH group than arbidol alone group. Furthermore, a lower level of SAA and CRP levels were found in the arbidol + LH group on the 7th day of admission. A recent study demonstrated that LH could significantly inhibit the SARS-COV-2 replication, affect virus morphology and exert anti-inflammatory activity in vitro.[24] All indicated that arbidol combined with LH therapy could significantly accelerate inflammation absorption and suppress inflammation caused by SARS-CoV-2. However, the serum cytokine level changes such as TNF-α, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10 in COVID 19 patients treated with arbidol and LH were not included in our study. We will conduct further research on these cytokine indicators in subsequent experiments.
In our study, 4 (10.0%) patients in the arbidol group and 10 (14.7%) patients in the arbidol + LH group received corticosteroid therapy, and no patients were transferred to ICU treatment. However, glucocorticoids could delay the clearance of coronavirus nucleic acids. Previous studies have reported that glucocorticoid could increase the risk of ICU admission and mortality for patients with hormone therapy.[25,26] The physical and biochemical examination of main organs and systems did not reveal any significant differences between arbidol treated and control groups, indicating good tolerability and safety of arbidol in humans. Treatment with arbidol and LH was well tolerated in our study.
When referring to the limitations of this study, the following points may exist: first, our review is a retrospective, nonrandomized study, the selection, and unmeasured confounding bias could not be excluded entirely, the credibility of the results need to be further confirmed. Then, this is a single-center study limited to a specific study population, the sample size was relatively small. Furthermore, this study only included patients with moderate and mild COVID-19, the clinical effects of the above drugs in the treatment of severe COVID-19 need further study.
5. Conclusion
Early antiviral would decrease the peak viral load and delay the progression of immunopathological damage. One possible implication of this is that arbidol combine with LH might benefit to delay the progression of lung lesions and lower the possibility of respiratory and gastrointestinal transmission for decreasing the viral load of COVID-19 and containing a high fecal concentration.
Author contributions
Design of the study: Lei Liu, Feng Shi, Chang Li, Data collection: Lei Liu, Pei Tu, Xiaoguang Li, Ming Zhang, Chang Li. Data analysis: Lei Liu, Feng Shi, and Ming Zhang. Writing: Lei Lei, and Feng Shi Lu. All authors read and approved the final manuscript.
Conceptualization: Lei Liu, Feng Shi, Li Chang.
Data curation: Pei Tu, Ming Zhang, Xiaoguang Li, Li Chang.
Formal analysis: Xiaoguang Li.
Methodology: Feng Shi, Chen Chen.
Resources: Chen Chen.
Software: Feng Shi.
Supervision: Chen Chen.
Writing – original draft: Lei Liu, Feng Shi.
Writing – review & editing: Lei Liu.
Glossary
Abbreviations: ALT = alanine transaminase, COVID-19 = coronavirus disease 2019, Cre = creatinine, CRP = C-reactive protein, EOS = Eosinophil, ESR = erythrocyte sedimentation rate, LH = Lianhuaqingwen, LY = Lymphocytes, NEUT = neutrophils, SAA = serum amyloid A, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, WBC = white blood cell.
References
- [1].Fung TS, Liu DX. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol 2019;73:529–57. [DOI] [PubMed] [Google Scholar]
- [2].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. [DOI] [PubMed] [Google Scholar]
- [4].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chu CM, Cheng VCC, Hung IFN, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004;59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res 2020;155:104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 2020;14:69–71. [DOI] [PubMed] [Google Scholar]
- [11].Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].World Health Organization. 2020. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 25 January 2020. [Google Scholar]
- [13].Wang Y, Kang H, Liu X, et al. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol 2020;92:538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020;92:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khamitov RA, Loginova S, Shchukina VN, et al. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol 2008;53:9–13. [PubMed] [Google Scholar]
- [16].Barnard DL, Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Fut Virol 2011;6:615–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 2020;14:64–8. [DOI] [PubMed] [Google Scholar]
- [18].Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect 2020;81:e21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect 2020;81:e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Peiris JS, Yuen KY, Osterhaus AD, et al. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431–41. [DOI] [PubMed] [Google Scholar]
- [21].Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, et al. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci U S A 2009;106:3455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leong HN, Chan KP, Oon LL, et al. Clinical and laboratory findings of SARS in Singapore. Ann Acad Med Singapore 2006;35:332–9. [PubMed] [Google Scholar]
- [24].Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020;156:104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757–67. [DOI] [PubMed] [Google Scholar]


