Supplemental Digital Content is available in the text
Keywords: ANO1, cancer, meta-analyze, prognosis
Abstract
Background:
Anoctamin-1 (ANO1) plays a pivotal role in cancer progression. A meta-analysis was conducted to assess the potential prognostic role of ANO1 in cancers.
Methods:
A total of 1760 patients from 7 eligible studies were included into the analysis. Pooled hazard ratios or odds ratios were extracted and calculated with a random-effects model, and analyses of heterogeneity bias were conducted.
Results:
Our results showed that over expression of ANO1 was significantly correlated with poor overall survival in all cancers (HR = 1.52; 95% CI: 1.19–1.92; P = .0006). Subgroup analysis indicated that there was a significant association between over expression of ANO1 and poor prognosis breast cancer (HR = 3.24; 95% CI: 1.74–6.04), head and neck squamous cell carcinoma (HR = 1.14; 95% CI: 1.00–1.30), esophageal squamous cell carcinoma (HR = 1.93; 95% CI: 1.07–3.50), gastric cancer (HR = 1.62; 95% CI: 1.12–2.34) and colorectal cancer (HR = 1.38; 95% CI: 1.03–1.85). In addition, over expression of ANO1 was not associated with TNM stage, histological grade, lymph node metastasis, tumor size, age and gender. However, ANO1 was significantly associated with human epidermal growth factor receptor 2, but not associated with progesterone receptor or estrogen receptor in breast cancer.
Conclusions:
Our results indicate that ANO1 can be a predictive factor for prognosis of cancer.
1. Introduction
Cancer which is a kind of disease that seriously endangers people's lives and health remains a increasing public health problem worldwide because the increasing tendency of overall morbidity over the past decades.[1,2] In recent years, despite there have been many new advances in cancer research, and the level of diagnosis and treatment has also been continuously improved, while the 5-year survival rate has not decreased significantly for cancers.[3] Therefore, there is an urgent need to explore reliable biomarkers for cancer diagnosis, prediction and prognosis.
Chloride channels play an important role in regulation of cell volume, transepithelial transport and membrane excitability.[4,5] In epithelial cells, calcium-activated chloride channels (CaCCs) are important for epithelial secretion in response to hypotonic stress,[5] and can lead to regulatory volume decrease (RVD)[6,7] which is a critical process that regulate cell volumes following osmotic perturbations.
Anoctamin-1 (ANO1) is confirmed as a CaCCs that is highly expressed in epithelial cells in 2008.[8–10] ANO1 which has 10 transmembrane segments with cytosolic amino and carboxyl termini[11] plays diverse roles in regulating epithelial fluid transport, gastrointestinal tract motility and saliva production, and ANO1 gene is located at chromosome 11q13 which is frequently amplified in many human cancers, including bladder cancer, breast cancer and head and neck squamous cell carcinoma (HNSCC).[12]
Studies have found that ANO1 over expressed in many types of tumors usually originated from epithelia including esophageal cancers, gastrointestinal stromal tumors (GISTs), HNSCC, lung cancer, breast cancer, gastric cancer, prostate cancer, ovarian cancer, oral squamous cell carcinoma, lung cancer, esophageal squamous cell carcinoma (ESCC), pancreatic cancer and hepatocellular carcinoma.[13–26] In recent years, studies have researched the role and mechanism of ANO1 in tumors from the perspective of molecular mechanisms, cellular levels, clinical tissues and blood. However, due to the large heterogeneity of tumors, the role and mechanism of ANO1 in tumors have not been fully elaborated. Although studies have found ANO1 has a important role in prediction and prognosis with poor survival of cancers, however, no studies have not yet summarized these studies, and the results of these studies are inconsistent and the sample size is small, therefore, the aim of this study is to make a comprehensive analysis on prediction and prognosis between ANO1 and cancers.
2. Methods
2.1. Search strategy and study selection
This study performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria. English studies were searched in the PubMed, Springer link, Cochrane and Web of Science by 2 researchers independently (updated on June 01, 2020) using the following search terms: (“ANO1” or “TMEM16A” or “TAOS2” or “DOG1”) and (“tumor” or “neoplasm” or “cancer” or “carcinoma”) and (“prognosis” or “prognostic”). We also manually searched the citation lists of relevant studies to identify additional eligible articles.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows:
-
1.
studies that evaluated the prognostic value of ANO1 in patients with cancers;
-
2.
studies in which ANO1 expression was detected in human tumor tissues by immunohistochemistry (IHC);
-
3.
studies that reported hazard ratios (HRs) and 95% CI for overall survival (OS);
-
4.
studies that classified tissues into 2 groups that high expression of ANO1 and low/negative expression of ANO1; and
-
5.
studies published in English.
Exclusion criteria were as follows:
-
1.
reviews, case reports, conference abstracts, letters to the editor, and laboratory studies;
-
2.
repeat analyses; and
-
3.
in sufficient data for further analysis.
2.3. Data extraction and quality assessment
Two researchers independently reviewed all studies, and disagreements between 2 researchers were resolved by consensus. The following data extracted from studies: first author, year of publication, region of study, cancer type, number of cases, TNM staging, detection method, cut-off value, survival outcomes, and median follow-up months. The quality of included studies was assessed by The Newcastle–Ottawa Scale (NOS), and studies with the score of ≥5 were considered high quality (Table S1, http://links.lww.com/MD/F612).
2.4. Statistical analysis
All the statistical analyses were calculated using RevMan software 5.3 (The Cochrane Collaboration, Oxford, UK) or Stata 12.0 (Stata Corp LP, College Station, TX, USA). The pooled HR and 95% CI were calculated to evaluate the association between ANO1 expression and OS of cancers. In addition, the pooled odds ratio (OR) and 95% CI were utilized to access the correlation between ANO1 expression and the pathological parameters of cancers. Heterogeneity was evaluated using the test of Higgins I2. The stability of the pooled results was validated using the sensitivity analysis by omitting each study. Two-tailed P values <.05 were considered statistically significant.
2.5. Ethics and dissemination
Ethical approval was not required in this secondary research evidence, and private information from individuals will not be published. Results from this study will be disseminated through peer-reviewed journals and conference reports.
3. Results
3.1. Study selection and demographic characteristics
The main characteristics of the 7 eligible studies are summarized in Table 1.[23,27–32] The initial search strategy identified 795 potentially relevant articles. After further screening, a total of 7 studies involving 1760 patients were finally included in this meta-analysis. The flow chart of the process of study selection is presented in Figure 1. ANO1 expression was detected by immunohistochemistry (IHC) in different cancer type involving in breast cancer, ESCC, HNSCC, gastric cancer and colorectal cancer. All the 7 studies researched the prognostic role of ANO1 in OS. HR with the corresponding 95% CI was directly obtained through COX analyses from 7 studies.
Table 1.
Main characteristics of the included studies.
| Author | Year | Country | Cancer type | Case | ANO1 Positive (%) | TNM stage | Reference method | Cut-off value (positive) | Outcome | MFU time (months) | NOS score |
| Bae et al | 2018 | Korea | Breast cancer | 139 | 71 (51) | I-IV | IHC | ≥5 (0–8) | OS | 154.1 | 8 |
| Jiang et al | 2019 | China | Colorectal cancer | 122 | 78 (64) | I-IV | IHC | ≥5 (0–8) | OS | NR | 7 |
| Liu et al | 2015 | China | Gastric cancer | 367 | 254 (69) | I-IV | IHC | >8 (0–16) | OS | NR | 6 |
| Rodrigo et al | 2015 | Spain | HNSCC | 357 | 78 (22) | I-IV | IHC | ≥1 (0–2) | OS | NR | 7 |
| Ruiz et al | 2012 | Germany | HNSCC | 225 | 18 (8) | NR | IHC | >0 (0–3) | OS | NR | 7 |
| Wu et al | 2017 | China | Breast cancer | 353 | 208 (59) | I-IV | IHC | ≥5% (0–100%) | OS | NR | 7 |
| Yu et al | 2019 | China | ESCC | 197 | 39 (20) | NR | IHC | ≥3 (0–7) | OS | 34 | 7 |
ESCC = esophageal squamous cell carcinoma, HNSCC = head and neck squamous cell carcinoma, IHC = immunohistochemistry, MFu = median Follow-up, NOS = Newcastle–Ottawa scale, NR = not reported, Os = overall survival.
Figure 1.
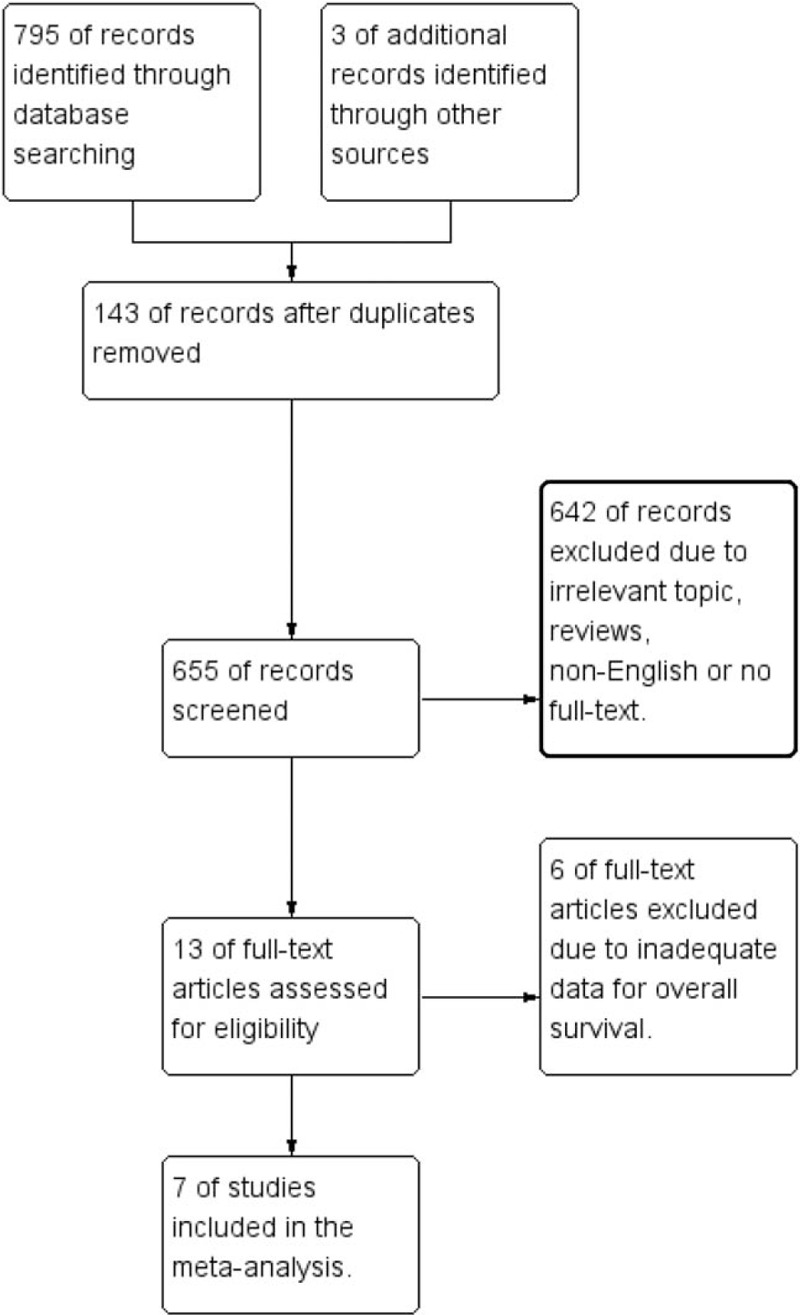
The flow diagram of the study selection process.
To ensure the accuracy of analysis results, we performed evaluation of the quality of included studies according to The Newcastle–Ottawa Scale (NOS), and results showed that the score of all 7 studies were ≥5 which was considered as high quality (see Table S1, http://links.lww.com/MD/F612, Supplemental Content, which evaluates the qualities of the included studies based on the Newcastle–Ottawa scale).
3.2. Evidence synthesis
3.2.1. Overall survival
The pooled results showed that over expression of ANO1 had a significant poor OS (HR = 1.52; 95% CI: 1.21–1.97; P = .0006). Owing to the obvious heterogeneity in the synthesis analysis (I2 = 68%, P = .004), a random-effects model was applied (Fig. 2A). In addition, when the subgroup analysis was completed according to cancer type (Fig. 2B), the pooled HR revealed an association between over expression of ANO1 and unfavorable prognosis in patients with breast cancer (HR = 3.24; 95% CI: 1.74–6.04; P = .0002), HNSCC (HR = 1.14; 95% CI: 1.00–1.30; P = .04), ESCC (HR = 1.93; 95% CI: 1.07–3.50; P = .03), gastric cancer (HR = 1.62; 95% CI: 1.12–2.34; P = .01), colorectal cancer (HR = 1.38; 95% CI: 1.03–1.85; P = .03).
Figure 2.
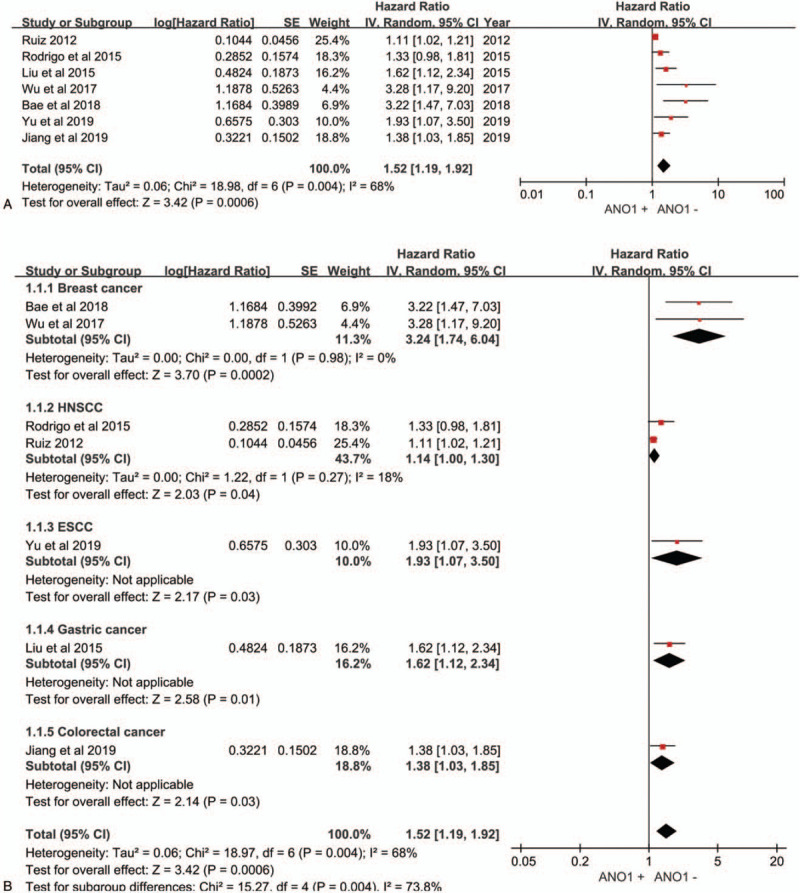
Forest plot of studies. (A) Forest plot of studies evaluating the correlation between ANO1 and overall survival in patients with cancers. (B) Forest plot describing the hazard ratios and their corresponding CIs by cancer type subgroups.
3.2.2. Pathological parameters
To explore the role of ANO1 as a biomarker in cancers, we also investigated the association between over expression of ANO1 and clinicopathological parameters of patients with cancer. As illustrated in Figure 3A-F, the combined data suggested that over expression of ANO1 had no association with the following parameters: TNM stage (III+IV vs I+II; OR = 1.79; 95% CI: 0.87–3.70; P = .12), histological grade (poor vs well or moderate; OR = 0.65; 95% CI: 0.13–3.19; P = .60), lymph node metastasis (positive vs negative; OR = 1.36; 95% CI: 0.53–3.49; P = .52), tumor size (>5 vs ≤5 cm; OR = 1.12; 95% CI: 0.76–1.65; P = .56), age (>50 vs ≤50 years; OR = 0.82; 95% CI: 0.47–1.42; P = .48) and gender (male vs female; OR = 1.50; 95% CI: 1.01–2.23; P = .05). However, ANO1 was significantly associated with human epidermal growth factor receptor 2 (HER2) (positive vs negative; OR = 1.98; 95% CI: 1.32–2.97; P = .001) (Fig. 4C), but not associated with estrogen receptor (ER) (positive vs negative; OR = 1.08; 95% CI: 0.75–1.54; P = .69) (Fig. 4A) or progesterone receptor (PR) (positive vs negative OR = 1.09; 95% CI: 0.60–1.96; P = .78) (Fig. 4B) in breast cancer. The details of the meta-analysis results are summarized in Table 2.
Figure 3.
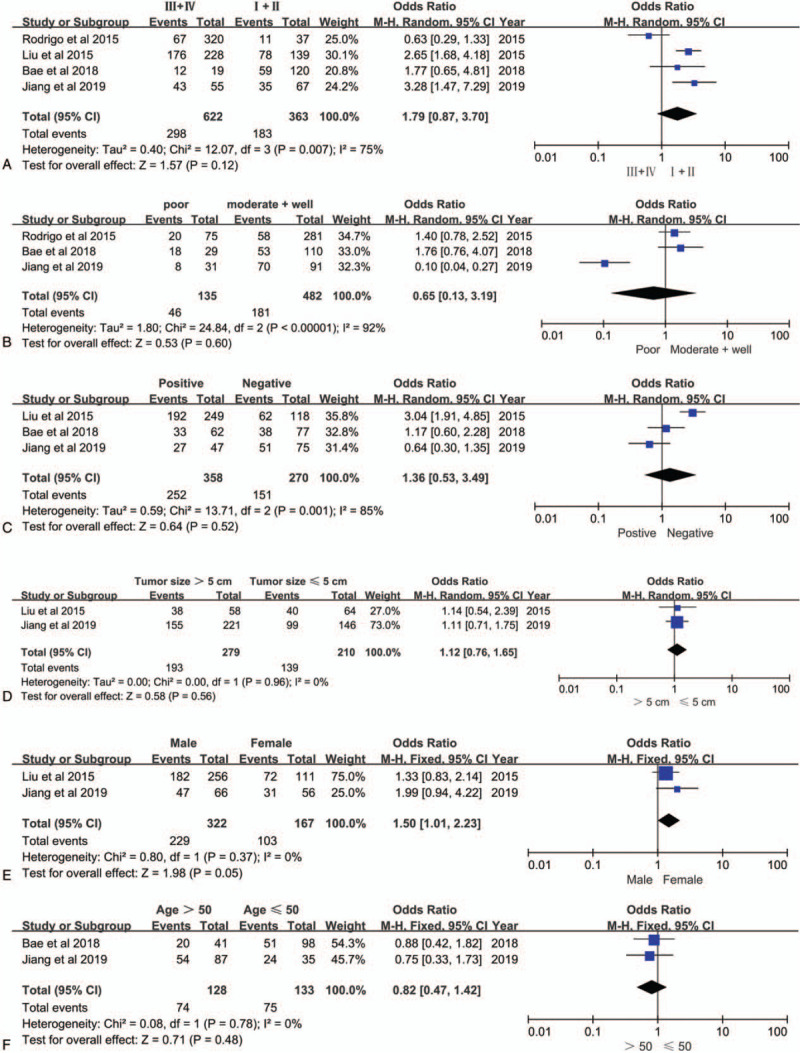
Forest plots of odds ratios for the associations between ANO1 and clinicopathological features in cancers. (A) TNM stage. (B) Histological grade. (C) Lymph node metastasis. (D) Tumor size. E, Gender. (F) Age.
Figure 4.
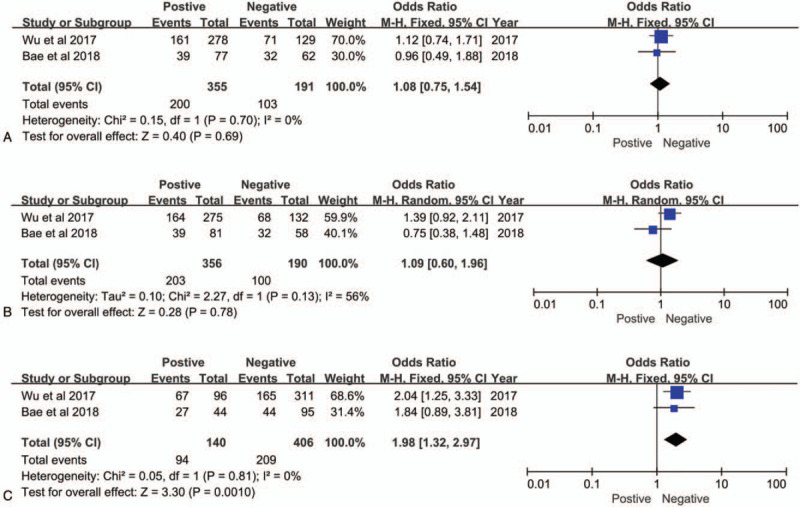
Forest plots of odds ratios for the associations between ANO1 and (A) ER. (B) PR. (C)HER-2.in breast cancer.
Table 2.
Main meta-analysis results of ANO1 overexpression in patients with cancers.
| Group (Number) | Number of studies | Number of patients | HR (95% CI) | P | Heterogeneity I2 (%) | P |
| Overall survival | 7 | 1858 | 1.52 (1.19–1.92) | ∗.0006 | 68 | .004 |
| Clinicopathological parameters | OR (95% CI) | |||||
| TNM stage (III+IV vs I+II) | 4 | 985 | 1.79 (0.87–3.70) | .12 | 75 | .007 |
| histological grade (poor vs well + moderate) | 3 | 617 | 0.65 (0.13– 3.19) | .60 | 92 | <.001 |
| lymph node metastasis (positive vs negative) | 3 | 628 | 1.36 (0.53–3.49) | .52 | 85 | .001 |
| tumor size (>5 vs ≤5 cm) | 2 | 489 | 1.12 (0.76–1.65) | .56 | 0 | .96 |
| ER (positive vs negative) | 2 | 546 | 1.08 (0.75–1.54) | .69 | 0 | .70 |
| PR (positive vs negative) | 2 | 546 | 1.09 (0.60–1.96) | .78 | 56 | .13 |
| HER2 (positive vs negative) | 2 | 546 | 1.98 (1.32–2.97) | ∗.001 | 0 | .81 |
| age (>50 vs ≤50 years) | 2 | 261 | 0.82 (0.47–1.42) | .48 | 0 | .78 |
| gender (male vs female) | 2 | 332 | 1.50 (1.01–2.23) | .05 | 0 | .37 |
HR = hazard ratio, OR = odds ratio.
3.3. Sensitivity analysis
Each single study was deleted to evaluate whether any individual study could affect the pooled HR for OS, and the result of random-effects sensitivity analysis was negative (Fig. 5). Therefore, these tests verified that the results were reliable.
Figure 5.
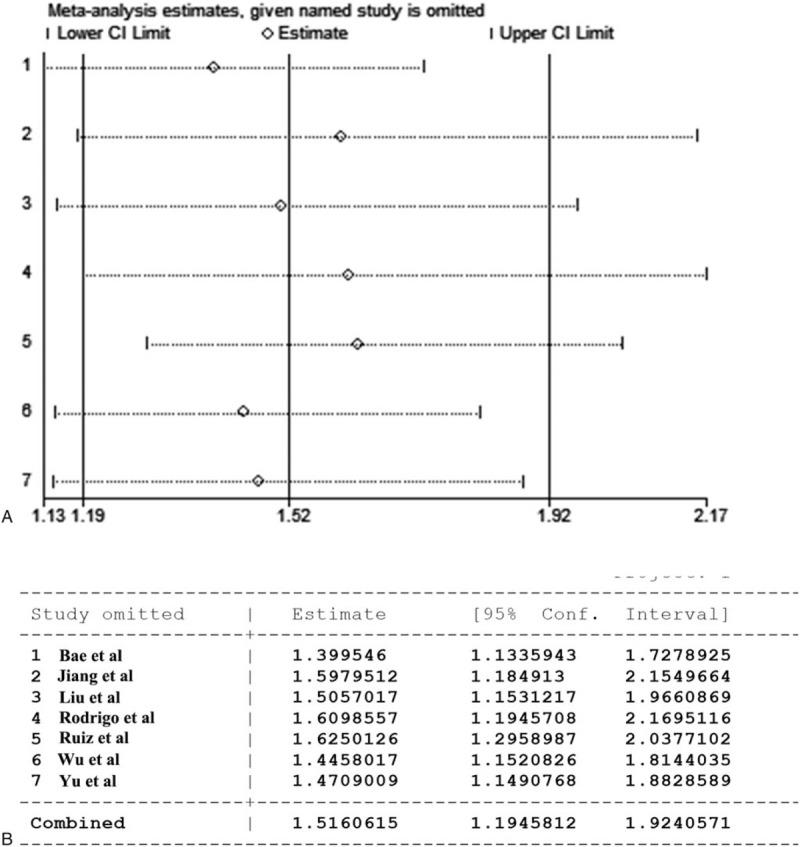
The random-effects sensitivity analysis of the overall survivals. (A)Graph of sensitivity analysis result. (B)Table of sensitivity analysis result.
4. Discussion
Research results that ANO1 over expressed in cancers originated from epithelial cell, and involved in the pathogenesis of cancer indicated that ANO1 is a promising marker and drug target for the diagnosis and treatment of tumors.[33–35] Furthermore, a number of studies have evaluated the role of ANO1 in guiding the prognosis of tumors. However, these studies have limitations due to the influence of factors such as different sample sizes, different races, and different inclusion criteria. This meta-analysis is the first comprehensive analysis of all 7 studies on the prognostic effect of ANO1 as a marker on tumors. In the present meta-analysis, we systematically evaluated the predictive effects of over expression of ANO1 for survival rate and pathological parameters from 7 independent studies involving 1760 patients with cancers. The similarity of these included studies is based on survival analysis to explore the potential prognostic role of ANO1 in cancer patients. The differences of these included studies mainly depend on different research objects and research methods, involving cancer type, sample size, population distribution, cut-off value and follow-up time.
Our results found that ANO1 was significantly associated with OS of cancer, and the obvious heterogeneity in the analysis (I2 = 68%, P = .004). The heterogeneity of this analysis result may be derived from the inherent heterogeneity of the tumor. The pooled of survival rates of different cancer types leads to the heterogeneity of the analysis results. To explore the heterogeneity of synthesis analysis, subgroup analysis was performed according to cancer type. The results of subgroup analysis showed that ANO1 has prediction effects on different types of cancer, and ANO1 had the strongest prognostic effect on breast cancer (HR = 3.24; 95% CI: 1.74–6.04; P = .0002). These results indicated that ANO1 is a promising predictor for OS of cancer. We further research the relationship between ANO1 and HER2, PR, ER in breast cancer. Our results also demonstrated that ANO1 expression is associated with human epidermal growth factor receptor 2, but not associated with progesterone receptor or estrogen receptor in breast cancer. These results indicate that there is an intrinsic relationship between the expression of ANO1 and HER2, and lay the foundation for the diagnosis and treatment of ANO1 combined with HER2 for breast cancer.
Our prognostic analysis results of clinical patients are consistent with previous research results. Epithelial cancers such as breast cancer often occur due to the amplification of the 11q13 gene region. Since the 11q13 locus contains the ANO1 gene, tumors originating from the epithelium are often accompanied by the amplification of the ANO1 gene.[36] However, some studies have also found that ANO1 forms a polymer with other molecules such as EGFR, and the polymer acts as a trans-acting factor to directly activate the amplification of 11q13.[18] Furthermore, in HNSCC and other cancers, it is found that cancer cell cycle regulation is closely related to ANO1.[37] In addition, ion channel properties that regulate the changes of water and chlorine to change the shape of cells to adapt to the migration and invasion of cancer cells.[38,39] More importantly, ERK, Ca-M, PI3K-Akt, TNF, and other signaling pathways can all regulate ANO1 or be regulated by ANO1.[13,14] The above factors lead to the proliferation and invasion of cancer cells, which leads to the decrease of clinical overall survival rate.
To further evaluate the predictive effect of ANO1 over expression on cancer clinical parameters, we comprehensively analyzed parameters of different types of tumors which is derived from epithelium, such as: TNM stage, lymph node metastasis, tumor size, tumor differentiation, age, gender. Although many studies have shown that ANO1 plays an important role in cancer through the mechanism of characteristics of ion channels and activation of cell signaling pathways, and the expression level of ANO1 is also highly correlated with clinical parameters,[13,17,23,29,31,40,41] but our pooled results that ANO1 was not correlated with tumor stage, histological grade, lymph node metastasis and tumor size is not consistent the previous studies. This seemingly contradictory result may be caused by the following reasons. First, the number of studies that meet the inclusion criteria for a comprehensive analysis is limited, and the conclusion of the pooled may be less effective and should be interpreted with caution. Second, all 7 eligible studies are retrospective studies, which may cause bias. Third, there is a high degree of undetected heterogeneity between subgroup analyses, which we believe may be due to a small number of studies and samples, different tumor types or cut-off values. However, since only aggregated data can be used, heterogeneity cannot be completely eliminated. More prospective studies are needed to further confirm the prognostic role of ANO1 expression in cancer survival and the relationship between ANO1 expression and clinicopathology.
Our research did not perform the test of publication bias to assess the stability of the results, because the number of articles that meet the inclusion criteria is small (7 studies), so the test of publication bias has its own limitations. While our study analyzed the heterogeneity of the results that originated from the clinicopathological classification, and sensitivity analysis also validated the stability of the pooled results.
In addition, in vitro diagnosis and liquid biopsy are effective ways to detect early cancer.[16] A number of studies have shown that ANO1 mRNA can amplified in peripheral blood mononuclear cells in the blood of patients with ovarian cancer and gastrointestinal stromal tumors, and as the tumor is removed, the level of ANO1 gene amplification is significantly reduced.[16,42] These results are consistent with our meta-analysis research results that ANO1 has a good predictive effect on cancer and is closely related to survival rate. This study provide statistical evidence that ANO1 is associated with unfavorable prognosis in various cancers. ANO1 can be a promising prognostic biomarker and a potential drug target for cancers.
Author contributions
Zt L conceived the experiments. Cx Z and Hn L contributed equally to this work. Hn L and Cx Z searched and analyzed the studies. Hn L, J G, Sm Y, and Zt L wrote and revised the main manuscript text, and analyzed the results. Cx Z and Xq C drew the figures and tables. All authors reviewed the manuscript.
Conceptualization: Zongtao Liu.
Data curation: Congxiao Zhang, Haini Li, Jing Gao, Xiaoqing Cui, Shengmei Yang, Zongtao Liu.
Formal analysis: Haini Li, Jing Gao, Shengmei Yang.
Investigation: Congxiao Zhang.
Methodology: Zongtao Liu.
Software: Haini Li, Zongtao Liu.
Writing – original draft: Congxiao Zhang, Haini Li, Jing Gao, Xiaoqing Cui, Zongtao Liu.
Writing – review & editing: Congxiao Zhang, Haini Li, Zongtao Liu.
Glossary
Abbreviations: ANO1 = Anoctamin-1, CaCCs = calcium-activated chloride channels, CI = confidence intervals, ER = estrogen receptor, ESCC = esophageal squamous cell carcinoma, GISTs = gastrointestinal stromal tumors, HER2 = human epidermal growth factor receptor 2, HNSCC = head and neck squamous cell carcinoma, HR = hazard ratio, OR = odds ratio, OS = overall survival, PR = progesterone receptor, RVD = regulatory volume decrease.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Ries LAG, J. L. Y. J., Keel GE, Eisner MP, Lin YD, Horner M-JD. SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988-2001. J. Patient and Tumor Characteristicsed., vol. 16. Bethesda, MD: NIH Pub. No. 07-6215, p133-144 (2007). [Google Scholar]
- [4].Chou CY, Shen MR, Wu SN. Volume-sensitive chloride channels associated with human cervical carcinogenesis. Cancer Res 1995;55:6077–83. [PubMed] [Google Scholar]
- [5].Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 2005;67:719–58. [DOI] [PubMed] [Google Scholar]
- [6].Furst J, et al. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. J Pflugers Archiv 2002;444:1–25. [DOI] [PubMed] [Google Scholar]
- [7].Jentsch TJ. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 2016;17:293–307. [DOI] [PubMed] [Google Scholar]
- [8].Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. J Science 2008;322:590–4. [DOI] [PubMed] [Google Scholar]
- [9].Schroeder BC, Cheng T, Jan YN, et al. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. J Cell 2008;134:1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. J Nature 2008;455:1210–5. [DOI] [PubMed] [Google Scholar]
- [11].Paulino C, et al. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. J Life 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schraml P, et al. Tissue microarrays for gene amplification surveys in many different tumor types. J Clinical Cancer Res 1999;5:1966–75. [PubMed] [Google Scholar]
- [13].Liu Z, et al. Inhibition of Ca (2+) -activated chloride channel ANO1 suppresses ovarian cancer through inactivating PI3K/Akt signaling. J Intern J Cancer 2019;144:2215–26. [DOI] [PubMed] [Google Scholar]
- [14].Britschgi A, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. J Proc Natl Acad Sci U S A 2013;110:E1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Song Y, et al. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-alpha signaling. J Cell Death Dis 2018;9:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li H, et al. Detection of ANO1 mRNA in PBMCs is a promising method for GISTs diagnosis. J Scientific reports 2019;9:9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu W, Lu M, Liu B, et al. Inhibition of Ca (2+)-activated Cl (-) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. J Cancer letters 2012;326:41–51. [DOI] [PubMed] [Google Scholar]
- [18].Bill A, et al. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. J Oncotarget 2015;6:9173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deng L, et al. Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. J Onco Targets Therapy 2016;9:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jia L, Liu W, Guan L, et al. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. J PLoS One 2015;10:e0136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li Q, et al. Circulating tumor cells as a prognostic and predictive marker in gastrointestinal stromal tumors: a prospective study. J Oncotarget 2016;7:36645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li YD. Effects of membrane protein ANO1 stable overexpression on laryngocarcinoma Hep-2 cells. J Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae 2014;36:20–4. [DOI] [PubMed] [Google Scholar]
- [23].Liu F, et al. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-beta signaling. J Oncotarget 2015;6:11585–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sauter DRP, Novak I, Pedersen SF, et al. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). J Pflugers Arch 2015;467:1495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shi ZZ, et al. Consistent and differential genetic aberrations between esophageal dysplasia and squamous cell carcinoma detected by array comparative genomic hybridization. J Clinical Cancer Res 2013;19:5867–78. [DOI] [PubMed] [Google Scholar]
- [26].Sui Y, et al. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. J PLoS One 2014;9:e115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bae JS, et al. Expression of ANO1/DOG1 is associated with shorter survival and progression of breast carcinomas. J Oncotarget 2018;9:607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ruiz C, et al. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. J PLoS One 2012;7:e43265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu H, et al. Ano1/TMEM16A over expression is associated with good prognosis in PR-Positive or HER2-negative breast cancer patients following tamoxifen treatment. J PLoS One 2015;10:e0126128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu Y, et al. Integrated analysis of genomic and transcriptomic profiles identified a prognostic immunohistochemistry panel for esophageal squamous cell cancer. J Cancer medicine 2020;9:575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang Y, et al. MicroRNA-144 suppresses aggressive phenotypes of tumor cells by targeting ANO1 in colorectal cancer. J Oncol Reports 2019;41:2361–70. [DOI] [PubMed] [Google Scholar]
- [32].Rodrigo JP, et al. Clinical significance of Anoctamin-1 gene at 11q13 in the development and progression of head and neck squamous cell carcinomas. J Scientific Reports 2015;5:15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guan L, Song Y, Gao J, et al. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. J Oncotarget 2016;7:78619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Crottes D, Jan LY. The multifaceted role of TMEM16A in cancer. J Cell Calcium 2019;82:102050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang H, et al. Cell-specific mechanisms of TMEM16A Ca (2+)-activated chloride channel in cancer. J Molecular Cancer 2017;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bill A, Alex Gaither L. The mechanistic role of the calcium-activated chloride channel ANO1 in tumor growth and signaling. Adv Exp Med Biol 2017;966:1–4. [DOI] [PubMed] [Google Scholar]
- [37].Kunzelmann K, Ousingsawat J, Benedetto R, et al. Contribution of anoctamins to cell survival and cell death. J Cancers (Basel) 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Becchetti A, Munaron L, Arcangeli A. The role of ion channels and transporters in cell proliferation and cancer. J Frontiers Physiol 2013;4:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cuddapah VA, Sontheimer H. Ion channels and transporters corrected in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol 2011;301:C541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cao Q, et al. MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J Exp Clin Cancer Res 2017;36:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shang L, et al. ANO1 protein as a potential biomarker for esophageal cancer prognosis and precancerous lesion development prediction. J Oncotarget 2016;7:24374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu H, et al. Clinical application of circulating tumor DNA in the genetic analysis of patients with advanced GIST. J Molecular Cancer Therapeutics 2018;17:290–6. [DOI] [PubMed] [Google Scholar]


