Abstract
Segmental multi-frequency bioelectrical impedance analysis (s-MFBIA) has been adopted recently to evaluate the volume of breast cancer-related lymphedema (BCRL). This procedure uses the segmental phase angle (s-PhA) as an indicator of cellular integrity. In the smaller-built Asian population, the BCRL often has a small volume difference and can be overlooked by tape circumference volume measurement (TVM). This study aimed to investigate the clinical feasibility of s-MFBIA for the assessment of lymphedema severity compared with TVM and evaluate the association between lymphedema severity and cellular integrity of the affected arm based on s-PhA values for a patient with mild- to moderate-degree BCRL.
Segmental PhA and extracellular water (ECW)/total body water (TBW) ratio of bilateral arms were measured using InBody S10, an s-MFBIA device, in 128 BCRL patients. Inter-limb volume ratio was measured using TVM. The inter-limb ECW/TBW ratio was correlated with inter-limb volume ratio. Inter-limb ECW/TBW ratio and inter-limb volume ratio were then correlated with inter-limb PhA ratio to demonstrate the association between lymphedema severity and arm cellular integrity.
The inter-limb ECW/TBW ratio and inter-limb volume ratio were positively correlated (r = 0.654, P < .001). The same result was obtained after adjusting for age, body mass index, postoperative survival, and duration of lymphedema (r = 0.636, 0.653, 0.652, and 0.648, P < .001). The inter-limb PhA ratio demonstrated significant negative correlation with inter-limb ECW/TBW ratio and inter-limb volume ratio (r = −0.896, −0.562, P < .001).
s-MFBIA has high consistency with the conventional TVM method, and its relation to cellular integrity by segmental PhA enables better understanding of the cellular state of the affected limb in mild- to moderate-degree BCRL. Therefore, it is clinically feasible for severity assessment and monitoring of mild- to moderate-degree BCRL in smaller-built Asian patients.
Keywords: bioimpedance analysis, breast-cancer related lymphedema, lymphedema
1. Introduction
Lymphedema is defined as swelling of the extremities followed by limb discomfort, heaviness, and tightness that significantly decreases the patients quality of life.[1–3] One of the representative mechanisms of lymphedema is an increase in extracellular fluid (ECF) caused by an accumulation of protein, fluid, and cellular debris at the interstitial space due to destruction and blockage of the lymphatic system, resulting in insufficient lymph drainage.[4,5] The causative factors of lymphedema are categorized into treatment- and non-treatment-related factors. Treatment-related factors that may cause lymphedema in breast cancer patients include axillary lymphadenectomy (ALND) and sentinel lymph node dissection (SLND), breast cancer surgery including axillary clearance, and adjuvant radiation therapy. In particular, the prevalence of lymphedema is higher in patients with breast cancer who undergo surgery, ranging from 6% to 30%.[1–3,6] Non-treatment-related factors include obesity, sedentary lifestyle, postoperative infection, and diabetes.[7]
Similar to cancer diagnosis cancer, the detection of lymphedema at the early stage is important to provide early treatment and prevent progression to secondary fibrosis and lipid deposition in the affected arm, which leads to poor prognosis.[8,9] In the smaller-built Asian population[10] (i.e., thinner arms), the lymphedema is often less significantly obvious and thus overlooked because of the small volume difference between arms. Therefore, diagnosing lymphedema in this population is challenging, and this can lead to delayed treatment. As such, an accurate and convenient tool for measuring the upper limb volume and diagnosing breast cancer-related lymphedema (BCRL) is needed.
Bioelectrical impedance analysis (BIA) has been recently adopted for measuring body fluid volume for assessment of body composition and fluid distribution.[4,11] BIA devices measure body resistance and reactance by determining electrical impedance from various electrical currents passing through different tissues,[5,12–14] particularly in accumulated regions such as in lymphedema.[5] Electric current applied to the body is first distributed to low resistivity water-containing areas in the body. The water encompasses ICW and ECW. Because the cell membrane acts as an insulator, current flows through the extracellular fluid because zero or low-frequency electrical currents prevent the current from traversing to the ICW. However, as the current frequency increases, the capacitive effect of the cell membrane decreases, and the electrical current passes through both the ECW and ICW.[5,13,14] According to Ohm law, the recorded voltage difference between both hands can be divided by the current intensity to obtain the resistance of the arm induced by the electrical currents[15] (Fig. 1-B). Based on this, segmental intracellular water (ICW), extracellular water (ECW), and total body water (TBW) values are measured in each limb, and the edema degree is calculated by comparing the ECW/TBW ratio of the affected and unaffected limbs. The electrical current flows through cell membranes acting as a capacitor and resulting in a phase shift known as the geometrically phase angle (PhA).[16] The PhA reflects changes in fluid balance, cellular membrane integrity, and cellular function, and is an indicator of cellular health.[17] Age, sex, and body mass index (BMI) in healthy adults are the main determinants of PhA. The PhA decreases with increasing age, because resistance increases with decreasing body water ratio, due to increasing fat mass, in older age. Men have a higher PhA than women due to a higher body muscle mass.[17] Thus, it is often used as a predictor of impaired clinical outcome and mortality in various diseases.[17,18]
Figure 1.
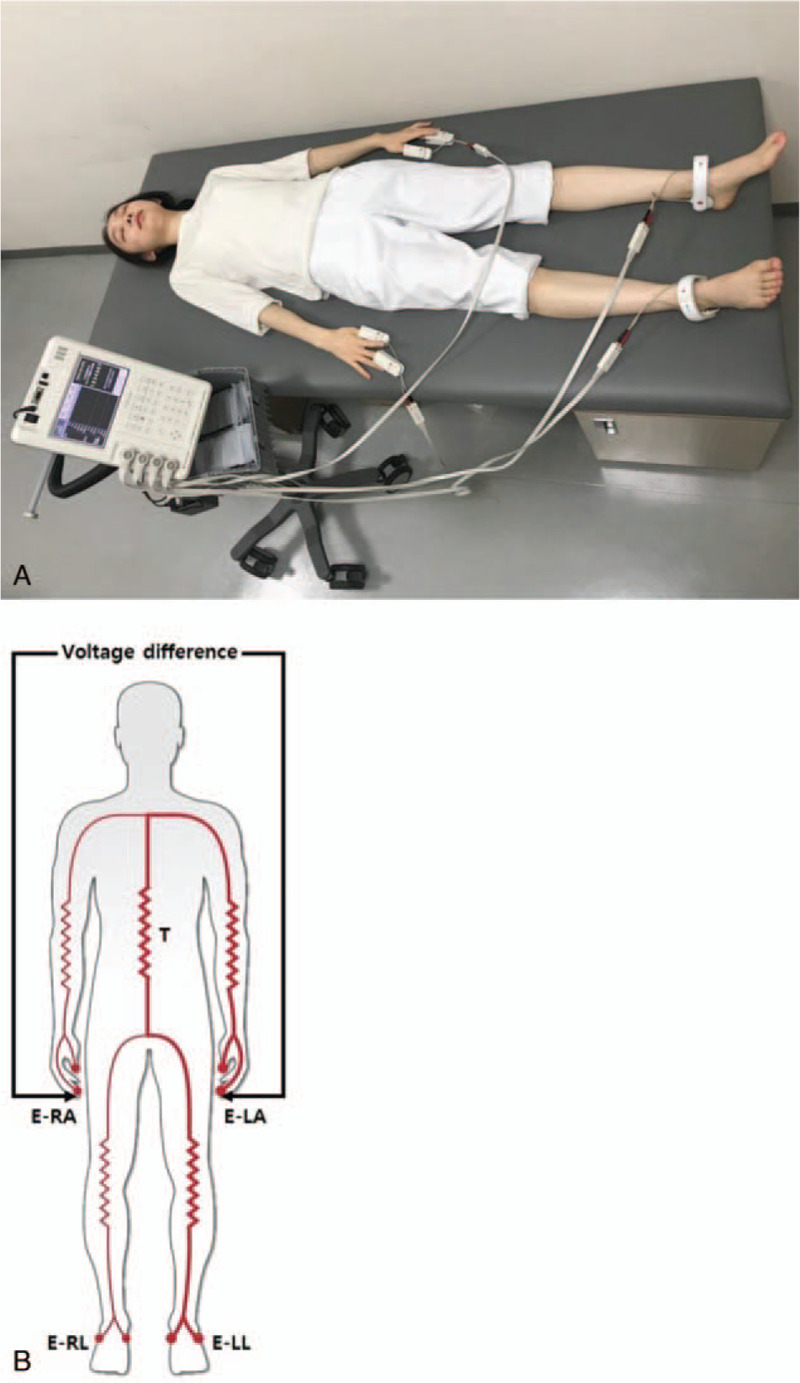
Bioelectrical impedance measurement using InBody S10 (A) Bioelectrical impedance measurement in supine position. The electrodes were attached to the thumb–middle finger and ankle of both extremities; (B) Pathway of electrical current on InBody S10. The electrical current flows through the trunk from finger to ankle and finger to ankle. E-LA = electrode of left arm, E-LL = electrode of left leg, E-RA = electrode of right arm, E-RL = electrode of right leg, T = trunk.
The incidence of clinically diagnosed BCRL ranges from 6% to 30%.[3] One of the most common techniques to diagnose BCRL is the tape circumferential volume measurement (TVM) for which assumption of the volume is made by principles of solid geometry.[13,19] It is an indirect method to assess total limb volume including ECF, which accounts for 25% of the whole mass, bone, muscle, fat, and all the soft tissues. Therefore, TVM is limited in the detection of early stage lymphedema owing to inaccuracy in measuring fluid, and since it is measured manually using a tape at an anatomical landmark, it has poor reliability.[20–22]
Currently, many researchers use a 2-cm circumferential difference measured by TVM between both limbs as the criterion for lymphedema diagnosis.[23] However, it was reported BCRL can be underestimated in Asian women with thinner arms when assessed using the 2-cm circumferential difference criterion.[23] Thus, in Asian patients treated for breast cancer, lymphedema should still be considered in those with a history of axillary lymphadenectomy (ALND) or sentinel lymph node dissection (SLND) and evidence of lymphatic drainage dysfunction even when there is no 2-cm circumferential difference or significantly visible swelling.[24] Accordingly, other reliable methods to overcome the limitations of TVM in Asian BCRL patients are needed.
Many diagnostic and observational studies of lymphedema have been conducted using impedance value analysis or TVM. However, the correlation of the affected arm ECW/TBW ratio and PhA measured via BIA with TVM has not been studied yet. Thus, this study aimed to investigate the clinical feasibility of segmental multi-frequency BIA (s-MFBIA) for lymphedema severity assessment compared with TVM. We also aimed to evaluate the association between lymphedema severity and cellular integrity of the affected arm by segmental PhA values for subjects with mild to moderate BCRL, especially in the Asian population who present with a small volume difference. We aimed to analyze whether BIA can replace TVM in the Asian population who present with a small volume difference that can be overlooked as TVM, and whether PhA can be an indicator of lymphedema severity and cellular integrity. To the best of our knowledge, this study is the first to analyze these 2 aspects.
2. Subjects and methods
2.1. Subjects
The subjects were patients who developed unilateral arm lymphedema following breast cancer surgery and were admitted to the Department of Physical Medicine and Rehabilitation, Inje University Haeundae–Paik Hospital, Republic of Korea between June 2015 and September 2016. The inclusion criteria were as follows:
-
1.
history of breast cancer surgery,
-
2.
history of ALND and/or SLND,
-
3.
history of radiotherapy (RT) and/or chemotherapy,
-
4.
impaired lymphatic flow and/or non-visualization of axillary lymph node in lymphangiography of the affected limb,
-
5.
self-reported edema compared with the opposite side,
-
6.
lymphedema stage I or II determined according to the International Society of Lymphology guidelines, and
-
7.
inter-limb volume ratio of less than 40%.
The exclusion criteria were
-
1.
inter-limb volume ratio of more than 40%,
-
2.
bilateral BCRL,
-
3.
lymphedema accompanied with other complications (e.g., current active infection, such as cellulitis and lymphangitis of affected arm),
-
4.
having an electrical medical (e.g., cardiac pacemaker, insulin pump) or monitoring device,
-
5.
history of trauma or surgery on the affected arm, and
-
6.
inability to hold a supine position for 15 minutes.
To avoid underestimation of mild-degree BCRL, the 2-cm circumferential difference criterion was not applied. This study was conducted in accordance with Inje University Haeundae–Paik Hospital institutional review boards research guidelines and proceeded after obtaining the relevant approval (2016–01–009–001) and patient informed consent.
2.2. Tape circumferential volume measurement
Circumference was measured at 5 cm and 10 cm proximal regions and 15 cm, 10 cm, and 5 cm distal regions from the elbow crease using a 1-cm width tapeline in both affected and unaffected limbs while the patient was in a sitting position with the forearms pronated (Fig. 2). All measurements were performed by a single experienced physiatrist to reduce any errors and biases. The tapeline was directly contacted with skin, without excessive pressure on the skin. The volumes of both arms were calculated from the circumference measurements using a truncated cone formula as follows:[2,3]
Figure 2.
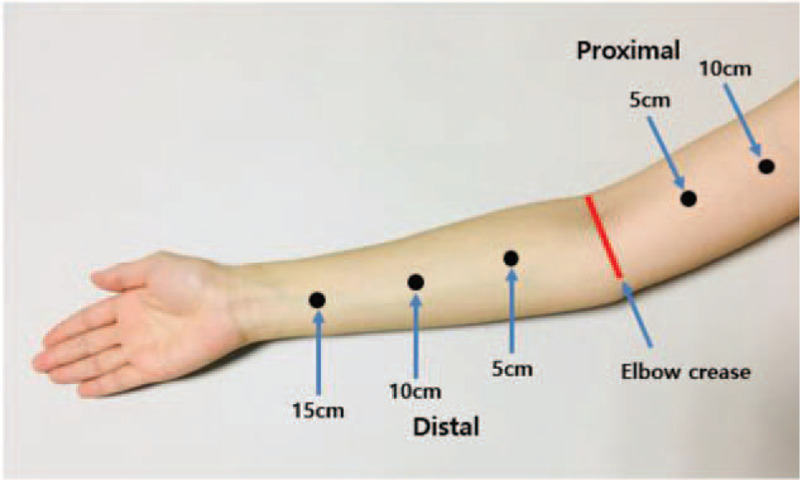
Measuring area for tape circumference volume measurement.
(V=volume/C1, C2=circumferences at the ends of the segment / h=distance between segment length)
The inter-limb volume ratio was determined as follows:
The severity of lymphedema based on the inter-limb volume ratio was graded and categorized according to the Consensus document of the International Society of Lymphology classification for the severity of unilateral limb lymphedema. The severity was categorized as mild, moderate, and severe when the excess volume of the affected arm is less than 20%, 20% to 40%, and more than 40% of the unaffected arm, respectively.[24]
2.3. Segmental multi-frequency bioelectrical impedance analysis
We used the InBody S10 for all BIA measurements. InBody S10 is a Food and Drug Administration-approved portable version of multi-frequency bioelectrical impedance plethysmograph body composition analyzer that enables measurement in supine position, eliminating the gravity effect of body fluid when standing.
2.3.1. Measurement of s-MFBIA using InBody S10
The measurement of s-MFBIA started with the preparation of the patient to accurately capture the impedance and PhA values. First, metal and electronic devices (necklace, bracelet, and cellular phone) were removed before measurement to avoid interference with the electrical current flow. Second, patients were maintained in the supine position for 15 minutes on a non-conducting surface to avoid the effects of gravity-induced water movement. Third, the arms were slightly abducted with elbows pronated (palms down) at about 15 degrees, and the legs were slightly abducted to the shoulder width (Fig. 1-A). Fourth, the skin was cleaned using wet electrolyte tissue paper to increase current conduction. After these precursory procedures, touch-type electrodes were attached to the tip of the thumb and middle finger of both hands and between the malleolus and the calcaneus of both feet. The impedance and PhA values of each segment (both arms, legs, trunk, and whole body) were obtained using the same method.
2.3.2. Inter-limb ECW/TBW ratio
An alternating intensity of electrical current was applied between the hand and foot of one side. The electrical current will pass through different tissues depending on the impedance. InBody S10 provides a ratio of segmental ECW/TBW about body segments (both arms, legs, trunks, and whole body) from the collected segmental impedance. The reference value of segmental ECW/TBW ratio in previous studies was 0.38–0.42 for normal subjects.[15,25,26] However, there is no reference value for segmental ECW/TBW ratio for lymphedema patients. As such, to determine the degree of edema, the inter-limb ECW/TBW ratio was calculated from the segmental ECW/TBW ratio of both affected and unaffected arms using the following formula:
2.3.3. Inter-limb PhA ratio
The PhA is automatically calculated by the BIA as arc tangent = resistance/reactance × (180 ° / π).[17] The s-MFBIA measures the segmental PhA from segmental resistance and reactance using various electrical current flows to determine impedance.[12] Based on previous studies, the normal phase angle was defined as 5 or more in men and 4.6 or more in women and low PhA as less than 5 in men and less than 4.6 in women.[16,27] To assess the cellular health including cell membrane integrity of affected arms in comparison to the unaffected arm, the inter-limb PhA ratio of the affected to unaffected arms was calculated as follows:
2.4. Test-retest reliability of InBody S10 measurement
To test the reliability and reproducibility of the measurements from the InBody S10 device, a test–retest s-MFBIA was performed on the first 39 patients recruited. BIA was measured 3 times at 5-minute intervals while maintaining the supine position after a 15-minute rest in the same position. The reliability and reproducibility were verified according to the segmental ECW/TBW ratio and inter-limb 1 kHz impedance ratio of both arms. After confirming the test–retest reliability, the first data of the 3 s-MFBIA measurements were used to obtain the inter-limb ECW/TBW ratio for correlation with TVM.
2.5. Statistical analysis
The measured values, inter-limb ECW/TBW ratio, inter-limb PhA ratio, and inter-limb volume ratio used in this study were square-transformed. For test–retest reliability, the intra-class correlation coefficient (ICC) with 95% confidence interval (CI) of both arms measured 3 times was calculated using values measured by InBody S10. For correlation of the inter-limb ECW/TBW ratio with the inter-limb PhA ratio and inter-limb volume ratio, Pearson correlation analysis was used. Adjusted analysis according to age, BMI, duration of after surgery, and duration of lymphedema was conducted using multiple linear regression, followed by acquiring partial correlation coefficient and partial regression plots for each cofactor. All statistical analyses were performed using SPSS software version 25.0 (IBM, Armonk, NY, USA). A P value of < .01 was considered significant.
3. Results
3.1. Patient characteristics
In total, 128 BCRL patients with a mean age of 52.85 ± 8.91 years were enrolled. The patient characteristics are presented in Table 1. Right- and left-sided lymphedema was diagnosed in 52.34% (n = 67) and 47.66% (n = 61) of the patients, respectively. The mean BMI was 24.76 ± 12.43 kg/m2, mean duration of after surgery was 51.88 ± 34.64 months, and mean duration of lymphedema was 20.26 ± 14.48 months.
Table 1.
Participant characteristics.
| Factors | Values |
| Participants (Number) | 128 |
| Age (years) | 52.85 ± 8.91 |
| Lesion side (Right: Left) | 67 (52.34%): 61 (47.66%) |
| Body mass index (kg/m2) | 24.76 ± 12.43 |
| Duration of After surgery (Months) | 51.88 ± 34.64 |
| Duration of Lymphedema (Months) | 20.26 ± 14.48 |
3.2. s-MFBIA and TVM measurements
The mean value of the segmental ECW/TBW ratio was 0.39 ± 0.01 on the affected side and 0.38 ± 0.01 on the unaffected side, with an inter-limb ECW/TBW ratio of 1.03 ± 0.02. The mean PhA value was 3.94 ± 0.76 on the affected side and 4.67 ± 0.67 on the unaffected side, with an inter-limb PhA ratio of 0.84 ± 0.11. The mean volume of each arm calculated via TVM was 375.16 ± 90.18 cc on the affected side and 327.46 ± 68.63 cc on the unaffected side, with an inter-limb volume ratio of 1.15 ± 0.12. In total, 69.5% (n = 89) and 30.5% (n = 39) of patients were classified to have mild and moderate degree lymphedema (Table 2).
Table 2.
Values of bioelectrical impedance analysis and tape circumferential volume measurements.
| Factors | Values | |
| Segmental ECW/TBW ratio (Mean ± SD) | Affected arm | 0.39 ± 0.01 |
| Unaffected arm | 0.38 ± 0.01 | |
| Inter-limb ECW/TBW ratio (Affected arm/Unaffected arm) | 1.03 ± 0.02 | |
| Phase angle (Mean ± SD) | Affected arm | 3.94 ± 0.76 |
| Unaffected arm | 4.67 ± 0.67 | |
| Inter-limb PhA ratio (Affected arm/Unaffected arm) | 0.84 ± 0.11 | |
| Tape circumferential volume measurement (Mean ± SD) | Affected arm (cc) | 375.16 ± 90.18 |
| Unaffected arm (cc) | 327.46 ± 68.63 | |
| Inter-limb volume difference (cc) (Affected arm-Unaffected arm) | 47.69 ± 42.86 | |
| Inter-limb volume ratio (Affected arm/Unaffected arm) | 1.15 ± 0.12 | |
| Classification of lymphedema (Number) | Mild degree (<20% volume difference) | 89 (69.53%) |
| Moderate degree (20%-40% volume difference) | 39 (30.47%) |
ECW = extracellular water, SD = standard deviation, TBW = total body water.
3.3. Correlations of the inter-limb volume ratio with the inter-limb ECW/TBW and PhA ratio
The inter-limb ECW/TBW ratio and inter-limb volume ratio were positively associated, with a Pearson correlation coefficient (r) of 0.654 (P < .001; Fig. 4A). The partial correlation coefficients (r∗) of the adjusted age, BMI, duration of after surgery, and duration of lymphedema were 0.636, 0.653, 0.652, and 0.648, respectively, showing positive linear correlations in multiple linear regression analysis (Fig. 5A, B, C, D). Positive linear correlation was observed when all factors were simultaneously adjusted (r∗ = 0.623, P < .001) (Fig. 5E). Inter-limb PhA ratio demonstrated significant negative correlations with inter-limb ECW/TBW ratio (r = −0.896, P < .001) and inter-limb volume ratio (r = −0.562, P < .001) (Fig. 4B, C). The inter-limb ECW/TBW ratio by s-MFBIA reflects the changes in TVM and depicts the severity of lymphedema. The inter-limb PhA ratio also changes according to the severity of lymphedema.
Figure 4.
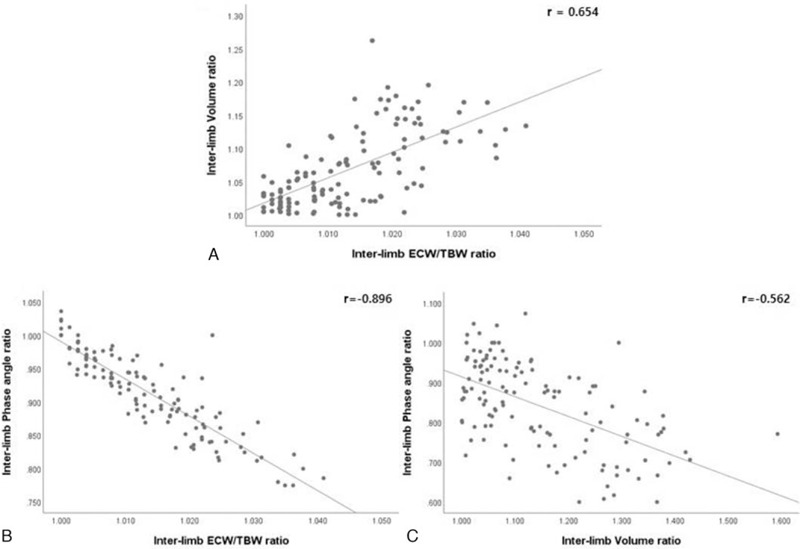
Correlations between the inter-limb ECW/TBW ratio, PhA ratio, and volume ratio. r (Pearson correlation coefficient) All measured values were converted to square root and used in the statistical analysis.
Figure 5.
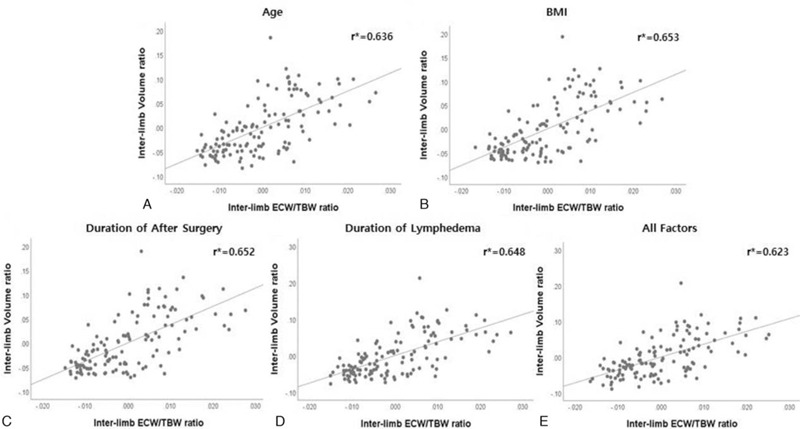
Correlations between the inter-limb ECW/TBW ratio and inter-limb volume ratio adjusted for age, BMI, postoperative survival, and duration of lymphedema: partial regression plots. r∗ (Partial correlation coefficient) All measured values were converted to square root and used in the statistical analysis.
3.4. Test–retest reliability of InBody S10 measurement
The mean values for each test session of the affected and unaffected arm segmental ECW/TBW ratio and the inter-limb 1-kHz impedance ratio are shown in Table 3. The ICC values of affected and unaffected arm ECW/TBW ratio and inter-limb 1-kHz impedance ratio were 0.998 (95% CI: 0.997–0.999), 0.997 (95% CI: 0.995–0.998), and 1.000 (95% CI: 0.997–1.000), respectively. An ICC value of 0.9 or higher[28] shows excellent reliability. Therefore, the InBody S10 measurement used in this study had excellent reliability and reproducibility.
Table 3.
Reliability test for InBody S10; intra-class correlation coefficient in test-retest.
| Test 1 | Test 2 | Test 3 | ICC | ||
| Factors | Mean ± SD | Mean ± SD | Mean ± SD | (95% CI) | P value |
| Affected arm ECW/TBW ratio | 0.390 ± 0.009 | 0.391 ± 0.009 | 0.391 ± 0.009 | 0.998 | <.001 |
| Unaffected arm ECW/TBW ratio | 0.381 ± 0.006 | 0.381 ± 0.006 | 0.381 ± 0.005 | 0.997 | <.001 |
| Inter-limb 1 kHz impedance ratio | 1.215 ± 0.203 | 1.218 ± 0.206 | 1.222 ± 0.208 | 1.000 | <.001 |
CI = confidence interval, ECW = extracellular water, ICC = intra-class correlation coefficient, SD = standard deviation, TBW = total body water.
4. Discussion
This study investigated the clinical feasibility of s-MFBIA for lymphedema severity assessment compared with TVM (Fig. 3). The inter-limb ECW/TBW ratio measured by s-MFBIA showed a strong positive correlation with inter-limb volume ratio. Further, the inter-limb PhA ratio showed a significant negative correlation with inter-limb volume ratio and inter-limb ECW/TBW ratio. s-MFBIA measurements were also highly consistent with TVM measurements. To our knowledge, this was the first study to use segmental ECW/TBW ratio measured by s-MFBIA in mild- to moderate-degree BCRL patients and to confirm the usefulness of PhA in BCRL (Fig. 5).
Figure 3.
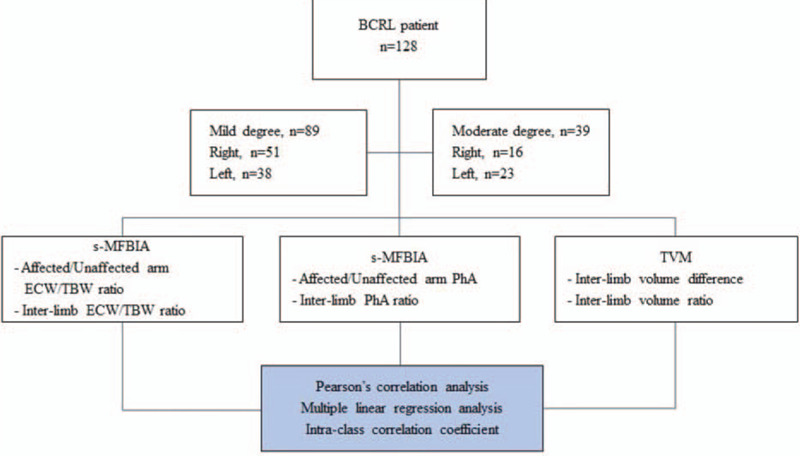
Flowchart of the study methodology.
There are various methods for detecting the limb volume changes in BCRL patients. Among them, TVM of the arm circumference at a specified distance from the anatomic landmark is now the most commonly used.[7,13,19] However, TVM has some technical limitations, including its inaccuracy and inconsistency due to positioning of the tape and various pressures applied to the skin during and between measurements. Hence, accurate measurements may be time consuming and cause discrepancy between measurers.[2,3] With respect to sensitivity of TVM, Su et al reported that only 8.1% (46/570 patients) of post-breast cancer treatment (surgery ± RT) patients were diagnosed with BCRL by TVM using the 2-cm circumference difference criterion. However, 28% (161/570 patients) of the BCRL patients in their study complained of actual arm swelling. Further, large studies have shown an incidence of up to 30%. These results imply that TVM using the 2-cm difference criterion can underestimate lymphedema in smaller-built populations such as oriental women.[23] Considering that lymphedema requires early diagnosis and appropriate treatment, a more precise method for evaluating mild- to moderate-degree BCRL is needed, particularly in patients with less volume differences between both arms such as smaller-built oriental women.
Water displacement volumetry is also considered a gold standard for assessing changes in limb volume owing to its high sensitivity[3,29,30]. However, many researchers regard this method as time-consuming, less portable, and non-hygienic, and should thus be avoided in patients with skin ulcer or cellulitis.[2,7,29] As an alternative method, skin tonometry, BIA, infrared optoelectronic volumetry, and computed tonometry have been suggested.[3,31–34] However, most of these methods cannot adequately isolate lymphedema as the overall limb volume measurement contains bone, muscle, fat, and all the soft tissues. On the other hand, the s-MFBIA enables quantification of the ECF volume specifically, thus making it applicable for detecting lymphedema.[35] Furthermore, s-MFBIA device such as InBody S10 is more useful for monitoring limb fluid status such as lymphedema by measuring the impedance of each body segment.[36]
In this study, ICC was analyzed to confirm the consistency or reproducibility of s-MFBIA using InBody S10, and the results showed high reliability. We found a positive linear correlation between the inter-limb volume ratio measured by conventional TVM method and the inter-limb ECW/TBW ratio measured by the s-MFBIA in patients with mild- to moderate-degree lymphedema, even after adjusting for age, BMI, duration of after surgery, and duration of lymphedema. These results indicate that s-MFBIA measurements are comparable to those by TVM, and thus s-MFBIA can replace TVM. Furthermore, s-MFBIA is easier to use and is a more useful tool for assessing mild- to moderate-degree lymphedema in patients with little difference in arm circumference.
Despite increased ECF volume in patients with lymphedema, in many cases, discrimination of the degree of edema by TVM is restricted due to simultaneous progression of the atrophy of subcutaneous tissues including muscle and fat.[20–22] Thus, as shown in this study, inter-limb ECW/TBW ratio measured by the s-MFBIA is a more useful for detecting the degree of edema in patients with less volume difference between both limbs, including those with mild- to moderate-degree BCRL. Further, s-MFBIA has the following advantages:
-
1.
obtaining consistent data,
-
2.
avoiding underestimation of lymphedema in oriental women with mild to moderate BCRL,
-
3.
is cost-effective, and
-
4.
is non-invasive.
Therefore, s-MFBIA can replace and is more optimal for the diagnosis and progress monitoring of lymphedema in oriental women.
Recent studies[16,27] have shown that low PhA measured by s-MFBIA is associated with nutritional risk and increased morbidity and mortality in various diseases. Further, PhA can be used as a prognostic indicator of survival in patients with poor functional status and cancer.[16,27,37] Low PhA is known to reflect low serum albumin level and is particularly interesting because it is a non-invasive, objective marker of nutritional and morbidity risk and can be measured directly and quickly.[16,27] In this study, we confirmed that both the TVM measurement of inter-limb volume ratio and the s-MFBIA measurement of inter-limb ECW/TBW ratio were negatively correlated with inter-limb PhA ratio. BCRL patients with increased inter-limb volume ratio and ECW/TBW ratio showed decreased inter-limb PhA ratio, and this may be related to increase in fluid collection. In addition, it is known that lymphostasis caused by damage of the lymphatic system causes events that alter cellular components, including infiltration of inflammatory cells into the dermis, fibrotic remodeling of tissues, and adipocyte hypertrophy or hyperplasia.[38–40] In this context, low PhA may well reflect an abnormal cellular state, and it can be predicted that PhA will decrease with increasing cellular abnormality such as increased cellular damage or cellular membrane permeability in patients with BCRL. Therefore, breast cancer patients with low PhA measured on s-MFBIA should be carefully monitored after treatment, even if they have no obvious signs and symptoms of edema. The PhA, together with segmental ECW/TBW ratio, may be useful for the assessment and prediction of lymphedema severity and cellular status in mild- to moderate-degree BCRL.
This study has some limitations. Most s-MFBIA devices using surface electrodes are contacted on the finger, palm, ankle, or sole. InBody S10 applies an alternative current of intensity through a surface electrode that contacts the thumb and middle finger, and the current is coupled to the wrist level and then flows to the trunk through the arm. These s-MFBIA devices have the disadvantage of inability to measure below the wrist level before the currents is coupled, and thus the hands are excluded from the edema measurements. Therefore, we excluded measurements below the wrist circumference for TVM in consideration of such limitation of s-MFBIA. Because most cases of lymphedema are accompanied by swelling of the hands, the measurement of lymphedema involving the hand through the s-MFBIA device can be considered incomplete. In the future, a large-scale sampling study will further enhance reliability of s-MFBIA and understanding of PhA in this patient group of mild to moderate BCRL.
5. Conclusion
s-MFBIA for assessment and monitoring of lymphedema severity is clinically feasible in mild- to moderate-degree BCRL. Its correlation to cellular integrity measured by segmental PhA ratio enables better understanding of the cellular state of the affected limb. Therefore, it may be a good alternative measuring tool to the conventional TVM method for mild- to moderate-degree BCRL patients, especially for the smaller-built Asian population. Our study findings have demonstrated that s-MFBIA is a promising evaluation method with excellent reliability and reproducibility in estimating the progress of lymphedema and the efficiency of treatment.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
Conceptualization: Woo-Jin Kim, Hwan-Kwon Do.
Data curation: Ji-Ho Park, Hwan-Kwon Do.
Formal analysis: Hwan-Kwon Do.
Supervision: Geun-Yeol Jo, Ji-Ho Park.
Writing – original draft: Hwan-Kwon Do.
Writing – review & editing: Woo-Jin Kim, Hwan-Kwon Do.
Glossary
Abbreviations: ALND = axillary lymphadenectomy, BCRL = breast cancer-related lymphedema, BIA = bioelectrical impedance analysis, BMI = body mass index, CI = confidence interval, ECF = extracellular fluid, ECW = extracellular water, ICC = intra-class correlation coefficient, ICW = intracellular water, PhA = phase angle, RT = radiotherapy, SLND = sentinel lymph node dissection, s-MFBIA = segmental multi-frequency BIA, TBW = total body water, TVM = tape circumference volume measurement.
References
- [1].Chen YW, Tsai HJ, Hung HC, et al. Reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil 2008;87:33–8. [DOI] [PubMed] [Google Scholar]
- [2].Taylor R, Jayasinghe UW, Koelmeyer L, et al. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther 2006;86:205–14. [PubMed] [Google Scholar]
- [3].Deltombe T, Jamart J, Recloux S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology 2007;40:26–34. [PubMed] [Google Scholar]
- [4].Kim L, Jeon JY, Sung IY, et al. Prediction of treatment outcome with bioimpedance measurements in breast cancer related lymphedema patients. Ann Rehabil Med 2011;35:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gaw R, Box R, Cornish B. Bioimpedance in the assessment of unilateral lymphedema of a limb: the optimal frequency. Lymphat Res Biol 2011;9:93–9. [DOI] [PubMed] [Google Scholar]
- [6].Ibrahim A, Alawad A. Risk factors and management of breast cancer-related lymphedema. Int J Med 2015;3:38–40. [Google Scholar]
- [7].Goel A, Agarwal J, Mehta S, et al. Arm lymphedema after treatment of breast cancer: Etiology, diagnosis, and management. Asian J Oncol 2015;1:77–83. [Google Scholar]
- [8].Niebuhr DW. Topic Brief: Effectiveness of Surveillance Measures for Early Detection and Interventions to Prevent Breast Cancer-Related Lymphedema. Agency for Healthcare Research and Quality. https://effectivehealthcare.ahrq.gov/system/files/docs/topic-brief-breast-cancer-lymphedema.pdf. Published October 2019. Accessed September 15 2020 [Google Scholar]
- [9].Melissa Agsalda-Garcia TS, Ryan Souza, Natalie Kamada, et al. Raman-enhanced spectroscopy (Respect) probe for childhood non-Hodgkin lymphoma. Sci Med J 2020;2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Webster J, Cornolo J. Comparison of European and Asian morphology. Proceedings of the 4th International Conference on 3D Body Scanning Technologies, Long Beach CA, USA, 19-20 November 2013;2013. [Google Scholar]
- [11].Lorenzo AD, Andreoli A. Segmental bioelectrical impedance analysis. Curr Opin Clin Nutr Metab Care 2003;6:551–5. [DOI] [PubMed] [Google Scholar]
- [12].Gunn SM, Halbert JA, Giles LC, et al. Bioelectrical phase angle values in a clinical sample of ambulatory rehabilitation patients. Dyn Med 2008;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rockson S. Bioimpedance analysis in the assessment of lymphoedema diagnosis and management. J Lymphoedema 2007;2:44–8. [Google Scholar]
- [14].Cornish B, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001;34:2–11. [PubMed] [Google Scholar]
- [15].Sartorio A, Malavolti M, Agosti F, et al. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur J Clin Nutr 2005;59:155–60. [DOI] [PubMed] [Google Scholar]
- [16].Kyle UG, Genton L, Pichard C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin Nutr 2013;32:294–9. [DOI] [PubMed] [Google Scholar]
- [17].Norman K, Stobaus N, Pirlich M, et al. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31:854–61. [DOI] [PubMed] [Google Scholar]
- [18].A. Kosvyra CM, Chouvarda I. Developing an integrated genomic profile for cancer patients with the use of NGS data. Emerg Sci J 2019;3.: [Google Scholar]
- [19].Ward LC. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol 2006;4:51–6. [DOI] [PubMed] [Google Scholar]
- [20].Choi YH, Seo KS. Correlation among bioimpedance analysis, sonographic and circumferential measurement in assessment of breast cancer-related arm lLymphedema. Lymphology 2014;47:123–33. [PubMed] [Google Scholar]
- [21].Gerber LH. A Review of measures of lymphedema. Cancer 1998;83: (12 Suppl American): 2803–4. [DOI] [PubMed] [Google Scholar]
- [22].Sander AP, Hajer NM, Hemenway K, et al. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther 2002;82:1201–12. [PubMed] [Google Scholar]
- [23].Liao S, Lee Y, Chen S, et al. Incidence and risk factors analysis of lymphedema. Tw J Phys Med Rehabil 2009;37:217–25. [Google Scholar]
- [24].International Society of L. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology 2013;46:1–1. [PubMed] [Google Scholar]
- [25].Klassen P, Mazariegos M, Deurenberg P, et al. Hydrational status assessed by bioelectrical impedance spectroscopy and dilution methods in patients with classical dengue fever. Ann N Y Acad Sci 2000;904:163–70. [DOI] [PubMed] [Google Scholar]
- [26].Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr 1992;11:199–209. [PubMed] [Google Scholar]
- [27].Kyle UG, Soundar EP, Genton L, et al. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin Nutr 2012;31:875–81. [DOI] [PubMed] [Google Scholar]
- [28].Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Megens AM, Harris SR, Kim-Sing C, et al. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil 2001;82:1639–44. [DOI] [PubMed] [Google Scholar]
- [30].Kaulesar Sukul DM, den Hoed PT, Johannes EJ, et al. Direct and indirect methods for the quantification of leg volume: comparison between water displacement volumetry, the disk model mthod and the frustum sign model method, using the correlation coefficient and the limits of agreement. J Biomed Eng 1993;15:477–80. [DOI] [PubMed] [Google Scholar]
- [31].Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg 1996;12:412–7. [DOI] [PubMed] [Google Scholar]
- [32].Chen H, O’Brien B, Pribaz J, et al. The use of tonometry in the assessment of upper extremity lymphoedema. Br J Plast Surg 1988;41:399–402. [DOI] [PubMed] [Google Scholar]
- [33].Cornish BH, Bunce IH, Ward LC, et al. Bioelectrical impedance for monitoring the efficacy of lymphoedema treatment programmes. Breast Cancer Res Treat 1996;38:169–76. [DOI] [PubMed] [Google Scholar]
- [34].Collins CD, Mortimer PS, D’Ettorre H, et al. Computed tomography in the assessment of response to limb compression in unilateral lymphoedema. Clin Radiol 1995;50:541–4. [DOI] [PubMed] [Google Scholar]
- [35].Dylke ES, Ward LC, Kilbreath SL. Standardized approach to lymphedema screening. Oncologist 2013;18:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ward LC. Segmental bioelectrical impedance analysis: an update. Curr Opin Clin Nutr Metab Care 2012;15:424–9. [DOI] [PubMed] [Google Scholar]
- [37].Oliveira CM, Kubrusly M, Mota RS, et al. The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J Ren Nutr 2010;20:314–20. [DOI] [PubMed] [Google Scholar]
- [38].Rockson SG, Keeley V, Kilbreath S, et al. Cancer-associated secondary lymphoedema. Nat Rev Dis Primers 2019;5:22. [DOI] [PubMed] [Google Scholar]
- [39].Daroczy J. Pathology of lymphedema. Clin Dermatol 1995;13:433–44. [DOI] [PubMed] [Google Scholar]
- [40].Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol 2017;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


