Abstract
We explored whether hysterectomy with or without bilateral oophorectomy was associated with the increasing incidence of diabetes mellitus (DM) in an East Asian population. This was a retrospective population-based cohort study that analyzed DM risk in Taiwanese women, using a health insurance research database of 1998 to 2013 containing nearly 1 million people. We identified 7088 women aged 30 to 49 years who had undergone hysterectomy with or without oophorectomy. The comparison group included 27,845 women without a hysterectomy who were randomly selected from the population and matched to women in the hysterectomy group by age (exact year) and year of the surgery. DM comorbidities were identified. The incidence and hazard ratios for DM were calculated with Cox proportional hazard regression models. The median ages of patients in the hysterectomy and comparison groups were both approximately 44 years. After a median 7.1 years of follow-up, the incidence of DM was 40% higher in the hysterectomized women as compared with the comparisons (9.12 vs 6.78/1000 person-years, P < .001), with an adjusted hazard ratio (aHR) of 1.37 (95% confidence interval [CI] = 1.23 –1.52). However, the DM risk was not increased in the women with hysterectomy plus oophorectomy (aHR=1.28, 95% CI = 0.93–1.76). Furthermore, among women aged 30 to 39 years, 40 to 49 years, the risk in hysterectomized women was higher than the comparisons (aHR = 1.75, 95% CI = 1.27–2.41; aHR = 1.33, 95% CI = 1.19–1.49, respectively). Our study provides essential and novel evidence for the association between hysterectomy and DM risk in women aged 30 to 49 years, which is relevant to these women and their physicians. Physicians should be aware of the increased DM risk associated with hysterectomy and take this into consideration when evaluating a patient for a hysterectomy. The current results might help gynecologists prevent DM and encourage diagnostic and preventive interventions in appropriate patients.
Keywords: cohort, diabetes mellitus, hysterectomy, menopause, oophorectomy
1. Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia caused by insulin resistance, inappropriate insulin secretion, and excessive glucagon secretion.[1] To diagnose DM, serum level of fasting glucose should be ≥126 mg/dL and 2 hour after 75 g glucose water intake ≥200 mg/dl.[2] The risk factors for DM are including obesity, family history, an unhealthy dietary mode, low level of education, decreased physical behavior, history of hypertension, gestational diabetes, and metabolic syndrome.[3] The sex hormones may be associated with the impairment of glucose metabolism. The higher level of endogenous testosterone or estrogen is associated with an increased risk of DM in women.[4,5] Polycystic ovary syndrome is also associated with an increased risk of DM in middle-aged women as it causes insulin resistance.[6]
Hysterectomy, a major cause of surgical menopause, is the most prevalent surgical gynecological intervention in the United States, China, and Taiwan (5.1–5.8, 1.12, and 2.68–3.03 per 1000 person-years, respectively).[7–10] It is performed to treat endometriosis, uterine myoma, uterine prolapse, and genital malignancies (cervical, endometrial, and ovarian cancer).[7–9] Oophorectomy causes an abrupt stop of endogenous estrogen production. This ovarian hormone is thought to be a regulator of insulin sensitivity, which might be related to DM occurrence.[11] Obviously, hysterectomy is associated with cardiovascular disease (CVD) and its pre-existing risk factors including metabolic syndrome (i.e., obesity, hyperlipidemia, and hypertension).[12–16]
Studies regarding the association of hysterectomy and oophorectomy with DM are limited and inconclusive. A previous study recruited 2597 postmenopausal women without DM as baseline. After a follow-up of 9.2 years, women who underwent both hysterectomy and bilateral salpingo-oophorectomy (BSO) showed a hazard ratio (HR) of 1.57 (95% confidence interval [CI]=1.03–2.41) for association of an elevated DM risk; but women with hysterectomy alone were not associated with DM risk (HR = 1.38, 95% CI = 0.94–2.04).[17] Another study however suggested BSO did not increase the DM risk in postmenopausal women underwent hysterectomy.[18] A cohort study also showed postmenopausal hysterectomized women were not associated with an increased risk of DM.[19]
The above studies were limited in the postmenopausal hysterectomized women. In premenopausal women, however, the ovary may still produce hormones. Hysterectomy and oophorectomy may hamper ovarian hormone production, prompting to menopause.[20,21] Thus, BSO may put women predispose to DM.[22] Moreover, oophorectomy may worsen glucose tolerance and insulin resistance.[23] Menopausal hormone therapy with estrogen and progesterone can decrease DM incidence.[24,25] Thus, we hypothesized that hysterectomy with or without oophorectomy may also cause endocrine dysfunction (alter sex hormones regulation) in premenopausal women, subsequently leading to the development of type 2 DM.
We aimed to determine whether hysterectomy for treating benign diseases, with or without BSO, increases the DM risk using the present population-based cohort study involving nationwide health insurance data from Taiwan.
2. Methods
2.1. Data source
The Taiwan National Health Insurance (NHI) program is a government-run program that was launched on March 1, 1995. It covers more than 99% of the population of Taiwan and has contracts with 93% of the country's medical institutions. Individuals were continuously enrolled in the NHI Research Database (NHIRD), and they did not have an opportunity for care outside of the program. The NHIRD is an electronic database that includes the registration files and original claims data of individuals insured under the NHI program (details available at http://nhird.nhri.org.tw/en/index.htm). All data are de-identified through encryption of the identification numbers of beneficiaries and medical facilities. We used a sub-dataset of 1 million people who were randomly selected from those in the NHIRD in 2000 to investigate the association between hysterectomy and DM. Complete NHIRD medical records from 1996 to 2013 were obtained by linking the scrambled identification of each insurant. Diseases were diagnosed using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. A disease diagnosis without valid supporting clinical findings might be considered medical fraud by the NHI program, and this might involve a penalty of 100 times the payment claimed by the physician or hospital that had provided the treatment. This study was approved by the Institutional Review Board of China Medical University and the Hospital Research Ethics Committee (IRB permit number: CMUH-104-REC2-115). Informed consent has been waived by the approval of the committee. All methods were performed in accordance with the relevant guidelines and regulations.
2.2. Study participants
The study included different patient groups. The hysterectomy group included hysterectomized women between 2000 and 2013. The index date was the date of hysterectomy. Women who had undergone oophorectomy before or after hysterectomy were not included in this study. In total, 12,763 women underwent hysterectomy between 2000 and 2013. Among these women, 472 who had undergone oophorectomy before or after hysterectomy and 3811 aged <30 years (too few patients) or >50 years (might experience menopause) were excluded (Fig. 1). Additionally, women who had any cancer diagnosis at baseline (n = 276) or within 1 year after hysterectomy (n = 330), had DM at baseline or within 1 year after the index date (n = 470), and were withdrawn from the database at baseline (n = 312) and were unmatched (n = 4) were excluded. The comparison group included women who did not undergo hysterectomy and did not have cancer or DM at baseline or within 1 year before the index date. The index date for the comparison group without oophorectomy was randomly assigned. The index date for the without hysterectomy with oophorectomy comparison subgroup was the oophorectomy date. The size of the comparison group was 4-fold greater than that of the hysterectomy, and both groups were propensity score-matched by age, hysterectomy date, urbanization, income, occupation, oophorectomy, and comorbidities listed in Table 1.
Figure 1.
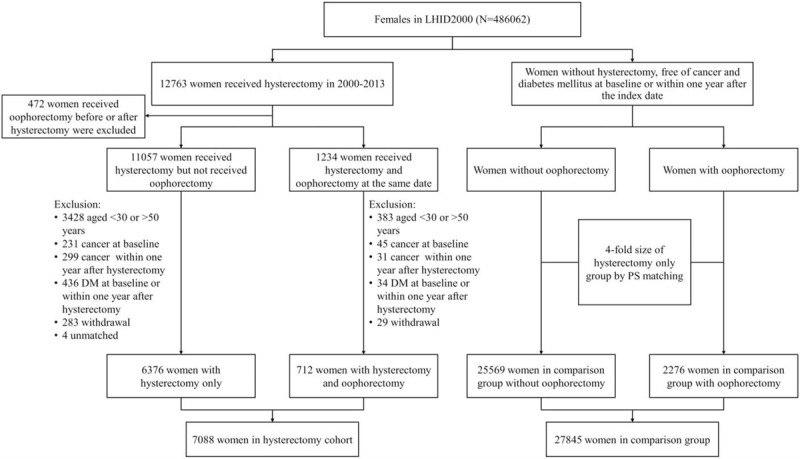
Study flow: the patient population enrolled from the National Health Insurance Research Database.
Table 1.
Baseline characteristics in women with and without hysterectomy.
| Hysterectomy(n = 7088) | Matched cohort(n = 27845) | P-value | |
| Age, yr | |||
| 30–39 | 1226 (17.3) | 5487 (19.7) | <.0001 |
| 40–49 | 5862 (82.7) | 22358 (80.3) | |
| Median (Q1, Q3) | 44.2 (41.3, 46.7) | 44.3 (41.0, 47.2) | .0008 |
| Urbanization | |||
| 1 | 2328 (32.8) | 9245 (33.2) | .4041 |
| 2 | 2228 (31.4) | 8548 (30.7) | |
| 3 | 1214 (17.1) | 4683 (16.8) | |
| 4 | 1318 (18.6) | 5369 (19.3) | |
| Income | |||
| 0–15000 | 1587 (22.4) | 6587 (23.7) | .0003 |
| 15000–30000 | 4121 (58.1) | 15452 (55.5) | |
| ≥30000 | 1380 (19.5) | 5806 (20.9) | |
| Occupation | |||
| White collar | 3887 (54.8) | 15738 (56.5) | <.0001 |
| Blue collar | 2712 (38.3) | 9938 (35.7) | |
| Other | 489 (6.9) | 2169 (7.8) | |
| Follow-up duration, years | |||
| Median (Q1, Q3) | 7.1 (3.8, 10.7) | 7.1 (4.4, 10.1) | .0001 |
| Oophorectomy | 712 (10.0) | 2276 (8.2) | <.0001 |
| Comorbidity, n (%) | |||
| Menopause | 361 (5.1) | 1434 (5.1) | .8466 |
| Hypertension | 832 (11.7) | 2933 (10.5) | .0035 |
| Hyperlipidemia | 630 (8.9) | 2304 (8.3) | .0962 |
| CKD | 50 (0.7) | 198 (0.7) | .9596 |
| CAD | 389 (5.5) | 1395 (5.0) | .1025 |
| Heart failure | 52 (0.7) | 191 (0.7) | .6662 |
| Stroke | 220 (3.1) | 835 (3.0) | .6444 |
| Depression | 503 (7.1) | 1835 (6.6) | .1277 |
| Insomnia | 2927 (41.3) | 11008 (39.5) | .0068 |
P-values were calculated by Chi-squared tests for categorical variables, such as age groups, urbanization, level of income, occupation, oophorectomy, and comorbidities.
For continuous variables, median age and follow-up duration, the P-values were calculated by Wilcoxon rank-sum tests.
P-values less than .05 were considered significant.
CAD = coronary artery disease, CKD = chronic kidney disease.
2.3. Outcome measures
The assessed outcome was DM (ICD-9-CM: 250). To improve the validity of the DM diagnosis, we considered patients with DM as those who made at least 2 clinic visits for DM within 1 year. For every patient, the assessment ended at DM diagnosis, death, withdrawal from the NHI program, or December 1, 2013, whichever was first. We followed the DM occurrence after 1 year of hysterectomy due to DM happening within 1 year of hysterectomy may be not related to the surgical procedure.
2.4. Sociodemographic characteristics and comorbidities
The sociodemographic characteristics assessed in this study were age, residential area urbanization, income, and occupation. Urbanization was divided into 4 levels according to the population density (people/km2) of the residential area (most urbanized [level 1] to least urbanized [level 4]). Occupations were classified as white-collar (government or office work), blue-collar (manual labor), and others (retired, unemployed, or low income). Income was divided into 3 levels according to income-related NHI premiums New Taiwan dollars (NT$ ≥30000, NT$ 15000–29999, and NT$ 1–14999). Detailed descriptions of the assessments of urbanization, occupation, and income have been previously published.[26]
Type 2 DM patients have risk factors such as endothelial dysfunction, vascular inflammation, and hyperlipidemia;[27] they will cause cardiovascular complications,[28] chronic kidney disease (CKD),[29] and hypertension.[30] Besides, DM patients are also found to have higher risk for depression and sleep disorders.[31,32] Menopause may be associated with a rapid progression of glucose intolerance.[33] Therefore, we accounted for baseline comorbidities, namely menopause (ICD-9-CM: 627.2), hypertension (ICD-9-CM: 401–405), hyperlipidemia (ICD-9-CM s: 272), (CKD; ICD-9-CM: 585, 586, 588.8, and 588.9), coronary artery disease (CAD; ICD-9-CM: 410–414), heart failure (ICD-9-CM: 428), stroke (ICD-9-CM s: 430–438), depression (ICD-9-CM: 296.2, 296.3, 296.82, 300.4, 309.0, 309.1, 309.28, and 311), and insomnia (ICD-9-CM: 780).
2.5. The number of clinic visits
We considered the number of clinic visits as confounding factors. We counted the clinic visits and hospitalizations involving the gynecology and Endocrine Departments.
2.6. Statistical analysis
For between-group (hysterectomy group vs comparison group) comparisons of categorical and continuous variables, the chi-squared test and Wilcoxon rank-sum test were applied, respectively. The proportional hazards assumption was examined by adding an interaction term, group∗follow-up time, in the Cox proportional hazards model to test whether the 2 groups have the same effect on hazard at any point in time. A comparison of hysterectomy and matched cohort satisfied the proportional hazards assumption (P = .0811). Multivariable Cox proportional hazard regression models were used to calculate HRs with 95% CIs, and the variables considered were age, urbanization, income, occupation, oophorectomy, and all aforementioned comorbidities. Interactions between comorbidities and hysterectomy were examined by putting the interaction term, hysterectomy∗the comorbidity, in the Cox regression model. Due to the large sample size in the database, we estimated the power of this study will be more than 80%. We did a retrospective power analysis. We used the POWER procedure of SAS software to calculate the power. To calculate the power, 3 key values were input: the observation numbers, hazard rates, and follow-up duration. The hazard rate refers to the rate of events for a group of a given time. Kaplan–Meier survival curves were used to assess the cumulative incidences of DM in the 2 groups, and differences between the groups were assessed using the log-rank test. All statistical analyses were performed using SAS (version 9.4; SAS Institute INC., Cary, NC). A 2-tailed P-value <.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
The present study enrolled 7088 women who underwent a hysterectomy in the hysterectomy group and 27,845 who did not undergo a hysterectomy in the comparison group. The median patient ages in the hysterectomy and comparison groups were both approximately 44 years (Table 1). The distributions of urbanization levels, incomes, occupations, and clinic visits did not differ between the groups (Table 1, Fig. 2). The frequency of comorbidities in the hysterectomy group was also no different from that in the comparison group.
Figure 2.
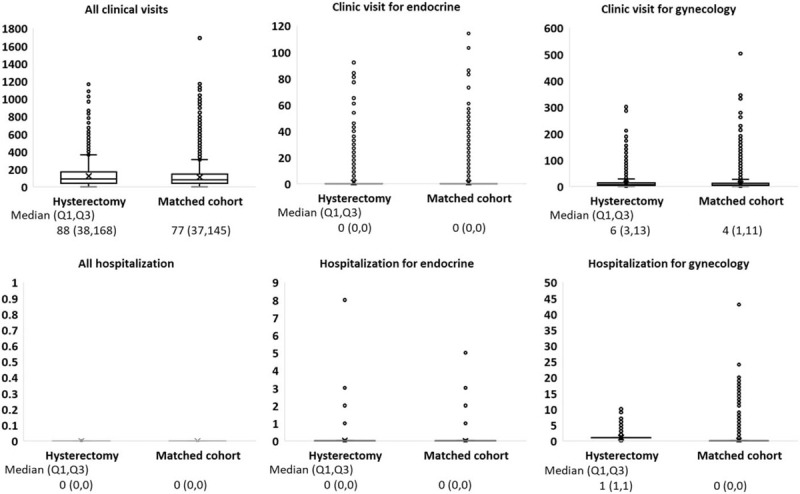
Box plot shows the distribution of all clinic visits, all hospitalization, clinic visit for gynecology, hospitalization for gynecology, clinic visit for endocrine, hospitalization for endocrine.
3.2. DM risk in hysterectomy and comparison groups
During median follow-up periods of 7.1 years, 462 women in the hysterectomy group and 1389 women in the comparison group developed DM, respectively (Table 2). Kaplan–Meier analysis revealed that the cumulative DM incidence in the hysterectomy group was higher than that in the comparison group (9.12 vs 6.78 per 1000 person-years; log-rank test, P < .0001; Fig. 3). The DM risk in the hysterectomy group was significantly higher than that in the comparison group in multivariable Cox proportional hazard regression models (adjusted HR [aHR] = 1.37, 95% CI = 1.23–1.52) (Table 2). The following variables: age, urbanization, income, occupation, oophorectomy, menopause, hypertension, CKD, CAD, heart failure, stroke, depression, and insomnia, were put in the Cox regression model for adjustment.
Table 2.
Risk of diabetes mellitus in women with hysterectomy compared with the matched cohort.
| N | Event | Person-yr | Incidence per 1000 person-years | Adjusted∗ HR with 95% CI | P-value | |
| All | ||||||
| Matched cohort | 27845 | 1389 | 205001.383 | 6.78 | 1 (reference) | |
| Hysterectomy | 7088 | 462 | 50666.6639 | 9.12 | 1.37 (1.23, 1.52) | <.0001 |
| All | ||||||
| Matched cohort without oophorectomy | 25569 | 1322 | 189082.39 | 6.99 | 1 (reference) | |
| Matched cohort with oophorectomy | 2276 | 67 | 15918.9925 | 4.21 | 0.79 (0.61, 1.01) | .0575 |
| Hysterectomy only | 6376 | 423 | 46030.7953 | 9.19 | 1.35 (1.21, 1.51) | <.0001 |
| Hysterectomy with oophorectomy | 712 | 39 | 4635.86858 | 8.41 | 1.28 (0.93, 1.76) | .1314 |
| All | ||||||
| Non-menopause | 33138 | 1714 | 241898.387 | 7.09 | 1 (reference) | |
| Menopause | 1795 | 137 | 13769.6591 | 9.95 | 0.92 (0.77, 1.10) | .3499 |
| Age 30–39 | ||||||
| Matched cohort | 5575 | 122 | 41115.8001 | 2.97 | 1 (reference) | |
| Hysterectomy | 1227 | 55 | 9860.83504 | 5.58 | 1.75 (1.27, 2.41) | .0007 |
| Age 40–49 | ||||||
| Matched cohort | 22270 | 1267 | 163885.582 | 7.73 | 1 (reference) | |
| Hysterectomy | 5861 | 407 | 40805.8289 | 9.97 | 1.33 (1.19, 1.49) | <.0001 |
Model was adjusted for age, urbanization, income, occupation, oophorectomy, and comorbidities listed in Table 1.
HR = hazard ratio.
Figure 3.
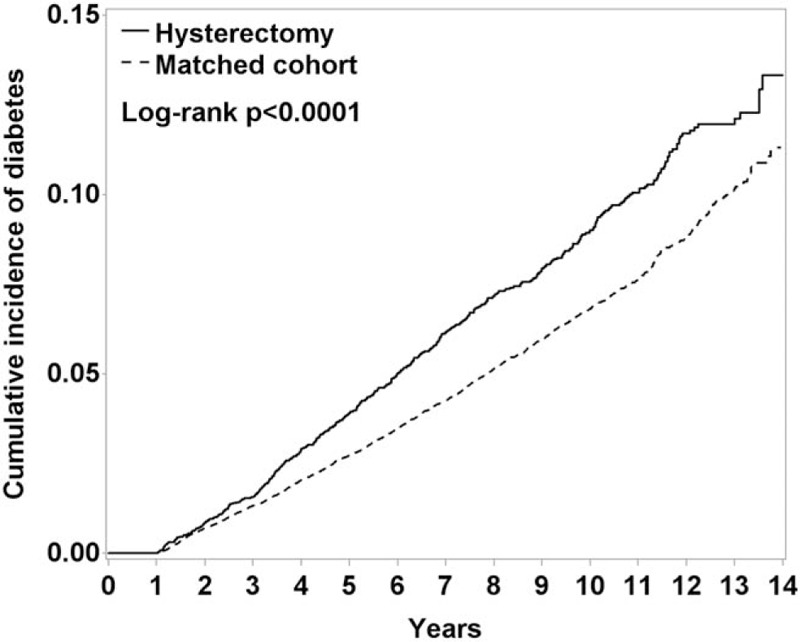
Kaplan–Meier curves showing the cumulative diabetes mellitus incidence in the hysterectomy group (dashed line) and comparison group (solid line) matched by age and comorbidities.
3.3. Effect of oophorectomy on the DM risk in the hysterectomy group
The DM risk in the hysterectomy without oophorectomy subgroup was significantly higher than that in the comparison group (aHR = 1.35, 95% CI = 1.21–1.51) (Table 2). However, the DM risk in the hysterectomy with oophorectomy was not significantly higher than that in the comparison group (aHR = 1.28, 95% CI = 0.93–1.76). Notably, the DM risk was not significant in the without hysterectomy with oophorectomy comparison subgroup (aHR = 0.79, 95% CI = 0.61–1.01).
3.4. Stratified analysis of menopause on DM risk
We compared the DM risk stratified by menopausal status. There was no significant risk for DM after menopause (aHR = 0.92, 95% CI = 0.77–1.10) (Table 2).
3.5. Effect of age on DM risk in the hysterectomy group
We compared the DM risk between the hysterectomy and comparison groups stratified by age. With regard to women aged 30 to 39 years and 40 to 49 years, there was a significant difference between the groups (aHR = 1.75, 95% CI = 1.27–2.41, aHR = 1.33, 95% CI = 1.19–1.49, respectively) (Table 2).
3.6. Effect of comorbidities on DM risk in the hysterectomy group
We compared the DM risk between the hysterectomy and comparison groups stratified by comorbidities (menopause, hypertension, hyperlipidemia, CKD, CAD, heart failure, stroke, obesity, depression, and insomnia) (Table 3). For the comorbidities like hypertension, CAD, stroke, depression, and insomnia, no matter with or without any one of these comorbidities, the hysterectomy group patients were associated with a higher risk of DM than the comparison group. For without the other comorbidities (menopause, hyperlipidemia, CKD, and heart failure), the hysterectomy group were also associated a higher risk of DM than the comparison group. Thus, patients who underwent hysterectomy and with one of these comorbidities (hypertension, CAD, stroke, depression, and insomnia) should be more cautious on diabetes.
Table 3.
Interaction between different comorbidities and hysterectomy on the risk of diabetes mellitus.
| Hysterectomy | Comparison group | adjusted HR | Interaction | ||||||||
| N | Event | person-yr | Incidence per 1000 person-yr | N | Event | person-yr | Incidence per 1000 person-yr | P | |||
| Menopause | No | 6727 | 429 | 47991.272 | 8.94 | 26411 | 1285 | 193907.12 | 6.63 | 1.36 (1.22, 1.52) | .93 |
| Yes | 361 | 33 | 2675.3922 | 12.3 | 1434 | 104 | 11094.267 | 9.37 | 1.37 (0.92, 2.04) | ||
| Hypertension | No | 6256 | 350 | 45356.783 | 7.72 | 24912 | 1056 | 184780.82 | 5.71 | 1.36 (1.21, 1.54) | .75 |
| Yes | 832 | 112 | 5309.8809 | 21.1 | 2933 | 333 | 20220.567 | 16.5 | 1.34 (1.08, 1.67) | ||
| Hyperlipidemia | No | 6458 | 409 | 46916.441 | 8.72 | 25541 | 1213 | 189969.51 | 6.39 | 1.36 (1.21, 1.52) | .50 |
| Yes | 630 | 53 | 3750.2231 | 14.1 | 2304 | 176 | 15031.871 | 11.7 | 1.34 (0.98, 1.83) | ||
| CKD | No | 7038 | 459 | 50346.921 | 9.12 | 27647 | 1379 | 203634.05 | 6.77 | 1.37 (1.23, 1.52) | .94 |
| Yes | 50 | 3 | 319.74264 | 9.38 | 198 | 10 | 1367.3374 | 7.31 | 1.88 (0.40, 8.91) | ||
| CAD | No | 6699 | 416 | 47949.506 | 8.68 | 26450 | 1278 | 195116.45 | 6.55 | 1.34 (1.20, 1.05) | .47 |
| Yes | 389 | 46 | 2717.1581 | 16.9 | 1395 | 111 | 9884.9281 | 11.2 | 1.63 (1.15, 2.31) | ||
| Heart failure | No | 7036 | 459 | 50352.26 | 9.12 | 27654 | 1369 | 203768.35 | 6.72 | 1.38 (1.24, 1.54) | .19 |
| Yes | 52 | 3 | 314.40383 | 9.54 | 191 | 20 | 1233.0294 | 16.2 | 0.49 (0.13, 1.81) | ||
| Stroke | No | 6868 | 440 | 49127.159 | 8.96 | 27010 | 1330 | 198917.22 | 6.69 | 1.36 (1.22, 1.52) | .69 |
| Yes | 220 | 22 | 1539.5044 | 14.3 | 835 | 59 | 6084.1588 | 9.70 | 1.68 (1.02, 2.77) | ||
| Depression | No | 6585 | 427 | 47924.271 | 8.91 | 26010 | 1296 | 193777.4 | 6.69 | 1.36 (1.21, 1.51) | .42 |
| Yes | 503 | 35 | 2742.3929 | 12.8 | 1835 | 93 | 11223.986 | 8.29 | 1.62 (1.09, 2.41) | ||
| Insomnia | No | 4161 | 294 | 34630.615 | 8.49 | 16837 | 911 | 137236.41 | 6.64 | 1.28 (1.13, 1.47) | .12 |
| Yes | 2927 | 168 | 16036.049 | 10.5 | 11008 | 478 | 67764.972 | 7.05 | 1.56 (1.31, 1.86) | ||
∗Model was adjusted for age, urbanization, income, occupation, oophorectomy, and comorbidities listed in Table 1.
CAD = coronary artery disease, CKD = chronic kidney disease, HR = hazard ratio.
3.7. The power of this study
The hazard rates of the 2 groups: 9.12 and 6.78 for hysterectomy and the comparison groups, respectively. The observation number: 7088 and 27,845 for hysterectomy and the comparison groups, respectively. And follow-up duration was 14 years for both 2 groups. Finally, we get a power 99%, which is greater than 80%.
4. Discussion
The present retrospective cohort study found that the risk of developing DM was high in hysterectomized women aged 30 to 39 and 40 to 50 years (aHR = 1.75, 95% CI = 1.27–2.41; aHR = 1.33, 95% CI = 1.19–1.49). The incidences of DM in this age group without and with hysterectomy were 6.78 and 9.12 per 1000 person-years, respectively. These incidences were higher than those in Western countries (3.4 per 1000 person-years).[34] Additionally, our study found that oophorectomy did not affect the DM risk in hysterectomized women (aHR = 1.25, 95% CI = 0.91–1.72).
Menopause causes changes in body composition as well as metabolic and sex hormone profiles.[35,36] Declines in ovarian hormone levels negatively influence glucose metabolism by altering not only pancreatic beta-cell function[37] but also glucose transportation.[38] This is because of the impairment of the phosphatidylinositol 3 kinase/Akt signaling pathway, which causes GLUT4 translocation to the plasma membrane. Moreover, hepatic glucose output decreases because of gluconeogenesis suppression.[39] Women with a short reproductive lifespan have a high DM risk because of reduced endogenous estrogen levels.[17,40]
With regard to surgical menopause, patients typically undergo hysterectomy combined with oophorectomy as the primary prevention approach for ovarian cancer.[41] This approach causes a sharp iatrogenic decline in estrogen production and early menopause, indicating it differs from those of natural menopause. Several studies have revealed the effects of post-hysterectomy menopause-related sudden estrogen loss on glycemic regulation. Hysterectomy combined with oophorectomy can cause carbohydrate metabolism impairment and eventually insulin-resistant glucose tolerance.[23,42,43] A previous study showed that hysterectomy with or without oophorectomy in postmenopausal women was associated with an increased DM risk (HR = 1.13, 95% CI = 1.06–1.21).[18] Oophorectomy might not increase the DM risk in postmenopausal hysterectomized women. In the present study, hysterectomized women, without oophorectomy, were associated with an increased DM risk (HR = 1.5, 95% CI = 1.21–1.51). The effect of hysterectomy combined with oophorectomy was not obvious (aHR = 1.28, 95% CI = 0.93–1.76), probably because most women who underwent hysterectomy were near menopausal age (40–49 years) so that they potentially had compromised ovarian function. Moreover, we also found menopause was not associated with a higher risk of DM (aHR = 0.92, 95% CI = 0.77–1.10).
In the National Health and Nutrition Examination Survey I follow-up study, the DM risk was 57% higher in women who underwent both hysterectomy and bilateral oophorectomy when compared with women who experienced natural menopause (95% CI = 1.03–2.41).[17] In women who underwent hysterectomy alone, no significant DM risk was observed after adjusting for relevant confounders.[17] However, the ovarian function might be disrupted because of a decrease in ovarian blood supply after hysterectomy.[13,40] Our study, with factor control including the exclusion of patients with DM history and adjustment of other cardiovascular risk factors, demonstrated that women with hysterectomy alone were associated with DM risk. Therefore, the potential for increased DM risk should be considered before hysterectomy in premenopausal women.
The association of hysterectomy with DM risk may be attributed to 3 possible reasons:
The first reason is the decrease in estrogen after hysterectomy[44] or oophorectomy.[15,17] It is hypothesized that the ovarian hormone decrease is associated with glucose intolerance. BSO causes dramatic decreases in both estrogen and androgen levels;[20]; estrogen protects glucose metabolism,[45] and hormone therapy lowers the incidence of DM in postmenopausal women.[46] Although reduced estrogen levels may increase the DM risk, reduced androgen levels may decrease or neutralize the DM risk.[47] The maintenance of circulating peripheral tissue-derived estrogen after BSO may help reduce DM risk; therefore, BSO may not be associated with DM risk. However, our study failed to show that BSO was a risk factor for DM.
There was a nearly 2-fold risk of ovarian failure after hysterectomy (HR = 1.92, 95% CI = 1.29–2.86).[44] A prospective cohort study showed that the menopause age was 4 years earlier in a hysterectomy cohort than in a non-hysterectomy cohort.[21] Early menopause can increase the androgen proportion and decrease sex hormone-binding globulin levels.[48] Decreased estrogen production after hysterectomy can increase the incidence of DM and glucose intolerance.[42] In our study, hysterectomy alone was associated with an increased risk of DM.
The second reason is the indication for hysterectomy. The indication might be a risk factor for DM development. For example, obesity is linked to dysfunctional uterine bleeding[49] and uterine myoma,[50] and it is a risk factor for DM development. This implied that obesity was not the only factor in DM development after hysterectomy. The higher obesity and hypertension prevalence in the hysterectomy group could be due to the indication of the hysterectomy. Over 60% of hysterectomies were performed because of uterine fibroids, which are associated with cardiovascular risk factors in particular hypertension but also obesity and diabetes. However, obesity was underreported in the database. Therefore, we did not analyze the influence of obesity in this study.
The third reason is the possible surveillance bias. Usually, after hysterectomy, women visit the clinic often for follow-up of their postoperative condition. Therefore, the chance of DM diagnosis is high in women who have undergone a hysterectomy. In this study, we found that the numbers of clinic visit and hospitalizations were higher in the hysterectomy group than in the comparison group. However, a previous study showed that the number of clinic visits was not different between hysterectomy and comparison cohorts.[18] The surveillance bias could be eliminated by adjustment of the visit number.
The present study has several notable strengths. First, data were collected from 23 million people in Taiwan, and 1 million people with generalized ethnicities were selected randomly. Second, this population-based study included all hysterectomies for benign diseases during a 14-year period. Selection bias and incidence-prevalence bias were limited. Finally, both independent variables (presurgical cardiovascular risk factors) and dependent variables (case-control conditions) were available in the medical diagnosis system.
However, several limitations of our study need to be considered. First, population variables were clarified through ICD-9-CM codes, which could have caused diagnostic errors. Nevertheless, a previous report suggested consistency between diagnostic code use and manual diagnosis,[51] and thus, ICD-9-CM codes are considered appropriate for detecting actual risk factors or diseases. Another shortage of using diagnostic codes was not entirely reflected in the comorbidities prevalence. Third, obesity, smoking/drinking, and body mass index data were underreported or not recorded in the database. High body mass index is considered a risk factor for DM. Lastly, although the comparison group was propensity-score-matched, some women in the hysterectomy group have no matched comparisons. There were some distributions of variables not quite similar in both groups. Nevertheless, we adjusted those variables in the later analysis.
In conclusion, our study provides essential and novel evidence for the association between hysterectomy and DM risk in middle-aged women, which is relevant to these women and their physicians. Physicians should be aware of the increased DM risk associated with hysterectomy and take this into consideration when evaluating a patient for a hysterectomy. The current results will help gynecologists prevent DM and encourage diagnostic and preventive interventions in appropriate patients.
Author contributions
CHC wrote the manuscript; DCD wrote the manuscript, was responsible for the concept design, and supervised the project; WC and IJT wrote the manuscript and were responsible for statistical analyses; JHW was responsible for the concept design and statistical analysis; CYH and SZL supervised the project, and all authors approved the final manuscript. DCD is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Conceptualization: Dah-Ching Ding.
Data curation: Ching-Hsiang Chiang, Weishan Chen, I-Ju Tsai, Dah-Ching Ding.
Formal analysis: Weishan Chen, I-Ju Tsai, Jen-Hung Wang, Dah-Ching Ding.
Funding acquisition: Weishan Chen, Chung Y. Hsu, Shinn-Zong Lin, Dah-Ching Ding.
Investigation: Ching-Hsiang Chiang, Weishan Chen, I-Ju Tsai, Dah-Ching Ding.
Methodology: Ching-Hsiang Chiang, Weishan Chen, I-Ju Tsai.
Supervision: Chung Y. Hsu, Jen-Hung Wang, Shinn-Zong Lin, Dah-Ching Ding.
Validation: Chung Y. Hsu, Jen-Hung Wang, Shinn-Zong Lin, Dah-Ching Ding.
Visualization: Chung Y. Hsu, Shinn-Zong Lin.
Writing – original draft: Ching-Hsiang Chiang, Weishan Chen, I-Ju Tsai, Dah-Ching Ding.
Writing – review & editing: Dah-Ching Ding.
Glossary
Abbreviations: aHR = adjusted hazard ratio, BSO = bilateral salpingo-oophorectomy, CAD = coronary artery disease, CKD = chronic kidney disease, DM = diabetes mellitus, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = NHI Research Database, NT = New Taiwan dollars.
References
- [1].Blair M. Diabetes mellitus review. Urol Nurs 2016;36:27–36. [PubMed] [Google Scholar]
- [2].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34: (Suppl 1): S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bellou V, Belbasis L, Tzoulaki I, et al. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One 2018;13:e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim C, Halter JB. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep 2014;16:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007;50:2076–84. [DOI] [PubMed] [Google Scholar]
- [6].Jaliseh HK, Tehrani FR, Behboudi-Gandevani S, et al. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril 2017;108:1078–84. [DOI] [PubMed] [Google Scholar]
- [7].Ding DC, Chu TY, Chang YH. Trend changes in the proportion of minimal invasive hysterectomies over a five-year period: a single-center experience. Tzu Chi Med J 2012;24:136–8. [Google Scholar]
- [8].Wu MP, Huang KH, Long CY, et al. Trends in various types of surgery for hysterectomy and distribution by patient age, surgeon age, and hospital accreditation: 10-year population-based study in Taiwan. J Minim Invasive Gynecol 2010;17:612–9. [DOI] [PubMed] [Google Scholar]
- [9].Falcone T, Walters MD. Hysterectomy for benign disease. Obstet Gynecol 2008;111:753–67. [DOI] [PubMed] [Google Scholar]
- [10].Liu F, Pan Y, Liang Y, et al. The epidemiological profile of hysterectomy in rural Chinese women: a population-based study. BMJ Open 2017;7:e015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36: (Suppl 1): S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Matthews KA, Gibson CJ, El Khoudary SR, et al. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: study of women's health across the nation. J Am Coll Cardiol 2013;62:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Michelsen TM, Dørum A, Cvancarova M, et al. Association between hysterectomy with ovarian preservation and cardiovascular disease in a Norwegian population-based sample. Gynecol Obstet Invest 2013;75:61–7. [DOI] [PubMed] [Google Scholar]
- [14].Ingelsson E, Lundholm C, Johansson ALV, et al. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011;32:745–50. [DOI] [PubMed] [Google Scholar]
- [15].Li PC, Tsai IJ, Hsu CY, et al. Risk of hyperlipidemia in women with hysterectomy-a retrospective cohort study in Taiwan. Sci Rep 2018;8:12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ding DC, Tsai IJ, Hsu CY, et al. Risk of hypertension after hysterectomy: a population-based study. BJOG 2018;125:1717–24. [DOI] [PubMed] [Google Scholar]
- [17].Appiah D, Winters SJ, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care 2014;37:725–33. [DOI] [PubMed] [Google Scholar]
- [18].Luo J, Manson JE, Urrutia RP, et al. Risk of diabetes after hysterectomy with or without oophorectomy in postmenopausal women. Am J Epidemiol 2017;185:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laughlin-Tommaso SK, Khan Z, Weaver AL, et al. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause 2018;25:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kotsopoulos J, Shafrir AL, Rice M, et al. The relationship between bilateral oophorectomy and plasma hormone levels in postmenopausal women. Horm Cancer 2015;6:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG 2005;112:956–62. [DOI] [PubMed] [Google Scholar]
- [22].Dørum A, Tonstad S, Liavaag AH, et al. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: a controlled, population-based study (HUNT-2). Gynecologic Oncol 2008;109:377–83. [DOI] [PubMed] [Google Scholar]
- [23].Riant E, Waget A, Cogo H, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009;150:2109–17. [DOI] [PubMed] [Google Scholar]
- [24].Margolis KL, Bonds DE, Rodabough RJ, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia 2004;47:1175–87. [DOI] [PubMed] [Google Scholar]
- [25].Bonds DE, Lasser N, Qi L, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia 2006;49:459–68. [DOI] [PubMed] [Google Scholar]
- [26].Lin SM, Yang SH, Liang CC, et al. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: a population-based cohort study. Osteoporos Int 2018;29:153–62. [DOI] [PubMed] [Google Scholar]
- [27].Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 2018;34:575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sarwar N, Gao P, Seshasai SR, et al. Emerging risk factors collaboration diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nowakowska M, Zghebi SS, Ashcroft DM, et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med 2019;17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Waeber B, Feihl F, Ruilope L. Diabetes and hypertension. Blood Press 2001;10:311–21. [DOI] [PubMed] [Google Scholar]
- [31].de Groot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–30. [DOI] [PubMed] [Google Scholar]
- [32].Khandelwal D, Dutta D, Chittawar S, et al. Sleep disorders in type 2 diabetes. Indian J Endocrinol Metab 2017;21:758–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim C, Edelstein SL, Crandall JP, et al. Menopause and risk of diabetes in the diabetes prevention program. Menopause 2011;18:857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nguyen QM, Xu JH, Chen W, et al. Correlates of age onset of type 2 diabetes among relatively young black and white adults in a community: the Bogalusa Heart Study. Diabetes Care 2012;35:1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop+ 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Karvonen-Gutierrez CA, Park SK, Kim C. Diabetes and menopause. Curr Diab Rep 2016;16:20. [DOI] [PubMed] [Google Scholar]
- [37].Gannon M, Kulkarni RN, Tse HM, et al. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab 2018;15:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Suba Z. Low estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditure. J Diabetes Metab 2013;04: [Google Scholar]
- [39].Rines AK, Sharabi K, Tavares CDJ, et al. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov 2016;15:786–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brand JS, van der Schouw YT, Onland-Moret NC, et al. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 2013;36:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sarrel PM, Sullivan SD, Nelson LM. Hormone replacement therapy in young women with surgical primary ovarian insufficiency. Fertil Steril 2016;106:1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pirimoglu ZM, Arslan C, Buyukbayrak EE, et al. Glucose tolerance of premenopausal women after menopause due to surgical removal of ovaries. Climacteric 2011;14:453–7. [DOI] [PubMed] [Google Scholar]
- [43].Zhu L, Martinez MN, Emfinger CH, et al. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab 2014;306:E1188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moorman PG, Myers ER, Schildkraut JM, et al. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol 2011;118:1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mauvais-Jarvis F, Manson JE, Stevenson JC, et al. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev 2017;38:173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Navarro G, Allard C, Xu W, et al. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015;23:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Torréns JI, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of Women's Health Across the Nation. Menopause 2009;16:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nouri M, Tavakkolian A, Mousavi SR. Association of dysfunctional uterine bleeding with high body mass index and obesity as a main predisposing factor. Diabetes Metab Syndr 2014;8:1–2. [DOI] [PubMed] [Google Scholar]
- [50].Pandey S, Bhattacharya S. Impact of obesity on gynecology. Womens Health 2010;6:107–17. [DOI] [PubMed] [Google Scholar]
- [51].Babalola EO, Bharucha AE, Melton LJ, 3rd, et al. Utilization of surgical procedures for pelvic organ prolapse: a population-based study in Olmsted County, Minnesota, 1965-2002. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


