Abstract
To evaluate the learning curve of percutaneous endoscopic transforaminal lumbar discectomy (PETLD) from the novice stage to the proficient stage, we performed retrospective study for patients with lumbar disc herniation who underwent PETLD performed by a single surgeon and evaluated the surgeon's learning curve and the effect of surgical proficiency on outcomes.
A total of 48 patients who underwent PETLD at the lower lumbar level (L3–S1) with a minimum 1-year follow-up were enrolled. The learning curve of the surgeon was assessed using cumulative study of operation time and linear regression analyses to reveal the correlation between operation time and case series number.
Because the cutoff of familiarity was 25 cases according to the cumulative study of operation time, the patients were allocated into two groups: early group (n = 25) and late group (n = 23). The clinical, surgical, and radiological outcomes were retrospectively evaluated and compared between the two groups.
According to linear regression analyses, the operation time was obtained using the following formula: operation time (minutes) = 69.925–(0.503 × [case number]) (P < .001).
As expected, the operation time was significantly different between the two groups (mean 66.00 ± 11.37 min in the early group vs 50.43 ± 7.52 min in the late group, P < .001). No differences were found between the two groups in demographic data and baseline characteristics. Almost all clinical outcomes (including pain improvement and patient satisfaction), surgical outcomes (including failure, recurrence, and additional procedure rates), and radiological outcomes (including change of disc height and sagittal angles) did not differ between the two groups.
However, the late group demonstrated a more favorable postoperative volume index of the remnant disc (362.91 mm3 [95% confidence interval, 272.81–453.02] in the early group vs 161.14 mm3 [95% confidence interval, 124.31–197.97] in the late group, P < .001), and a lower complication rate related to exiting nerve root (16.0% in the early group vs 0% in the late group, P = .045).
The learning curve of PETLD is not as difficult as that of other minimally invasive spine surgery technique. Although the overall outcomes were not different between the groups, the risks of incomplete decompression and exiting root injury-related complication were higher in the novice stage.
Keywords: discectomy, endoscopy, intervertebral disc displacement, percutaneous
1. Introduction
Percutaneous endoscopic transforaminal lumbar discectomy (PETLD) is a full endoscopic spine surgery for disc herniation in the lumbar spine, and has been popular worldwide as an alternative minimally invasive technique to open lumbar microdiscectomy since the 2000s.[1] Many previous comparative studies have reported that PETLD is not inferior to open microdisectomy in terms of the clinical results and full endoscopic surgery is better than open surgery in the aspect of minimal invasiveness.[2–10]
However, the major concerns with PETLD include its difficult learning curve and fear of failure, complications, or recurrence after surgery, particularly in the surgeon's novice stage. A definite threshold exists for a skillful surgical technique in terms of the small percutaneous single-port entry via transforaminal approach and the unfamiliar two-dimensional surgical view utilizing a narrow and magnified endoscopic view. Accordingly, some authors have suggested that the learning curve of a surgeon for PETLD, that is whether the surgeon is a beginner or an expert in PETLD, might affect the clinical outcomes.[11–13]
Although several studies about the learning curve of PETLD have been published, there are only a few methodical reports about the learning curve of PETLD by a single surgeon from the novice stage to the proficient stage and about the effect of surgical proficiency on outcomes.[11,14,15] In this study, we aimed to analyze the learning curve of PETLD by a single surgeon and to compared the outcomes based on the level of surgical skill in patients with disc herniation of the lower lumbar spine (L3–S1).
2. Materials and methods
2.1. Patient selection and surgical indication
The study was approved by the institutional review board of our institute (GBIRB2020-301). The ethics committee waived the requirement for informed consent owing to the retrospective nature of the study, and all data were fully anonymized before access by the authors.
One surgeon at a single institute started to perform PETLD from September 2014 after an intensive fellowship of 4 weeks and several cadaveric training courses for full endoscopic surgery. The indications for PETLD were as follows:
-
1.
persistent low back pain and leg pain despite sufficient conservative treatment or at least 6 weeks,
-
2.
severe pain making daily life activities impossible, or
-
3.
severe paresis of motor grade ≤3.
The charts of all 64 patients who underwent PETLD from September 2014 to August 2017, performed by a single surgeon, were retrospectively reviewed. The data showed that the average interval between cases was 0.56 month, which was not too long.
To minimize the influence of various surgical difficulties and patient selection bias, the exclusion criteria were set as follows:
-
1.
multi-level surgery,
-
2.
PETLD at the upper lumbar spine (L1–L2 or L2–L3),
-
3.
foraminal or extraforaminal disc herniation,
-
4.
revision surgery of the index level,
-
5.
history of previous lumbar spine surgery, and
-
6.
insufficient follow-up duration (<1 year) or incomplete medical records.
After the exclusion of 16 of the 64 patients, the remaining 48 patients were enrolled as the final study cohort. The patients in the final cohort were divided into an early group (n = 25) and a late group (n = 23) based on the cutoff case of familiarity of 25 cases according to the cumulative study of operation time (Fig. 1).
Figure 1.
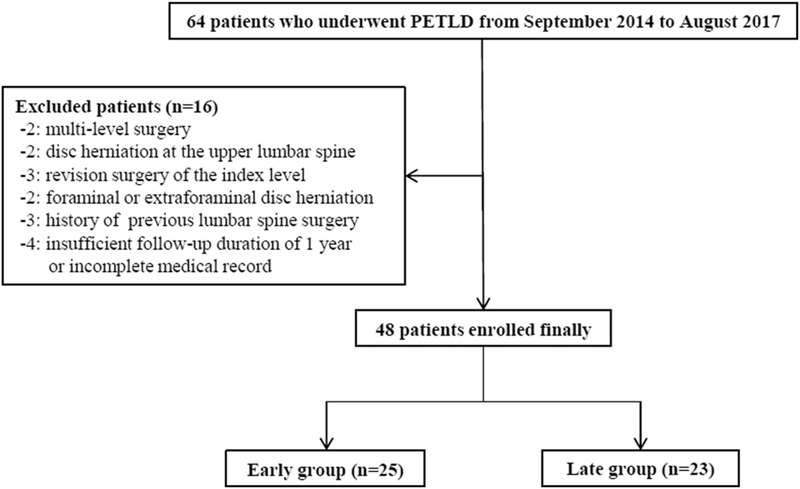
Patient selection.
2.2. Operative technique
All patients were placed in the prone position with decreased abdominal pressure followed by local anesthesia using lidocaine and slight sedation using intravenous injection of sedative drugs.
An about 0.5-cm skin incision was made at the extreme far lateral side from the midline (about 10–15 cm far away from the midline) according to the anatomical variation and surgical plan. Insertion of a discogram needle to the target disc and discography with indigo carmine were performed under fluoroscopic guidance. After serially inserting a guidewire, a serial dilator, and an obturator, a working cannula and an endoscope (Vertebris system [Richard Wolf, Knittlingen, Germany] or Joimax system [Joimax, Irvine, CA]) were inserted. Under the endoscopic view with continuous irrigation, disc space evacuation, removal of the ruptured disc, and penetration of the posterior longitudinal ligament to confirm the decompressed thecal sac or traversing nerve root were performed on a case-by-case basis. Radiofrequency (Elliquence Int; Hewlett, NY) was used for bleeding control and annuloplasty. The surgeon sometimes performed a foraminoplasty or pediculectomy using a high-speed drill (Primado 2; Nakanishi Inc, Tochigi, Japan) according to the preoperative plan and the patient's anatomy.
Finally, the wound was closed using one- or two-point subcutaneous sutures and skin tape.
The decision of equipment to use, bone work, the degree of intervertebral disc evacuation, and penetration of the posterior longitudinal ligament were determined depending on pre-operative planning and/or intra-operative findings.
2.3. Learning curve evaluation based on operation time
The authors assessed the learning curve by analyzing the procedure time. The preparation time (including patient positioning, draping, and setting of surgery), the operation time (defined as from skin incision to wound closure), and total operation time determined (sum of preparation time and operation time) were assessed. The operation time was collected according to case number and cumulatively analyzed to determine the cutoff value of familiarity. Furthermore, linear regression analyses were performed to reveal the linear correlation between operation time and case series number.
2.4. Outcome evaluation
According to the cumulative analysis of operation time, the final cohort was divided into two groups: the early group, which included earlier cases in the novice stage, and the late group, which included later cases in the proficient stage.
Demographic data, including age, sex, occupation, smoking habit, alcohol drinking habit, and body mass index; symptom-related baseline characteristics, including symptom duration, history of previous nerve block, trauma history, and presence of weakness; and magnetic resonance imaging (MRI)-related baseline characteristics, including degree of disc degeneration according to Pfirrmann grade,[16] side of pathogenic lesion (right, central, or left), type of disc herniation (migrated or non-migrated), and volume of ruptured disc, were assessed and compared between the two groups. The volume of ruptured disc was evaluated as the volume index determined by as the product of the largest width, length, and height of the ruptured disc fragment on MRI.
Detailed surgical techniques, including foraminoplasty, pediculectomy, and penetration of the posterior longitudinal ligament, and operation time were compared between the two groups.
Surgical outcomes, including intraoperative blood loss, duration of hospital stay and return to work, surgical failure (conversion to open surgery or failure of removal of the ruptured disc), surgical complication, recurrence, and additional procedure (revision surgery or additional nerve block) during 1 year after surgery, were assessed and compared between the two groups. Intraoperative blood loss was indirectly evaluated using preoperative and postoperative hemoglobin levels. Immediate postoperative MRI was performed in all patients to confirm the decompression of the nerve root and to check for any postoperative complication. To evaluate the degree of decompression and surgical efficacy, the volume of the remnant disc was analyzed using the same method of volume index determination as in the preoperative evaluation.
Clinical outcome was assessed using visual analogue scale (VAS) scores of low back pain and leg pain. Data were collected preoperatively, 1 week postoperatively, and 1 year postoperatively. Patient satisfaction was surveyed using Odom's criteria at 1 week and 1 year after surgery.
Plain neutral radiography, including dynamic radiography, was performed preoperatively and at 1 year after surgery to evaluate the change in average disc height and lumbar alignment. Segmental angle and range of motion at the surgery level, and total lumbar lordosis (measured using Cobb's method) were used to evaluate the radiological outcomes in the two groups. Average disc height was obtained from the average anterior and posterior disc heights at the surgery level in the standing position. Thereafter, the average disc height was calibrated with the anteroposterior diameter of the L5 body to eliminate the fluctuating magnification effect of radiography, as follows: average disc height (mm)/anteroposterior diameter of the L5 body (mm) × 100.
All radiological parameters were assessed by two researchers who were blinded to group allocation. If there was any disagreement about qualitative parameters between the researchers, a conclusion was reached by consensus discussion. Quantitative parameters were determined as the average of the measurements of the two researchers. To confirm the reproducibility of quantitative measurement, two spine surgeons (SS and BRY) independently measured parameters. The reliability levels of quantitative radiological parameters were assessed using interrater correlation coefficients, the values of which were ≥0.90 between observers.
2.5. Statistical analysis
Data management and statistical analysis were performed using SPSS version 23.0 (SPSS Inc, Chicago, IL). Pearson's chi square test, independent t test, non-parametric Mann–Whitney U test, one-way analysis of variance, and interrater correlation coefficient test were used according to the characteristics of the factors. A cumulative study and linear regression analysis were performed to analyze the learning curve based on operation time. The results are expressed as means ± standard deviations or mean and 95% confidence interval (CI) depending on the data distribution. Statistical significance was considered for P values < .05.
3. Results
3.1. Learning curve of PETLD based on operation time
The mean preparation time, mean operation time (from skin incision to closure), and mean total operation time (sum of preparation time and operation time) were 20.46 ± 8.79, 58.54 ± 12.42, and 77.23 ± 14.99 min among all patients. A trend of decreasing operation time was observed according to the accumulation of case series or surgical experience of the surgeon. In the cumulative study, the cumulative average operation time showed a plateau after 25 cases. The mean operation time was most significantly different between the earlier 25 cases and the later 23 cases (66.00 ± 11.37 min in the early group vs 50.43 ± 7.52 min in the late group; P < .001, independent t test) (Fig. 2).
Figure 2.
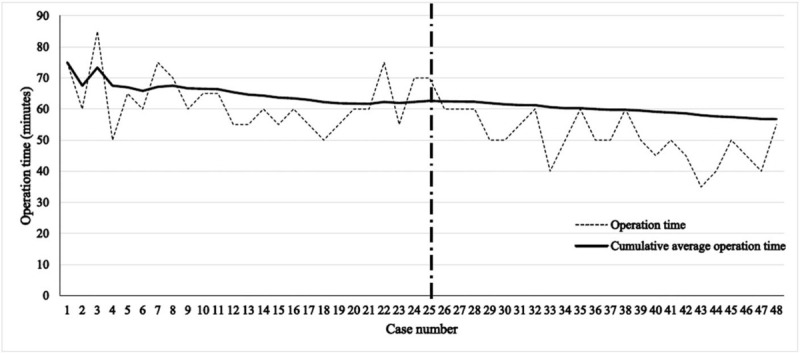
Operation time according to case series number.
According to linear regression analysis, the operation time was calculated as 69.925 – (0.503 × [case number]) (P < .001). This equation suggests that the operation time definitely decreases with the accumulation of operations. Furthermore, the proportional constant, −0.503, means that the operation time continuously decreased even in cases in which the surgeon had limited surgical experience (Fig. 3).
Figure 3.
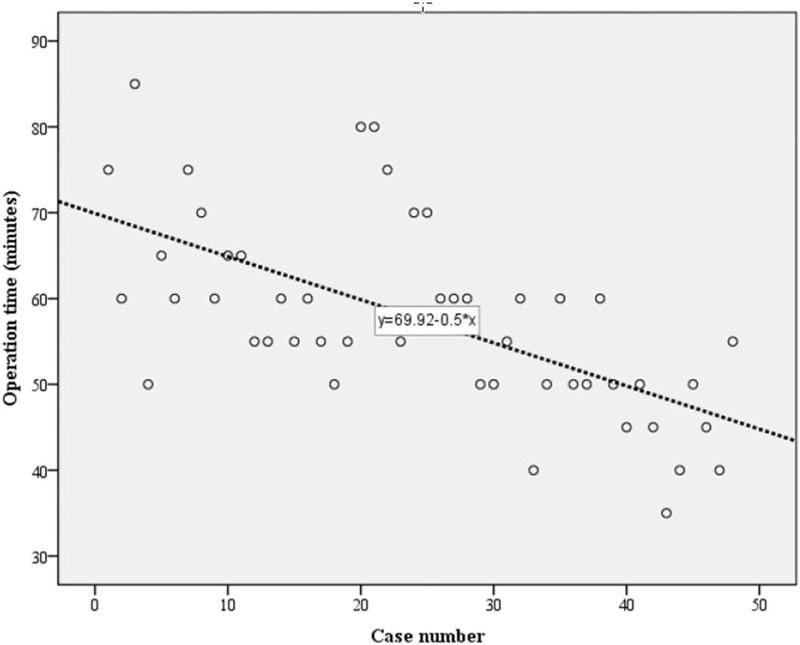
Linear and log regression analyses. Operation time (minutes) = 69.925–(0.503 × [case number]) (P < .001).
3.2. Baseline characteristics in the two groups
On the basis of the significant cutoff value of operation time in the cumulative analysis of operation time, the cohort was divided cohort into two groups: the early group comprising the earlier 25 cases in the novice level and the late group comprising the later 23 cases in the proficient level.
All demographic data and baseline characteristics related to clinical symptoms and MRI findings did not significantly differ between the two groups (Table 1).
Table 1.
Demographic data and baseline characteristics in the two groups.
| Early (n = 25) | Late (n = 23) | P | |
| Sex, male/female | 16/9 | 15/8 | .930∗ |
| Age (years) | 38.44 ± 12.19 | 39.17 ± 18.23 | .870† |
| Occupation, white collar/blue collar/others | 13/6/9 | 9/4/10 | .534∗ |
| Smoking | 14 | 8 | .141∗ |
| Alcohol | 16 | 9 | .108∗ |
| Height (cm) | 168.58 ± 7.61 | 168.13 ± 11.95 | .877† |
| Weight (kg) | 70.25 ± 13.15 | 71.68 ± 19.99 | .769† |
| Body mass index (kg/m2) | 24.65 ± 3.95 | 25.04 ± 4.32 | .750† |
| Symptom duration (days) | 141.32 (95% CI, 46.32–236.32) | 75.26 (95% CI, 29.73–120.79) | .215‡ |
| Previous block | 12 | 15 | .230∗ |
| Trauma | 2 | 1 | .602∗ |
| Weakness | 9 | 9 | .823∗ |
| Surgery level, L3–L4/L4–L5/L5–S1 | 3/22/0 | 2/19/2 | .749∗ |
| Pfirrmann grade, III/IV/V | 21/4/0 | 15/7/1 | .326∗ |
| Side, right/central/left | 9/5/11 | 8/1/14 | .222∗ |
| Type of ruptured disc, migrated/non-migrated | 6/19 | 6/17 | .868∗ |
| Volume index of the ruptured disc (mm3) | 1054.74 (95%CI, 841.46–1268.01) | 769.23 (95%CI, 488.78–1049.68) | .233‡ |
Volume index of the ruptured disc = anteroposterior × transverse × sagittal diameter of the ruptured fragment.
CI = confidence interval.
Pearson's chi square test.
Independent t test.
Non-parametric Mann–Whitney U test.
3.3. Surgical outcomes in the two groups
The detailed surgical techniques were not different between the two groups. However, as expected, the preparation, operation, and total operation times were significantly shorter in the late group (mean 23.88 ± 9.77, 66.00 ± 11.37, and 86.48 ± 14.15 min in the early group vs 16.74 ± 5.76, 50.43 ± 7.52, and 67.17 ± 7.66 min in the late group, respectively; P < .001, independent t test) (Table 2).
Table 2.
Surgical technique and operation time in the two groups.
| Early (n = 25) | Late (n = 23) | P | |
| Surgical technique | |||
| Foraminoplasty | 2 | 3 | .701∗ |
| Pediculotomy | 1 | 1 | .952∗ |
| Penetration of the posterior longitudinal ligament | 16 | 22 | .203∗ |
| Procedure time | |||
| Preparation time (min) | 23.88 ± 9.77 | 16.74 ± 5.76 | .004† |
| Operation time (min) | 66.00 ± 11.37 | 50.43 ± 7.52 | <.001† |
| Total operation time (min) | 86.48 ± 14.15 | 67.17 ± 7.66 | <.001† |
Pearson's chi square test.
Independent t test.
Almost all surgical outcomes including blood loss based on preoperative and postoperative hemoglobin levels, hospital stay, return to work, surgical failure, surgical complication rate, recurrence rate, and additional procedure rate were not different between the two groups. However, the late group demonstrated a lower rate of complication related to exciting nerve root (16.0% in the early group vs 0% in the late group; P = .045, Pearson's chi square test) and a more favorable postoperative volume index of the remnant disc (362.91 [95%CI, 272.81–453.02] in the early group vs 161.14 [95%CI, 124.31–197.97] in the late group; P < .001, non-parametric Mann–Whitney U test) (Table 3). The complications related to exiting nerve root in the early group included two patients with transient dysesthesia of the concordant dermatome, one patient with transient minor weakness of the knee, and one patient with permanent weakness of the knee (grade I immediately after surgery and grade III at 1 year after surgery).
Table 3.
Surgical outcomes in the two groups.
| Early (n = 25) | Late (n = 23) | P | |
| Preoperative hemoglobin (g/dL) | 14.03 ± 1.47 | 14.30 ± 1.33 | .500∗ |
| Postoperative hemoglobin (g/dL) | 13.78 ± 1.72 | 13.79 ± 1.44 | .978∗ |
| Hospital stay (days) | 3.40 ± 1.47 | 4.00 ± 2.68 | .336∗ |
| Return-to-work (days) | 10.32 ± 4.85 | 14.52 ± 11.09 | .091∗ |
| Failure | 3 (12.0%) | 1 (4.3%) | .155† |
| Conversion to open | 0 | 1 | .292† |
| Remnant disc | 3 | 0 | .086† |
| Complication | 6 (24.0%) | 2 (8.7%) | .155† |
| Exiting root related | 4 | 0 | .045† |
| Dura tearing | 1 | 1 | .952† |
| Others | 1 (headache during procedure) | 1 (transient mild dorsiflexion weakness) | .952† |
| Recurrence | 1 (4.0%) | 3 (13.0%) | .393† |
| Additional procedure | 4 (16.0%) | 9 (39.1%) | .072† |
| Revision surgery | 1 | 4 | .129† |
| Nerve block | 3 | 4 | .553† |
| Post-operative volume index of the remnant disc (mm3) | 362.91 (95%CI, 272.81–453.02) | 161.14 (95%CI, 124.31–197.97) | <.001‡ |
Independent t test.
Pearson's chi square test.
Non-parametric Mann–Whitney U test.
3.4. Clinical outcomes and radiological outcomes between the two groups
Unexpectedly, the clinical outcomes including the improvement of VAS for low back pain or leg pain and the patients’ satisfaction according to Odom's criteria were not significantly different between the two groups (Table 4).
Table 4.
Clinical outcomes between in the two groups.
| Early (n = 25) | Late (n = 23) | P | |
| VAS back pain | |||
| Pre-operative | 5.80 ± 1.47 | 5.30 ± 1.58 | .266∗ |
| 1 week | 2.36 ± 0.71 | 2.61 ± 1.27 | .436∗ |
| 1 year | 1.72 ± 0.77 | 1.13 ± 0.87 | .155∗ |
| ANOVA | <.001† | ||
| VAS leg pain | |||
| Pre-operative | 7.76 ± 1.20 | 7.39 ± 1.31 | .313∗ |
| 1 week | 2.44 ± 1.39 | 3.13 ± 2.11 | .185∗ |
| 1 year | 1.56 ± 0.69 | 1.30 ± 0.52 | .585∗ |
| ANOVA | <.001† | ||
| Odom's criteria (1 week) | .100‡ | ||
| Excellent | 16 | 10 | |
| Good | 8 | 10 | |
| Fair | 1 | 3 | |
| Poor | 0 | 0 | |
| Odom's criteria (1 year) | .354‡ | ||
| Excellent | 18 | 18 | |
| Good | 6 | 3 | |
| Fair | 1 | 2 | |
| Poor | 0 | 0 |
ANOVA = analysis of variance, VAS = visual analogue scale.
Independent t test.
ANOVA.
Pearson's chi square test.
Moreover, the radiological outcomes including the decrease of disc height, neutral segmental angle in the surgery level, range of motion in the surgery level, and total lumbar lordosis were not different between the two groups at 1 year after surgery (Table 5).
Table 5.
Radiological outcomes in the two groups.
| Early (n = 25) | Late (n = 23) | P | |
| Disc height, calibrated | |||
| Preoperative | 26.59 ± 4.05 | 25.21 ± 4.38 | .288∗ |
| 1 year | 24.69 ± 3.32 | 23.11 ± 3.83 | .200∗ |
| Segmental angle (°) | |||
| Preoperative | 8.11 ± 6.53 | 8.76 ± 6.76 | .313∗ |
| 1 year | 9.03 ± 5.43 | 9.76 ± 5.45 | .185∗ |
| Range of motion (°) | |||
| Preoperative | 5.96 ± 5.12 | 9.54 ± 6.84 | .100∗ |
| 1 year | 5.66 ± 2.48 | 7.52 ± 7.95 | .448∗ |
| Total lumbar lordosis (°) | |||
| Preoperative | 39.11 ± 12.05 | 38.41 ± 8.97 | .838∗ |
| 1 year | 39.36 ± 10.05 | 35.65 ± 10.14 | .277∗ |
Independent t test.
4. Discussion
PETLD is considerably different from conventional microdiscectomy because of the different access trajectory and required equipment.[11,17] The obstacles in starting PETLD include the rather different access method via the transforaminal Kambin's triangle,[18] difficulty in the insertion of the needle and the working cannula into the exact target site, use of a narrow and magnified two-dimensional endoscopic vision, presence of a vague or obscured view in case of uncontrolled bleeding during surgery, fear of iatrogenic durotomy and nerve injury, uncertainty of successful decompression, or concern about recurrence. These barriers may pose challenges to a surgeon in the beginner stage and may result in a difficult learning curve and unfavorable clinical outcomes. To overcome this difficult learning curve, the surgeon needs to accumulate at least a certain number of cases to become adjusted to the PETLD procedure.[11]
Operation time is a major parameter in evaluating the technical competency of surgeons.[19] The trend of operation time is an effective statistical tool that is commonly used to assess whether a trainee has achieved acceptable proficiency. The surgeon's comfort and technical proficiency is correlated to a decrease in procedure length in chronological case series, and the traditional evaluation of the learning curve has focused on operation time according to the number of cases.[20]
In our study, as the number of cases accumulated, the operation time shortened as a result of familiarity with the surgical technique. The cumulative analysis identified a threshold of 25 cases after which the operation time was nearly consistent. In other words, the operation time decreased from a mean 66.00 min to mean 50.43 min (decrease of 23.6%) after the initial 25 cases, and approached an asymptote from the 26th case. On the basis of the asymptote point, we assumed that the difficulty of the learning curve of PETLD is similar to 10 to 30 cases in previous studies or in other minimally invasive spinal surgeries, such as microsurgery using a tubular retractor.[11,17,21–23] On the basis of the 23.6% decrease of operation time and the proportional constant of −0.503 in the formula of operation time, the rate of decline was not steep compared with the 23% to 58% decrease in operation time during the initial series of cases between the 10th and 30th case.[11,17,21–24] This finding implies that the entry barriers for beginners in PETLD are similar to those in other techniques.
Another clinically relevant parameter used to assess surgeon proficiency through the learning curve is the failure rate, complication rate, and clinical outcome. Most of the failure cases and surgery-related complications usually occurred within the novice stages of the learning process in minimally invasive spine surgery.[20] The lack of clear anatomic knowledge or orientation and unfamiliarity with new instruments seem to be significant limitations, and these may result in serious injury to neurological structures or unintended adverse events in the initial series of patients.[25] Many studies on minimally invasive spine surgery have reported that the complication rate is higher and the clinical outcome is poorer at the beginner level than at the expert level.[12,21,26,27]
According to our findings, both the surgical outcomes, including failure rate, complication rate, and recurrence rate, and the clinical outcomes were similar between the early and late groups. However, the degree of decompression was unfavorable in the early group compared with the late group based on postoperative MRI findings. This finding implies that the surgical proficiency in removing pathogenic lesions is improved by the accumulation of cases. In addition, the rate of complication related to exiting nerve root was higher in the early group than in the late group. This finding suggests that the risk of exiting nerve root injury or irritation during the transforaminal approach is higher in the novice level. On the basis of these results, PETLD is not easy to perform and is not safe in the novice level, although the overall outcomes in the novice level were comparable to those in the proficient level.
This study had several limitations. Because of its retrospective design, it was impossible to control for all variations. Nevertheless, we attempted to minimize errors by precluding variables affecting the results. In addition, the number of patients in this study was relatively small and the study was conducted by one surgeon at a single institute. However, this study could maintain the consistent quality of follow-up evaluation and exclude diversity of surgeon's technique.
To the best of our knowledge, this is the first study to evaluate the learning curve and related outcomes of PETLD by a single surgeon. More complete studies with a prospective design and a larger number of patients are required to verify our results.
5. Conclusion
On the basis of operation time and outcomes, the learning curve of PETLD is not as difficult as that of other minimally invasive spine surgeries and the previous reports on full endoscopic spine surgery. However, the complication related to exiting nerve root injury/irritation during approach and relatively insufficient decompression is concerning points in the novice level. Incompetence is inevitable in the early stage of learning a PETLD; thus, sufficient training is mandatory for a novice surgeon.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
SS has full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. JC designed the study protocol and supervised. SS and BRY managed the literature searches and summaries of previous related works and wrote the first draft of the manuscript. All authors provided revision for intellectual content and final approval of the manuscript.
Conceptualization: Seong Son, Yong Ahn, Joon Cho.
Data curation: Byung Rhae Yoo, Jong Myung Jung.
Formal analysis: Seong Son, Byung Rhae Yoo.
Investigation: Seong Son, Byung Rhae Yoo.
Methodology: Seong Son.
Project administration: Seong Son, Joon Cho.
Resources: Seong Son.
Software: Seong Son.
Supervision: Sang Gu Lee, Woo Kyung Kim, Joon Cho.
Validation: Yong Ahn, Sang Gu Lee, Woo Kyung Kim, Jong Myung Jung.
Visualization: Seong Son.
Writing – original draft: Seong Son.
Writing – review & editing: Seong Son, Joon Cho.
Glossary
Abbreviations: CI = confidence interval, MRI = magnetic resonance imaging, PETLD = percutaneous endoscopic transforaminal lumbar discectomy, VAS = Visual Analogue Scale.
References
- [1].Ruetten S, Komp M, Godolias G. Full-endoscopic interlaminar operation of lumbar disc herniations using new endoscopes and instruments. Orthop Praxis 2005;10:527–32. [Google Scholar]
- [2].Choi K-C, Shim H-K, Kim J-S, et al. Cost-effectiveness of microdiscectomy versus endoscopic discectomy for lumbar disc herniation. Spine J 2019;19:1162–9. [DOI] [PubMed] [Google Scholar]
- [3].Kim M, Lee S, Kim HS, et al. A comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for lumbar disc herniation in the Korean: a meta-analysis. Biomed Res Int 2018;2018:9073460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Phan K, Xu J, Schultz K, et al. Full-endoscopic versus micro-endoscopic and open discectomy: a systematic review and meta-analysis of outcomes and complications. Clin Neurol Neurosurg 2017;154:1–2. [DOI] [PubMed] [Google Scholar]
- [5].Ruan W, Feng F, Liu Z, et al. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg 2016;31:86–92. [DOI] [PubMed] [Google Scholar]
- [6].Choi KC, Kim JS, Park CK. Percutaneous endoscopic lumbar discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Physician 2016;19:E291–300. [PubMed] [Google Scholar]
- [7].Ahn SS, Kim SH, Kim DW, et al. Comparison of outcomes of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for young adults: a retrospective matched cohort study. World Neurosurg 2016;86:250–8. [DOI] [PubMed] [Google Scholar]
- [8].Chen HC, Lee CH, Wei L, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar surgery for adjacent segment degeneration and recurrent disc herniation. Neurol Res Int 2015;2015:791943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee DY, Shim CS, Ahn Y, et al. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for recurrent disc herniation. J Korean Neurosurg Soc 2009;46:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee SH, Chung SE, Ahn Y, et al. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med 2006;73:795–801. [PubMed] [Google Scholar]
- [11].Lee DY, Lee SH. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir (Tokyo) 2008;48:383–8. discussion 388–389. [DOI] [PubMed] [Google Scholar]
- [12].Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery 2015;76:372–80. discussion 380–371; quiz 381. [DOI] [PubMed] [Google Scholar]
- [13].Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician 2018;21:E105–12. [PubMed] [Google Scholar]
- [14].Wang B, Lu G, Patel AA, et al. An evaluation of the learning curve for a complex surgical technique: the full endoscopic interlaminar approach for lumbar disc herniations. Spine J 2011;11:122–30. [DOI] [PubMed] [Google Scholar]
- [15].Wang H, Huang B, Li C, et al. Learning curve for percutaneous endoscopic lumbar discectomy depending on the surgeon's training level of minimally invasive spine surgery. Clin Neurol Neurosurg 2013;115:1987–91. [DOI] [PubMed] [Google Scholar]
- [16].Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. [DOI] [PubMed] [Google Scholar]
- [17].McLoughlin GS, Fourney DR. The learning curve of minimally-invasive lumbar microdiscectomy. Can J Neurol Sci 2008;35:75–8. [DOI] [PubMed] [Google Scholar]
- [18].Kambin P, Casey K, O’Brien E, et al. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg 1996;84:462–7. [DOI] [PubMed] [Google Scholar]
- [19].Dagash H, Chowdhury M, Pierro A. When can I be proficient in laparoscopic surgery? A systematic review of the evidence. J Pediatr Surg 2003;38:720–4. [DOI] [PubMed] [Google Scholar]
- [20].Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review. Clin Orthop Relat Res 2014;472:1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini–open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine 2008;9:560–5. [DOI] [PubMed] [Google Scholar]
- [22].Tan J, Zheng Y, Gong L, et al. Anterior cervical discectomy and interbody fusion by endoscopic approach: a preliminary report. J Neurosurg Spine 2008;8:17. [DOI] [PubMed] [Google Scholar]
- [23].Scheufler KM, Kirsch E. Percutaneous multilevel decompressive laminectomy, foraminotomy, and instrumented fusion for cervical spondylotic radiculopathy and myelopathy: assessment of feasibility and surgical technique. J Neurosurg Spine 2007;7:514–20. [DOI] [PubMed] [Google Scholar]
- [24].Son S, Ahn Y, Lee SG, et al. Learning curve of percutaneous endoscopic interlaminar lumbar discectomy versus open lumbar microdiscectomy at the L5-S1 level. PLoS One 2020;15:e0236296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McAfee PC, Phillips FM, Andersson G, et al. Minimally invasive spine surgery. Spine (Phila Pa 1976) 2010;35: (26 Suppl): S271–273. [DOI] [PubMed] [Google Scholar]
- [26].Rong LM, Xie PG, Shi DH, et al. Spinal surgeons’ learning curve for lumbar microendoscopic discectomy: a prospective study of our first 50 and latest 10 cases. Chin Med J 2008;121:2148–51. [PubMed] [Google Scholar]
- [27].Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine 2007;32:537–43. [DOI] [PubMed] [Google Scholar]


