Abstract
Stem cell-based products (SCPs) are an emerging field of veterinary medicine that focuses on the regeneration, repair, or replacement of damaged tissues or organs. However, there are some issues in applying the traditional regulatory guideline for the approval of SCPs as veterinary medicinal products. This article describes the positions of Korea, US, and EU regarding SCPs, and compares the regulatory guidelines of each country for their safety evaluation. Although there are some differences in the regulatory guidelines, similar considerations in identifying the quality of SCPs and their safety has adopted. Overall, these guidelines need to be harmonized among countries.
Keywords: Stem cell-based products, veterinary medicinal products, regulatory guidelines, quality, safety
INTRODUCTION
Stem cells have the ability of self-renewal and differentiation. It can be widely used in regenerative medicine to repair the bone, cartilage, and skin [1,2]. Stem cell-based products (SCPs) are rapidly developing challenges for the assurance of consistent and safe approaches to making such it available to treat of a disease or physical injury. As living drugs, SCPs are complex in terms of their structure, content, mode of action, and distribution, all of which face marketing authorization challenges for regulatory authorities, manufacturers, and clinical veterinarians. Unlike small-molecule drugs, there are no standardized safety test methods, and few references and guidelines are yet available in this field. Because there is substantial diversity of SCPs based on cell's origin, cell processing methods, target disease, target animals, application sites, and application methods. Each manufacturer or researcher uses different material sources, assays, equipment, and techniques to develop products. SCPs itself should not be evaluated in isolation. The whole process, including selection of donor cells, raw materials, validation, assays, storage, shipment, and delivery, as well as factors involving the recipient such as dose, frequency, and route of administration will need to be considered to assess the safety of SCPs [3]. Although some countries have regulatory polices about stem cell therapeutics for animals, there are differences between across the country. In this article, we provide an overview of the safety assessment guidelines for SCPs established by the Animal and Plant Quarantine Agency (APQA), Korea; Center for Veterinary Medicine (CVM), US; and Committee for Medicinal Products for Veterinary Use (CVMP), EU. Furthermore, we propose some considerations for improving stem cell therapy regulations to promote the steadfast growth of the well-regulated stem cell therapies abreast of the evolution of stem cell sciences and technologies. This article is intended to facilitate the rapid development and availability of SCPs to improve of the quality of life and treatment of companion animals suffering from incurable diseases.
APQA GUIDELINES PERTAINING TO STEM CELL-BASED VETERINARY MEDICINAL PRODUCTS
The APQA established the “Guideline on Safety Evaluation of Cell-based Medicinal Products for Animal Use” to ensure the safe use of stem cell therapies for animals in June 2018 [4]. The guideline covers the following regulations:
• Manufacturing processes related to cell origin, cell collection, freezing, thawing, cell culture, filling of cells, storage conditions and periods, specific screening information on the cell donor animals, and evidence of the absence of biologic contamination.
• Stability testing to confirm whether formulation, storage conditions, storage period, and transportation container and transport procedures influence stability or specifications.
• Toxicity studies to demonstrate safety, such as acute, subacute, and chronic toxicity testing; reproduction toxicity testing; mutagenicity (or genotoxicity) testing; carcinogenicity (or tumorigenicity) testing; local toxicity testing (or local tolerance testing); immunotoxicity testing (or confirmatory testing of immune system alteration); and other special toxicity testing.
• Pharmacological action testing to determine the mechanism of action and efficacy, such as efficacy testing, general pharmacological testing, and testing for absorption, distribution, metabolism, and excretion.
• Clinical trials (including target animal safety testing) to verify the efficacy and assess safety in target animals.
• Standard test methods such as chromosomal karyotyping to evaluate genomic stability; tumorigenicity testing to assess tumorigenic potential; confirmatory test of biodistribution and kinetics to confirm engraftment, proliferation (or differentiation), and persistence of stem cells in tissues; and target animal safety testing.
GUIDELINES FOR CELL-BASED PRODUCTS FOR ANIMAL USE ESTABLISHED BY THE FOOD AND DRUG ADMINISTRATION'S CVM
In June 2015, the CVM released the guidance “Cell-Based Products for Animal Use,” which includes “animal stem cell-based products (ASCPs)” [5]. The guidance describes categories of ASCPs, preapproval requirements, and post-approval responsibilities.
-
• ASCPs is categorized products from different species (xenogeneic), other individuals of the same species (allogeneic), and those from the same individual (autologous). Autologous cells were divided into two types: type I and type II.
- Type I cells were defined as those that are more than minimally manipulated (for example, by cell expansion, differentiation, and addition of purified trophic factors), intended for non-homologous use, intended for use in a food-producing animal and dependent on the metabolic activity of its living cells for effect, and combine with/modified by another article, drug, or device.
- Type II cells are those that are minimally manipulated (for example, by centrifugation and cryopreservation), intended for homologous use, intended for use in non-food-producing animals, and are not combined with other materials.
-
• A new animal drug application (NADA) for xenogeneic, allogeneic, and autologous type I are as follows:
- Evaluation documents of safety and efficacy including tumorigenicity, immunogenicity, donor selection criteria, transmission of adventitious agents, long-term safety, cell survival, biodistribution, ectopic tissue formation.
- Information of chemistry, manufacturing, and controls (CMC) must meet current good manufacturing practice (cGMP) and include standard operating procedures (SOPs) about tissue handling and cellular isolation for reliable, consistent, preserve cellular function and integrity, and prevent contamination.
- The requirement for investigational use of new animal drugs refer to 21 CFR 551 that is New Animal Drugs for Investigational Use.
• Although an approved NADA is required for a type II product, the product is considered a lower risk to human and animal safety; therefore, it is a lower enforcement priority.
• Manufacturers is responsible for the post-approval of SCPs with respect to the manufacturing facilities, labeling, safety and efficacy records.
It is recommended that manufacturers contact the CVM during the product development process to discuss the types of data and information that will be required to demonstrate the safety and efficacy of products in the NADA applications.
GUIDANCE FOR STEM CELL-BASED MEDICINES FOR VETERINARY USE ESTABLISHED BY THE EUROPEAN MEDICINES AGENCY'S CVMP
The CVMP has issued guidance on stem cell-based medicines for veterinary use, which was organized in the form of eight specific questions and answers in June 2017 [6] and primarily addresses sterility-related aspects of the stem cell product:
• The product must be developed using qualified and tested raw materials and by a validated aseptic manufacturing process.
• Tissue collection should only be performed by experienced/well-trained veterinarians by using aseptic techniques in a clean room and the procedures for tissue collection, packing, and shipping should be performed according to defined procedures.
• Vials and stoppers containing raw materials such as biological materials (for example, blood, sera, cells, and tissues) and synthetic materials (for example, media and medium supplements) that are in direct contact with the product should be compliance with the regulation.
• All manufacturing procedures should be systemized to prevent compromised quality or risk of contamination. Procedures for cleaning and sanitation should be well described and only qualified/trained personnel should be involved in production.
• Testing for sterility should be conducted at every step, including all stages of cell handling, product to cryopreservation, and prior to packing/finished product of the production process is strongly recommended.
• Alternative methods such as suitable (rapid) microbiological testing may be acceptable if justified. However, it is the responsibility of the manufacturer to demonstrate the suitability of these methods and to ensure the comparability of results with the outcomes of techniques recommended by the European Medicines Agency.
• Polymerase chain reaction would be inadequate for comprehensive testing of bacteriological contamination because the spectrum of bacteria and fungi is huge.
• The presence of endotoxins is a valid safety concern; therefore, it is important to test at the level of the active substance and the finished product.
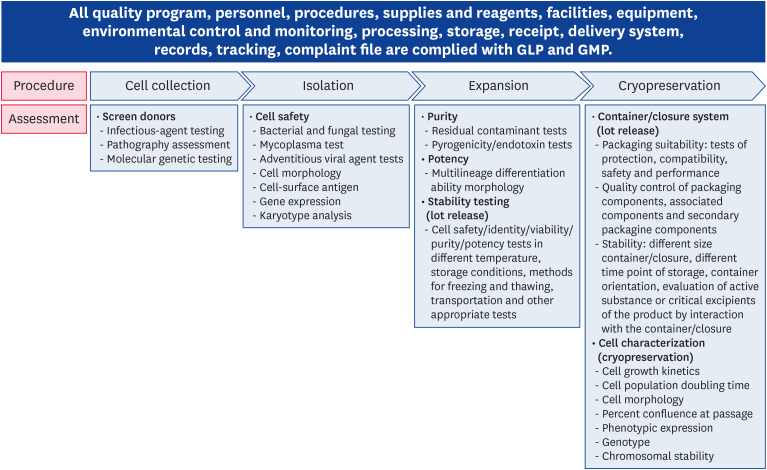
Stem cell therapy has been an important topic of discussion worldwide. However, there are inherent difficulties associated with SCPs involving living cells because several variations can occur in many steps, from cell collection to therapy. Furthermore, variations are complex because multiple bioactive factors can affect SCPs formulation and influence each other in the administration site's microenvironment. In vitro studies are recommended throughout the manufacturing process to evaluate the process itself and to ensure the consistency and quality of the product [7] (Fig. 1). This figure shows that the primary concern associated with stem cell therapy is quality control during the manufacturing procedure. SCPs recognized that conventional nonclinical safety testing was not always feasible because of the above cellular characteristics. Since the studies using laboratory animals are limited, it is thought that the study plan should be different for the following studies. Although the terminology is different in the guidelines for safety and efficacy testing of SCPs by country, it has been investigated that the test items for producing safe products are similar (Table 1). For example, reproductive toxicity studies were required only when a potential exposure for fertility animals and tumorigenicity testing is appropriated instead of carcinogenicity testing. The World Health Organization should collaborate with the different international groups active in human cell-based therapy with the goal of providing a standard guideline to promote the development of safe and effective cell therapies within an international regulatory framework [3]. In conclusion, we recognized that there is a need for interactions and discussions with international regulatory groups to cope with the scientific and regulatory challenges posed by SCPs. Consensus guidelines must be developed and eventual standards to ensure the final product's consistency, safety, and efficacy.
Fig. 1. The flow chart represents recommended assessments in the main steps in stem cell-based products development from cell collection, manufacturing to cryopreservation and lot release. There is suggestive assessment for individual steps in the squares under the flow chart.
GLP, Good Laboratory Practice; GMP, Good Manufacturing Practice.
Table 1. Regulatory considerations for safety and efficacy of stem cell-based products for animal use in the APQA, CVM, and CVMP.
| Country (Authority) | Non-clinical research | Clinical research | ||
|---|---|---|---|---|
| Korea (APQA) | • The finished SCPs are required to meet GMP. | • The finished SCPs are required to meet GMP. | ||
| • Safety studies (under GLP) | • Clinical trials (under GCP) (including target animal safety testing) | |||
| - Acute/Subacute/Chronic toxicity testing | ||||
| - Reproduction toxicity testing | ||||
| - Mutagenicity (genotoxicity) testing | ||||
| - Carcinogenicity (tumorigenicity) testing | ||||
| - Local toxicity (local tolerance) testing | ||||
| - Immunotoxicity testing (confirmatory testing of immune system alteration) | ||||
| - Other special toxicity testing | ||||
| US (FDA CVM) | • The finished SCPs are required to meet GMP. | • The finished SCPs are required to meet GMP. | ||
| • Safety studies (under GLP) | • Clinical investigations (under GCP) (e.g., use in client-owned animals) | |||
| - Tumorigenicity testing | ||||
| - Immunogenicity testing | ||||
| - Donor selection criteria | ||||
| - Long term-safety testing | ||||
| - Ectopic tissue formation testing | ||||
| EU (EMA CVMP) | • The finished SCPs are required to meet GMP. | • The finished SCPs are required to meet GMP. | ||
| • Safety studies (under GLP) | • Clinical trials (under GCP) | |||
| - Single/repeat dose toxicity testing | - Target animal tolerance testing | |||
| - Reproductive toxicity testing | - Clinical field trial | |||
| - Genotoxicity testing | ||||
| - Carcinogenicity testing | ||||
SCPs, stem cell-based products; GMP, good manufacturing practice; GLP, good laboratory practice; GCP, good clinical practice; APQA, Animal and Plant Quarantine Agency; FDA CVM, The Food and Drug Administration's Center for Veterinary Medicine; EMA CVMP, European Medicines Agency's Committee for Medicinal Products for Veterinary Use.
Footnotes
Funding: This project was supported by research funds from Animal and Plant Quarantine Agency, Republic of Korea.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Ku HO.
- Investigation: Ku HO, Jeon BS.
- Writing - original draft: Jeon BS.
- Writing - review & editing: Ku HO, Yi H.
References
- 1.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22(7):833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 2.Xing F, Li L, Zhou C, Long C, Wu L, Lei H, et al. Regulation and directing stem cell fate by tissue engineering functional microenvironments: scaffold physical and chemical cues. Stem Cells Int. 2019;2019:2180925. doi: 10.1155/2019/2180925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petricciani J, Hayakawa T, Stacey G, Trouvin JH, Knezevic I. Scientific considerations for the regulatory evaluation of cell therapy products. Biologicals. 2017;50:20–26. doi: 10.1016/j.biologicals.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Ku HO, Yi H, Park YI, Jeon BS, Kang HG, Kim YS, et al. Guideline on safety evaluation of cell-based medicinal products for animal use. J Vet Sci. 2019;20(2):e14. doi: 10.4142/jvs.2019.20.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Cell-Based Products for Animal Use. Report N˚: FDA-2014-D-0634, June, 2015 [Internet] Silver Spring: The Food and Drug Administration's (FDA) Center for Veterinary Medicine (CVM); [Updated 2018]. [Accessed 2020 Apr 20]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-218-cell-based-products-animal-use. [Google Scholar]
- 6.European Medicines Agency. Questions and Answers on Allogeneic stem cell-based products for veterinary use: specific questions on sterility-Revision 1. Report N˚: EMA/CVMP/ADVENT/751229/2016-Rev.1, June 20, 2019 [Internet] Amsterdam: European Medicines Agency's (EMA) Committee for Medicinal Products for Veterinary Use (CVMP); [Updated 2019]. [Accessed 2020 Apr 20]. https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-allogeneic-stem-cell-based-products-veterinary-use-specific-questions-sterility_en.pdf. [Google Scholar]
- 7.Vestergaard HT, D'Apote L, Schneider CK, Herberts C. The evolution of nonclinical regulatory science: advanced therapy medicinal products as a paradigm. Mol Ther. 2013;21(9):1644–1648. doi: 10.1038/mt.2013.175. [DOI] [PubMed] [Google Scholar]