Abstract
Background
Scalding burn injuries can occur in everyday life but occur more frequently in young children. Therefore, it is important to develop more effective burn treatments.
Objectives
This study examined the effects of bee venom (BV) stimulation on scalding burn injury-induced nociception in mice as a new treatment for burn pain.
Methods
To develop a burn injury model, the right hind paw was immersed temporarily in hot water (65°C, 3 seconds). Immediately after the burn, BV (0.01, 0.02, or 0.1 mg/kg) was injected subcutaneously into the ipsilateral knee area once daily for 14 days. A von Frey test was performed to assess the nociceptive response, and the altered walking parameters were evaluated using an automated gait analysis system. In addition, the peripheral and central expression changes in substance P (Sub P) were measured in the dorsal root ganglion and spinal cord by immunofluorescence.
Results
Repeated BV treatment at the 2 higher doses used in this study (0.02 and 0.1 mg/kg) alleviated the pain responses remarkably and recovered the gait performances to the level of acetaminophen (200 mg/kg, intraperitoneal, once daily), which used as the positive control group. Moreover, BV stimulation had an inhibitory effect on the increased expression of Sub P in the peripheral and central nervous systems by a burn injury.
Conclusions
These results suggest that a peripheral BV treatment may have positive potency in treating burn-induced pain.
Keywords: Burns, pain, bee venom, analgesia, substance P
INTRODUCTION
Burn injuries and their associated symptoms are major causes of mortality and postmortem disorders worldwide, and more than 300,000 people a year have died from various types of burns since 2009 [1]. According to global reports, burns are common among children, accounting for approximately 40–60 percent of all hospitalized burn patients [2]. Among the types of burns, scalding injuries by hot liquids occur more frequently in young children under 9 years of age. This specific injury is more serious because it can occur in everyday life, such as boiling water or hot water in the bathroom [3]. Therefore, it is important to develop a more effective burn treatment to reduce after-effect disorders and symptoms.
Bee venom (BV) is a mixture of natural toxins produced by the western honeybee (Apis mellifera) used widely as an oriental traditional medicine for various diseases. Recently, it has been used in various treatments in complementary alternative medicine [4]. Several studies have examined the biological and pharmacological activities of BV and confirmed various effects, such as radiation prevention, anti-inflammatory, antibacterial, antiviral, and anti-cancer effects [5,6]. In addition, it has been reported to be effective in relieving inflammatory pain, such as arthritis and neuropathic pain [7,8].
The expression of substance P (Sub P) is increased in almost all currently identified inflammatory cytokines, and plays a major role in neurogenic inflammation, a local inflammatory response to certain types of infections or injuries [9,10,11]. Moreover, it is a neurotransmitter and a regulator of the pain factors that transmit the signals related to pain to the central nervous system [12]. Previous studies have shown that in various pain animal models, increased Sub P expression in the peripheral nervous system and the central nervous system plays a major role in the pain path or causes pain itself [13,14,15,16]. Based on these findings, this study examined the effects of repeated BV stimulation on pain, locomotion, and tissue damage caused by scalding burns, and further on the Sub P manifestation of the peripheral and central nervous system.
MATERIALS AND METHODS
Animals
Male ICR mice (Samtako, Korea), weighing 20 to 25 g at 5 weeks of age, were used in this experiment. All animal experiments were performed according to the policy of the Chungnam National University regarding the use and care of animals. Furthermore, this study was conducted with the approval of the Animal Experiment Ethics Committee of Chungnam National University (approval number: CNU-00919). The animals were housed in a standard environment consisting of a 12 h light/dark cycle, constant room temperature (maintained between 20 and 25°C), and 40–60% humidity. Food and water were supplied ad libitum.
Scalding burn model
The normal baseline values of the paw withdrawal frequency were measured one day before burn induction. To induce a scalding burn, the mice were anesthetized by an intraperitoneal (i.p.) injection of 2-2-2 tribromoethanol (250 mg/kg; Sigma, USA) dissolved in 2.5% of a methyl butane solution. After the animal was deeply anesthetized, the right hind paw was immersed temporarily in 65 ± 0.5°C of hot water for 3 sec. After inducing a burn, the mice were placed on a heating pad until they recovered from anesthesia.
BV stimulation
The BV from A. mellifera (BV [0.01, 0.02, and 0.1 mg/kg, 50 µL; Sigma]) was dissolved in a 0.9% physiological saline solution and administered subcutaneously daily into the ipsilateral knee area from the day of burn induction for 14 days. The control group was administered the same volume of 0.9% of saline solution subcutaneously. Acetaminophen (Sigma) dissolved in 0.9% physiological saline was used as a positive drug for a symptomatic treatment, and 100 µL was administered intraperitoneally at a dose of 200 mg/kg using the same schedule as BV.
Mechanical allodynia assay
To assess the nociceptive responses to innocuous mechanical stimuli (mechanical allodynia), the paw withdrawal response frequency was measured using 1.0 g of von Frey filament (North Coast Medical, USA). The mice were placed on a metal mesh grid under a plastic chamber, and the von Frey filament was applied to each hind paw from underneath the metal mesh flooring. The von Frey filament was applied 10 times to each hind paw with 10-sec time intervals, and the number of paw-withdrawal responses was counted. The results of mechanical behavior testing in each experimental animal are expressed as a percentage withdrawal response frequency, representing the percentage of paw withdrawals out of a maximum of 10 times.
CatWalk automated gait analysis
The changes in motor functions during walking were assessed by measuring the gait parameters using the automated gait analysis system. The CatWalk XT system (Noldus Information Technology, The Netherlands) has been used to evaluate the gait parameters in pain models, such as osteoarthritis [17] or neuropathic pain [18,19,20]. When mice walk freely through a dark tunnel, the downward pressure of each paw illuminates fluorescent light from the glass platform, and a video camera detects and records these light signals. The CatWalk XT software then analyzes various gait parameters automatically, such as paw print area, duty cycle, and stance phase. In this study, 2 parameters (print area and single stance) were examined to determine the antinociceptive effect of BV on burn-injured mice. The print area was measured by calculating the surface area contacted to the glass floor, and the single stance is the part of a step cycle, indicating the time (seconds) each paw touched the glass plate while walking. All CatWalk data was gained by calculating the percentage changes between the ipsilateral and contralateral hind paws (i.e., normal mice showed approximately 50%, which means the ratio of ipsilateral vs. contralateral was 50:50).
Immunofluorescence
Immunofluorescence was performed at one and 7 days after burn induction. The mice were anesthetized with 2-2-2 tribromoethanol and perfused transcardially with heparinized phosphate-buffered saline (PBS, pH 7.4), followed by perfusion with 4% paraformaldehyde for 10 min. Ipsilaterally, the dorsal root ganglions (DRGs) and spinal cord of the lumbar enlargement (L4–L6) regions were extracted immediately and immersed in the same fixative solution overnight. After fixation, the tissues were immersed in a 30% sucrose solution with PBS until they sank to the bottom and were frozen after embedding in a Surgipath FSC 22 frozen section compound. (Leica Biosystems Richmond Inc., USA). The compound-embedded tissue arrays were sliced into 10-μm sections, which were then attached to silane-coated slide glasses. To rinse the embedding compound, sections were washed 3 times with PBS solution for 10 min each time. Nonspecific binding was blocked with 3% bovine serum albumin in PBST for 1 h at room temperature. After blocking, the sections were incubated with 1:1,000 dilution of anti-Sub P antibody (#ab14184; Abcam, UK) in the blocking solution overnight at 4°C. They were then washed 3 times with PBST for 10 min each. After washing, the sections were incubated with a 1:500 dilution of Cy3-conjugated secondary antibody (Jackson ImmunoResearch Inc., USA) for one hour at room temperature. Stained sections were mounted with VECTASHIELD (Vector Laboratories Inc., USA) and analyzed using an Axiophot microscope (Carl Zeiss, Germany).
Data analysis
The data are expressed as the mean ± standard error of the mean. The level of statistical significance was determined using an unpaired Student's t-test for the comparisons between 2 means and by an analysis of variance followed by a Dunnett' test for multiple comparisons. Graph pad Prism 6 (Graph Pad Software, Inc., USA) was used for statistical analysis. A p value < 0.05 was considered significant.
RESULTS
Effects of BV stimulation on burn-induced nociceptive responses
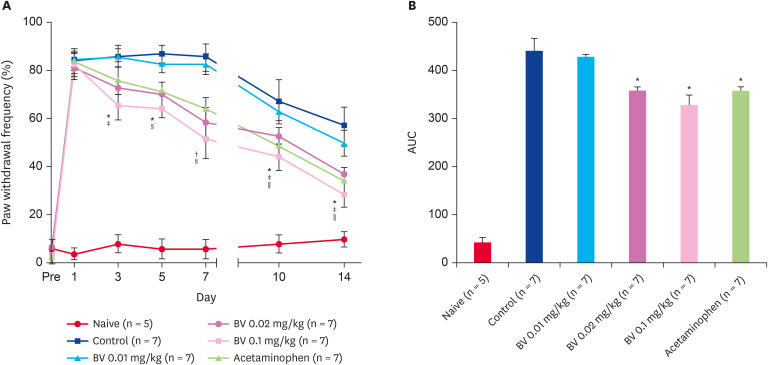
Hot water scalding burn injuries produced dramatic mechanical allodynia, as shown in Fig. 1A. Burn-induced mechanical allodynia peaked after induction, which plateaued by day 7 and began to decrease thereafter. Repeated BV stimulation at doses of 0.02 and 0.1 mg/kg significantly reduced the increase in mechanical allodynia from day 3 after injection. In addition, the acetaminophen administration group, as a positive control, also showed a significant decrease in mechanical allodynia from 10 days after injection. On the other hand, the lowest dose used in this study, 0.01 mg/kg BV, did not show antinociceptive effects (Fig. 1A and B).
Fig. 1. Antinociceptive effect of subcutaneously BV stimulation on burn-induced mechanical allodynia. (A) Scalding burn-induced pain response significantly attenuated by repetitive BV administration over time. (B) The area under curve data also showed the same tendency.
*p < 0.05 BV 0.02 mg/kg vs. Control; †p < 0.05 and ‡p < 0.005 BV 0.1 mg/kg vs. Control; §p < 0.05 Acetaminophen vs. Control; ∥p < 0.05 compared to the control group.
BV, bee venom; AUC, area under the curve.
Effects of BV stimulation on burn-induced locomotion parameter changes
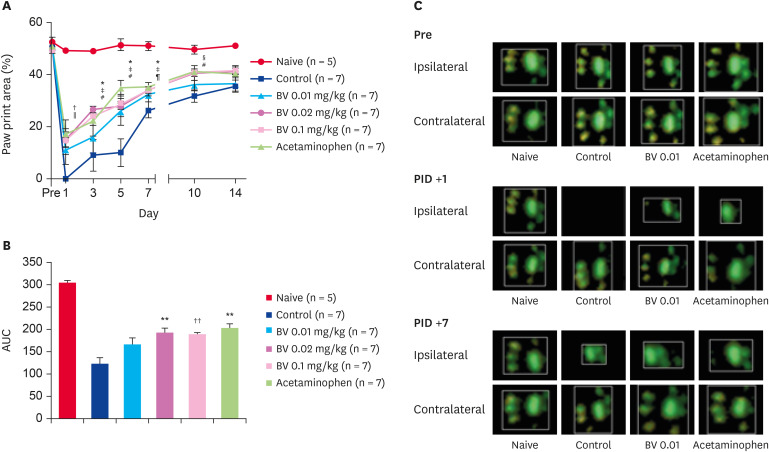
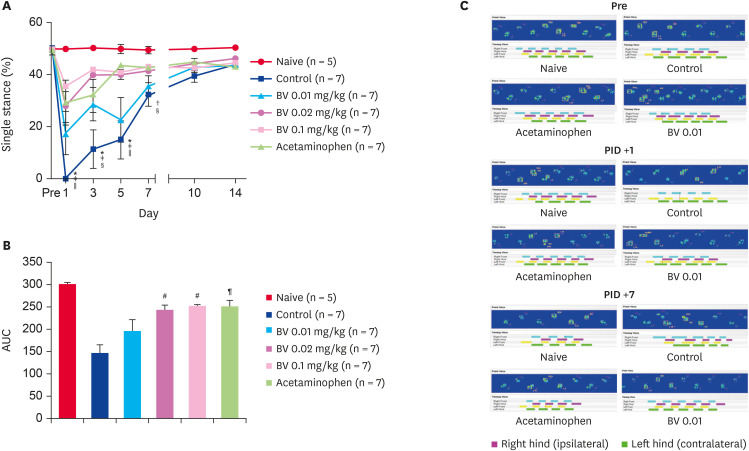
In the paw print area, scalding burns caused a decrease in the ipsilateral paw print area to 0% while walking in the control group administered the BV vehicle. Repeated BV stimulation at doses of 0.02 and 0.1 mg/kg gradually recovered the paw print area one day after injection. Moreover, the acetaminophen administration group used as a positive control also showed significant recovery in the paw print area 3 days after injection. On the other hand, the lowest dose used in this study, 0.01 mg/kg of BV, did not show a print area recovery effect (Fig. 2). Similar to the paw print area, scalding burns also caused a decrease in the single ipsilateral stance to 0% during the walk in the control group, which had been administered the BV vehicle. Repeated BV stimulation at doses of 0.02 and 0.1 mg/kg and i.p. acetaminophen administration gradually recovered the single stance from one day after treatment. The lowest dose of BV stimulation (0.01 mg/kg) did not affect the print area in a single stance (Fig. 3).
Fig. 2. Recovery effect of subcutaneously BV stimulation on burn-induced paw print area. (A) Scalding burn-induced reduction of paw print area significantly recovered by repetitive BV administration over time. (B) The area under curve data also showed the same tendency. (C) The difference in the actual print area shown in the ipsilateral paw over time.
*p < 0.05 and †p < 0.01 BV 0.02 mg/kg vs. Control; ‡p < 0.05, §p < 0.01 and ∥p <0.001 BV 0.1 mg/kg vs. Control; ¶p < 0.05, #p < 0.01 Acetaminophen vs. Control; **p < 0.01 and ††p < 0.001 compared with control group.
BV, bee venom; AUC, area under the curve; PID, post-induction day.
Fig. 3. Recovery effect of subcutaneously BV stimulation on burn-induced single stance. (A) Scalding burn-induced reduction of single stance significantly recovered by repetitive BV administration over time. (B) The area under curve data also showed the same tendency. (C) The difference of actual single stance shown in ipsilateral paw over time. The length of each colored bar represents the time the corresponding paw touched the ground.
*p < 0.01 BV 0.02 mg/kg vs. Control; †p < 0.05 and ‡p < 0.001 BV 0.1 mg/kg vs. Control; §p < 0.05 and ∥p < 0.01 Acetaminophen vs. Control; ¶p < 0.01 and #p < 0.001 compared with control group.
BV, bee venom; AUC, area under the curve; PID, post-induction day.
Effect of BV stimulation on the burn-induced degree of tissue damage
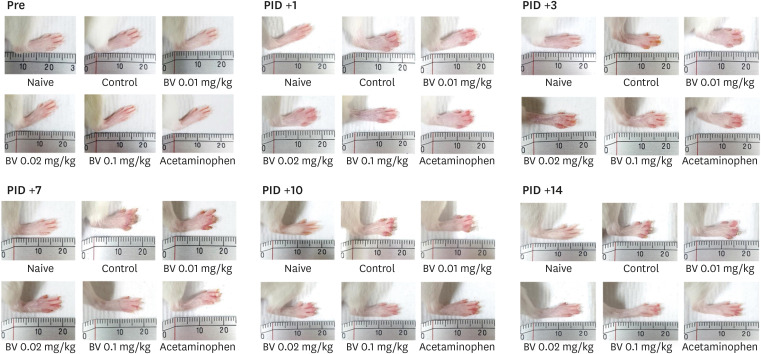
The effects of BV stimulation on the degree of tissue damage degree caused by burns were examined by taking images of the affected area of the ipsilateral paw after inducing the burn. Damage to the ipsilateral paw was produced from one day after the burn induction. On the other hand, the repeated BV treatments of 0.02 and 0.01 mg/kg doses and acetaminophen administration reduced the tissue damage significantly over time. As shown in the figure, on the seventh day after burn induction, the difference in tissue damage between the group treated with BV and the control group was the greatest (Fig. 4).
Fig. 4. Protection effect of subcutaneously BV stimulation on burn-induced tissue damage. After burn induction, significant tissue damage was observed, which increase gradually over time. The effective doses of subcutaneous BV stimulation used in this study protected tissue damage.
BV, bee venom; PID, post-induction day.
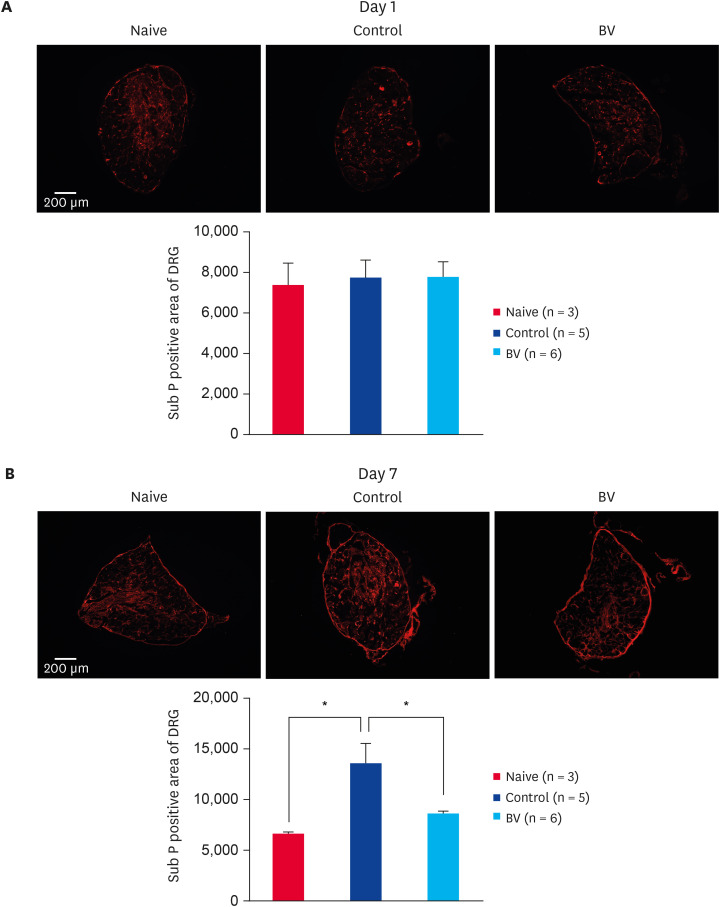
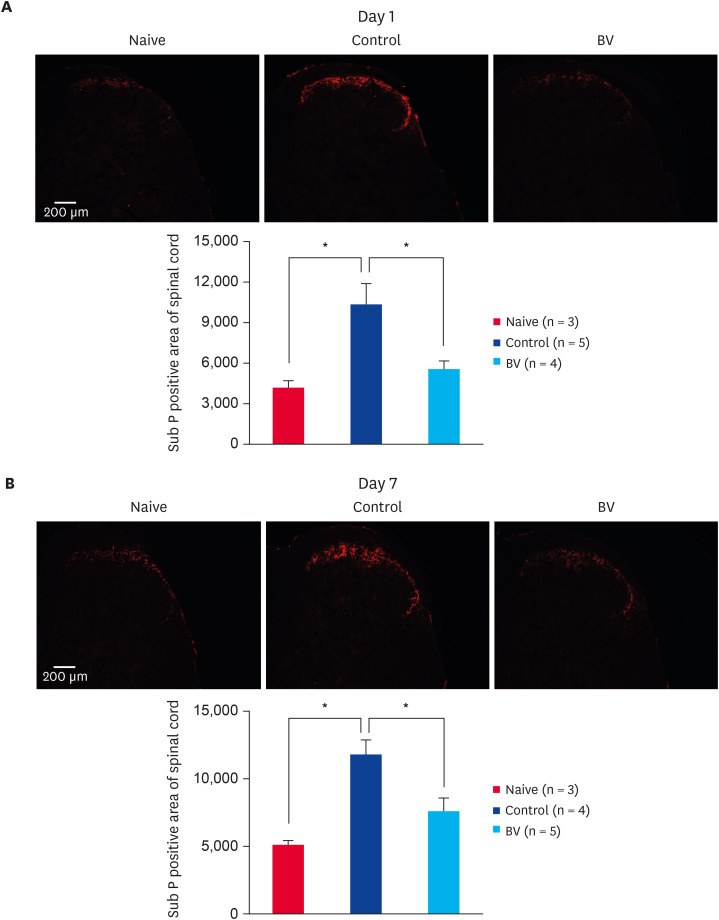
Effects of BV stimulation on burn-induced changes of Sub P in DRG and spinal cord
Immunofluorescence staining one day after inducing the scalding burn revealed no changes in the expression of Sub P in the ipsilateral DRG (Fig. 5A). On the other hand, at 7 days after burn induction, the expression of Sub P was increased significantly in the control group. Repeated BV stimulation at a dose of 0.1 mg/kg reduced the increase in Sub P expression in DRG significantly (Fig. 5B). Moreover, in the case of the spinal cord, the level of Sub P expression was increased dramatically in the dorsal spinal area at 1 (Fig. 6A) and 7 (Fig. 6B) days of after-burn induction. Repeated BV stimulation reduced the increase in Sub P expression in the spinal cord significantly (Fig. 6).
Fig. 5. Inhibitory effect of subcutaneously BV stimulation on Sub P expression in peripheral nervous systems. (A) Scalding burn-induced expression of Sub P in DRG has no changes one day after burn induction. (B) Scalding burn-induced increase in Sub P expression in the DRG was significantly suppressed by repetitive BV administration 7 days after burn induction.
BV, bee venom; Sub P, substance P; DRG, dorsal root ganglion.
*p < 0.001 compared to the control group.
Fig. 6. Inhibitory effect of subcutaneously BV stimulation on Sub P expression in central nervous systems. (A) Scalding burn-induced increase of Sub P expression in the spinal cord was significantly suppressed by the BV administration since 1 day after burn-induction. (B) Scalding burn-induced increase of Sub P expression in the spinal cord dorsal area was significantly suppressed by the repetitive BV administration for 7 days.
BV, bee venom; Sub P, substance P.
*p < 0.001 compared to the control group.
DISCUSSION
Previous studies have reported the antinociceptive effects of BV in various pain models, such as arthritis [7], chemotherapy-induced peripheral neuropathy [21], and chronic constriction injury models [22]. On the other hand, repetitive subcutaneous BV stimulation inhibited the pain mechanism caused by scalding burns. First, repetitive stimulation of BV on the ipsilateral knee area reduced the burn-induced nociceptive behavior in the mechanical allodynia test significantly, and the recovery of gait parameters in catwalk gait analysis supports the antinociceptive effect of treatment. In addition, BV stimulation reduced significantly the degree of tissue damage that occurs over time after the burn. Furthermore, the BV treatment reduced the burn-induced expression of Sub P in the DRG and spinal cord significantly. These results suggest that local BV stimulation has central and peripheral effects.
Scalding burns are thermal burns caused by heated liquids, such as boiling water or steam. In most cases, first- or second-degree burns are encountered, but prolonged contact with heat sources can cause third-degree burns. Typically, naked skin immersed in water at 60°C can cause burns within 3 seconds, and at 57°C, it takes 10 sec and 1.5 to 2 min at 52°C hot water [23]. The burn model used in this study was exposed to hot water at 65°C for 3 sec, resulting in tissue damage, including the common symptoms of burn injuries, such as redness, skin peeling, edema, and pain. Higher doses (0.02 and 0.1 mg/kg) of BV reduced the level of tissue damage significantly over time; the differences were most pronounced on the seventh day after the induction of burns.
In many injuries, including tissue damage, peripheral Sub P acts as a trigger to initiate the cytokines associated with inflammation, which is involved in all processes related to the nociceptive pathways via the neurokinin receptors [24]. In addition, pain intolerance after an inflammatory state has a significant effect on peripheral sensitization; other mechanisms are also involved. The Sub P-neurokinin receptor signal in the periphery and spinal cord acts as a co-transmitter that induces long-term changes in spinal excitation, which are collectively known as ‘central sensitization’ [25,26]. Certain pain relief mechanisms of BV are still unclear, but several mechanisms have been proposed. Previous studies reported the following mechanisms: activation of the spine α2-adrenoceptor [22,27], reduction of c-fos expression [28], and blocking of the N-methyl-d-aspartate receptor [29]. The present study showed that BV stimulation reduced the expression of Sub P significantly in DRG and spinal dorsal horn. In addition, Sub P was inhibited, suggesting an antinociceptive mechanism, leading to a decrease in neurokinin-1 signaling. Interestingly, the immunofluorescence results obtained one day after the burn injury showed no change in the expression of Sub P in the DRG. One day after the burn injury, the unchanged DRG expression suggests the significant transport of extrinsic Sub P to the peripheral (DRG) after its release from the periphery in response to the initial burn injury.
In conclusion, repetitive subcutaneous BV stimulation has potent antinociceptive effects in a scalding burn-induced pain mice model. This antinociceptive mechanism may inhibit the pain-producing pathways by suppressing the peripheral and central Sub P expression. These results are expected to improve the understanding of the anti-pain mechanism of BV stimulation and suggests that BV treatment may be effective in controlling pain for patients suffering from burn injuries.
Footnotes
Funding: This research was supported by the Chungnam National University and the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2018R1D1A1B07051069).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kim HW
- Data curation: Kang DW, Choi JG, Kim J.
- Formal analysis: Kang DW.
- Funding acquisition: Kim HW.
- Investigation: Kang DW.
- Methodology: Kang DW, Choi JG, Kim J.
- Project administration: Kim HW.
- Supervision: Park JB, Lee JH, Kim HW.
- Validation: Choi JG, Kim J.
- Visualization: Kang DW.
- Writing - original draft: Kang DW.
- Writing - review & editing: Park JB, Lee JH, Kim HW.
References
- 1.Mock C, Peck M, Krug E, Haberal M. Confronting the global burden of burns: a WHO plan and a challenge. Burns. 2009;35(5):615–617. doi: 10.1016/j.burns.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen NL, Ngo MD. Profile and outcome of burn injuries amongst preschool children in a developing country. Ann Burns Fire Disasters. 2019;32(4):267–271. [PMC free article] [PubMed] [Google Scholar]
- 3.Zou K, Wynn PM, Miller P, Hindmarch P, Majsak-Newman G, Young B, et al. Preventing childhood scalds within the home: overview of systematic reviews and a systematic review of primary studies. Burns. 2015;41(5):907–924. doi: 10.1016/j.burns.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Kim SG, Kim IS, Lee HD. Standardization of the manufacturing process of bee venom pharmacopuncture containing melittin as the active ingredient. Evid Based Complement Alternat Med. 2018;2018:2353280. doi: 10.1155/2018/2353280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee G, Bae H. Bee venom phospholipase A2: yesterday's enemy becomes today's friend. Toxins (Basel) 2016;8(2):48. doi: 10.3390/toxins8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115(2):246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Kwon YB, Lee JD, Lee HJ, Han HJ, Mar WC, Kang SK, et al. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90(3):271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SY, Roh DH, Kwon YB, Kim HW, Seo HS, Han HJ, et al. Acupoint stimulation with diluted bee venom (apipuncture) potentiates the analgesic effect of intrathecal clonidine in the rodent formalin test and in a neuropathic pain model. J Pain. 2009;10(3):253–263. doi: 10.1016/j.jpain.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Donkin JJ, Turner RJ, Hassan I, Vink R. Substance P in traumatic brain injury. Prog Brain Res. 2007;161:97–109. doi: 10.1016/S0079-6123(06)61007-8. [DOI] [PubMed] [Google Scholar]
- 10.Palma C, Manzini S. Substance P induces secretion of immunomodulatory cytokines by human astrocytoma cells. J Neuroimmunol. 1998;81(1-2):127–137. doi: 10.1016/s0165-5728(97)00167-7. [DOI] [PubMed] [Google Scholar]
- 11.Rameshwar P, Gascon P, Ganea D. Immunoregulatory effects of neuropeptides. Stimulation of interleukin-2 production by substance P. J Neuroimmunol. 1992;37(1-2):65–74. doi: 10.1016/0165-5728(92)90156-f. [DOI] [PubMed] [Google Scholar]
- 12.De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 13.Irie Y, Tsubota M, Maeda M, Hiramoto S, Sekiguchi F, Ishikura H, et al. HMGB1 and its membrane receptors as therapeutic targets in an intravesical substance P-induced bladder pain syndrome mouse model. J Pharmacol Sci. 2020;143(2):112–116. doi: 10.1016/j.jphs.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Qin X, Lu X, Jiang J. Effects of inhibiting the PI3K/Akt/mTOR signaling pathway on the pain of sciatic endometriosis in a rat model. Can J Physiol Pharmacol. 2019;97(10):963–970. doi: 10.1139/cjpp-2019-0156. [DOI] [PubMed] [Google Scholar]
- 15.Santos FM, Silva JT, Rocha IR, Martins DO, Chacur M. Non-pharmacological treatment affects neuropeptide expression in neuropathic pain model. Brain Res. 2018;1687:60–65. doi: 10.1016/j.brainres.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Zucoloto AZ, Manchope MF, Borghi SM, Dos Santos TS, Fattori V, Badaro-Garcia S, et al. Probucol ameliorates Complete Freund's Adjuvant-induced hyperalgesia by targeting peripheral and spinal cord inflammation. Inflammation. 2019;42(4):1474–1490. doi: 10.1007/s10753-019-01011-3. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto S, Nakamura J, Ohtori S, Orita S, Nakajima T, Omae T, et al. Pain-related behavior and the characteristics of dorsal-root ganglia in a rat model of hip osteoarthritis induced by mono-iodoacetate. J Orthop Res. 2017;35(7):1424–1430. doi: 10.1002/jor.23395. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CY, Sheu ML, Cheng FC, Chen CJ, Su HL, Sheehan J, et al. Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system. J Neurosurg. 2014;120(1):250–262. doi: 10.3171/2013.9.JNS13353. [DOI] [PubMed] [Google Scholar]
- 19.Huehnchen P, Boehmerle W, Endres M. Assessment of paclitaxel induced sensory polyneuropathy with “Catwalk” automated gait analysis in mice. PLoS One. 2013;8(10):e76772. doi: 10.1371/journal.pone.0076772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrinten DH, Hamers FF. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102(1-2):203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Lee Y, Kim W, Lee K, Bae H, Kim SK. Analgesic effects of bee venom derived phospholipase A2 in a mouse model of oxaliplatin-induced neuropathic pain. Toxins (Basel) 2015;7(7):2422–2434. doi: 10.3390/toxins7072422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SY, Roh DH, Park JH, Lee HJ, Lee JH. Activation of spinal α2-adrenoceptors using diluted bee venom stimulation reduces cold allodynia in neuropathic pain rats. Evid Based Complement Alternat Med. 2012;2012:784713. doi: 10.1155/2012/784713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry L. Mosby's dictionary of medicine, nursing & health professions - seventh edition Mosby Mosby's dictionary of medicine, nursing & health professions - Seventh edition 2272 Mosby 9780723433934 0723433933. Nurs Stand. 2006;20(22):36. doi: 10.7748/ns2006.02.20.22.36.b426. [DOI] [PubMed] [Google Scholar]
- 24.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87(1):3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Li WW, Guo TZ, Shi X, Sun Y, Wei T, Clark DJ, et al. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience. 2015;310:73–90. doi: 10.1016/j.neuroscience.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, et al. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009;13(3):253–262. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Jeon C, Lee JH, Jang JU, Quan FS, Lee K, et al. Suppressive effects of bee venom acupuncture on paclitaxel-induced neuropathic pain in rats: mediation by spinal α2-adrenergic receptor. Toxins (Basel) 2017;9(11):351. doi: 10.3390/toxins9110351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu SX, Wang W, Wang YY, Ni TS, Li YQ, Yew DT. C-fos antisense oligodeoxynucleotide decreases subcutaneous bee venom injection-induced nociceptive behavior and fos expression in the rat. Neurosignals. 2002;11(4):224–230. doi: 10.1159/000065430. [DOI] [PubMed] [Google Scholar]
- 29.Kang SY, Roh DH, Yoon SY, Moon JY, Kim HW, Lee HJ, et al. Repetitive treatment with diluted bee venom reduces neuropathic pain via potentiation of locus coeruleus noradrenergic neuronal activity and modulation of spinal NR1 phosphorylation in rats. J Pain. 2012;13(2):155–166. doi: 10.1016/j.jpain.2011.10.012. [DOI] [PubMed] [Google Scholar]