Abstract
This study presents a comprehensive systematic review and meta-analysis of randomized controlled trials (RCTs) on Chlorella vulgaris (C. vulgaris) supplementation and liver function biomarkers. Pertinent studies were identified using Scopus, ISI Web of Science, PubMed, and Cochrane library databases up to August 2020. Mean differences were pooled using a random-effects model. Pooling 7 RCTs together showed that C. vulgaris supplementation led to a significant reduction of serum aspartate aminotransferase (AST) levels (weighted mean difference [WMD], −9.15 U/L; 95% confidence interval [CI], −16.09, −2.21), but not alanine aminotransferase (ALT) or alkaline phosphatase (ALP) levels compared to the placebo consumption. Subgroup-analysis indicated that C. vulgaris supplementation had more effect on AST decreasing among non-alcoholic fatty liver disease patients (WMD, −16.42 U/L; 95% CI, −29.75, −3.09) than others. Furthermore, subgroup analysis based on kind of compression showed that C. vulgaris supplementation significantly decreased ALT levels (WMD, −4.65 U/L; 95% CI, −8.88, −0.42) compared with the placebo, but not metformin consumption. It seems that C. vulgaris supplementation mainly affects AST levels rather than ALT and ALP levels, however, as mentioned the effect of C. vulgaris on those enzymes might be context-dependent. Therefore, further investigations with a large number of patients as well as on different disorders are necessary and can provide more definitive evidence.
Keywords: Chlorella vulgaris, Liver, Systematic review, Meta-analysis
INTRODUCTION
Chlorella vulgaris (C. vulgaris) is a genus of small spherical unicellular green algae that naturally exist in freshwater environments. Its name is derived from the Greek word “chloros” meaning green and the Latin suffix “-ella” meaning small [1]. Chlorella belongs to the phylum Chlorophyta and the class Trebouxiophyceae. Among different species of this genus, C. vulgaris is the most known and studied one [2]. C. vulgaris has traditionally been used as a food source only in Japan and Taiwan. But now, it has been marketed as a nutraceutical product in different forms such as tablets, capsules, powders, and extracts all over the world [3].
C. vulgaris provides 42%–58% proteins, 5%–40% lipids, and 12%–55% carbohydrates per its dry weight. Interestingly, this microscopic alga contains all essential amino acids as well as all essential fatty acids and is a good source of dietary fiber [4]. It also has many kinds of vitamins and minerals such as thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, biotin, folic acid, cobalamin, ascorbic acid, retinol, tocopherols, sodium, potassium, calcium, magnesium, phosphorous, copper, zinc, manganese, iodine, and iron [5,6,7]. Also, this functional food has beneficial pigments including chlorophyll, beta-carotene, astaxanthin, canthaxanthin, violaxanthin, lutein, and pheophytin [4].
Due to the aforementioned nutritional composition, C. vulgaris has been reported to have antioxidant, anti-inflammatory, and immunomodulatory properties [8,9,10]. This alga has shown favorable effects on different health conditions, such as hyperlipidemia, hyperglycemia, obesity, depression, anxiety, and cancer; therefore, it is regarded as a multifunctional dietary supplement [11]. Importantly, C. vulgaris supplementation not only has health-promoting benefits but also has a good record of safety and even aids detoxification. These features have made researchers investigate its possibly protective effects on hepatocytes, especially in patients suffering from non-alcoholic fatty liver disease (NAFLD) [12]. However, the effectiveness of this bioactive compound in humans is not fully elucidated. It has been well established that, the hepatocytes are in the frontline against oxidative stress, and thus may be affected more [13]. In this regard, the serum levels of different enzymes including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) have been correlated with the extent of oxidative damage [14,15].
Although growing evidence from animal and human studies suggests that C. vulgaris can be a promising hepatoprotective agent, the obtained results are neither consistent nor conclusive [16,17,18,19]. It is expected that dietary supplements like C. vulgaris would decrease the plasma levels of these enzymes, corresponding to their antioxidant properties. Considering the above-mentioned notes, the current literature review and meta-analysis based on clinical trial studies was performed to investigate whether C. vulgaris supplementation can alter the aforementioned liver enzymes in the context of different human disorders. Therefore, we aimed to conduct a systematic review and meta-analysis of published randomized controlled trials (RCTs) to assess the effect of C. vulgaris supplementation on liver enzymes as indicators of hepatocellular function. To the best of our knowledge, the present study is the first one of its kind.
MATERIALS AND METHODS
Literature search and selection
Current systematic review and meta-analysis were conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement [20]. A comprehensive and systematic literature search was carried out through the Scopus, the Cochrane library, PubMed, and Web of Science databases until 5 August 2020. In the search strategy, we used medical subject heading (MeSHs), abstract, and keywords but the language and date restrictions were not used. Systematic search was performed using the following search terms (((“Chlorella”[Mesh] OR “Chlorella”[tiab] OR “Chlorellas”[tiab]) AND ((“Non-alcoholic Fatty Liver Disease”[Mesh] OR “Non-alcoholic Fatty Liver Disease”[tiab] OR “Nonalcoholic Fatty Liver Disease”[tiab] OR “NAFLD”[tiab] OR “Nonalcoholic Fatty Liver Disease”[tiab] OR “Nonalcoholic Fatty Liver”[tiab] OR “Nonalcoholic Fatty Livers”[tiab] OR “Nonalcoholic Fatty Livers”[tiab] OR “Nonalcoholic Steatohepatitis”[tiab] OR “Nonalcoholic Steatohepatitis”[tiab])) OR (“Alanine Transaminase”[Mesh] OR “Alanine Transaminase”[tiab] OR “Glutamic-Alanine Transaminase”[tiab] OR “Alanine Aminotransferase”[tiab] OR “Glutamic-Pyruvic Transaminase”[tiab] OR “SGPT”[tiab])) OR (“Aspartate Aminotransferases”[Mesh] OR “Aspartate Aminotransferases”[tiab] OR “Aspartate Apoaminotransferase”[tiab] OR “Aspartate Transaminase”[tiab] OR “Glutamic-Oxaloacetic Transaminase”[tiab] OR “Glutamate Aspartate Transaminase”[tiab] OR “SGOT”[tiab])) OR (“Alkaline Phosphatase”[Mesh] OR “Alkaline Phosphatase”[tiab])). Electronic database searches were completed along with reference lists and citation hand searches. The research process was conducted by 2 authors (Sajjad Moradi and Mohammad Hosein Farzaei) independently and in duplicate. Any disagreements in this regard were resolved through discussion with the third researcher (Niloofar Hemati).
Eligibility criteria
Two investigators selected eligible articles separately by reading titles, abstracts and whenever required the full-text of the publications. All human RCTs (either parallel or cross-over designs) which reported the efficacy of C. vulgaris supplementation on liver enzymes including ALT, AST, and ALP were considered. Following studies were excluded: 1) RCTs with treatment duration less than 2 weeks, 2) studies without any comparing control group. To keep away from overlapping, we included studies with larger participants. Disagreements regarding the study selection process were resolved by face to face discussion. Description of population, intervention, comparator, and outcome is displayed in Table 1.
Table 1. Description of population, intervention, comparator and outcome.
| Characteristics | Description |
|---|---|
| Population | Healthy and patients subjects |
| Intervention | Chlorella vulgaris |
| Comparison | Placebo |
| Outcome | Aspartate aminotransferase, alanine aminotransferase or alkaline phosphatase |
Data extraction
The following data were extracted from the full-text of included studies using a pre-designed abstraction form: first author's specification, publication year, location of the study, study design and blinding, total sample size, patient characteristics (age, gender, and diseases) type and the dose of the intervention of Chlorella and placebo, study duration and the ultimate result of ALT, AST, and ALP comparisons. When the data were reported at multiple measurements, only the outcomes at the end of the intervention were included in the analysis. In cases of lack of relevant data, we contacted the corresponding authors via e-mail to get their help. The whole process of data extraction was undertaken independently by 2 investigators (Sajjad Moradi and Mohammad Hosein Farzaei) to minimize potential errors. If there was a disagreement, it was resolved by consensus.
Quality assessment of studies
We had used Cochrane Collaboration's tools for quality assessment of studies [21]. The tool separates a judgment about the risk of bias from a description of the support for that judgment, for a series of items covering different domains of bias. The method of quality assessment was mentioned in our previous study [22]. The quality assessment results for each article are shown in Table 2.
Table 2. Risk of bias assessment for included randomized controlled clinical trials.
| Domain | Lee et al. [25] | Panahi et al. [7] | Miyazawa et al. [27] | Ebrahimi-Mameghani et al. [17] | Talebi Pour et al. [28] | Chitsaz et al. [30] | Ebrahimi-Mameghani et al. [19] | Vakili et al. [29] |
|---|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | + | + | + | + | + | + | + | + |
| Allocation concealment (selection bias) | ? | ? | + | ? | ? | + | ? | + |
| Blinding of participants and personnel (performance bias) | + | − | + | + | − | + | + | + |
| Blinding of outcome assessment (detection bias) | + | − | + | + | − | − | − | − |
| Incomplete outcome data (attrition bias) | + | + | + | + | + | − | + | − |
| Selective reporting (reporting bias) | ? | − | ? | ? | − | ? | ? | ? |
| Score | 4 | 2 | 5 | 4 | 2 | 3 | 4 | 3 |
| Overall quality | Good | Fair | Good | Good | Fair | Good | Good | Good |
Meta-analysis of data
To analyze the effect size for ALT, AST, and ALP, the mean change and its standard deviation for intervention and control groups as the comparison groups were extracted. A random-effect model was used to calculate weighted mean differences (WMDs) with 95% confidence intervals (CIs). Between-study heterogeneity was tested by Cochran's Q test and quantified by I2 statistic. A subgroup analysis based on the health status (NAFLD patient and others) and kind of compression (metformin and placebo) was performed to detect potential sources of heterogeneity. Between subgroup, heterogeneity was assessed using a fixed-effect model. Sensitivity analysis was conducted by removing each study one by one and recalculating the pooled evaluations. Begg's rank correlation test and Egger's regression asymmetry test were performed for detecting potential publication bias. Statistical analysis was conducted using STATA, version 11.2 (Stata Corp., College Station, TX, USA). The statistical significant value was defined as p values < 0.05.
RESULTS
Selection and identification of studies
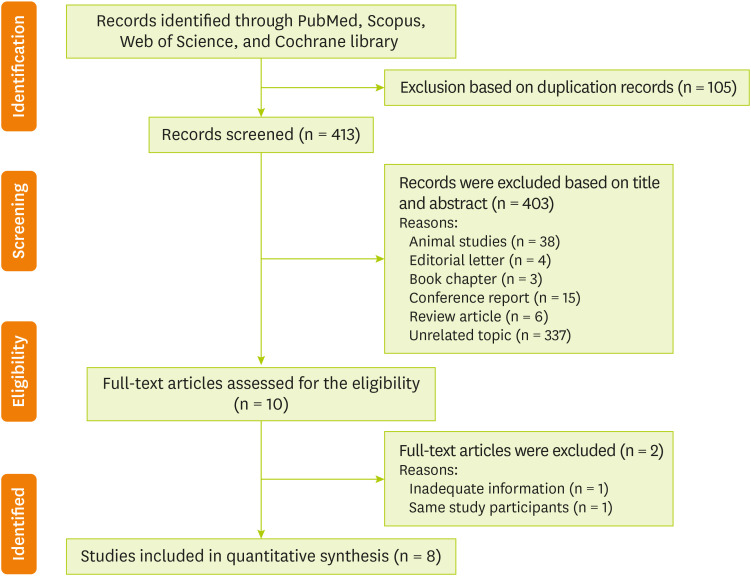
Out of the initial 518 articles that were obtained by electronic and hand search (105 duplicates) 403 were excluded because according to our inclusion criteria, they were unrelated to the present meta-analysis. After reading the full text of the remaining 10 papers [7,17,23,24,25,26,27,28,29,30], 2 studies did not meet the desired criteria [23,24]. In total, 8 eligible RCTs with 8 treatment arms were included in our final analysis [7,17,25,26,27,28,29,30]. A flow chart describing the systematic search and study selection process is shown in Figure 1.
Figure 1. PRISMA flowchart describing the study's systematic literature search and study selection.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Characteristics of studies
The main characteristics of the included studies in the present meta-analysis are described in Table 3. Overall, 8 effect sizes were extracted from 8 RCTs which included a total of 432 subjects, out of which 211 subjects were in the C. vulgaris group and 221 belonged to the control group. The mean age of participants in these studies ranged from 20 to 57 years. Five of this RCTs were conducted on NAFLD patient [11,17,26,28,30] and other conducted on metabolic syndrome patient [25], type 2 diabetic patient [29] and healthy participants [27]. All the RCTs used a parallel study design. These studies were published between the years 2012 and 2019. The RCTs were conducted in Iran [11,17,26,28,29,30], Japan, [27] and Taiwan [25]. The dose of C. vulgaris ranged from 300 mg to 8 g/day and 7 studies of included studies were used alone C. vulgaris as an intervention [11,17,26,27,28,29,30]. Just one study was used C. vulgaris plus another plant extractive as an intervention [25]. The duration of intervention also varied from 8 to 12 weeks. According to Cochrane scores, except 2 studies [11,28], other studies were classified as high-quality studies (score = 3) [17,25,26,27,29,30]. The result of the quality assessment is reported in Table 2.
Table 3. Main characteristics of included studies.
| Author | Year | Country | Sample size (intervention/placebo) | Target population | Mean age (yr) | Mean BMI | RCT design (blinding) | Duration (wk) | Dose of Chlorella | Comparison | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al. [25] | 2012 | Taiwan | 106 (54/52) | Metabolic syndrome patient | 52.00 ± 10.00 | 27.20 ± 3.50 | Parallel (double) | 12 | 1.5 g/d plus other plant extractive | Placebo (NR) | There were no significant differences in the changes in liver functions, including serum AST, ALT, Alk-P |
| Panahi et al. [7] | 2012 | Iran | 54 (21/33) | NAFLD patient | 51.00 ± 7.94 | 31.22 ± 4.75 | Parallel (no) | 12 | 1.5 g/d | Placebo (metformin) | Serum ALT, AST, Alk-P were reduced only in the Chlorella group but not placebo group |
| Miyazawa et al. [27] | 2013 | Japan | 12 (6/6) | Healthy | 57.00 ± 7.00 | 23.50 ± 3.40 | Parallel (double) | 8 | 8 g/d | Placebo (NR) | In the Chlorella supplemented group, serum Alk-P after a total of 2 months of treatment were significantly lower than the placebo group |
| Ebrahimi-Mameghani et al. [17] | 2013 | Iran | 70 (35/35) | NAFLD patient | 20–50 | > 30 | Parallel (double) | 8 | 300 mg/d | Placebo (NR) | The differences Alk-P between the 2 groups were statistically significant. A significant reduction in serum Alk-P was observed but not AST or ALT |
| Talebi Pour et al. [28] | 2015 | Iran | 60 (30/30) | NAFLD patient | 38.00 ± 10.00 | 27.00 ± 4.00 | Parallel (no) | 8 | 1.5 g/d | Placebo (metformin and vitamin E) | Serum ALT and AST were significantly reduced after 8 weeks Chlorella supplementation in comparison with placebo group |
| Chitsaz et al. [30] | 2016 | Iran | 40 (20/20) | NAFLD patient | 42.80 ± 8.58 | 27.03 ± 1.68 | Parallel (no) | 8 | 1 g/d | Placebo (NR) | Mean of changes of ALT was significant but not for AST or Alk-P in comparison with placebo group |
| Ebrahimi-Mameghani et al. [19] (a subset of 2013 study) | 2017 | Iran | 70 (35/35) | NAFLD patient | 20–50 | > 30 | Parallel (double) | 8 | 300 mg/d | Placebo (NR) | Serum concentrations of ALT and AST decreased significantly after intervention in Chlorella vulgaris treated group, while no change in placebo-treated group was occurred |
| Vakili et al. [29] | 2019 | Iran | 20 (10/10) | Type 2 diabetic patient | 56.80 ± 4.21 | > 25 | Parallel (no) | 8 | 600 mg/d | Placebo (NR) | A significant difference was observed among control and Chlorella group after 8 weeks training on the enzymes AST, ALT, Alk-P |
BMI, body mass index; RCT, randomized controlled trial; NR, not reported; AST, aspartate transaminase; ALT, alanine transaminase; Alk-P: alkaline phosphatase; NAFLD, non-alcoholic fatty liver disease.
Effects of C. vulgaris supplementation on liver enzymes
Effects of C. vulgaris on AST
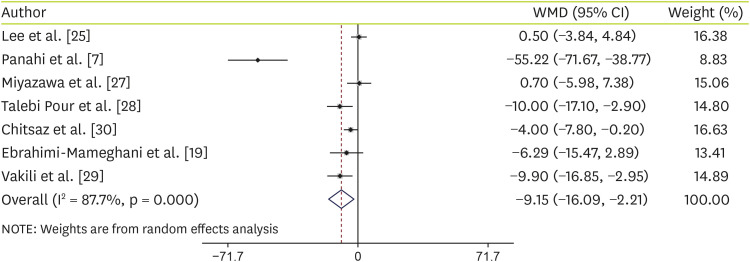
Forest plots summarizing the efficacy of C. vulgaris supplementation on AST are shown in Figure 2. Pooling 7 RCTs (8 treatment arms) together showed that C. vulgaris supplementation led to a significant reduction of serum AST levels (WMD, −9.15 U/L; 95% CI, −16.09, −2.21) (Figure 2). High heterogeneity was observed in the studies (I2 = 87.7%; p < 0.001). For determining the sources of heterogeneity, subgroup analysis was conducted according to the health status (NAFLD patient and others) and kind of compression (metformin and placebo). Subgroup-analysis indicated that Chlorella supplementation had more effect on AST decreasing among NAFLD patients (WMD, −16.42 U/L; 95% CI, −29.75, −3.09) than others including healthy people, patients with type 2 diabetes and metabolic syndrome participants (WMD, −2.58 U/L; 95% CI, −8.90, 3.75). However, there was no significant differences between metformin (WMD, −31.98 U/L; 95% CI, −76.27, 12.32) or placebo (WMD, −3.25 U/L; 95% CI, −6.92, 0.42) compressions (Table 4).
Figure 2. Forest plot of the comparison of the effects of Chlorella versus placebo on AST.
WMD, weighted mean difference; CI, confidence interval; AST, aspartate transaminase.
Table 4. Effect of Chlorella supplementation on other liver enzymes.
| Liver enzymes | Subgroup | No. of studies | Effect size* | 95% CI | I2 (%) | p for heterogeneity | |
|---|---|---|---|---|---|---|---|
| AST | Health status | ||||||
| NAFLD patient | 4 | −16.42 | −29.75, −3.09 | 91.7 | < 0.001 | ||
| Others | 3 | −2.58 | −8.90, 3.75 | 70.7 | 0.030 | ||
| Kind of compression | |||||||
| Metformin | 2 | −31.98 | −76.27, 12.32 | 95.9 | < 0.001 | ||
| Placebo | 5 | −3.25 | −6.92, 0.42 | 51.3 | 0.080 | ||
| ALT | Health status | ||||||
| NAFLD patient | 4 | 9.26 | −7.01, 25.53 | 93.4 | < 0.001 | ||
| Others | 3 | −4.41 | −11.45, 2.64 | 61.2 | 0.070 | ||
| Kind of compression | |||||||
| Metformin | 2 | 32.16 | −32.19, 96.51 | 96.9 | < 0.001 | ||
| Placebo | 5 | −4.65 | −8.88, −0.42 | 51.0 | 0.080 | ||
| ALP | Health status | ||||||
| NAFLD patient | 3 | −4.06 | −18.60, 10.47 | 84.2 | < 0.001 | ||
| Others | 3 | −8.95 | −33.46, 15.56 | 73.2 | 0.020 | ||
CI, confidence interval; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; NAFLD, non-alcoholic fatty liver disease.
*Calculated by random-effects model.
Effects of C. vulgaris on ALT
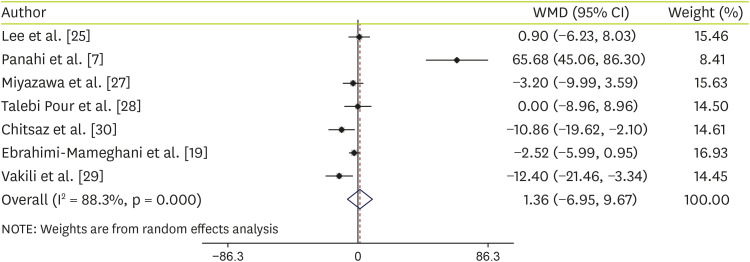
However, our results suggested that C. vulgaris supplementation did not indicate any significant effect on ALT levels (WMD, 1.36 U/L; 95% CI, −6.95, 9.67) (Figure 3). Although high heterogeneity was identified among the studies (I2 = 88.3%, p < 0.001). For determining the sources of heterogeneity, subgroup analysis was conducted according to the health status (NAFLD patient and others) and kind of compression (metformin and placebo). Subgroup analysis based on kind of compression showed that C. vulgaris supplementation significantly decreased ALT levels (WMD, −4.65 U/L; 95% CI, −8.88, −0.42) compared with the placebo, but not Metformin concumption (WMD, 32.16 U/L; 95% CI, −32.19, 96.51) (Table 4). Although, other subgroup analysis based on health status illustrated that C. vulgaris supplementation did not have any effect on serum ALT levels among NAFLD patients (WMD, 9.26 U/L; 95% CI, −7.01, 25.53) and others including healthy people, and patients with type 2 diabetes or metabolic syndrome (WMD, −4.41 U/L; 95% CI, −11.45, 2.64) (Table 4).
Figure 3. Subgroup analysis to assess the effects of Chlorella versus placebo on ALT.
WMD, weighted mean difference; CI, confidence interval; ALT, alanine transaminase.
Effects of C. vulgaris on ALP
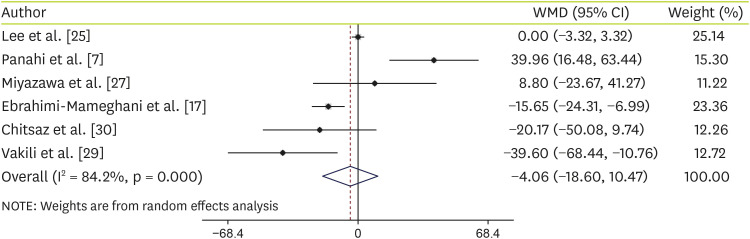
In the same results, our outcomes demonstrated that C. vulgaris supplementation did not have any effect on serum ALP levels (WMD, −4.06 U/L; 95% CI, −18.60, 10.47) (Figure 4). In addition, other subgroup analysis based on health status illustrated that C. vulgaris supplementation did not have any effect on serum ALP levels in NAFLD patients (WMD, −4.06 U/L; 95% CI, −18.60, 10.47) or others including healthy people, and patients with type 2 diabetes and metabolic syndrome (WMD: −8.95 U/L; 95% CI, −33.46, 15.56) (Table 4).
Figure 4. Forest plot of the comparison of the effects of Chlorella versus placebo ALP.
WMD, weighted mean difference; CI, confidence interval; ALP, alkaline phosphatase.
Publication bias and sensitivity analysis
The sensitivity analysis revealed that evaluated overall effect sizes for AST, ALT and ALP were not substantially changed after removing each study. Egger's weighted regression tests and Begg's rank correlation were used to assess the publication bias. The results of Begg's test demonstrated no publication bias for AST (p = 0.09), ALT (p = 0.88) and ALP (p = 0.85). Besides, the outcomes of Egger's test revealed no publication bias for AST (p = 0.06), ALT (p = 0.34) and ALP (p = 0.74).
Side effects
In toxicological studies conducted on animals and humans, no toxic or side effects were reported for C. vulgaris [11]. Included studies did not report any allergic or serious adverse events during administration among patients with NAFLD [11,17,26,28,30], metabolic syndrome [25], type 2 diabetes [29] and healthy participants [27] (dosage intervention from 300 mg/day to 8 g/day). However, rare potential adverse effects for C. vulgaris were reported in some case reports in Chlorella consumer with kidney failure [31] Although, the C. vulgaris supplementation is safe and well-tolerated in routine dosage, it is better to consider an allergy risk assessment before administration.
DISCUSSION
The findings of the present study showed that C. vulgaris could significantly decrease the levels of AST. Interestingly, the most significant changes were observed among patients with NAFLD rather than other diseases. However, according to our results, the consumption of C. vulgaris only slightly decreased both ALT and ALP levels but the differences were not significant different compared to the placebo consumption.
It is obvious that the effect of C. vulgaris on liver enzymes was not the same among patients with different kinds of diseases, and was more probably context-dependent. For instance, C. vulgaris could improve serum levels of AST rather than ALP and ALT in the majority of the patients with NAFLD, but, treatment with C. vulgaris has significantly reduced ALT levels rather than AST in patients with hepatitis C virus (HCV) infection. It is well demonstrated that ALT and AST levels strikingly increased in acute hepatic injuries, and the ALT remained elevated in chronic injuries [32]. Therefore, it might be assumed that in conditions like NAFLD, C. vulgaris partially could improve the hepatic function by decreasing AST levels. However, in other conditions like infection with HCV, the C. vulgaris could results in more promising effects by reducing ALT levels. Moreover, it should be noted that ALT and ALP are found predominantly in the liver, but AST is found in the liver, skeletal muscle, heart, kidneys, the brain as well as red blood cells [33], thus the effect of C. vulgaris on these enzymes must have been context-dependent.
Although the putative mechanisms by which C. vulgaris could decrease plasma levels of liver enzymes are not clearly defined, it is likely that this compound exerts its protective effects at least partially by reducing patients' weight, changing lipid profiles (decreasing both cholesterol and triglyceride levels), improving fasting blood sugar levels through decreasing insulin resistance and probably scavenging free radicals produced as a result of different oxidation processes, and thus protecting the tissue from damage [17,19,30,34].
Previous studies suggested that the C. vulgaris protects liver cells by affecting insulin resistance as a biomarker involved in the onset of NAFLD [19]. The proposed mechanisms are as follows the C. vulgaris supplementation reduces plasma non-esterified fatty acid concentration which increases improvement in glucose homeostasis and noticeable reduction serum glucose concentrations [35,36]. Besides, the C. vulgaris administration induces activation of insulin signaling pathways [37] and subsequently decreases insulin resistance [19]. Furthermore, according to the literature and the results of previous studies, there are other mechanisms by which antioxidant dietary supplements could affect plasma levels of liver enzymes. In this regard, the previous experiences with some antioxidants have demonstrated that the function of both antioxidant enzymatic systems including catalase, superoxide dismutase, NADPH, and glutathione peroxidase (GSH-Px), and non-enzymatic electron receptors such as GSH in deals with oxidative stress are improved by dietary antioxidant supplements and resulted in decreased levels of liver enzymes [38,39,40].
Regardless of the NAFLD, the therapeutic effects of C. vulgaris on liver enzymes have also been investigated in the context of other disorders. Lee et al. [25] have examined the effect of C. vulgaris in complex with some other plant-extractive compounds on lipid profile in subjects with metabolic syndrome. Although, this group found a significant reduction in the levels of serum fasting triglyceride among the subjects that received plant extractives, no significant alterations in the levels of ALT, AST, and ALP were observed between treatment and placebo groups. Miyazawa et al. [27] have also assessed the potential preventive effect of C. vulgaris supplementation on oxidative stress induced by phospholipid hydroperoxide (PLOOH) in erythrocyte membranes. After 2 months of treatment, the erythrocyte PLOOH concentrations were found to be lower than those concentrations before supplementation. Furthermore, they showed that C. vulgaris has no effect on the serum values of ALP in treatment group compared to the controls. Azocar et al. [24] investigated the efficacy of C. vulgaris supplementation in adult patients with HCV infection. The levels of ALT and AST was found to be decreased among the majority of patients who received C. vulgaris, however only the ALT levels was significantly reduced before and after treatment. The results of this study also showed that the HCV viral load was also decreased together with improvement in the levels of AST and ALT. Therefore, C. vulgaris exhibited beneficial effects in patients with HCV infection. Recently, Vakili et al. [29] have examined the effect of C. vulgaris supplementation on the levels of liver enzymes among women with type 2 diabetes. Significant differences were observed in serum AST and ALP levels between patients who received C. vulgaris for 8 weeks and controls.
It is probable that the C. vulgaris has also exerted similar effects among the studies, but further research is required to establish this claim. We propose that future researches should be more focused on:
• Determining the best effective doses of Chlorella supplementation on liver enzymes management
• Clinical trials on the C. vulgaris supplementation on different levels of fatty liver disease
• Clinical trials on the C. vulgaris supplementation on other liver health parameters including liver size, hepatic steatosis, or hepatic fibrosis
• Assessing the possible interactions between C. vulgaris supplementation and well-known fatty liver disease treatments, by both experimental and clinical studies
There are some limitations in our study. First, high statistical heterogeneity was detected among the studies. However, we used a subgroup analysis based on intervention duration to find the potential sources of heterogeneity. Second, the outcomes of the present systematic review and meta-analysis are in agreement with a small number of studies. Therefore, the results should be interpreted with caution.
CONCLUSIONS
Collectively, according to the result of this study, C. vulgaris supplementation could significantly decrease the levels of AST. Interestingly, the most significant changes were observed among patients with NAFLD rather than other diseases. Further investigations with a large number of patients as well as on different disorders are necessary and can provide more definitive evidence.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Phukan MM, Chutia RS, Konwar BK, Kataki R. Microalgae Chlorella as a potential bio-energy feedstock. Appl Energy. 2011;88:3307–3312. [Google Scholar]
- 2.Blinová L, Bartošová A, Gerulová K. Cultivation of microalgae (Chlorella vulgaris) for biodiesel production. Res Pap Fac Mater Sci Technol Slovak Univ Technol. 2015;23:87–95. [Google Scholar]
- 3.Camacho F, Macedo A, Malcata F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: a short review. Mar Drugs. 2019;17:312. doi: 10.3390/md17060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safi C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev. 2014;35:265–278. [Google Scholar]
- 5.Yeh KL, Chang JS. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: implications for biofuels. Biotechnol J. 2011;6:1358–1366. doi: 10.1002/biot.201000433. [DOI] [PubMed] [Google Scholar]
- 6.Tokuşoglu Ö, üUnal MK. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana . J Food Sci. 2003;68:1144–1148. [Google Scholar]
- 7.Panahi Y, Pishgoo B, Jalalian HR, Mohammadi E, Taghipour HR, Sahebkar A, Abolhasani E. Investigation of the effects of Chlorella vulgaris as an adjunctive therapy for dyslipidemia: results of a randomised open‐label clinical trial. Nutr Diet. 2012;69:13–19. [Google Scholar]
- 8.Lee SH, Kang HJ, Lee HJ, Kang MH, Park YK. Six-week supplementation with Chlorella has favorable impact on antioxidant status in Korean male smokers. Nutrition. 2010;26:175–183. doi: 10.1016/j.nut.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Haidari F, Homayouni F, Helli B, Haghighizadeh MH, Farahmandpour F. Effect of chlorella supplementation on systematic symptoms and serum levels of prostaglandins, inflammatory and oxidative markers in women with primary dysmenorrhea. Eur J Obstet Gynecol Reprod Biol. 2018;229:185–189. doi: 10.1016/j.ejogrb.2018.08.578. [DOI] [PubMed] [Google Scholar]
- 10.Kwak JH, Baek SH, Woo Y, Han JK, Kim BG, Kim OY, Lee JH. Beneficial immunostimulatory effect of short-term Chlorella supplementation: enhancement of natural killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial) Nutr J. 2012;11:53. doi: 10.1186/1475-2891-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panahi Y, Darvishi B, Jowzi N, Beiraghdar F, Sahebkar A. Chlorella vulgaris: a multifunctional dietary supplement with diverse medicinal properties. Curr Pharm Des. 2016;22:164–173. doi: 10.2174/1381612822666151112145226. [DOI] [PubMed] [Google Scholar]
- 12.Bagherniya M, Nobili V, Blesso CN, Sahebkar A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol Res. 2018;130:213–240. doi: 10.1016/j.phrs.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Jadeja RN, Devkar RV, Nammi S. Oxidative stress in liver diseases: pathogenesis, prevention, and therapeutics. Oxid Med Cell Longev. 2017;2017:8341286. doi: 10.1155/2017/8341286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muriel P, Gordillo KR. Role of oxidative stress in liver health and disease. Oxid Med Cell Longev. 2016;2016:9037051. doi: 10.1155/2016/9037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Świderska M, Maciejczyk M, Zalewska A, Pogorzelska J, Flisiak R, Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic Res. 2019;53:841–850. doi: 10.1080/10715762.2019.1635691. [DOI] [PubMed] [Google Scholar]
- 16.Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, Bresalier RS, Goodman M, Bostick RM, Baron JA. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384–1392. doi: 10.1093/cid/cit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi-Mameghani M, Aliashrafi S, Javadzadeh Y, AsghariJafarabadi M. The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promot Perspect. 2014;4:107–115. doi: 10.5681/hpp.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun HG, Lee JK. Chlorella ethanol extract induced phase II enzyme through NFE2L2 (nuclear factor [erythroid-derived] 2-like 2, NRF2) activation and protected ethanol-induced hepatoxicity. J Med Food. 2015;18:182–189. doi: 10.1089/jmf.2014.3159. [DOI] [PubMed] [Google Scholar]
- 19.Ebrahimi-Mameghani M, Sadeghi Z, Abbasalizad Farhangi M, Vaghef-Mehrabany E, Aliashrafi S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: beneficial effects of supplementation with microalgae Chlorella vulgaris: a double-blind placebo-controlled randomized clinical trial. Clin Nutr. 2017;36:1001–1006. doi: 10.1016/j.clnu.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asis M, Hemmati N, Moradi S, Nagulapalli Venkata KC, Mohammadi E, Farzaei MH, Bishayee A. Effects of resveratrol supplementation on bone biomarkers: a systematic review and meta-analysis. Ann N Y Acad Sci. 2019;1457:92–103. doi: 10.1111/nyas.14226. [DOI] [PubMed] [Google Scholar]
- 23.Aliashrafi S, Ebrahimi Mameghani M, Kakaie F, Javadzadeh Y, Asghari Jafarabadi M. The effect of microalgae Chlorella vulgaris supplementation on inflammatory factors in non-alcoholic fatty liver disease (NAFLD): a double-blind randomized clinical trial. J Mazandaran Univ Med Sci. 2014;24:113–121. [Google Scholar]
- 24.Azocar J, Diaz A. Efficacy and safety of Chlorella supplementation in adults with chronic hepatitis C virus infection. World J Gastroenterol. 2013;19:1085–1090. doi: 10.3748/wjg.v19.i7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee IT, Lee WJ, Tsai CM, Su IJ, Yen HT, Sheu WH. Combined extractives of red yeast rice, bitter gourd, chlorella, soy protein, and licorice improve total cholesterol, low-density lipoprotein cholesterol, and triglyceride in subjects with metabolic syndrome. Nutr Res. 2012;32:85–92. doi: 10.1016/j.nutres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahimi-Mameghani M, Sadeghi Z, Abbasalizad Farhangi M, Vaghef-Mehrabany E, Aliashrafi S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: beneficial effects of supplementation with microalgae Chlorella vulgaris: a double-blind placebo-controlled randomized clinical trial. Clin Nutr. 2017;36:1001–1006. doi: 10.1016/j.clnu.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa T, Nakagawa K, Takekoshi H, Higuchi O, Kato S, Kondo M, Kimura F, Miyazawa T. Ingestion of Chlorella reduced the oxidation of erythrocyte membrane lipids in senior Japanese subjects. J Oleo Sci. 2013;62:873–881. doi: 10.5650/jos.62.873. [DOI] [PubMed] [Google Scholar]
- 28.Talebi Pour B, Jameshorani M, Salmani R, Chiti H. The effect of Chlorella vulgaris vs. Artichoke on patients with non-alcoholic fatty liver disease (NAFLD): a randomized clinical trial. J Adv Med Biomed Res. 2015;23:36–44. [Google Scholar]
- 29.Vakili J, Amir Sasan R, Ordibazar F. Effect of 8 weeks endurance training with Chlorella vulgaris supplementation on liver enzymes levels in women with type 2 diabetes. Med J Tabriz Univ Med Sci. 2019;40:88–97. [Google Scholar]
- 30.Chitsaz M, Mozaffari-Khosravi H, Salman-Roghani H, Zavar-Reza J, Lotfi M. Effect of Chlorella vulgaris vs. Spirulina supplementation on lipid profile and liver function in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Int J Probiotics Prebiotics. 2016;11:127–136. [Google Scholar]
- 31.Rzymski P, Jaśkiewicz M. Microalgal food supplements from the perspective of Polish consumers: patterns of use, adverse events, and beneficial effects. J Appl Phycol. 2017;29:1841–1850. doi: 10.1007/s10811-017-1079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wroblewski F. The clinical significance of alterations in transaminase activities of serum and other body fluids. Adv Clin Chem. 1958;1:313–351. [PubMed] [Google Scholar]
- 34.Vijayavel K, Anbuselvam C, Balasubramanian MP. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol Cell Biochem. 2007;303:39–44. doi: 10.1007/s11010-007-9453-2. [DOI] [PubMed] [Google Scholar]
- 35.Jong-Yuh C, Mei-Fen S. Potential hypoglycemic effects of Chlorella in streptozotocin-induced diabetic mice. Life Sci. 2005;77:980–990. doi: 10.1016/j.lfs.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Cherng JY, Shih MF. Improving glycogenesis in Streptozocin (STZ) diabetic mice after administration of green algae Chlorella . Life Sci. 2006;78:1181–1186. doi: 10.1016/j.lfs.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 37.Mizoguchi T, Takehara I, Masuzawa T, Saito T, Naoki Y. Nutrigenomic studies of effects of Chlorella on subjects with high-risk factors for lifestyle-related disease. J Med Food. 2008;11:395–404. doi: 10.1089/jmf.2006.0180. [DOI] [PubMed] [Google Scholar]
- 38.Olorunnisola OS, Bradley G, Afolayan AJ. Protective effect of Tulbaghia violacea Harv. on aortic pathology, tissue antioxidant enzymes and liver damage in diet-induced atherosclerotic rats. Int J Mol Sci. 2012;13:12747–12760. doi: 10.3390/ijms131012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Zhou W, Wang B, Zhu H, Ye L, Feng M. Antioxidant effect of apolipoprotein A-I on high-fat diet-induced non-alcoholic fatty liver disease in rabbits. Acta Biochim Biophys Sin (Shanghai) 2013;45:95–103. doi: 10.1093/abbs/gms100. [DOI] [PubMed] [Google Scholar]
- 40.de Assis AM, Rech A, Longoni A, Rotta LN, Denardin CC, Pasquali MA, Souza DO, Perry ML, Moreira JC. Ω3-Polyunsaturated fatty acids prevent lipoperoxidation, modulate antioxidant enzymes, and reduce lipid content but do not alter glycogen metabolism in the livers of diabetic rats fed on a high fat thermolyzed diet. Mol Cell Biochem. 2012;361:151–160. doi: 10.1007/s11010-011-1099-4. [DOI] [PubMed] [Google Scholar]