Abstract
Introduction Mucociliary clearance (MCC) is the first line of defense of the pulmonary system. Mucociliary clearance impairment may lead to increased risk of respiratory infections, lung injury, pulmonary repair problems, chronic dysfunctions and progression of respiratory diseases.
Objective To characterize the MCC of active and passive smokers and individuals with chronic obstructive pulmonary disease (COPD) and compare the MCC behaviors between men and women of different age groups.
Methods Patients with COPD (current smokers and ex-smokers) and apparently healthy individuals (current smokers, passive smokers and nonsmokers) were evaluated. All of the subjects underwent lung function and MCC evaluation (saccharin transport test [STT]). Smokers (with or without COPD) were questioned about the smoking history.
Results A total of 418 individuals aged 16 to 82 years old, of both genders, were evaluated. The STT values of active and passive smokers were statistically higher than those of the control group ( p < 0.01). Men of the control group had lower values of STT than active smokers (9.7 ± 7.1 and 15.4 ± 10.1 minute, respectively, p < 0.01). In addition, higher MCC velocity was observed in women that are current smokers (11.7 ± 6.8 minute) compared with men (15.4 ± 10.1 minute) in this group ( p = 0.01). Among the younger age groups (< 50 years old), only passive smokers presented higher STT in relation to the control group.
Conclusion Passive and active smoking are factors that influence negatively the MCC, and passive smokers may present losses of this mechanism at a younger age. Additionally, male smokers present worse MCC than male nonsmokers.
Keywords: mucociliary clearance, saccharin, chronic obstructive pulmonary disease, smoking, passive smoking
Introduction
The human airway is lined by a ciliated cylindrical pseudostratified epithelium and a layer of mucus that is produced by submucosal glands and goblet cells scattered over the epithelium. 1 This integrated system of cilia and mucus characterizes the mucociliary clearance (MCC), the first line of defense of the pulmonary system.
Mucociliary clearance captures harmful particles, pathogens and toxins in the mucus layer, and removes them from the airways by the ciliary beat. Thus, disorders that affect mucus quantity, quality and/or ciliary beat may lead to impaired MCC and ultimately to obstruction and inflammation of small airways, increased risk of respiratory infections, lung injuries, lung repair problems, chronic dysfunctions and progression of respiratory diseases. 2
Age and gender are factors directly related to MCC. 3 4 5 Studies show an inverse relationship between MCC and age, which may predispose the elderly to respiratory infections. 4 5 6 7 8 Regarding gender, some studies report that women have a better MCC than men, perhaps due to anatomical differences in which women have shorter bronchi. 7 9 10 11 Yet, some conditions may change the frequency of ciliary beating in MCC, such as exposure to tobacco smoke 12 and environmental pollution, 4 as well as respiratory tract disorders such as chronic obstructive pulmonary disease (COPD). 13 The thousands of toxic substances contained in cigarette smoke directly affect the ciliogenesis process in the maturation and differentiation stage. 14 They also induce apoptosis, causing death of respiratory ciliated cells earlier than expected, 15 and stimulate mechanically the axonemes, increasing the frequency of the ciliary beat. 16 In specific conditions such as in the presence of COPD, the frequency of the ciliary beat may be impaired. 13
An intact nasosinusal system is important to promote quality of life and prevent respiratory diseases. 17 A normal MCC can represent integrity in the ciliary beat, ideal conditions of bronchial secretion and reflects on the perfect interaction between these two components. 1 18 However, it is still unclear in the literature which clearance rate characterizes healthy subjects, smokers, passive smokers and patients with chronic conditions such as COPD. The present study aimed to characterize the MCC of active and passive smokers, individuals with COPD (smokers and nonsmokers) and to compare the values with a reference sample of healthy volunteers. In addition, the influence of age and gender on MCC was evaluated.
Methods
Study Design
In the present study, participants of both genders and > 16 years old were included if they presented any of the following conditions: 1) patients with COPD and current smokers participating in a pulmonary rehabilitation program, 2) patients with COPD and ex-smokers participating in a pulmonary rehabilitation program, 3) current smokers participating in a smoking cessation program, 4) passive smokers (family members of the current smokers) and 5) nonsmoking volunteers. In addition, they must have one of the following characteristics: 1) spirometry alteration post bronchodilator (FEV 1 /FVC < 0.70) with current smoking history or; 2) spirometry alteration post bronchodilator (FEV 1 /FVC < 0.70) with previous smoking history or; 3) conventional cigarette smoker > 5 years or; 4) living with smokers for > 1 year or; 5) have never smoked or lived with smokers.
Subjects were excluded if they presented a history of nasal trauma or surgery, deviated nasal septum, upper airway inflammatory process (self-reported) or did not understand/cooperate with the procedures and methods of the study.
The participants were submitted to an evaluation process including: questionnaire to obtain personal data, pulmonary function test and nasal MCC assessment. Current smokers and current smokers with COPD were asked about their smoking history and, lastly, current and passive smokers were evaluated about the carbon monoxide levels in the expired air (monoximetry). All of the evaluations were performed in the morning, to avoid the influence of the circadian rhythm on MCC.
The data presented in the present study originates from other cohorts, all of which were approved by the institutional review board. (#18/2011; #07152212.0.0000.5402; and #00849812.0.0000.5402).
Carbon Monoxide in Exhaled air (COex) – Monoximetry
The carbon monoxide in exhaled air (COex) levels were assessed in current and passive smokers to confirm their abstinence/exposure to cigarette smoking in the 12 hours prior to the testing. 19
To conduct this evaluation, subjects were instructed to inhale deeply and remain in apnea for 15 seconds, and then perform a complete and slow expiration on the mouthpiece of the monoximeter (Micro Medical Ltda., Rochester, Kent, United Kingdom). The device measures the carbon monoxide in exhaled air in parts per million.
Pulmonary Function Test – Spirometry
Spirometry was performed to assess the pulmonary function of the subjects. The test was conducted using a portable spirometer Spirobank G (Medical International Research USA, Inc.-Waukesha, Wisconsin/USA) following the criteria to pulmonary function tests established by the Brazilian Society of Pneumology and Tisiology. 20 Interpretation of data followed the guidelines of the American Thoracic Society and of the European Respiratory Society. 21 Finally, results (post bronchodilator) were compared with reference values specific for the Brazilian population. 22
Nasal Mucociliary Clearance – Saccharin Transport Test (STT)
The MCC was assessed in ambient temperature between 22 and 27°C and air humidity between 50 and 60%. The participants remained seated with their heads slightly extended to ∼ 10°. The test was started by introducing ∼ 250 µg of granulated saccharin using a plastic straw, under the visual control of the evaluator, to ∼ 2 cm inside the right nostril. The time from the introduction of saccharin until the first perception of a sweet taste in the mouth was recorded. 23 Subjects were instructed to do not use medications such as anesthetics, barbiturate anesthetics, tranquilizers and antidepressants, and to avoid drinking alcoholic beverages and caffeine-based substances within a minimum of 12 hours before the STT measurement. 4
Data Analysis
The data analysis was conducted using the statistical software GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Data normality was tested by the Shapiro-Wilk test. One-way analysis of variance (ANOVA) or the Kruskal-Wallis test was used for the comparisons between the five groups as well as to compare COex values between current smokers and passive smokers (three groups). Post-hoc tests were done using either the Bonferroni or Dunn tests. The Student t -test or Mann-Whitney test was done to compare the smoking history (pack-years) and the STT values between men and women. Pearson or Spearman tests were conducted to investigate correlations. The chi-squared test was used to analyze categorical data. The significance level adopted in the present study was 5%.
Results
A total of 418 subjects were evaluated and grouped according to the smoking habit, passive exposure to cigarette smoke and diagnosis of COPD. Also, to better investigate factors that could influence MCC, the individuals were regrouped according to gender and age (≤ 50 and > 50 years old).
Table 1 shows the characterization of the sample. It is possible to observe that individuals with COPD, regardless of smoking habit, were older and presented worse lungs compared with individuals without disease.
Table 1. Anthropometric data, lung function, saccharine transport test, and pack/years of the groups evaluated.
| Total ( n = 418) | Control ( n = 128) | Current smokers ( n = 175) | Passive smokers ( n = 30) | COPD current smokers ( n = 11) | COPD ex-smokers ( n = 74) | p-value | |
|---|---|---|---|---|---|---|---|
| Gender (M/F) | 220/198 | 52/76 | 80/95 | 9/21 | 9/2 | 48/26 | < 0.01 |
| Age (years) | 48 [38–59] | 43 [36–48] | 47 [36–55] | 42.5 [35.75–50] | 59 [50–64] | 67 [60.75–73.25] | < 0.01 c.d.g.h.i |
| -Weight (kg) | 72.15 [61.15–82.8] | 73.05 [60.4–84] | 73 [62–83.2] | 76.35 [65.75–94.25] | 69.50 [64.6–72.7] | 67.55 [59.18–78.83] | 0.04 i |
| Height (m) | 1.65 [1.57–1.72] | 1.65 [1.59–1.72] | 1.66 [1.57–1.72] | 1.66 [1.56–1.74] | 1.67 [1.55–1.73] | 1.65 [1.55–1.69] | 0.53 |
| BMI (Kg/m 2 ) | 26.13 [23.19–29.43] | 26.48 [23.12–29.42] | 26.05 [23.59–29.78] | 27.53 [24.54–32.09] | 24.34 [21.72–27.7] | 24.79 [21.8–28.48] | 0.05 |
| FVC (% predict) | 94.85 ± 17.88 | 103.1 ± 13.45 | 97.51 ± 12.88 | 95.71 ± 16.4 | 90.33 ± 29.27 | 74.58 ± 18.29 | < 0.01 a.d.g.i.j |
| FEV 1 (% predict) | 88.86 ± 23.01 | 101.4 ± 12.37 | 96.41 ± 12.3 | 93.41 ± 14.9 | 67.96 ± 24.02 | 50.52 ± 15.92 | < 0.01 a.b.c.d.f.g.h.i.j |
| FEV 1 /FVC (%) | 80.6 [72.9–83.93] | 82.15 [78.5–85] | 82.20 [79.2–84.1] | 81.65 [76.73–85.23] | 61.1 [55.3–67.2] | 52.60 [43.98–61.35] | < 0.01 c.d.f.g.h.i |
| STT (minutes) | 10.48 [6.83–16.33] | 8.52 [5.54–13.91] | 10.87 [7.29–17] | 12.07 [9.37–19.39] | 16.47 [8.25–20.15] | 10.83 [7.85–15.85] | < 0.01 a.b |
| Pack/years† | 24.4 [15–41] | 24 [15–40] | 50 [18–80] | 0.02* | |||
| COex (ppm)‡ | 7.64 ± 5.52 | 8.33 ± 5.34 | 2.5 ± 3.02 | 10.55 ± 5.83 | < 0.01 e,h |
Abbreviations: COex, carbon monoxide in exhaled air; COPD, chronic obstructive pulmonary disease; F, female; FEV 1 , forced expiratory volume in the first second; FVC, forced vital capacity; M, male; ppm, parts per million; STT, saccharin transport test.
a, b, c, d, e, f, g, h, i, j: statistical differences detected in the post-hoc (a: Control versus Current smokers, b: Control versus Passive smokers, c: Control versus COPD smokers; d: Control versus COPD ex-smokers, e: Current smokers versus p assive smokers, f: Current smokers versus COPD current smokers, g: Current smokers versus COPD ex-smokers, h: Passive smokers versus COPD current smokers, i: Passive smokers versus COPD ex-smokers, j: COPD current smokers versus COPD ex-smokers).
* statistical difference detected in the comparison between current smokers and COPD current smokers groups.
†:for this variable the sample n was 186 (contemplating the groups of current smokers).
‡: for this variable the sample n was 216 (contemplating the groups of current and passive smokers).
Data are expressed as mean ± standard deviation or median [25–75] in parametric and non-parametric variables, respectively.
Fig. 1 depicts the values of STT among the five groups. Although the control group presented lower values of STT, statistically significant differences were only found between control (10.55 ± 7.33 minutes) and current (13.41 ± 8.67 minutes) and passive smokers (13.72 ± 5.87 minutes) ( p < 0.01 for both).
Fig. 1.
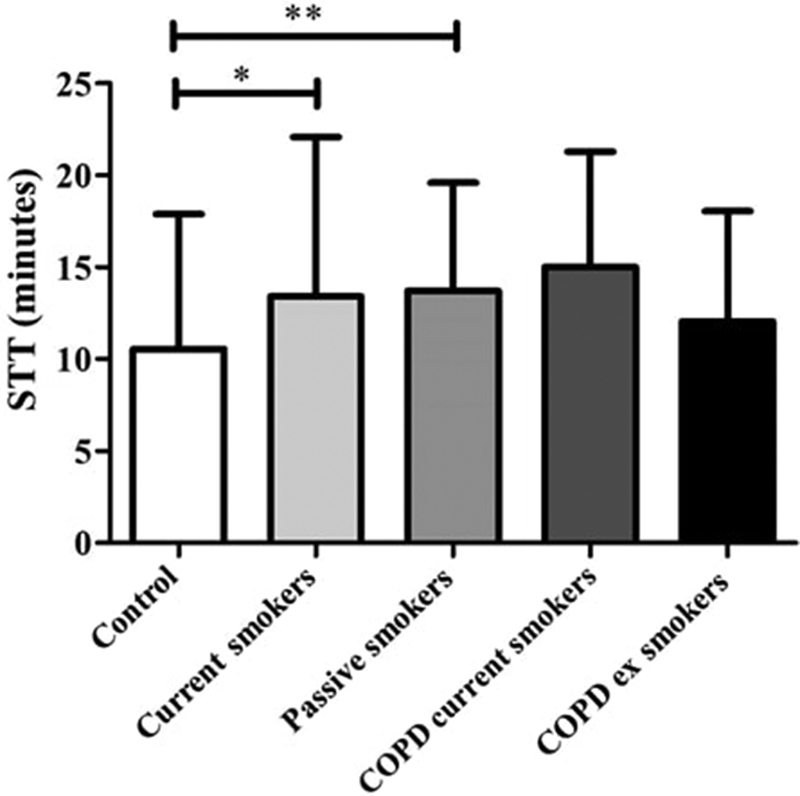
Comparison of the saccharin transit test between the groups evaluated (*: difference detected in Dunn post-hoc between control and current smokers groups; **: difference detected in Dunn post-hoc between control and passive smokers groups); STT: saccharin transport test; COPD: chronic obstructive pulmonary disease.
Fig. 2 describes the comparisons of STT between groups, according to gender. Statistical difference was only observed among men ( p < 0.01) between control (9.72 ± 7.14 minutes) and current smokers (15.45 ± 10.15 minutes) groups. In addition, better MCC was observed in women current smokers (11.69 ± 6.77 minutes) compared with men in this group (15.45 ± 10.15 minutes) ( p = 0.01). There were no significant differences in MCC between genders in the remaining groups.
Fig. 2.
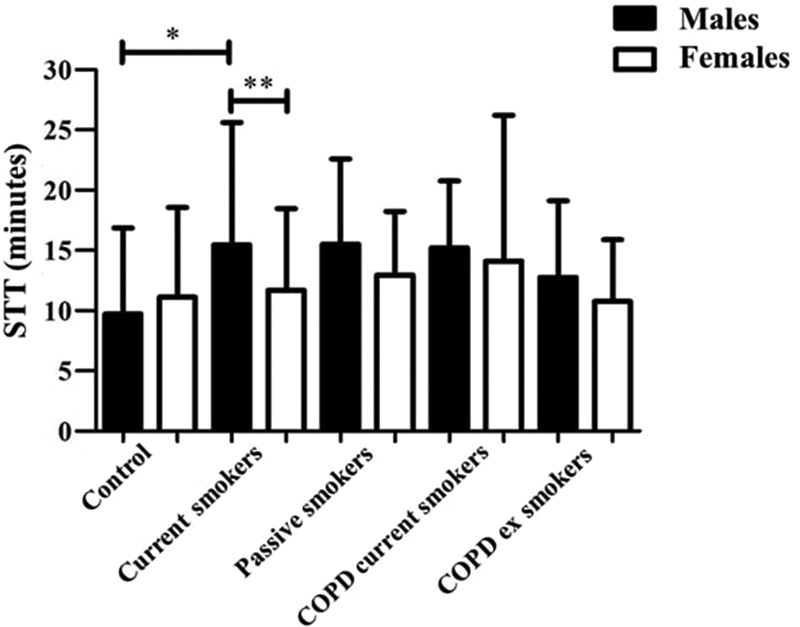
Comparison of the saccharin transit test among the five groups in the different genders (*: difference detected in Dunn post-hoc between male in the control and current smokers groups; **: p = 0.01 in the comparison between male and female current smokers); STT: saccharin transport test; COPD: chronic obstructive pulmonary disease.
Regarding the comparison of STT between younger and older participants, differences were observed only in younger participants between the control and passive smokers groups ( Fig. 3 ).
Fig. 3.
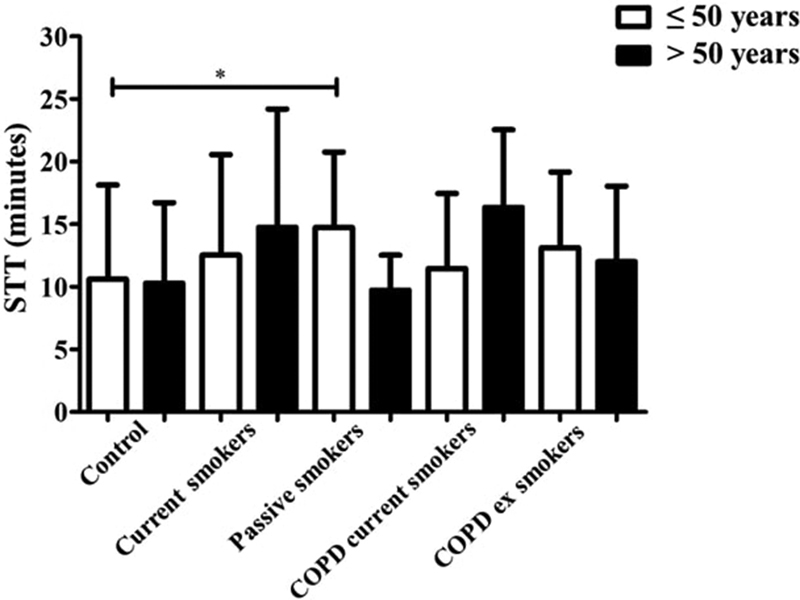
Comparison of the saccharin transit test among the five groups, classified by age range ≤ and > 50 years old (*: difference detected in Dunn post-hoc between the control and passive smokers ≤ 50 years old); STT: saccharin transport test; COPD: chronic obstructive pulmonary disease.
No significant correlations were found between STT and spirometric variables (FVC % of prediction, FEV 1% of prediction and FEV 1 /FVC %). Yet, among current smokers, it was not possible to find any relation between smoking history (age at which started smoking, cigarettes per day, years of smoking or pack/years) and STT (p > 0.05).
Discussion
The results of the present study show that current and passive smokers present higher STT values compared with the control group. These results confirm previous evidence of studies including smaller samples that MCC efficiency is compromised in these individuals. 24 25
The optimal functioning of the respiratory defense mechanism is dependent on the integrity, number and beating of cilia, and adequate biological properties of mucus. 26 However, cigarette smoke (more specifically the constituent substances - phenol, formaldehyde, acrolein and potassium cyanide) has already been described as an important harmful agent for these two components (cilia and mucus) as they are capable of causing cytological and functional modifications, resulting in the damage of the MCC, with stasis of secretion and predisposition for respiratory infections. 27 This theory is strengthened by findings from previous studies, 28 29 in which active and passive smokers have worse STT values (and therefore greater susceptibility to respiratory infections), and may be a consequence of the deficit in the MCC of those subjects exposed to cigarette smoking.
Longer STT was found in the group of COPD smokers. A possible explanation for this may lie on the characteristics of the cilia epithelium. Hessel et al 30 described that the length of the cilia epithelium in both large and small airways of nonsmokers is bigger than the length in healthy smokers and even bigger than the length in individuals with COPD. Although the values were visually discrepant, no statistical difference was observed, possibly due to the low sample size of this group. In line with this, Ito et al observed that chronic pulmonary patients, when smokers, present higher STT compared with ex-smokers. 31
Some studies 32 33 suggest that there is impairment in the MCC of patients with COPD, regardless of the use of cigarettes, mainly attributing this impairment to the decline of the ciliary beating. However, in the present study, similar transportability was observed between subjects with COPD and other groups.
In the comparative analyzes among the younger participants (≤ 50 years old), only passive smokers presented higher STT than the control group. Intriguingly, this finding leads us to assume that individuals passively exposed to cigarette smoke present impairments in MCC even before active smokers. However, this result should be interpreted with caution, since the time of exposure to cigarette smoke among the passive smokers of the present study is unknown.
In the investigation of a population of young smokers (18 to 35 years old), Nicola et al 12 observed that they presented lower STT when compared with nonsmokers of the same age. However, abstinence was not requested before the MCC evaluation, suggesting that this response could be associated with the protection mechanism, with acceleration of ciliary beating frequency to expel the toxic agents of cigarette smoke, demonstrating integrity of this mechanism in young smokers, as in the present study. Among the older participants (> 50 years old), a statistical difference ( p = 0.04) between the groups in the STT values was observed. Between-group differences, however, were not shown, likely due to the skewed distribution of data.
Some studies indicate that there is an inverse relation between MCC and age. 5 9 However, no correlation of age and STT was observed with the general sample of the present study (r = 0.09 and p = 0.06). Regarding the differences between men and women, the literature presents many divergences, even in healthy and nonsmokers individuals. Proença de Oliveira-Maul et al 3 performed STT in 79 nonsmokers and did not find significant differences between men and women. Other authors 7 11 observed better MCC in women, also healthy and nonsmokers. However, there are few studies investigating the influence of gender on MCC in other populations. Uzeloto et al 34 investigated smokers and did not observe differences in the values of STT between men and women. In this study, women smokers had more efficient transport than men smokers. Yet, a comparison of the MCC by gender between the groups was done, making it possible to observe similar STT among women. However, among men, smokers showed slower MCC than the control group. These findings demonstrate a loss of this mechanism previously in men smokers.
A larger STT in the COPD smokers group (mean of 15 minutes) was observed. However, statistical differences were not observed, likely due to the low sample size of this group. The lack of quantification of passive cigarette exposure in the passive smoking group is also a limiting factor in the present study. Another limitation identified was the method used to evaluate MCC. The STT presents some disadvantages compared with rhinocintigraphy, which presents more accurate results since it calculates the exact rate of MCC by the velocity of radioactive material that is inserted into the nasal cavity and measured by a gamma camera. 35 Furthermore, STT results are dependent on the gustatory sensation of the evaluated individual. Although rhinocintigraphy is a reliable and easily reproducible method, it is expensive and exposes the subject to a dose of radiation.
Conclusion
In conclusion, passive and active smoking are factors that negatively influence MCC. Passive young smokers may present impairments of this mechanism. Additionally, male smokers present worse MCC than male nonsmokers.
Compliance with Ethical Standards
Funding Statement
Funding This study was funded by the São Paulo Research Foundation (FAPESP, in the Portuguese acronym) (grant number 2014/11970–3).
Conflicts of Interests The authors have no conflicts of interests to declare.
Ethical Approval
All of the procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The data presented in the present study originates from other cohorts, all of which were approved by the institutional review board. (#18/2011; #07152212.0.0000.5402; and #00849812.0.0000.5402). Informed consent was obtained from all individual participants included in the study.
References
- 1.Trindade S HK, de Mello Júnior J F, Mion O G. Métodos de estudo do transporte mucociliar. Rev Bras Otorrinolaringol. 2007;73:704–712. [Google Scholar]
- 2.Sears P R, Yin W N, Ostrowski L E. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2015;309(02):L99–L108. doi: 10.1152/ajplung.00024.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proença de Oliveira-Maul J, Barbosa de Carvalho H, Goto D M. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest. 2013;143(04):1091–1097. doi: 10.1378/chest.12-1183. [DOI] [PubMed] [Google Scholar]
- 4.Plaza Valía P, Carrión Valero F, Marín Pardo J, Bautista Rentero D, González Monte C. [Saccharin test for the study of mucociliary clearance: reference values for a Spanish population] Arch Bronconeumol. 2008;44(10):540–545. [PubMed] [Google Scholar]
- 5.Paul P, Johnson P, Ramaswamy P, Ramadoss S, Geetha B, Subhashini A S. The effect of ageing on nasal mucociliary clearance in women: a pilot study. ISRN Pulmonol. 2013;2013:1–5. [Google Scholar]
- 6.Puchelle E, Zahm J M, Bertrand A. Influence of age on bronchial mucociliary transport. Scand J Respir Dis. 1979;60(06):307–313. [PubMed] [Google Scholar]
- 7.Armengot M, Barona R, Garín L, Basterra J. [The influence of age, sex and circadian rhythms on the nasal mucosal in the mucociliary clearance] An Otorrinolaringol Ibero Am. 1993;20(06):581–588. [PubMed] [Google Scholar]
- 8.Ho J C, Chan K N, Hu W H. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163(04):983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 9.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J. 2005;26(04):609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 10.Gerrard C S, Gerrity T R, Yeates D B. The relationships of aerosol deposition, lung size, and the rate of mucociliary clearance. Arch Environ Health. 1986;41(01):11–15. doi: 10.1080/00039896.1986.9935759. [DOI] [PubMed] [Google Scholar]
- 11.Bennett W D, Laube B L, Corcoran T. Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv. 2013;26(03):157–164. doi: 10.1089/jamp.2011.0909. [DOI] [PubMed] [Google Scholar]
- 12.Nicola M L, Carvalho H B, Yoshida C T. Young “healthy” smokers have functional and inflammatory changes in the nasal and the lower airways. Chest. 2014;145(05):998–1005. doi: 10.1378/chest.13-1355. [DOI] [PubMed] [Google Scholar]
- 13.Tilley A E, Walters M S, Shaykhiev R, Crystal R G. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamashiro E, Xiong G, Anselmo-Lima W T, Kreindler J L, Palmer J N, Cohen N A. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23(02):117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 15.Lan M Y, Ho C Y, Lee T C, Yang A H. Cigarette smoke extract induces cytotoxicity on human nasal epithelial cells. Am J Rhinol. 2007;21(02):218–223. doi: 10.2500/ajr.2007.21.2966. [DOI] [PubMed] [Google Scholar]
- 16.Navarrette C R, Sisson J H, Nance E, Allen-Gipson D, Hanes J, Wyatt T A. Particulate matter in cigarette smoke increases ciliary axoneme beating through mechanical stimulation. J Aerosol Med Pulm Drug Deliv. 2012;25(03):159–168. doi: 10.1089/jamp.2011.0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borin A, Abib E, Jr, Araujo C I, Martinez L L, Rodrigues H. Standardizing selection criteria in nasal medication studies. Rev Bras Otorrinolaringol (Engl Ed) 2009;75(06):872–878. doi: 10.1016/S1808-8694(15)30552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith D J, Gaffney E A, Blake J R.Modelling mucociliary clearance Respir Physiol Neurobiol 2008163(1-3):178–188. [DOI] [PubMed] [Google Scholar]
- 19.SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(02):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 20.Sociedade Brasileira de Pneumologia e Tisiologia (SBPT) . Diretrizes para testes de função pulmonar. J Pneumol. 2002;28:S1–S238. [Google Scholar]
- 21.ATS/ERS Task Force . Miller M R, Hankinson J, Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(02):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Pereira C AC, Barreto S P, Simões J G, Pereira F WL, Gerstler J G, Nakatani J. Valores de referência para espirometria em uma amostra da população brasileira adulta. J Bras Pneumol. 1992;18:10–22. [Google Scholar]
- 23.Ramos E M, Vanderlei L C, Ito J T. Acute mucociliary clearance response to aerobic exercise in smokers. Respir Care. 2015;60(11):1575–1584. doi: 10.4187/respcare.04093. [DOI] [PubMed] [Google Scholar]
- 24.Habesoglu M, Demir K, Yumusakhuylu A C, Yilmaz A S, Oysu C. Does passive smoking have an effect on nasal mucociliary clearance? Otolaryngol Head Neck Surg. 2012;147(01):152–156. doi: 10.1177/0194599812439004. [DOI] [PubMed] [Google Scholar]
- 25.Freire A PCF, Ramos D, Leite M R. Influence of time and frequency of passive smoking exposure on mucociliary clearance and the autonomic nervous system. Respir Care. 2016;61(04):453–461. doi: 10.4187/respcare.04398. [DOI] [PubMed] [Google Scholar]
- 26.Fahy J V, Dickey B F. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliuca G, Rosato C, Martellucci S. Cytologic and functional alterations of nasal mucosa in smokers: temporary or permanent damage? Otolaryngol Head Neck Surg. 2015;152(04):740–745. doi: 10.1177/0194599814566598. [DOI] [PubMed] [Google Scholar]
- 28.Petanová J. [Smoking and immunity] Cas Lek Cesk. 2017;156(01):6–8. [PubMed] [Google Scholar]
- 29.Tobacco Control Committee of the European Respiratory Society . Jayes L, Haslam P L, Gratziou C G. SmokeHaz: Systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest. 2016;150(01):164–179. doi: 10.1016/j.chest.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Hessel J, Heldrich J, Fuller J. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One. 2014;9(01):e85453. doi: 10.1371/journal.pone.0085453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito J T, Ramos D, Lima F F. Nasal mucociliary clearance in subjects with COPD after smoking cessation. Respir Care. 2015;60(03):399–405. doi: 10.4187/respcare.03266. [DOI] [PubMed] [Google Scholar]
- 32.Yaghi A, Dolovich M B. Airway epithelial cell cilia and obstructive lung disease. Cells. 2016;5(04):e40. doi: 10.3390/cells5040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaghi A, Zaman A, Cox G, Dolovich M B. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir Med. 2012;106(08):1139–1147. doi: 10.1016/j.rmed.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Uzeloto J S, Ramos D, Freire A PCF, Christofaro D GD, Ramos E MC. Nasal mucociliary transportability of male and female smokers. Rev Bras Otorrinolaringol (Engl Ed) 2017;17:S1808–S8694. [Google Scholar]
- 35.Di Giuda D, Galli J, Calcagni M L. Rhinoscintigraphy: a simple radioisotope technique to study the mucociliary system. Clin Nucl Med. 2000;25(02):127–130. doi: 10.1097/00003072-200002000-00010. [DOI] [PubMed] [Google Scholar]


