Abstract
Natural killer (NK) cells are a specialised population of innate lymphoid cells (ILCs) that help control local immune responses. Through natural cytotoxicity, production of cytokines and chemokines, and migratory capacity, NK cells play a vital immunoregulatory role in the initiation and chronicity of inflammatory and autoimmune responses. Our understanding of their functional differences and contributions in disease settings is evolving owing to new genetic and functional murine proof‐of‐concept studies. Here, we summarise current understanding of NK cells in several classic autoimmune disorders, particularly in rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE) and type 1 diabetes mellitus (T1DM), but also less understood diseases such as idiopathic inflammatory myopathies (IIMs). A better understanding of how NK cells contribute to these autoimmune disorders may pave the way for NK cell‐targeted therapeutics.
Keywords: natural killer cells, autoimmune disease, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes mellitus, idiopathic inflammatory myopathies
In this review, we summarise current understanding of NK cells in several classic autoimmune disorders, particularly in rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE) and type 1 diabetes mellitus (T1DM), but also less understood diseases such as idiopathic inflammatory myopathies. We also discuss how a better understanding of the contribution of NK cells to these autoimmune disorders may pave the way for NK cell‐targeted therapeutics.
Introduction
Autoimmune diseases are caused by inappropriate reactivity of adaptive immune cells towards self‐antigens and comprise highly heterogenous conditions. These diseases can be organ‐specific (i.e. multiple sclerosis and type 1 diabetes) or systemic (i.e. rheumatoid arthritis, systemic lupus erythematosus, and idiopathic inflammatory myopathies). While a complex interplay of genetic and environmental factors is thought to give rise to these discrete conditions, aberrant immune and inflammatory responses drive their initiation, progression and chronicity. Autoreactive T cells and autoantibody‐producing B cells (plasma cells) are key upstream drivers of autoimmune diseases. 1 , 2 Innate immune cells such as neutrophils, monocytes and macrophages are well‐described effector cells mediating tissue damage and inflammation in targeted organs. 3 , 4 However, other innate effector cells, including natural killer (NK) cells and other innate lymphoid cell (ILC) subsets, are also found in inflamed tissues and may regulate immune dysfunction and inflammation. 5
NK cells are bone marrow‐derived granular cells with classic lymphoid cell morphology. 6 The steady‐state NK cell reservoir includes blood, secondary lymphoid organs (spleen, lymph nodes and tonsils) and non‐lymphoid tissues (i.e. liver, uterus). In addition to the active recruitment of circulating NK cells, NK cells in non‐lymphoid organs (e.g. liver, skin, kidney and intestine) comprise a subset of tissue‐resident NK cells that develop from local precursors. 7 Regardless of their origin, NK cells are geared for rapid modulation of immune responses. 7
The ontogeny of NK cells has been extensively investigated. NK cells are generated from common lymphoid progenitors (which also give rise to T cells, B cells and other ILC subsets). They, however, do not rely on the thymic reservoir, but rather derive from bone marrow haemopoiesis and are dependent on the cytokine IL‐15. 8 , 9 NK cells and ILCs also differ from other lymphoid cells as they do not express somatically rearranged receptors and thus lack antigen specificity. 6 In contrast, NK cells possess both cytotoxicity and cytokine‐producing properties, resembling CD8+ T cells and CD4+ T helper (Th) cells, respectively, and share similar transcription factor dependency (i.e. T‐bet and Eomes). 10 Through cytotoxicity, cytokine production and migratory capacity, NK cells represent a highly versatile immune subset and contribute to many physiological and pathological settings, including protection against intracellular pathogens, anti‐tumor immunity, maternal‐foetal immune tolerance, graft rejection, chronic autoinflammatory diseases and autoimmune disorders. 11 , 12 , 13 , 14 , 15 While the functional contributions of NK cells in infection and malignancy are clear, there have been mixed and often contradictory findings in autoimmune disease settings (discussed below). These discrepancies may relate to the different tissue niche or source of NK cells analysed, variable experimental systems, stage of disease, as well as the intrinsic disease heterogeneity. Here, we review current knowledge of NK cell biology in some major autoimmune diseases and discuss the future immunotherapeutic potential of NK cells.
NK cell subsets
In humans, NK cells are commonly defined as CD3– CD56+ NKp46+ mononuclear cells, which can be further classified into CD56dim or CD56bright subsets. 16 , 17 In mice, NK cells lack CD56 expression. Murine NK cells are commonly defined as CD3– NK1.1+ NKp46+ CD49b+, and are further classified CD11b+ CD27– and CD11b– CD27+ subsets. 18 , 19 , 20 High‐dimensional single‐cell analysis confirms that the two murine NK subsets represent conserved counterparts of human NK cell subsets. 17
The CD56dim NK cells are predominantly found in the blood and represent a highly cytotoxic subset. They express high levels of inhibitory killer immunoglobulin‐like receptors (KIRs), components of cytolytic granules (perforin and granzymes), and FcγRIIIA (CD16a+), which collectively mediate antibody‐dependent cell‐dependent cytotoxicity (ADCC). 6 , 21 , 22 In contrast, the CD56bright NK cells are abundant in secondary lymphoid tissues such as lymph nodes and tonsils, 21 , 23 in inflamed tissues and in the decidua during pregnancy. 24 , 25 , 26 CD56bright NK cells express lower levels of KIRs, cytolytic granules, and CD16 than CD56dim NK subset, but have higher levels of cytokine receptors and inhibitory receptor CD94/NKG2A. Correspondingly, the CD56bright NK subset is less cytotoxic but more efficient at cytokine and chemokine production than the CD56dim subset. 21 , 27 CD56dim and CD56bright subsets also express discrete cytokine and chemokine receptors, which contribute to their temporal regulation in discrete niche. 21
It should be noted that the cytolytic or cytokine‐producing capacity of NK cells is not necessarily restricted to a specific subset. For example, presumed cytokine‐producing CD56bright NK cells may acquire cytolytic activity equal to, if not stronger than, CD56dim NK cells upon appropriate stimulation through cytokines or activating receptors. 28 , 29 Conversely, CD56dim NK cells can also produce IFN‐γ upon contact with target cells. 22 Although cell‐surface markers can aid in identifying NK cell subsets in various anatomical locations, this does not necessarily define the functional phenotype of NK cells in different physiological settings. Furthermore, the bilateral system with CD56 as a classical means to separate NK subpopulations is becoming outdated. Both single‐cell transcriptomic and mass cytometric analyses have revealed remarkable phenotypic diversity of NK cell subpopulations in human peripheral blood and in primary tumors. 30 , 31 , 32
NK cell activation and licensing
In the steady state, peripheral NK cells are relatively quiescent but can rapidly respond to an array of germline encoded activating and inhibitory cell‐surface receptors. Sensing and signal integration downstream of these receptors allow NK cells to discriminate ‘altered self’ from ‘normal self’ and thereby play a critical role in host defence, while also keeping the potentially self‐destructive activity of NK cells in check. 33 Examples of NK‐activating receptors include the KIR S family (or the corresponding murine homolog Ly49D and Ly49H), FcγRIII, CD94‐NKG2C complex, NKG2D, NKp46, IgG‐like receptor 2B4/CD244, adhesion molecules DNAM‐1/CD226, lymphocyte function‐associated antigen (LFA‐1), as well as various cytokine and chemokine receptors. 33 Cytokines that regulate NK cell function include IL‐15, IL‐18, IL‐12, IL‐23, and type I interferons (IFNs). 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41
Inhibitory NK cell receptors that recognise either classical or non‐classical major histocompatibility complex class I (MHC‐I) proteins balance NK‐activating signals. Example of NK‐inhibiting receptors include the human KIR L family (or the corresponding murine homolog Ly49A and Ly49C), CD94‐NKG2A complex, and TIGIT. 33 According to the ‘missing self‐hypothesis', ubiquitously expressed MHC‐I ligands on host cells spares normal host cells from NK cell‐mediated lysis, while the loss or downregulated level of MHC‐I in infected or transformed cells renders host cells ‘foreign’ and thus susceptible to NK‐mediated killing. 42 Paradoxically, the engagement of host MHC‐I with inhibitory receptors is also necessary for NK cells to gain full functional competence (NK cell licensing). 42 Without such signals, unlicensed NK cells from MHC‐I‐deficient mice demonstrate diminished cytolytic and cytokine‐producing functions relative to NK cells from MHC‐I‐sufficient hosts. 43 , 44 , 45 , 46
NK cells are tightly controlled to prevent inappropriate responses against host cells. 33 In keeping with the importance of fine‐tuning NK cells in immune homeostasis, several autoinflammatory conditions have been associated with mutations in NK cell activating receptors. 14 NK‐activating and ‐inhibitory receptors can also be harnessed therapeutically. For example, blockade of the inhibitory NKG2A receptor promotes anti‐tumor immunity by enhancing the cytotoxic effector function of NK cells and CD8+ T cells. 47 More recently, a first‐in‐class trifunctional agonistic antibody targeting two activating receptors (NKp46 and CD16‐mediated ADCC) and a tumor antigen on cancer cells augmented NK‐mediated tumor killing in preclinical murine models of cancer. 48
Certain combinations of KIR and HLA molecules may predispose individuals to, or protect them from, inflammatory diseases. The expression of KIR S family members (e.g. KIR2DS1, KIR2DS2 and KIR3DS1), particularly when paired with certain HLA ligands, is associated with increased susceptibility to psoriatic arthritis, 49 , 50 , 51 rheumatoid vasculitis, 52 and SLE. 53 However, these reports were observational and further functional studies are warranted to establish the disease‐promoting role of these KIR S isoforms in NK cells across autoimmune disorders. It is important to note that a lower level of NK inhibitory receptors does not necessarily translate to heightened autoreactivity. Rather, these ‘unlicensed’ NK cells may be rendered hyporesponsive to further stimulation by activating receptors. Conversely, elevated levels of inhibitory receptors could contribute to potent ‘licensing’ effects in NK cells in inflammatory settings. Indeed, patients with ulcerative colitis, 54 Crohn’s disease 55 and spondyloarthropathy 56 , 57 express more inhibitory KIRs (i.e. KIR2DL2/3 and KIR3DL1/2) and their cognate ligands compared with healthy controls. Spondyloarthropathy is associated with HLA‐B27, 56 , 57 tends to form heavy chain homodimers (so called B272). B272 can in turn interact with KIR3DL2 expressed by NK cells and T cells. Such interactions were shown to promote the survival and effector functions of KIR3DL2‐expressing NK cells and T cells, including enhanced NK cell cytotoxicity and T cell production of IL‐17. 56 , 57 Furthermore, genetically ‘licensed’ individuals, determined by the presence of KIR2DL2/3 and homozygosity for HLA‐C1, exhibit augmented CD4+ T cell proliferation and Th17 differentiation, compared with those from unlicensed individuals. 58
Immune regulatory functions of NK cells
Natural killer cells are implicated in both protective and pathogenic immunity, depending on the type and stage of the immune response, the target organ and NK subsets analysed. A well‐recognised function of NK cells is contact‐dependent cytotoxicity. This occurs through release of cytolytic granules containing proteases, granzymes, and perforins into target cells via a lytic synapse, and through the induction of caspase‐dependent apoptosis of target cells via the engagement of natural cytotoxicity receptors (NCRs), NKG2D, CD16a, LFA‐1, DNAM‐1, FAS ligands, and TNF‐related apoptosis‐inducing ligand (TRAIL). 28 , 29 , 48 , 59 , 60 , 61 , 62 , 63 Rapid NK cell‐mediated killing of infected or transformed cells is an important protective function against intracellular pathogens and malignancy, respectively. 64 Self‐directed attack by cytotoxic NK cells could also aggravate pathology as has been observed in experimental autoimmune encephalomyelitis (EAE). 65 , 66 NK cells can also modulate immune responses by directly killing other immune cells such as monocytes/macrophages, 67 , 68 , 69 , 70 neutrophils, 59 , 71 and CD4+ Th17 and T follicular helper (Tfh) cell subsets. 29 , 61 , 63 , 72 , 73 , 74 , 75 , 76
Natural killer cells secrete cytokines and chemokines, orchestrating interactions with other immune cells. For example, NK cell‐derived IFN‐γ is essential for early Th1 priming in draining lymph nodes. 77 Abundant IFN‐γ production by NK cells has also been associated with the pathogenesis of several inflammatory disorders such as SLE 78 , 79 and psoriasis. 80 Furthermore, NK cells found in target non‐lymphoid tissues, such as the inflamed joint of RA patients, 81 , 82 the central nervous system (CNS) of MS patients, 83 and the inflamed skin of psoriasis patients, 80 were found to produce high levels of pro‐inflammatory cytokines including IFN‐γ and tumor necrosis factor‐α (TNF‐α). In addition to IFN‐γ and TNF‐α, NK cells may also secrete other cytokines such as granulocyte/macrophage‐colony stimulating factor (GM‐CSF), macrophage‐colony stimulating factor (M‐CSF), IL‐5, IL‐10, IL‐13, and chemokines CCL3, CCL4, CCL5, IL‐8, RANTES and XCL1. 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92
The protective effect of NK cells in autoimmune disorders is thought to occur by the downregulation of autoreactive adaptive immune responses. NK cell ‘degeneration’, defined as numerical and/or functional deficits (reduced cytotoxicity), has been extensively documented in peripheral blood of patients with many inflammatory autoimmune diseases including RA, 93 MS, 83 T1DM, 94 SLE, 95 Sjogren’s syndrome 96 and idiopathic inflammatory myopathies. 97 , 98 , 99 These findings have also been observed in animal models of autoimmune diseases such as the collagen‐induced arthritis model for RA, 100 a model of systemic juvenile idiopathic arthritis 69 and the non‐obese diabetes (NOD) model for T1DM. 101 , 102 Reduced cytotoxicity of NK cells in these settings is thought to impair the restraint of pathogenic immune cells, while reduced circulating NK cell numbers might reflect enhanced recruitment to sites of inflammation. Enrichment of NK cells has been found in the synovial joints of RA patients, 25 , 26 , 81 the kidneys of patients with lupus nephritis, 103 cerebrospinal fluid and brain lesions of MS patients, 83 , 104 acute psoriatic plaques, 80 and fibrotic lungs of patients with anti‐synthetase syndrome. 98 These tissue NK cells have upregulated expression of tissue‐homing chemokine receptors. 26 , 80 , 81 , 103
Natural killer cell ‘degeneration’ does not necessarily portend autoimmunity. NK‐deficient (Mcl1fl/fl:Ncr1Cre) mice, 105 or patients with congenital deficiency in NK cells, 106 do not develop spontaneous autoimmunity, most likely because of compensatory tolerogenic mechanisms and immune checkpoints. Studies using anti‐NK1.1 or anti‐asialoGM1 monoclonal antibodies to deplete NK cells in mice have produced mixed results (discussed below). In the following sections we discuss how NK cells, which only account for a small fraction of total lymphocytes, nevertheless contribute to the outcomes of inflammatory and autoimmune diseases.
NK cells in rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that is characterised by persistent joint inflammation, cartilage damage, and bone erosion. 107 Studies using the autoimmune collagen‐induced arthritis (CIA) model of RA identify Th17 cells and germinal centre‐dependent humoral responses as key drivers of disease, 108 , 109 , 110 while innate immune cells (neutrophils, monocytes/macrophages and NK cells), fibroblast‐like synovial cells and bone‐resorbing osteoclasts cause joint inflammation and destruction. 111 , 112 , 113 , 114
In RA, the frequency of NK cells is increased in peripheral blood of patients 81 , 115 but these NK cells consistently display impaired effector functions such as reduced IFN‐γ production and decreased cytotoxicity. 115 , 116 , 117 , 118 This may be due to upregulation of inhibitory receptors such as CD161 117 and NKG2A, 119 or downregulation of activating receptors such as CD16. 116 Deficient cytotoxic function of peripheral NK cells is thought to contribute to the early phase of autoimmune arthritis. Robust CIA induction was observed following adoptive transfer of collagen type II‐specific CD4+ T cells and B cells into Rag2–/– Prf1–/– hosts (lacking T and B cells, and perforin‐deficient NK cells), but not into Rag2–/– hosts (lacking T and B cells, but with perforin‐sufficient NK cells). 119 Although ex vivo experiments demonstrated NK‐mediated lysis of arthritogenic Th17 and Tfh cells, 119 this may not reflect a physiological scenario because other immune checkpoints (i.e. T regulatory cells) are absent. IFN‐γ produced by NK cells is also thought to inhibit arthritis, both in the passive transfer autoantibody‐induced arthritis 120 and the autoimmune CIA 100 models. However, NK‐mediated inhibition of passive transfer autoantibody‐induced arthritis is only apparent following CpG‐oligonucleotide stimulation. 120 In the CIA model, NK‐derived IFN‐γ is thought to limit Th17 differentiation as NK cells depletion with anti‐asialoGM1 at priming phase led to the expansion of Th17 cells and mild exacerbation of CIA at disease onset. 100 A more sustained exacerbation of CIA was similarly observed following anti‐NK1.1‐mediated depletion, but this appears to be due to NK T cell depletion as CD1d–/– (NK T‐deficient) mice also develop worse CIA. 121
Abundant NK cells are present in RA synovium and most harbour a unique CD56bright phenotype. 25 , 26 , 115 , 122 RA synovial NK cells are CD69+ NKp44+, indicative of their activated state, but are perforinlow. 25 , 26 , 115 , 122 They also upregulate surface expression of inhibitory CD94‐NKG2A, 123 which strongly inhibits NK cell production of IFN‐γ and TNF and also restrains cytotoxicity upon binding to its ligand, HLA‐E. 25 , 119 , 122 Unlike circulating NK cells, synovial NK cells have low KIR expression, 25 , 26 , 81 but express chemokine receptors, such as CCR5, CXCR3 and CCR1, which may facilitate their preferential recruitment into RA synovium. 26 , 81 Given the low cytotoxicity and IFN‐γ production of synovial NK cells, 115 these NK cells likely contribute to local joint inflammation by producing other pro‐inflammatory mediators. Reciprocal activation of joint‐infiltrating CD56bright NK cells and CD14+ inflammatory monocytes has also been suggested in RA. 25 , 124 Murine studies identify joint NK cells as sources of M‐CSF and RANKL that promote the differentiation of bone‐resorbing osteoclasts 89 (Figure 1a). In contrast to earlier studies, 100 the depletion of NK cells using anti‐asialoGM1 attenuated both joint inflammation and bone erosion in the CIA model. 89 These studies demonstrate the limitations of antibody depletion of NK, 125 , 126 which can be further confounded by the dynamics of autoimmune responses.
Figure 1.
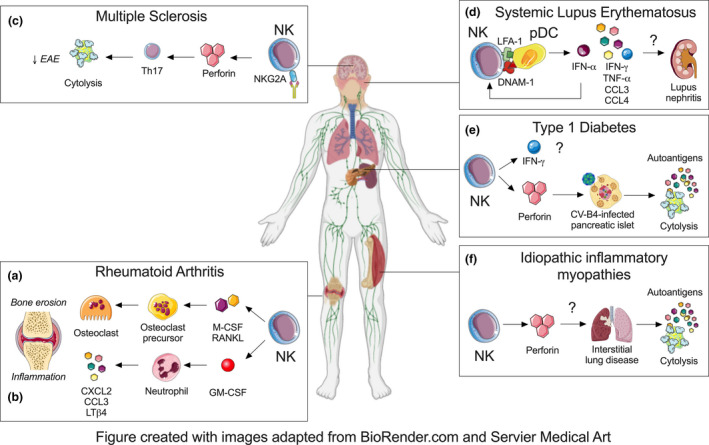
NK cell involvement in autoimmune inflammatory diseases. NK cells exacerbate RA by secreting soluble mediators such as (a) M‐CSF and RANKL that drive the differentiation of bone‐eroding osteoclasts and (b) GM‐CSF that promotes the production of pro‐inflammatory mediators by joint‐infiltrating neutrophils. (c) NK cells do not appear to play a dominant role in MS but boosting their cytotoxic function with anti‐NKG2A may eliminate encephalitogenic Th17 cells and alleviate disease in the EAE model. (d) NK cells may promote SLE through their interaction with pDCs via LFA‐1 and DNAM‐1 that enhances the production of cytokines and chemokines such as IFN‐α, IFN‐γ, TNF‐α, IL‐6, IL‐8, CCL3 and CCL4. NK cells are also found in kidney of lupus nephritis patients but it remains unclear if NK cells and their cytokine dysfunction contribute to tissue pathology. (e) NK cells could contribute to the generation of autoantigens through excessive killing of CV‐B4‐infected pancreatic β islets. However, other functions of NK cells such as IFN‐γ production remain unclear and future studies are required to capture phenotypic and functional diversity of NK cells in both CV‐B4‐associated and sterile T1DM subtypes. (f) Alveolar NK cells are thought to give rise to autoantigens such as histidyl tRNA synthetase following respiratory insults in anti‐synthetase syndrome. Future studies are needed to evaluate whether similar numerical and functional changes in NK cells occur in the discrete subtype of IIM.
Others suggest additional mechanisms by which NK cells can regulate local joint inflammation. For example, IL‐15‐activated NK cells trigger apoptosis of mature bone‐resorbing osteoclasts via LFA‐1, DNAM‐1 and TRAIL. 127 Another study demonstrates the degranulation of an NK cell line in response to RA fibroblast‐like synoviocytes, upon stimulation via NKG2D, DNAM‐1, NKp46 and NKp44 receptors. 123 These studies, however, were limited to in vitro observations using blood NK cells and monocyte‐derived osteoclasts 127 or an NK cell line 123 and contradict reports showing low cytotoxicity of synovial NK cells. 115 , 116 , 117 , 118 Synovial NK‐derived IFN‐γ has also been thought to limit arthritis by inhibiting Th17 polarisation in the CIA model, 100 but this seems questionable given the low IFN‐γ production by RA synovial NK cells. 115 More recently, we reported that synovial joint NK cells propagate joint inflammation by secreting the pro‐inflammatory cytokine GM‐CSF 91 (Figure 1b). This occurs in an IL‐18‐dependent manner, and independently of their cytotoxic function or IFN‐γ production. 91 GM‐CSF, in turn, signals to joint‐infiltrating myeloid cells such as neutrophils to upregulate pro‐inflammatory CXCL2, CCL3 and LTβ4, thereby sustaining immune cell recruitment into inflamed joints. 91 To our knowledge, this was the first study to define how joint NK cells participate in local inflammatory cascades in the effector phase of autoantibody‐induced arthritis.
In summary, synovial NK cells and peripheral NK cells may perform distinct immune functions in RA. Current evidence shows that synovial NK cells aggravate arthritis through the production inflammatory mediators such as GM‐CSF, M‐CSF and RANKL, thereby priming effector myeloid cells. In contrast, peripheral NK cells in secondary lymphoid tissues may exert a protective role in RA by virtue of IFN‐γ production and cytotoxicity against arthritogenic immune cells. Future studies should carefully consider the possibility of inherent differences in NK cells at anatomical locations. Better understanding of the NK‐activating and ‐inhibitory repertoire in RA could inform NK‐based therapies.
NK cells in multiple sclerosis
Multiple sclerosis (MS) is an inflammatory neurodegenerative disease characterised by autoreactive T cell‐induced demyelination of the CNS, leading to progressive neurological deficits. 128 Experimental autoimmune encephalomyelitis (EAE) is a widely used model for MS and is induced by active immunisation or passive transfer of myelin oligodendrocyte glycoprotein antigen‐specific T cells. 129 Disease induction in both MS patients and EAE model is driven by Th17 cells, particularly the GM‐CSF‐producing subset, 130 , 131 , 132 and to some extent by autoantibodies. 133
In relapsing‐remitting MS, a reduction in peripheral NK cell activity was found to coincide with clinical relapse. 134 Ex vivo cytokine‐activated NK cells demonstrate potent lytic capacity towards autologous CD4+ T cells following the engagement of surface receptors including NKp30, NKp46, DNAM‐1, NKG2D, LFA‐1 and TRAIL. 28 , 29 , 61 , 83 Peripheral NK cells from untreated MS patients, however, were less efficient in suppressing autologous CD4+ T cells compared with healthy controls. 29 , 83 Compromised cytotoxicity in MS NK cells may result from upregulated T cell expression of HLA‐E, the ligand for the inhibitory receptor NKG2A, 29 or downregulated DNAM‐1/CD155‐mediated NK cell priming. 83 Elevated levels of HLA‐E have also been detected in the cerebrospinal fluid and CNS plaques of MS patients, which correlated with reduced NK cytotoxicity and higher disease activity. 135 Collectively, these studies suggest compromised NK‐mediated removal of myelin‐reactive T cells could contribute to MS. This functional deficiency might reflect resistance of autoreactive T cells to the cytolytic activity of NK cells, rather than an intrinsic NK cell defect.
The involvement of NK cell in MS remains contentious. NK cell depletion with anti‐NK.1.1 antibody exacerbated murine EAE. 65 , 72 Similarly, mice deficient in the CX3CR1 chemokine receptor, which directs NK cell recruitment to the CNS, showed worse EAE, while inducible NK cell expansion with IL‐2 attenuated EAE. 65 In these settings, the protective role for NK cells in EAE development is thought to be mediated by CNS‐recruited NK cells and to occur through indirect suppression of Th17 responses via microglia 65 or killing of CNS‐infiltrating CCR2+ Ly6C+ inflammatory monocytes. 68 In support of the notion of protective effects for NK cells in MS, treatment with biological disease‐modifying agents, 136 , 137 , 138 is associated with restoration of circulating NK cell number. However, these studies are limited to correlative observations and may not reveal the true regulatory functions of NK cells.
In an earlier study, NK cell depletion with either anti‐NK.1. or anti‐asialoGM1 was shown to reduce EAE severity. 139 These discrepancies might be explained by differences in immunisation and antibody dose. In a more recent and comprehensive report, passive transfer of encephalitogenic 2D2 transgenic Th17 cells into mice treated with anti‐NK1.1 antibody, or into mice with NKp46‐lineage specific depletion of T‐bet (Tbx21fl/fl:NKp46Cre), resulted in significantly reduced EAE. This finding underscores the importance of NK1.1+ NKp46+ ILCs (i.e. NK cells, ILC1 and/or ILC3 subsets). 140 Further, passive transfer of encephalitogenic 2D2 Th17 cells induced EAE of comparable incidence and severity in NK‐sufficient and NK‐deficient (Eomesfl/fl:NKp46Cre) mice. 140
Taken together, NK cells do not appear to have a significant physiological role in Th17‐induced autoimmune neuroinflammation. Nevertheless, manipulation of NK cell function through inhibitory or activating receptors may still offer a viable therapeutic strategy in MS. For example, pharmacological blockade of the inhibitory NKG2A or its ligands Qa‐1, was shown to enhance NK‐mediated killing of autoreactive CD4+ T cells, skew Th1/Th17 responses towards a non‐pathogenic Th2 response, and ameliorate EAE 73 , 141 (Figure 1c).
NK cells in systemic lupus erythematosus
Systemic Lupus Erythematosus (SLE) is a chronic, systemic autoimmune disease and substantial clinical heterogeneity makes it one of the most therapeutically challenging autoimmune disorder. 142 Hallmarks of SLE include hyperactivation of type I IFN responses, sustained production of multiple autoantibodies against nuclear autoantigens, and immune complex formation in various organs (i.e. skin, kidney, lung, blood, joint, and CNS), leading to tissue inflammation and damage, such as lupus nephritis. In addition to autoimmune T and B cells, plasmacytoid dendritic cells (pDCs) play a prominent role in lupus through type I IFN production, namely IFN‐α, downstream of innate immune recognition of self‐DNA and ‐RNA through toll‐like receptors (TLRs). 143 , 144
In SLE, both the absolute number and frequency of NK cells are diminished in the peripheral blood of patients, especially in those with active disease or with severe clinical manifestations such as lupus nephritis and thrombocytopenia. 95 , 145 , 146 , 147 Some studies found no difference in the proportions of NK cell subsets, 78 , 145 while others observed an increased frequency of CD56bright NK cells. 148 These discrepancies might be explained by immunosuppressive therapies the patients were receiving. Phenotypic alterations in SLE NK cells include increased expression of CD69, NKp46, CD86, and OX40/CD134, 78 , 146 , 148 , 149 suggesting a dysfunctional state. CD69 expression on NK cells appears to correlate with disease activity. 149 Other changes include upregulation of inhibitory CD94‐NKG2A and reduced CD16. 78 However, the expression of other NK receptors, including NKG2C, NKG2D and KIR family members, is less definitive, likely reflecting clinical heterogeneity of SLE. 78 , 149 , 150
NK cells might contribute to protection against SLE by eliminating DCs, 146 , 150 but peripheral NK cell cytotoxicity is impaired in SLE patients irrespective of disease activity. 95 , 145 , 146 , 148 , 149 , 151 This impaired cytotoxicity might, at least partially, result from an intrinsic NK cell defect, because first‐degree relatives of SLE patients showed similar impairment of NK‐mediated killing compared with healthy donors. 151 Alternative explanations include inhibition by anti‐lymphocyte antibodies, 95 , 152 reduced IL‐2Rβ expression on NK cells, 95 and/or a defective NK response to IL‐15. 149 , 153 Autoantibodies to CD94 and KIRs have been described in SLE and may contribute to the reduced cytotoxic function of NK cells and increase the risk of lupus nephritis. 154 , 155
Natural killer cells have been implicated in the pathogenesis of SLE through their interactions with pDCs. 150 , 156 , 157 NK cells augment IFN‐α production by immune complex‐activated pDCs through the secretion of CCL4 and cell–cell interaction in an LFA‐1‐ and DNAM‐1‐dependent manner 156 (Figure 1d). In turn, pDC‐derived IFN‐α is essential for NK cell development, maturation and IFN‐γ production. 150 , 158 Bidirectional activation of these two cell types establishes a highly inflammatory milieu containing abundant cytokines and chemokines, including IFN‐α, IFN‐γ, TNF‐α, IL‐6, IL‐8, CCL3 and CCL4 157 , 159 (Figure 1d). Other murine studies support a pathogenic role for NK cells in lupus. In the TLR7 transgenic and FcγRIIB–/– murine models of SLE, chronic TLR7 signalling has been associated with extended survival of NK cells and proliferation of immature NK cells. 146 , 160 An atypical NK cell subset that possesses both NK‐ and DC‐like functions has also been reported. 160 These unique NK/DC co‐express NK1.1, CD11c, CD122 and MHC‐II, respond to IL‐15 stimulation, produce type I and II IFNs, are highly proliferative, and demonstrate both cytotoxic and antigen‐presenting functions. Remarkably, adoptive transfer of these atypical NK/DC cells to wildtype mice induces lupus‐like autoimmunity. 160 Consistent with this finding, a similar subset of CD3– CD56+ HLA‐DR+ CD11c+ NK cells have been identified in SLE patients, although their precise role remains to be defined. 146 In another study, 147 a subset of proliferating Ki67+ NK cells were identified in SLE patients and was associated with more severe disease, active nephritis and a lowered total NK cell number. Whether these HLA‐DR+ CD11c+ or Ki67+ NK cells truly represent the human counterpart of pathogenic murine NK:DCs in SLE remains to be determined. 147 , 160 More recently, inducible expansion of cytotoxic lymphocytes with an IL‐15 superagonist led to the exacerbation of lupus parameters in mice. However, this was shown to be driven primarily by CD8+ T cell expansion and not NK cells, 161 arguing against a direct pathogenic role of NK cells in lupus, at least in the MRL/lpr lupus model.
Lupus nephritis is a serious manifestation of SLE and is often studied as a model for organ involvement. Single‐cell RNA sequencing revealed an abundance of both CD56dim CD16+ and CD56bright CD16– NK subsets in kidney biopsies of patients with lupus nephritis, 103 but what role these NK cells play has not been characterised (Figure 1d). Similarly, in murine MRL/lpr and MRL/MpJ models of SLE, NK cells are actively recruited into kidneys during the early phases of disease. 79 , 150 These observational studies await functional evidence to confirm the involvement of NK cells in the pathogenesis of SLE.
In summary, interaction of peripheral NK cells with pDCs in a reciprocal manner could contribute to an exaggerated systemic inflammatory response in SLE, that is characterised by type I and type II IFN responses. Further investigations are needed to determine if interfering such NK‐pDC crosstalk is therapeutically beneficial and to define if organ‐infiltrating NK cells play an immune regulatory function in lupus nephritis.
NK cells in type 1 diabetes mellitus
Type 1 diabetes mellitus (T1DM) is probably mediated by CD8+ T cells, which selectively destroy pancreatic β cells, causing insulin deficiency and hyperglycaemia. 162 Inflamed islets are infiltrated by cytotoxic CD8+ T cells. CD4+ T cells, B cells, and NK cells have also been found. 163 , 164 , 165
Studies characterising NK cell numbers and activity in human T1DM demonstrate that NK cell deficiency is universal. A decrease in peripheral blood NK cell number was observed both in patients with newly onset T1DM 94 and those with long‐standing disease. 166 Furthermore, functional deficiencies of NK cells in long‐standing diabetic patients have been reported. Although blood NK cells displayed a hyperactivated state (IFN‐γ‐producing) at disease onset, these cells expressed lower levels of activating receptors (i.e. NKG2D, NKp30 and NKp46) and had decreased IFN‐γ expression and NKG2D‐dependent cytolytic activity in the chronic phase of disease, compared with control subjects. 94 , 166
A disease‐promoting effect of NK cells in diabetes is proposed based on studies in the NOD mice. NK cells infiltrate into the pancreas prior to T cells and have an activated phenotype, with enhanced proliferation and spontaneous IFN‐γ production and degranulation 101 , 102 , 167 (Figure 1e). The proportion and numbers of NK cell infiltrating the pancreas positively correlated with autoimmune responses in NOD mice, and depletion of NK cells significantly inhibited anti‐CTLA‐4‐induced exacerbation of diabetes. 168 In this study, early islet destruction was proposed to be due to NK cell‐derived IFN‐γ. 168 However, transgenic NOD mice expressing a dominant negative IFN‐γ receptor on β cells had a similar incidence of diabetes compared to non‐transgenic mice, 169 indicating that any direct effect of IFN‐γ on β cells is dispensable for diabetes, at least in the NOD model.
Abundant NK cell infiltration and degranulation were reported during the evolution of destructive insulitis in low‐dose streptozotocin‐induced diabetes model. 167 Genetic deletion or blockade of NKp46 abrogated the development of diabetes in NOD mice. A subsequent study, however, queried the role of NK cells in spontaneous diabetes in NOD mice because anti‐NK1.1‐induced NK cell depletion caused only a slight delay in the onset of full‐blown disease. 102
Interestingly, destruction of pancreatic β cells by NK cells and the subsequent development of T1DM can occur following enterovirus infection. Animal and human studies have highlighted the role of NK cells in mediating group B4 coxsackieviruses (CV‐B4)‐induced autoimmunity against islet cells. 164 , 170 In chronic infection, CV‐B4‐infected islet cells can downregulate the surface expression of HLA class I molecules on β cells and avoid recognition and killing by cytotoxic T cells. These islets remain susceptible to NK cell‐mediated elimination 170 but β cell apoptosis could lead to the release of potential autoantigens that may trigger autoreactivity and cause further β cell destruction 171 (Figure 1e). Consistently, NK cell depletion using anti‐asialo‐GM1 reduced early CVB4‐induced insulitis and islet destruction in SOCS1‐Tg NOD mice. 170 NK cells may therefore contribute to CV‐B4‐associated T1DM through excessive killing of infected pancreatic β islet cells.
In sum, current studies suggest that NK cells could contribute to the generation of autoantigens in enterovirus‐associated T1DM through pancreatic β islet killing. However, the role of NK cells in sterile T1DM is unknown. Future studies are required to capture phenotypic and functional diversity of NK cells in both CV‐B4‐associated and sterile T1DM subtypes.
NK cells in idiopathic inflammatory myopathies
Idiopathic inflammatory myopathies (IIMs) comprise a group of uncommon chronic inflammatory autoimmune diseases affecting skeletal muscles. IIMs have been further classified into several subtypes based on differences in immunopathology, including polymyositis, dermatomyositis, anti‐synthetase syndrome, immune‐mediated necrotising myopathy, sporadic inclusion body myositis and nonspecific myositis. 172 IIMs are characterised by chronic muscle inflammation and destruction, leading to muscle fibre degeneration and weakness. The causes of IIMs are unclear but autoreactive CD8+ T cells, CD4+ T cells and/or autoantibodies have all been identified in muscle biopsies. 173
As in other autoimmune disorders, reductions in the number and frequency of circulating NK cells have been observed in active dermatomyositis, 174 and levels normalise with disease remission. 99 , 175 An increased number of NK cells have been reported in affected muscles of juvenile dermatomyositis patients early in the disease course, 176 but not in adult dermatomyositis patients. 177 Impaired cytotoxicity of NK cells has been reported in dermatomyositis, which may be related to compromised PLCγ2 signalling and a defect in calcium flux. 99 Whether and how NK cells contribute to dermatomyositis remains unclear.
A role for NK cells in the pathogenesis of anti‐synthetase syndrome has been hypothesised, specifically in the generation of autoantigens (Figure 1f). Anti‐synthetase syndrome is defined by the presence of autoantibodies against tRNA synthetases, most commonly histidyl tRNA synthetase, and characteristic clinical features include myositis and extramuscular manifestations (i.e. interstitial lung disease and arthritis). 178 Although anti‐synthetase syndrome is conceptualised as a myopathy, mounting evidence suggests disease induction likely occurs in the lungs. Epidemiologically, anti‐synthetase syndrome is strongly associated with prior respiratory insults, 179 , 180 and an immunogenic, granzyme B‐cleavable of histidyl tRNA synthetase has been identified in alveolar epithelium. 181 While NK cells are scarce in the inflamed muscles of patients with anti‐synthetase syndrome, they are greatly expanded in affected lungs and express granzyme B. 98 The number of circulating NK cells in active anti‐synthetase syndrome patients is comparable to healthy controls, but there is a higher frequency of NK cells expressing granzyme B. These NK cells display a mature CD57hi phenotype but low levels of NKp30 activating receptor. 98 This pattern appears to be specific to anti‐synthetase syndrome, as the percentage of differentiated CD57+ NK cells in the circulation of sporadic inclusion body myositis and immune‐mediated necrotising myopathy patients is comparable to healthy controls. 182 Whether alveolar NK cells contribute to the initiation of autoimmunity through the generation of immunogenic peptides of histidyl tRNA synthetase in the anti‐synthetase syndrome warrants further investigation (Figure 1f).
Compared to other autoimmune disorders described above, NK cells remain less well defined in IIMs. At least in anti‐synthetase syndrome, alveolar NK cells are thought to give rise to autoantigens such as histidyl tRNA synthetase following respiratory insults. However, it is unknown whether this occurs through excessive cytolysis of alveolar epithelial cells and/or granzyme B‐mediated cleavage of histidyl tRNA synthetase. Future studies are needed to evaluate whether similar numerical and functional changes in NK cells occur in the discrete subtype of IIM.
NK cell therapy in autoimmunity
Natural killer cells may hold significant therapeutic potential in autoimmune diseases. First, blocking or agonistic antibodies that target activating, inhibitory and/or cytokine receptors could be used to potentiate cytotoxic activity against autoreactive immune cells, or to suppress the production of pathogenic cytokines such as GM‐CSF, IFN‐g and TNF‐α (Figure 2a). For example, anti‐NKG2A blocks an NK inhibitory receptor and potentiates NK cells cytotoxicity towards pathogenic Th17 and Tfh, alleviating EAE, 73 , 141 as well as CIA. 119 Second, engineering of chimeric antigen receptor (CAR) NK cells could eliminate autoreactive immune cells in a ‘targeted’ manner (Figure 2b). This approach follows the use of CD19‐targeted CAR T cell therapy to deplete autoreactive B cells in murine lupus. 183 Subsequently, CAR NK cells expressing PD‐L1 were shown to eliminate PD‐1hi Tfh cells ex vivo and in a humanised mouse model of lupus‐like disease. 184 Alternatively, engineering of a multifunctional engager that incorporates combination of antibodies targeting antigens expressed by autoreactive immune cells and activating/inhibitory receptors could facilitate NK cell interaction and cytolytic activity (Figure 2c). Multifunctional NK cell engagers have recently demonstrated for cancer immunotherapy. 48
Figure 2.
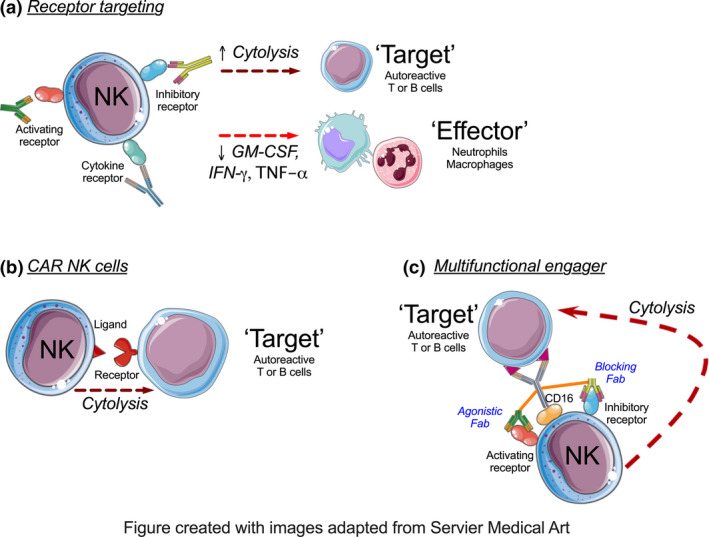
Therapeutic potential of NK‐based therapy in autoimmune disorders. (a) Blocking or agonistic antibodies target cell‐surface receptors of NK cells to potentiate their cytotoxicity against autoreactive immune cells, or suppress the production of pathogenic cytokines. (b) Engineering of chimeric antigen receptor (CAR) could direct NK cells to eliminate autoreactive immune cells. (c) A multifunctional engager combining antibodies that target antigens expressed by autoreactive cells and activating/inhibitory receptors on NK cells could facilitate NK cell interaction and cytolytic activity towards target autoimmune lymphocytes.
Conclusions and perspectives
Considerable evidence suggests that numerical and/or functional deficits in NK cells are common in a variety of inflammatory autoimmune diseases. However, the mechanisms underpinning this abnormality may differ between diseases. It remains contentious whether NK cell cytotoxic function constitutes an immune checkpoint by direct elimination of autoreactive immune cells or is merely a consequence of disease. Recent appreciation of NK cells’ heterogeneity in cancer studies calls for a more careful interpretation of past studies in autoimmune disease settings. The integration of new technical advances such as single‐cell transcriptomic and mass cytometry may similarly reveal an underappreciated phenotypic diversity of NK cells in autoimmune diseases.
Studies defining the role of NK cells in cancer have paved the way for NK cell‐based cancer immunotherapies. We believe detailed understanding of the temporal and spatial functions of NK cells in autoimmune inflammatory diseases could similarly offer opportunities to target NK cells in concert with other immune‐modifying therapies. While several KIR‐HLA haplotypes have been genetically associated with discrete autoimmune disorders, caution should be exercised when drawing conclusions from observational and ex vivo studies using patient samples. These novel hypotheses must be accompanied by proof‐of‐principle experiments at functional protein level with genetic and pharmacologic in vivo models, such as NK‐specific conditional knockout mice or KIR isoform‐specific antibodies. Such information may also be relevant in understanding immunotherapy‐associated rheumatic adverse events, particularly with the increasing use of immune checkpoint inhibitors and introduction of NK‐based cancer immunotherapies. Whether NK cell dysfunction contributes to the development of these complex autoimmune‐like conditions merits further investigation.
Conflict of Interest
FSFG is a consultant for Biotheus Inc.
Author Contributions
Yuyan Yang: Writing‐original draft; Writing‐review & editing. Jess Day: Writing‐review & editing. Fernando Souza‐Fonseca‐Guimaraes: Writing‐review & editing. Ian Wicks: Writing‐review & editing. Cynthia Louis: Conceptualization; Supervision; Visualization; Writing‐original draft; Writing‐review & editing.
Acknowledgments
IPW is supported by NHMRC Program Grant #1113577, NHMRC Practitioner Fellowship #1154325, and The Reid Charitable Trusts. FSFG is funded by The University of Queensland, a National Health and Medical Research Council (NHMRC) of Australia grant (#1140406), and a grant (#1158085) awarded through the Priority driven Collaborative Cancer Research Scheme and funded by Cure Cancer Australia with the assistance of Cancer Australia.
Contributor Information
Ian P Wicks, Email: wicks@wehi.edu.au.
Cynthia Louis, Email: louis.c@wehi.edu.au.
References
- 1. Bluestone JA, Bour‐Jordan H, Cheng M, Anderson M. T cells in the control of organ‐specific autoimmunity. J Clin Invest 2015; 125: 2250–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 2015; 125: 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex‐mediated neutrophil recruitment and tissue injury. Circulation 2009; 120: 2012–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Funes SC, Rios M, Escobar‐Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology 2018; 154: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castellanos JG, Longman RS. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest 2019; 129: 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caligiuri MA. Human natural killer cells. Blood 2008; 112: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ‐specific features of natural killer cells. Nat Rev Immunol 2011; 11: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huntington ND. The unconventional expression of IL‐15 and its role in NK cell homeostasis. Immunol Cell Biol 2014; 92: 210–213. [DOI] [PubMed] [Google Scholar]
- 9. Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol 2016; 16: 112–123. [DOI] [PubMed] [Google Scholar]
- 10. Gordon SM, Chaix J, Rupp LJ et al The transcription factors T‐bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 2012; 36: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moffett‐King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002; 2: 656–663. [DOI] [PubMed] [Google Scholar]
- 12. Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol 2013; 31: 163–194. [DOI] [PubMed] [Google Scholar]
- 13. Simonetta F, Alvarez M, Negrin RS. Natural killer cells in graft‐versus‐host‐disease after allogeneic hematopoietic cell transplantation. Front Immunol 2017; 8: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandenhaute J, Wouters CH, Matthys P. Natural killer cells in systemic autoinflammatory diseases: a focus on systemic juvenile idiopathic arthritis and macrophage activation syndrome. Front Immunol 2019; 10: 3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther 2013; 15: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peritt D, Robertson S, Gri G et al Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol 1998; 161: 5821–5824. [PubMed] [Google Scholar]
- 17. Crinier A, Milpied P, Escaliere B et al High‐dimensional single‐cell analysis identifies organ‐specific signatures and conserved NK cell subsets in humans and mice. Immunity 2018; 49: 971–986 e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiossone L, Chaix J, Fuseri N et al Maturation of mouse NK cells is a 4‐stage developmental program. Blood 2009; 113: 5488–5496. [DOI] [PubMed] [Google Scholar]
- 19. Walzer T, Blery M, Chaix J et al Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci USA 2007; 104: 3384–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S, Iizuka K, Kang HS et al In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 2002; 3: 523–528. [DOI] [PubMed] [Google Scholar]
- 21. Poli A, Michel T, Theresine M et al CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN‐γ on activation. Proc Natl Acad Sci USA 2011; 108: 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fehniger TA, Cooper MA, Nuovo GJ et al CD56bright natural killer cells are present in human lymph nodes and are activated by T cell‐derived IL‐2: a potential new link between adaptive and innate immunity. Blood 2003; 101: 3052–3057. [DOI] [PubMed] [Google Scholar]
- 24. Vacca P, Vitale C, Montaldo E et al CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci USA 2011; 108: 2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dalbeth N, Gundle R, Davies RJ et al CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol 2004; 173: 6418–6426. [DOI] [PubMed] [Google Scholar]
- 26. Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum 2002; 46: 1763–1772. [DOI] [PubMed] [Google Scholar]
- 27. Jacobs R, Hintzen G, Kemper A et al CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 2001; 31: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen N, Odum N, Urso B, Lanier LL, Spee P. Cytotoxicity of CD56bright NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA‐1 and TRAIL and dampened via CD94/NKG2A. PLoS One 2012; 7: e31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laroni A, Armentani E, Kerlero de Rosbo N et al Dysregulation of regulatory CD56bright NK cells/T cells interactions in multiple sclerosis. J Autoimmun 2016; 72: 8–18. [DOI] [PubMed] [Google Scholar]
- 30. Smith SL, Kennedy PR, Stacey KB et al Diversity of peripheral blood human NK cells identified by single‐cell RNA sequencing. Blood Adv 2020; 4: 1388–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horowitz A, Strauss‐Albee DM, Leipold M et al Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5: 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Andrade LF, Lu Y, Luoma A et al Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 2019; 41–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31: 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fehniger TA, Shah MH, Turner MJ et al Differential cytokine and chemokine gene expression by human NK cells following activation with IL‐18 or IL‐15 in combination with IL‐12: implications for the innate immune response. J Immunol 1999; 162: 4511–4520. [PubMed] [Google Scholar]
- 35. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T‐lymphocyte‐independent induction of interferon gamma by an intracellular parasite and induces resistance in T‐cell‐deficient hosts. Proc Natl Acad Sci USA 1993; 90: 6115–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chaix J, Tessmer MS, Hoebe K et al Cutting edge: Priming of NK cells by IL‐18. J Immunol 2008; 181: 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL‐12/15/18‐preactivated NK cells against established tumors. J Exp Med 2012; 209: 2351–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans‐presenting interleukin 15. Immunity 2007; 26: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo . J Immunol 2008; 180: 1592–1597. [DOI] [PubMed] [Google Scholar]
- 41. Teng MW, Andrews DM, McLaughlin N et al IL‐23 suppresses innate immune response independently of IL‐17A during carcinogenesis and metastasis. Proc Natl Acad Sci USA 2010; 107: 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity 2006; 24: 249–257. [DOI] [PubMed] [Google Scholar]
- 43. Kim S, Poursine‐Laurent J, Truscott SM et al Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005; 436: 709–713. [DOI] [PubMed] [Google Scholar]
- 44. Anfossi N, Andre P, Guia S et al Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006; 25: 331–342. [DOI] [PubMed] [Google Scholar]
- 45. Belanger S, Tu MM, Rahim MM et al Impaired natural killer cell self‐education and "missing‐self" responses in Ly49‐deficient mice. Blood 2012; 120: 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 1991; 253: 199–202. [DOI] [PubMed] [Google Scholar]
- 47. Andre P, Denis C, Soulas C et al Anti‐NKG2A mAb is a checkpoint inhibitor that promotes anti‐tumor immunity by unleashing both T and NK cells. Cell 2018; 175: 1731‐1743 e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gauthier L, Morel A, Anceriz N et al Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019; 177: 1701‐1713 e1716. [DOI] [PubMed] [Google Scholar]
- 49. Martin MP, Nelson G, Lee JH et al Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig‐like receptor genes in the absence of specific HLA‐C alleles. J Immunol 2002; 169: 2818–2822. [DOI] [PubMed] [Google Scholar]
- 50. Nelson GW, Martin MP, Gladman D et al Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig‐like receptor combinations in psoriatic arthritis. J Immunol 2004; 173: 4273–4276. [DOI] [PubMed] [Google Scholar]
- 51. Chandran V, Bull SB, Pellett FJ et al Killer‐cell immunoglobulin‐like receptor gene polymorphisms and susceptibility to psoriatic arthritis. Rheumatology (Oxford) 2014; 53: 233–239. [DOI] [PubMed] [Google Scholar]
- 52. Yen JH, Moore BE, Nakajima T et al Major histocompatibility complex class I‐recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med 2001; 193: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Akhtari M, Farazmand A, Mahmoudi M et al Analysis of killer cell immunoglobulin‐like receptors and their human leukocyte antigen‐ligands gene polymorphisms in Iranian patients with systemic lupus erythematosus. Lupus 2016; 25: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 54. Jones DC, Edgar RS, Ahmad T et al Killer Ig‐like receptor (KIR) genotype and HLA ligand combinations in ulcerative colitis susceptibility. Genes Immun 2006; 7: 576–582. [DOI] [PubMed] [Google Scholar]
- 55. Hollenbach JA, Ladner MB, Saeteurn K et al Susceptibility to Crohn's disease is mediated by KIR2DL2/KIR2DL3 heterozygosity and the HLA‐C ligand. Immunogenetics 2009; 61: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chan AT, Kollnberger SD, Wedderburn LR, Bowness P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin‐like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum 2005; 52: 3586–3595. [DOI] [PubMed] [Google Scholar]
- 57. Bowness P, Ridley A, Shaw J et al Th17 cells expressing KIR3DL2+ and responsive to HLA‐B27 homodimers are increased in ankylosing spondylitis. J Immunol 2011; 186: 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin L, Ma C, Wei B et al Human NK cells licensed by killer Ig receptor genes have an altered cytokine program that modifies CD4+ T cell function. J Immunol 2014; 193: 940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thoren FB, Riise RE, Ousback J et al Human NK Cells induce neutrophil apoptosis via an NKp46‐ and Fas‐dependent mechanism. J Immunol 2012; 188: 1668–1674. [DOI] [PubMed] [Google Scholar]
- 60. Schuster IS, Wikstrom ME, Brizard G et al TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014; 41: 646–656. [DOI] [PubMed] [Google Scholar]
- 61. Jiang W, Chai NR, Maric D, Bielekova B. Unexpected role for granzyme K in CD56bright NK cell‐mediated immunoregulation of multiple sclerosis. J Immunol 2011; 187: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol 2019; 10: 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cerboni C, Zingoni A, Cippitelli M et al Antigen‐activated human T lymphocytes express cell‐surface NKG2D ligands via an ATM/ATR‐dependent mechanism and become susceptible to autologous NK‐ cell lysis. Blood 2007; 110: 606–615. [DOI] [PubMed] [Google Scholar]
- 64. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 2018; 18: 671–688. [DOI] [PubMed] [Google Scholar]
- 65. Hao J, Liu R, Piao W et al Central nervous system (CNS)‐resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med 2010; 207: 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Q, Sanai N, Jin WN et al Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci 2016; 19: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nedvetzki S, Sowinski S, Eagle RA et al Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 2007; 109: 3776–3785. [DOI] [PubMed] [Google Scholar]
- 68. Jiang W, Li D, Han R et al Acetylcholine‐producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc Natl Acad Sci USA 2017; 114: E6202–E6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vandenhaute J, Avau A, Filtjens J et al Regulatory role for NK cells in a mouse model of systemic juvenile idiopathic arthritis. J Immunol 2019; 203: 3339–3348. [DOI] [PubMed] [Google Scholar]
- 70. Bellora F, Castriconi R, Dondero A et al The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc Natl Acad Sci USA 2010; 107: 21659–21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bernson E, Christenson K, Pesce S et al Downregulation of HLA class I renders inflammatory neutrophils more susceptible to NK cell‐induced apoptosis. Front Immunol 2019; 10: 2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med 1997; 186: 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lu L, Ikizawa K, Hu D et al Regulation of activated CD4+ T cells by NK cells via the Qa‐1‐NKG2A inhibitory pathway. Immunity 2007; 26: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature 2011; 481: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rydyznski C, Daniels KA, Karmele EP et al Generation of cellular immune memory and B‐cell immunity is impaired by natural killer cells. Nat Commun 2015; 6: 6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rydyznski CE, Cranert SA, Zhou JQ et al Affinity maturation is impaired by natural killer cell suppression of germinal centers. Cell Rep 2018; 24: 3367–3373 e3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin‐Fontecha A, Thomsen LL, Brett S et al Induced recruitment of NK cells to lymph nodes provides IFN‐gamma for T(H)1 priming. Nat Immunol 2004; 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 78. Hervier B, Beziat V, Haroche J et al Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon‐γ production in patients with active disease. Arthritis Rheum 2011; 63: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 79. Spada R, Rojas JM, Barber DF. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol 2015; 98: 479–487. [DOI] [PubMed] [Google Scholar]
- 80. Ottaviani C, Nasorri F, Bedini C et al CD56brightCD16– NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol 2006; 36: 118–128. [DOI] [PubMed] [Google Scholar]
- 81. Yamin R, Berhani O, Peleg H et al High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci Rep 2019; 9: 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Al‐Mossawi MH, Chen L, Fang H et al Unique transcriptome signatures and GM‐CSF expression in lymphocytes from patients with spondyloarthritis. Nat Commun 2017; 8: 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gross CC, Schulte‐Mecklenbeck A, Runzi A et al Impaired NK‐mediated regulation of T‐cell activity in multiple sclerosis is reconstituted by IL‐2 receptor modulation. Proc Natl Acad Sci USA 2016; 113: E2973–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cuturi MC, Anegon I, Sherman F et al Production of hematopoietic colony‐stimulating factors by human natural killer cells. J Exp Med 1989; 169: 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Warren HS, Kinnear BF, Phillips JH, Lanier LL. Production of IL‐5 by human NK cells and regulation of IL‐5 secretion by IL‐4, IL‐10, and IL‐12. J Immunol 1995; 154: 5144–5152. [PubMed] [Google Scholar]
- 86. Smyth MJ, Zachariae CO, Norihisa Y et al IL‐8 gene expression and production in human peripheral blood lymphocyte subsets. J Immunol 1991; 146: 3815–3823. [PubMed] [Google Scholar]
- 87. Bluman EM, Bartynski KJ, Avalos BR, Caligiuri MA. Human natural killer cells produce abundant macrophage inflammatory protein‐1 alpha in response to monocyte‐derived cytokines. J Clin Invest 1996; 97: 2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dorner BG, Smith HR, French AR et al Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J Immunol 2004; 172: 3119–3131. [DOI] [PubMed] [Google Scholar]
- 89. Soderstrom K, Stein E, Colmenero P et al Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA 2010; 107: 13028–13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut‐Landmann S. IL‐17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014; 40: 117–127. [DOI] [PubMed] [Google Scholar]
- 91. Louis C, Souza‐Fonseca‐Guimaraes F, Yang Y et al NK cell‐derived GM‐CSF potentiates inflammatory arthritis and is negatively regulated by CIS. J Exp Med 2020; 217: e20191421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bottcher JP, Bonavita E, Chakravarty P et al NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018; 172: 1022–1037 e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pridgeon C, Lennon GP, Pazmany L et al Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56<sup>bright</sup>, CD9<sup>4brigh</sup>t, CD1<sup>58negative</sup> phenotype. Rheumatology (Oxford) 2003; 42: 870–878. [DOI] [PubMed] [Google Scholar]
- 94. Rodacki M, Svoren B, Butty V et al Altered natural killer cells in type 1 diabetic patients. Diabetes 2007; 56: 177–185. [DOI] [PubMed] [Google Scholar]
- 95. Park YW, Kee SJ, Cho YN et al Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum 2009; 60: 1753–1763. [DOI] [PubMed] [Google Scholar]
- 96. Struyf NJ, Snoeck HW, Bridts CH, De Clerck LS, Stevens WJ. Natural killer cell activity in Sjogren's syndrome and systemic lupus erythematosus: stimulation with interferons and interleukin‐2 and correlation with immune complexes. Ann Rheum Dis 1990; 49: 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gonzalez‐Amaro R, Alcocer‐Varela J, Alarcon‐Segovia D. Natural killer cell activity in dermatomyositis‐polymyositis. J Rheumatol 1987; 14: 307–310. [PubMed] [Google Scholar]
- 98. Hervier B, Perez M, Allenbach Y et al Involvement of NK cells and NKp30 pathway in antisynthetase syndrome. J Immunol 2016; 197: 1621–1630. [DOI] [PubMed] [Google Scholar]
- 99. Throm AA, Alinger JB, Pingel JT et al Dysregulated NK cell PLCγ2 signaling and activity in juvenile dermatomyositis. JCI Insight 2018; 3: e123236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lo CK, Lam QL, Sun L et al Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin‐17 production. Arthritis Rheum 2008; 58: 2700–2711. [DOI] [PubMed] [Google Scholar]
- 101. Brauner H, Elemans M, Lemos S et al Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol 2010; 184: 2272–2280. [DOI] [PubMed] [Google Scholar]
- 102. Beilke JN, Meagher CT, Hosiawa K et al NK cells are not required for spontaneous autoimmune diabetes in NOD mice. PLoS One 2012; 7: e36011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Arazi A, Rao DA, Berthier CC et al The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol 2019; 20: 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rodriguez‐Martin E, Picon C, Costa‐Frossard L et al Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol 2015; 180: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sathe P, Delconte RB, Souza‐Fonseca‐Guimaraes F et al Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun 2014; 5: 4539. [DOI] [PubMed] [Google Scholar]
- 106. Mace EM, Orange JS. Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front Immunol 2016; 7: 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 108. Dahdah A, Habir K, Nandakumar KS et al Germinal center B cells are essential for collagen‐induced arthritis. Arthritis Rheumatol 2018; 70: 193–203. [DOI] [PubMed] [Google Scholar]
- 109. Murphy CA, Langrish CL, Chen Y et al Divergent pro‐ and antiinflammatory roles for IL‐23 and IL‐12 in joint autoimmune inflammation. J Exp Med 2003; 198: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol 2009; 39: 2040–2044. [DOI] [PubMed] [Google Scholar]
- 111. Monach PA, Nigrovic PA, Chen M et al Neutrophils in a mouse model of autoantibody‐mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function‐associated antigen 1. Arthritis Rheum 2010; 62: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Misharin AV, Cuda CM, Saber R et al Nonclassical Ly6C– monocytes drive the development of inflammatory arthritis in mice. Cell Rep 2014; 9: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast‐like synoviocytes. Nat Rev Rheumatol 2020; 16: 316–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hasegawa T, Kikuta J, Sudo T et al Identification of a novel arthritis‐associated osteoclast precursor macrophage regulated by FoxM1. Nat Immunol 2019; 20: 1631–1643. [DOI] [PubMed] [Google Scholar]
- 115. Lin SJ, Hsu CY, Kuo ML et al Phenotypic and functional characterization of natural killer cells in rheumatoid arthritis‐regulation with interleukin‐15. Sci Rep 2020; 10: 5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Aramaki T, Ida H, Izumi Y et al A significantly impaired natural killer cell activity due to a low activity on a per‐cell basis in rheumatoid arthritis. Mod Rheumatol 2009; 19: 245–252. [DOI] [PubMed] [Google Scholar]
- 117. Richter J, Benson V, Grobarova V et al CD161 receptor participates in both impairing NK cell cytotoxicity and the response to glycans and vimentin in patients with rheumatoid arthritis. Clin Immunol 2010; 136: 139–147. [DOI] [PubMed] [Google Scholar]
- 118. Chalan P, Bijzet J, Kroesen BJ, Boots AM, Brouwer E. Altered natural killer cell subsets in seropositive arthralgia and early rheumatoid arthritis are associated with autoantibody status. J Rheumatol 2016; 43: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 119. Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen‐induced arthritis. Proc Natl Acad Sci USA 2011; 108: 14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wu HJ, Sawaya H, Binstadt B et al Inflammatory arthritis can be reined in by CpG‐induced DC‐NK cell cross talk. J Exp Med 2007; 204: 1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Teige A, Bockermann R, Hasan M et al CD1d‐dependent NKT cells play a protective role in acute and chronic arthritis models by ameliorating antigen‐specific Th1 responses. J Immunol 2010; 185: 345–356. [DOI] [PubMed] [Google Scholar]
- 122. de Matos CT, Berg L, Michaelsson J et al Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology 2007; 122: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nielsen N, Pascal V, Fasth AE et al Balance between activating NKG2D, DNAM‐1, NKp44 and NKp46 and inhibitory CD94/NKG2A receptors determine natural killer degranulation towards rheumatoid arthritis synovial fibroblasts. Immunology 2014; 142: 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang AL, Colmenero P, Purath U et al Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood 2007; 110: 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Victorino F, Sojka DK, Brodsky KS et al Tissue‐resident NK cells mediate ischemic kidney injury and are not depleted by anti‐Asialo‐GM1 antibody. J Immunol 2015; 195: 4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nishikado H, Mukai K, Kawano Y, Minegishi Y, Karasuyama H. NK cell‐depleting anti‐asialo GM1 antibody exhibits a lethal off‐target effect on basophils in vivo . J Immunol 2011; 186: 5766–5771. [DOI] [PubMed] [Google Scholar]
- 127. Feng S, Madsen SH, Viller NN et al Interleukin‐15‐activated natural killer cells kill autologous osteoclasts via LFA‐1, DNAM‐1 and TRAIL, and inhibit osteoclast‐mediated bone erosion in vitro . Immunology 2015; 145: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet 2018; 391: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 129. Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 2011; 164: 1079–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wagner CA, Roque PJ, Goverman JM. Pathogenic T cell cytokines in multiple sclerosis. J Exp Med 2020; 217: e20190460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Galli E, Hartmann FJ, Schreiner B et al GM‐CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nat Med 2019; 25: 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Komuczki J, Tuzlak S, Friebel E et al Fate‐mapping of GM‐CSF expression identifies a discrete subset of inflammation‐driving T helper cells regulated by cytokines IL‐23 and IL‐1β. Immunity 2019; 50: 1289–1304.e1286. [DOI] [PubMed] [Google Scholar]
- 133. Flach AC, Litke T, Strauss J et al Autoantibody‐boosted T‐cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc Natl Acad Sci USA 2016; 113: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kastrukoff LF, Lau A, Wee R et al Clinical relapses of multiple sclerosis are associated with 'novel' valleys in natural killer cell functional activity. J Neuroimmunol 2003; 145: 103–114. [DOI] [PubMed] [Google Scholar]
- 135. Morandi F, Venturi C, Rizzo R et al Intrathecal soluble HLA‐E correlates with disease activity in patients with multiple sclerosis and may cooperate with soluble HLA‐G in the resolution of neuroinflammation. J Neuroimmune Pharmacol 2013; 8: 944–955. [DOI] [PubMed] [Google Scholar]
- 136. Saraste M, Irjala H, Airas L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon‐beta. Neurol Sci 2007; 28: 121–126. [DOI] [PubMed] [Google Scholar]
- 137. Marastoni D, Buriani A, Pisani AI et al Increased NK cell count in multiple sclerosis patients treated with dimethyl fumarate: a 2‐year longitudinal study. Front Immunol 2019; 10: 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Putzki N, Yaldizli O, Maurer M et al Efficacy of natalizumab in second line therapy of relapsing‐remitting multiple sclerosis: results from a multi‐center study in German speaking countries. Eur J Neurol 2010; 17: 31–37. [DOI] [PubMed] [Google Scholar]
- 139. Winkler‐Pickett R, Young HA, Cherry JM et al In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol 2008; 180: 4495–4506. [DOI] [PubMed] [Google Scholar]
- 140. Kwong B, Rua R, Gao Y et al T‐bet‐dependent NKp46+ innate lymphoid cells regulate the onset of TH17‐induced neuroinflammation. Nat Immunol 2017; 18: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Leavenworth JW, Schellack C, Kim HJ et al Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti‐NKG2A F(ab')2 . Proc Natl Acad Sci USA 2010; 107: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014; 384: 1878–1888. [DOI] [PubMed] [Google Scholar]
- 143. Sisirak V, Ganguly D, Lewis KL et al Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med 2014; 211: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 2006; 25: 383–392. [DOI] [PubMed] [Google Scholar]
- 145. Henriques A, Teixeira L, Ines L et al NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol 2013; 32: 805–813. [DOI] [PubMed] [Google Scholar]
- 146. Cruz‐Gonzalez DJ, Gomez‐Martin D, Layseca‐Espinosa E et al Analysis of the regulatory function of natural killer cells from patients with systemic lupus erythematosus. Clin Exp Immunol 2018; 191: 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hudspeth K, Wang S, Wang J et al Natural killer cell expression of Ki67 is associated with elevated serum IL‐15, disease activity and nephritis in systemic lupus erythematosus. Clin Exp Immunol 2019; 196: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schepis D, Gunnarsson I, Eloranta ML et al Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology 2009; 126: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lin SJ, Kuo ML, Hsiao HS et al Activating and inhibitory receptors on natural killer cells in patients with systemic lupus erythematosis‐regulation with interleukin‐15. PLoS One 2017; 12: e0186223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Huang Z, Fu B, Zheng SG et al Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol 2011; 186: 3421–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Green MR, Kennell AS, Larche MJ et al Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol 2005; 141: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Goto M, Tanimoto K, Horiuchi Y. Natural cell mediated cytotoxicity in systemic lupus erythematosus: suppression by antilymphocyte antibody. Arthritis Rheum 1980; 23: 1274–1281. [DOI] [PubMed] [Google Scholar]
- 153. Baranda L, de la Fuente H, Layseca‐Espinosa E et al IL‐15 and IL‐15R in leucocytes from patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005; 44: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 154. Hagberg N, Theorell J, Hjorton K et al Functional anti‐CD94/NKG2A and anti‐CD94/NKG2C autoantibodies in patients with systemic lupus erythematosus. Arthritis Rheumatol 2015; 67: 1000–1011. [DOI] [PubMed] [Google Scholar]
- 155. Segerberg F, Lundtoft C, Reid S et al Autoantibodies to killer cell immunoglobulin‐like receptors in patients with systemic lupus erythematosus induce natural killer cell hyporesponsiveness. Front Immunol 2019; 10: 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hagberg N, Berggren O, Leonard D et al IFN‐α production by plasmacytoid dendritic cells stimulated with RNA‐containing immune complexes is promoted by NK cells via MIP‐1β and LFA‐1. J Immunol 2011; 186: 5085–5094. [DOI] [PubMed] [Google Scholar]
- 157. Hagberg N, Theorell J, Schlums H et al Systemic lupus erythematosus immune complexes increase the expression of SLAM family members CD319 (CRACC) and CD229 (LY‐9) on plasmacytoid dendritic cells and CD319 on CD56dim NK cells. J Immunol 2013; 191: 2989–2998. [DOI] [PubMed] [Google Scholar]
- 158. Guan J, Miah SM, Wilson ZS et al Role of type I interferon receptor signaling on NK cell development and functions. PLoS One 2014; 9: e111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hjorton K, Hagberg N, Israelsson E et al Cytokine production by activated plasmacytoid dendritic cells and natural killer cells is suppressed by an IRAK4 inhibitor. Arthritis Res Ther 2018; 20: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Voynova EN, Skinner J, Bolland S. Expansion of an atypical NK cell subset in mouse models of systemic lupus erythematosus. J Immunol 2015; 194: 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Reighard SD, Krishnamurthy D, Cevik H et al Immunomodulatory effects of cytokine‐induced expansion of cytotoxic lymphocytes in a mouse model of lupus‐like disease. Cytotherapy 2020. e‐pub ahead of print Oct 19; 10.1016/j.jcyt.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Peters L, Posgai A, Brusko TM. Islet‐immune interactions in type 1 diabetes: the nexus of beta cell destruction. Clin Exp Immunol 2019; 198: 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Dotta F, Censini S, van Halteren AG et al Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent‐onset type 1 diabetic patients. Proc Natl Acad Sci USA 2007; 104: 5115–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Leete P, Willcox A, Krogvold L et al Differential insulitic profiles determine the extent of beta‐cell destruction and the age at onset of type 1 diabetes. Diabetes 2016; 65: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 166. Qin H, Lee IF, Panagiotopoulos C et al Natural killer cells from children with type 1 diabetes have defects in NKG2D‐dependent function and signaling. Diabetes 2011; 60: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gur C, Porgador A, Elboim M et al The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 168. Poirot L, Benoist C, Mathis D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc Natl Acad Sci USA 2004; 101: 8102–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Thomas HE, Parker JL, Schreiber RD, Kay TW. IFN‐gamma action on pancreatic beta cells causes class I MHC upregulation but not diabetes. J Clin Invest 1998; 102: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Flodstrom M, Maday A, Balakrishna D et al Target cell defense prevents the development of diabetes after viral infection. Nat Immunol 2002; 3: 373–382. [DOI] [PubMed] [Google Scholar]
- 171. Vives‐Pi M, Rodriguez‐Fernandez S, Pujol‐Autonell I. How apoptotic beta‐cells direct immune response to tolerance or to autoimmune diabetes: a review. Apoptosis 2015; 20: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lundberg IE, de Visser M, Werth VP. Classification of myositis. Nat Rev Rheumatol 2018; 14: 269–278. [DOI] [PubMed] [Google Scholar]
- 173. Zong M, Lundberg IE. Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol 2011; 7: 297–306. [DOI] [PubMed] [Google Scholar]
- 174. O'Gorman MR, Bianchi L, Zaas D, Corrochano V, Pachman LM. Decreased levels of CD54 (ICAM‐1)‐positive lymphocytes in the peripheral blood in untreated patients with active juvenile dermatomyositis. Clin Diagn Lab Immunol 2000; 7: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ernste FC, Crowson CS, de Padilla CL, Hein MS, Reed AM. Longitudinal peripheral blood lymphocyte subsets correlate with decreased disease activity in juvenile dermatomyositis. J Rheumatol 2013; 40: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 176. Pachman LM, Fedczyna TO, Lechman TS, Lutz J. Juvenile dermatomyositis: the association of the TNF alpha‐308A allele and disease chronicity. Curr Rheumatol Rep 2001; 3: 379–386. [DOI] [PubMed] [Google Scholar]
- 177. Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol 1984; 16: 193–208. [DOI] [PubMed] [Google Scholar]
- 178. Selva‐O'Callaghan A, Pinal‐Fernandez I, Trallero‐Araguas E et al Classification and management of adult inflammatory myopathies. Lancet Neurol 2018; 17: 816–828. [DOI] [PubMed] [Google Scholar]
- 179. Chinoy H, Adimulam S, Marriage F et al Interaction of HLA‐DRB1*03 and smoking for the development of anti‐Jo‐1 antibodies in adult idiopathic inflammatory myopathies: a European‐wide case study. Ann Rheum Dis 2012; 71: 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Svensson J, Holmqvist M, Lundberg IE, Arkema EV. Infections and respiratory tract disease as risk factors for idiopathic inflammatory myopathies: a population‐based case‐control study. Ann Rheum Dis 2017; 76: 1803–1808. [DOI] [PubMed] [Google Scholar]
- 181. Levine SM, Raben N, Xie D et al Novel conformation of histidyl‐transfer RNA synthetase in the lung: the target tissue in Jo‐1 autoantibody‐associated myositis. Arthritis Rheum 2007; 56: 2729–2739. [DOI] [PubMed] [Google Scholar]
- 182. Dzangue‐Tchoupou G, Allenbach Y, Preusse C, Stenzel W, Benveniste O. Mass cytometry reveals an impairment of B cell homeostasis in anti‐synthetase syndrome. J Neuroimmunol 2019; 332: 212–215. [DOI] [PubMed] [Google Scholar]
- 183. Kansal R, Richardson N, Neeli I et al Sustained B cell depletion by CD19‐targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med 2019; 11: eaav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Reighard SD, Cranert SA, Rangel KM et al Therapeutic targeting of follicular T cells with chimeric antigen receptor‐expressing natural killer cells. Cell Rep Med 2020; 1: 100003. [DOI] [PMC free article] [PubMed] [Google Scholar]


