Abstract
Background
Autologous serum eye drops, produced by separation of liquid and cellular components of the patient’s blood, contain biological nutrients present in natural tears. The aim of this study was to analyse changes in conjunctival impression cytology with transfer and both lachrymal stability and flow tests in patients with dry eye disease after treatment with autologous serum eye drops.
Materials and methods
Conjunctival impression cytology and lachrymal flow and stability tests, namely Schirmer’s and tear break-up time, were prospectively studied in patients with dry eye disease before and 1 month after treatment with autologous serum eye drops.
Results
Twenty-four patients (23 women, mean age 53.8±12.6 years) were included in the study. Ten patients (41.7%) had moderate and six (25.0%) had severe dry eye disease. Five patients had rheumatoid arthritis. After treatment, the number and density of conjunctival goblet cells, their size, the size of their nuclei and the nucleus/cytoplasm ratio increased significantly (202.3±107.5 vs 210.1±100.9 cells/mm2, p<0.01). Seven of ten patients with grade 3 or 4 metaplasia had an improvement in the degree of metaplasia. Both Schirmer’s test and tear break-up time improved significantly in this subgroup of patients. In the multivariate study, the increase in conjunctival goblet cells was associated with the number of goblet cells and the size of the cytoplasm at baseline. No adverse reactions were noted.
Discussion
Treatment with autologous serum eye drops for 1 month was well tolerated and improved tear production, lachrymal flow and stability tests and conjunctival impression cytology with transfer, increasing the density of the goblet cells.
Keywords: dry eye disease, autologous serum, conjunctival impression cytology, goblet cells, squamous metaplasia
INTRODUCTION
Dry eye disease (DED) is a multifactorial condition of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play aetiological roles1. Discomfort and visual disturbance are the fundamental symptoms of DED, accompanied by other ocular symptoms such as dryness, photophobia, and irritation on the ocular surface. The prevalence ranges from 5–30% of the population, with the condition being more frequent in people over 50 years old and in postmenopausal women1,2. In Spain, more than 5 million people may suffer from DED, which can have a significant impact on quality of life and visual function, in addition to a major economic impact. DED is one of the main reasons for primary care and ophthalmology consultations.
The diagnosis of DED should be based on the results of various tests such as Schirmer’s test, the break-up time (BUT) test, and conjunctival impression cytology (CIC)2. CIC is a histological technique in which cells are collected from the surface of the conjunctiva by adhesion to a substrate, usually a filter of microporous synthetic material. These cells are processed either while still adhered to the substrate or after transfer to a slide, in both cases for the purpose of carrying out tincture and histochemical analyses or other types of evaluations. CIC can be used to determine the degree of squamous metaplasia of the conjunctival surface, which parallels the severity of the disease3,4.
Conventional therapies for DED include frequent application of either normal or enriched artificial tears, which humidify and lubricate the ocular surface1. However, to date, no ideal artificial tears containing all components present in natural tears have been developed, resulting in inadequate function of the tear film. Eye drops from autologous serum (AS), produced by separating liquid and cellular components from the patient’s blood, contain many of the biological nutrients and growth factors found in natural tears3. Clinical studies indicate that AS therapy may be effective in ocular surface pathologies and cases of DED that do not respond to conventional therapies5–17. The beneficial effect of AS may be multifactorial and data suggest that AS therapy may modify CIC in patients with DED, although evidence in this regard is limited.
In our study we used CIC with transfer, which is an innovative modality of CIC that includes cell collection from the conjunctiva and then uses a mode of transfer to a slide. This method seems to equal or surpass the end quality of the final samples obtained by other procedures. It is a minimally invasive, easily implemented, outpatient technique that enables any kind of analysis18.
The aim of this study was to determine the improvement of CIC with transfer in patients with DED, the degree of squamous metaplasia and any changes in the results of Schirmer’s test and the BUT after 1 month of treatment with 20% AS eye drops.
MATERIALS AND METHODS
A clinical trial to be carried out on 24 patients was approved by the Clinical Research and Ethics Committee of the General Hospital of Segovia, Spain (Act 07/2015). Inclusion criteria were adults with a confirmed diagnosis of DED and indication for AS eye drop therapy after ineffective conventional therapies. Patients were enrolled consecutively during 1 year. Patients with positive serology for hepatitis B or C virus, human immunodeficiency virus or syphilis, as well as those whose serum was haemolysed and/or lipaemic were excluded.
PREPARATION OF THE AUTOLOGOUS SERUM EYE DROPS
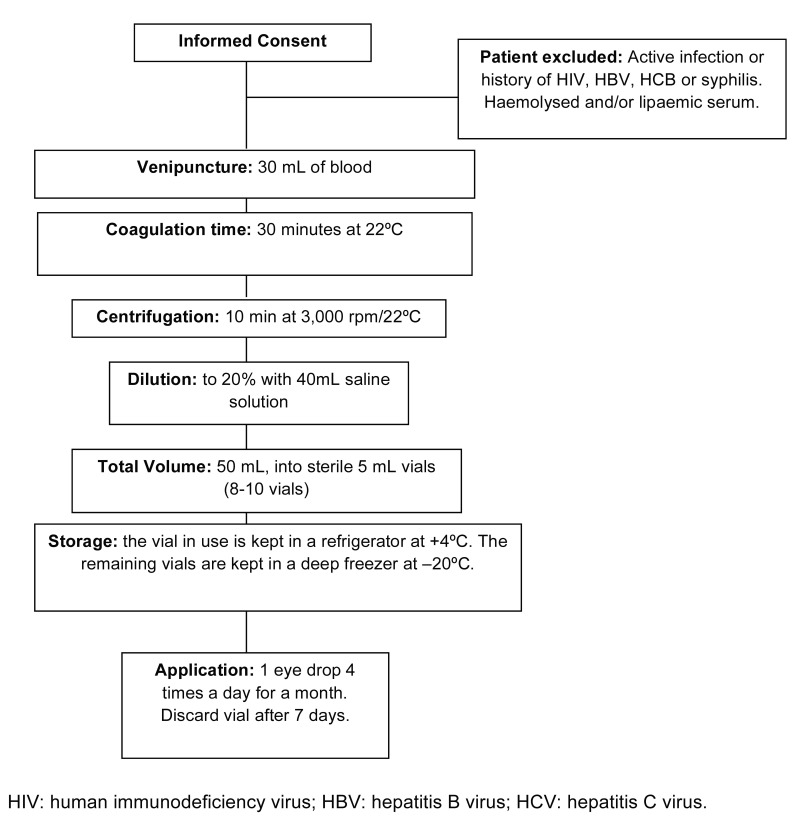
As summarised in Figure 1, 30–40 mL of total blood were extracted from each patient and distributed in three or four vacuum tubes with anticoagulant-free gel. The tubes were left to stand in a vertical position at 22 °C for 30 min. Each tube was labelled with the patient’s name and case history number, stating that it was blood serum for the preparation of AS eye drops.
Figure 1.
Preparation of autologous serum eye drops and their application in the Segovia General Hospital
At the Blood Bank Service, the total blood sample was processed using a centrifugation force of 3,100 g at 22 °C for 10 min. From each 10 mL aliquot of total blood, 5 mL of serum were obtained, without lysis of the cell elements. The serum obtained was sent to the Pharmacy Department for its individualised preparation at 20% in aseptic conditions under a laminar flow hood in order to prevent risks of cross-contamination. All material used for the preparation of AS eye drops was sterile and disposed of at completion of the process. Ten millilitres of serum from each patient were then diluted with 40 mL of 0.9% saline, thereby obtaining a total volume of 50 mL of diluted serum, with a final concentration of 20%. The resulting 50 mL of diluted AS was distributed into special sterile eye drop vials (5 mL per vial). Each vial was then labelled with a bar code, lot number, expiry date, the patient’s data, and preparation date. The eye drops in use were kept in a refrigerator at 4 °C, and the remainder in a deep freezer at −20 °C. Microbiological controls were carried out by sending 1 mL of the eye drops to the microbiology laboratory.
Each patient was subjected to a first CIC with transfer prior to treatment with 20% AS, which consisted of the administration of one drop of 20% AS, four times/day into each eye for 1 month. After this treatment, the CIC with transfer was repeated. Cytology was always performed by the principal investigator in the upper temporal region of each eye.
Information on the following variables was collected: age, sex, DED aetiology, DED clinical classification1 (1=mild, 2=moderate, 3=severe), Schirmer’s test (mm), BUT test (s), number of conjunctival goblet cells (GC) (cells/mm2), size of the GC (μm), density of the GC (quotient between number of GC present in 1,000 epithelial cells at 40× with a calibrated grid divided by 100)19, size of the nucleus and cytoplasm of the GC (μm), nucleus/cytoplasm (N/C) ratio and degree of squamous metaplasia (0–5)4.
CIC with transfer was carried out with a strip of adhesive filter. The sample was secured with a drop of acetone and stained with PAS-haematoxylin. GC morphology and the N/C ratio were determined by microscopic examination using a 40× magnification lens. The number of GC was quantified by manual counting using a calibrated grid and ten random 40× fields. The mean number of GC counted in the fields was multiplied by 40 to obtain the number of GC/mm219.
Sample size was calculated according to a probability sampling method, taking into account the number of conjunctival GC as the most representative variable of clinical improvement, as described in the literature5. Accepting an α error of 0.05, a power (1-β) of 0.8, a two-sided test and a 10% loss rate, 24 subjects were needed to detect a difference of 4 units or more5.
The statistical analysis was performed with the SPSS v24 package. Quantitative variables are described by their mean and standard deviation and qualitative variables as number and frequency. Wilcoxon’s signed rank test and Fisher’s exact test were used to compare quantitative and qualitative variables, respectively. Spearman’s correlation coefficient (rho) was used to calculate correlation between variables. A multiple linear regression model was developed, taking the change in the number of GC after treatment as a dependent variable. All baseline variables relating to the dependent variable were entered in the model with a p<0.20. Differences considered statistically significant were those with a level of significance of at least 95% (p<0.05).
RESULTS
Of the 24 patients analysed, 23 were women (95.8%). The mean age of the population was 53.8±12.6 years (range, 29–78 years). With regards to clinical severity, 41.7% of the patients (n=10) presented with moderate DED, 25% (n=6) with severe DED and 33.3% (n=8) with mild DED. Twenty-one percent of the patients (n=5) had rheumatoid arthritis.
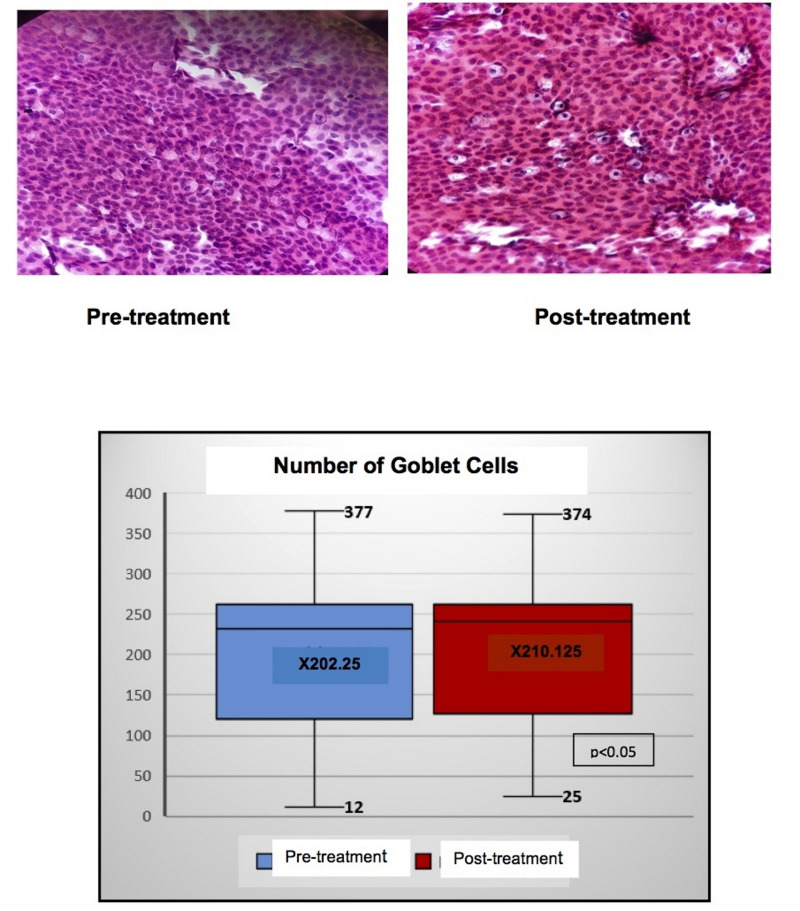
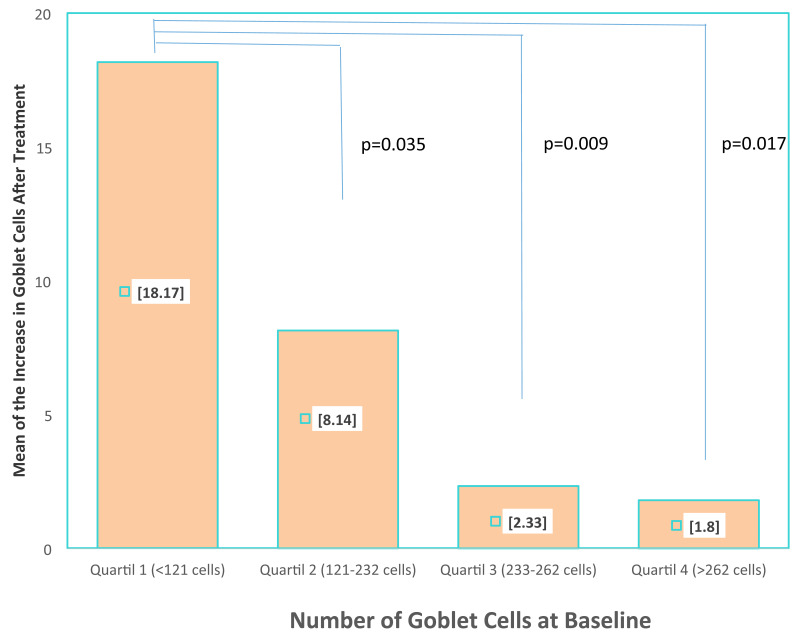
Tables I and II set out the comparisons and correlations of the quantitative variables obtained in the CIC with transfer, both before and after treatment. Statistically significant differences were found in the following variables: number, density and size of the GC as well as in the size of the nucleus and in the N/C ratio. Figure 2 shows the histological changes and the changes in the number and mean of the GC. On dividing the population into four quartiles according to the number of GC at baseline, it was observed that the mean increase in GC was significantly higher in those patients with a smaller number of GC at baseline (increase of 18.1±15 cells in the patients of quartile 1 vs 1.8±4.5 in those of quartile 4; p=0.017) (Figure 3).
Table I.
Comparison between quantitative variables determined by conjunctival impression cytology with transfer before and after treatment with autologous serum eye drops
| Variable | Pre-treatment mean (SD) |
Post-treatment mean (SD) |
Difference | p value* |
|---|---|---|---|---|
| Number GC | 202.3 (107.5) | 210.1 (100.9) | 7.8 | <0.01 |
| Size GC (μm) | 24.5 (3.3) | 30.8 (3.7) | 6.3 | <0.001 |
| Density GC | 2.39 (0.91) | 2.59 (0.72) | 0.2 | <0.05 |
| Size of the nucleus GC (μm) | 6.91 (1.69) | 7.71 (2.33) | 0.8 | <0.01 |
| Size of cytoplasm GC (μm) | 26.29 (4.52) | 26.62 (4.40) | 0.33 | >0.05 |
| N/C GC | 0.27 (0.08) | 0.29 (0.1) | 0.02 | <0.01 |
SD: standard deviation; GC: goblet cells; N/C GC: nucleus/cytoplasm ratio of goblet cells.
p value calculated by Wilcoxon’s signed rank test.
Table II.
Correlations between quantitative variables determined by conjunctival impression cytology with transfer before and after treatment with autologous serum eye drops
| Post-treatment variables | |||||
|---|---|---|---|---|---|
| Pre-treatment variables | Number of GC | Density of GC | Nucleus size GC (μm) | Cytoplasm size GC (μm) | Relation N/C GC |
| Number GC | 0.995 p<0.001 |
0.514 p<0.05 |
−0.564 p<0.05 |
||
| Size GC (μm) | |||||
| Density GC | 0.659 p<0.001 |
0.883 p>0.001 |
−0.555 p<0.001 |
0.639 p<0.001 |
−0.813 p>0.001 |
| Size of the nucleus GC (μm) | 0.920 P<0.001 |
0.736 p<0.001 |
|||
| Size of cytoplasm GC (μm) | 0.557 p<0.05 |
0.968 p<0.001 |
−0.594 p<0.05 |
||
| N/C GC | −0.546 p<0.05 |
−0.570 p<0.05 |
0.706 p<0.001 |
−0.592 p<0.05 |
0.935 p<0.001 |
GC: goblet cells; N/C GC: nucleus/cytoplasm ratio of goblet cells. Only statistically significant correlations are shown.
Figure 2.
Histological changes in the conjunctival impression cytology with transfer and difference in the number of goblet cells before and after treatment with autologous serum eye drops
Figure 3.
Comparison of the number of goblet cells after treatment with autologous serum eye drops dividing the patients into quartiles depending on the baseline goblet cell count
There were significant increases in the mean millimetres in Schirmer’s test and in the mean duration of the BUT test before and after treatment (Table III). At baseline, 16.7% of the patients (n=4) had grade 4 metaplasia, 25% (n=6) had grade 3, 41.7% (n=10) had grade 2 and 16.7% (n=4) had grade 1. After treatment, the degree of metaplasia in seven patients (29.2%) lowered by one level; the four patients with grade 4 metaplasia improved to grade 3, and three of the six patients with grade 3 metaplasia improved to grade 2. In total, seven out of ten patients with grade 3 or 4 metaplasia had an improvement in their degree of metaplasia by one level.
Table III.
Comparison of the results of Schirmer’s test and the tear break-up time test before and after treatment with autologous serum eye drops.
| Variable | Pre-treatment mean (SD) |
Post-treatment mean (SD) |
Difference | p value* |
|---|---|---|---|---|
| Schirmer’s test (mm) | 5.29 (2.49) | 6.62 (2.42) | 1.33 | <0.001 |
| BUT test (sec) | 10.87 (2.07) | 12.45 (2.06) | 1.58 | <0.01 |
SD: standard deviation; BUT: tear break-up time.
p value calculated by Wilcoxon’s signed rank test.
In univariable analyses, the statistically significant baseline variables directly related to the increase in the number of GC after treatment were: higher grade of metaplasia (r=0.489; p=0.015), poorer clinical classification (r=0.408; p=0.048) and higher baseline N/C ratio of the GC (r=0.542; p=0.006). The baseline variables related inversely and statistically significantly to the increase in the number of GC after treatment were: the baseline number of GC (r= −0.564; p=0.004), the baseline size of GC (r= −0.635; p=0.001), the baseline density of GC (r= −0.601; p=0.002) and the baseline size of the GC cytoplasm (r= −0.808; p<0.0001).
In the multivariable analysis the variables that remained in the model as predictors of a greater GC gain after treatment were a smaller number of GC (β= −0.279; 95% confidence interval [95%]= −0.539 to −0.019; p=0.037) and smaller size of the cytoplasm of the GC at baseline (β= −2.489; 95% CI = −3.733 to −1.244; p=0.001). All patients complied correctly with the treatment and no reaction or associated adverse effect was observed.
DISCUSSION
In this prospective study, carried out in patients with DED, the use of 20% AS eye drops for 1 month was associated with an improvement in CIC with transfer, the degree of squamous metaplasia and in flow and tear stability tests, without any side effects. CIC with transfer is an innovative and minimally invasive technique that allows a sample of the ocular surface to be obtained, different from that obtained with conventional CIC and only used in our study18.
The mechanisms of action of AS eye drops in DED are not completely clear8. AS contains substances with trophic effects that could act on epithelial dynamics, regulating the proliferation, migration and differentiation of the epithelial cells of the ocular surface7,8. In vitro studies with conjunctival epithelial cells have demonstrated a dose-dependent effect on the expression of mucins20. In addition, AS contains immunoglobulins and lysozymes with both bactericidal and bacteriostatic effects21. Vitamin A is the component of AS most related to the improvement in the degree of squamous metaplasia by stimulating the production of mucins, especially MUC5AC, and restoring the mucinic phase of the tear film21. GC are also sensitive to the action of vitamin A, which increases both the number and size of these cells. Furthermore, epithelial growth factor seems to mediate in the production of mucins and in the improvement of squamous metaplasia in patients treated with AS eye drops20. The beneficial effects of AS eye drops start to be observable between the first and second weeks of treatment, although subjective improvements begin practically from the second day. To determine whether or not a patient responds satisfactorily to the treatment, at least 3 weeks must pass from the beginning of the treatment7.
The histological changes in cells detected by CIC with transfer in our study included significant improvements in the number, size, density, size of the nucleus and N/C ratio of the GC after treatment with AS eye drops. These improvements were more evident in patients with severe DED than in those with moderate-mild DED. According to the grading system of squamous metaplasia proposed by Murube et al.4, which divides the condition into five pathological grades, in our study the severity of the metaplasia decreased one grade in patients with severe DED, and remained unchanged in patients with moderate-mild DED after treatment with 20% AS eye drops. We were unable to find previous studies showing, by means of CIC, an increase in the density of GC in patients with DED treated with 20% AS. By means of conventional biopsy of bulbar conjunctiva, Luna Martínez et al.5 showed a statistically significant decrease in the grade of squamous metaplasia and a statistically significant increase in the number of GC following treatment with 20% AS at a dose of one drop, four times a day, compared to treatment with carboxymethylcellulose adjusted to the same dosage. The results obtained in patients with severe DED were similar in all the studies, including ours5,6. However, unlike the other studies, only our study included cases of mild DED treated with AS eye drops.
Schirmer’s and BUT tests were used to evaluate the effects of AS on the ocular surface of the patients. Schirmer’s test has marked inter- and intra-variability. However, we do not have other tests instead of meniscometry in our hospital. In our study, both tests showed improvements, whereas Liu et al.6 only found an improvement in the BUT test. Noble et al.7 documented improvements in symptomatology and conventional CIC, but not in Schirmer’s test or the BUT.
In our opinion, the most suitable dose and concentration of AS eye drops to be used in the treatment of DED has not been defined. Published doses range from 1 drop/hour to three times a day, depending on the concentration of the AS eye drops used. In cases in which AS concentrations greater than 20% were used, the frequency of administration varied from every hour to every 3 or 4 hours20. Poon et al.8 showed that both 50% and 100% concentrations were more effective in improving the symptomatology. Cho et al.9 and Gus et al.10 compared the use of 50% AS vs 20% AS, without finding superior effectiveness of one over the other. However, the higher the AS concentration, the greater the risk of appearance of immunocomplex deposits. For this reason, Geerling et al.11 considered that AS at high concentrations (≥50%) is contraindicated in patients with corneal transplants because of a greater risk of rejection. Nonetheless, the response to AS in the treatment of corneal ulcers was dose-dependent, thus advocating more frequent blood collection so as to obtain a denser AS with a 50% concentration, which would enable better sealing of the corneal ulcer. In our study, we used 20% AS because this is the concentration that the majority of authors recommend as clinically effective and well tolerated by patients, allowing us to reduce the possible appearance of adverse effects and, thereby, achieving better adherence to treatment7,8,17,21–24.
There is a great variability in the process of preparation, storage and dispensing of AS eye drops. The amount of blood collected and the time from collection to centrifugation also varies, according to the articles published, from 30 min to 48 h. In our study, we collected 30 mL of blood and the time elapsed between collection and centrifugation was 30 min, which resembles the preparation described by Noble et al.7. Liu et al.12 observed that a coagulation time of at least 2 h prior to centrifugation was related to a greater effect on the migration and differentiation of epithelial cells, so they recommend leaving the test tubes in a rack in a vertical position at 22 °C for 2 h prior to centrifuging. The centrifugation force and time affect the amount of AS obtained, its purity, and the concentration of the factors related to cell migration and differentiation. These were not standardized in either of these studies. In our study the samples were prepared using a centrifugation force of 3,100 g for 10 min, a procedure that did not coincide with any of those in the research listed in the references.
This is because the processing of our blood serum samples intended for the preparation of AS eye drops complied with our Blood Bank’s protocol for the handling of samples, processed with an Ortoalresa centrifuge series Digtor21. There are also differences in the preparation of the AS eye drops depending on whether fresh or frozen serum is used. In our case fresh serum was prepared without freezing.
In our study the AS eye drops were kept in a refrigerator at 4 °C in the vial used and the rest in the freezer at −20 °C, as the components of the AS remain stable at temperatures of +4°C for 1 month, and at −20 °C for 3 months14. Sitaramamma et al.22 analysed the effect on the reduction of protein concentration (especially of IgA and lyzosome) of tear samples, when these were stored at temperatures such as −20 °C and −70 °C for 4 months. From this they inferred that frozen AS eye drops do not lose their properties.
We did not observe any toxic effects in our study. It is described in the literature that, in patients with DED and rheumatoid arthritis, there is some relationship between the activity of the rheumatoid factor and the existence of antibodies in the AS eye drops which could give rise to immunocomplex deposits, which might subsequently generate a secondary inflammatory reaction17. In our study, however, the patients diagnosed with rheumatoid arthritis and treated with AS eye drops had an increase in the density of the GC, without any toxic effects or inflammatory reaction. Harloff et al.23 demonstrated that the AS of the patients who had rheumatoid arthritis in the active phase and who were subjected to immunosuppressive treatment had lower concentrations of both fibronectin and transforming growth factor-β. Pasukkijwatama et al.24, however, compared the epitheliotropic effects of the AS of patients with autoimmune DED versus the AS of patients with non-autoimmune DED, without finding any significant differences24. They concluded that the use of AS eye drops is not contraindicated in patients with autoimmune diseases, such as rheumatoid arthritis. The limitations of our study are its small sample size and the absence of a control arm with placebo or an active comparator. The collection of the patients entailed a period of 1 year, since the use of AS eye drops for the treatment of DED continues to be limited, at least in our hospital, as it is not the first therapeutic option. The changes in the CIC with transfer, however, are observable and appear consistent with those found by other authors5,6.
CONCLUSIONS
In patients with DED, treatment with 20% AS eye drops significantly improves tear production, lachrymal flow and stability tests and the findings of CIC with transfer. The proposed treatment for DED patients based on 20% AS eye drops appears to be very promising with good results. The improvements are more significant in patients with severe DED.
Footnotes
AUTHORSHIP CONTRIBUTIONS
SLVC was involved in all stages of the production of the manuscript. She helped to design the study, collect and analyse the statistical data, and both write and prepare the manuscript for submission. ESM helped to design the study, collect and analyse the statistical data, and both write and prepare the manuscript for submission. JGF helped to write and prepare the manuscript for submission. FJG-M helped to design the study, collect and analyse the statistical data, and both write and prepare the manuscript for submission.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–74. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pan Q, Angelina A, Marrone M, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017;2:CD009327. doi: 10.1002/14651858.CD009327.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murube J, Rivas L. Impression cytology on conjunctiva and cornea in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:115–27. doi: 10.1177/112067210301300201. [DOI] [PubMed] [Google Scholar]
- 5.Luna Martínez I, Aguilar Montes G, Estrada Hernández MR, Reyes Castro MM. Histopathologic findings in patients with dry eye syndrome due to autoimmune disease treated with autologous serum. Rev Mex Oftalmol. 2015;89:37–42. [Google Scholar]
- 6.Liu Y, Hirayama M, Cui X, et al. Effectiveness of autologous serum eye drops combined with punctal plugs for the treatment of Sjögren syndrome-related dry eye. Cornea. 2015;34:1214–20. doi: 10.1097/ICO.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 7.Noble BA, Loh RS, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–52. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001;85:1188–97. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YK, Huang W, Kim GY, Lim BS. Comparison of autologous serum eye drops with different diluents. Curr Eye Res. 2013;38:9–17. doi: 10.3109/02713683.2012.720340. [DOI] [PubMed] [Google Scholar]
- 10.Gus PI, Marinho D, Zelanis S, et al. A case-control study on the oxidative balance of 50% autologous serum eye drops. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/9780193. 9780193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467–74. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Hartwig D, Harloff S, et al. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005;243:706–14. doi: 10.1007/s00417-004-1106-5. [DOI] [PubMed] [Google Scholar]
- 13.Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum aplicattion in Sjogren’s syndrome. Br J Ophthalmol. 1999;83:390–5. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-García JS, García-Lozano I, Rivas L, Martínez-Garchitorena J. Use of autologous serum in ophthalmic practice. Arch Soc Esp Oftalmol. 2007;82:9–20. doi: 10.4321/s0365-66912007000100004. [DOI] [PubMed] [Google Scholar]
- 15.Marks DC, van der Meer PF. Serum eye drops: a survey of international production methods. Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Vox Sang. 2017;112:310–17. doi: 10.1111/vox.12502. [DOI] [PubMed] [Google Scholar]
- 16.Mukerji N, Sinha R, Vajpayee RB. Role of autologous serum in persistent epithelial defects. Br J Ophthalmol. 2002;86:832. doi: 10.1136/bjo.86.7.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali TK, Gibbons A, Cartes C, et al. Use of autologous serum tears for the treatment of ocular surface disease from patients with systemic autoimmune diseases. Am J Ophthalmol. 2018;189:65–70. doi: 10.1016/j.ajo.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Minaya Martínez, Francisco. Doctoral Thesis. Malaga University. Ofthalmology Department; Dec, 2001. [Accessed on 01/01/2015]. Conjunctiva impression cytology with trasfer: thecnique, computerized study of image and clinical application. Avaible at: https:/www.educacion.es/teseo/mostrarRef.do?ref=265098. [Google Scholar]
- 19.Yeo ACH, Carkeet A, Carney LG, Yap MKH. Relationship between goblet cell density and tear function tests. Ophthalmic Physiol Opt. 2003;23:87–94. doi: 10.1046/j.1475-1313.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 20.Hori Y, Spurr-Michaud S, Russo CL, et al. Differential regulation of membrane-associated mucins in yhe huma ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114–22. doi: 10.1167/iovs.03-0903. [DOI] [PubMed] [Google Scholar]
- 21.López-García JS, García-Lozano I, Rivas L, et al. Effects of autologous serum eye drops on conjunctival expression of MUC5AC in patients wirh ocular surface disorders. Cornea. 2016;35:336–41. doi: 10.1097/ICO.0000000000000726. [DOI] [PubMed] [Google Scholar]
- 22.Sitaramamma T, Shivaji S, Rao GN. Effect of storage on protein concentration of tear samples. Curr Eye Res. 1998;17:1027–35. doi: 10.1076/ceyr.17.10.1027.5241. [DOI] [PubMed] [Google Scholar]
- 23.Harloff S, Hartwig D, Kasper K, et al. Epitheliotrophic capacity of serum eye drops from healthy donors versus serum from immunosuppressed patients with rheumatoid arthritis. Klin Monbl Augenheilkd. 2008;225:200–6. doi: 10.1055/s-2008-1027199. [DOI] [PubMed] [Google Scholar]
- 24.Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr Eye Res. 2011;36:775–81. doi: 10.3109/02713683.2011.587935. [DOI] [PubMed] [Google Scholar]