In patients with COVID-19 pneumonia, severe anaemia on hospital admission is uncommon1. However, haemoglobin (Hb) concentration tends to progressively decline during the course of the disease. Both sepsis and hypoxia have profound effects on red cell morphology, rheology and survival, likely contributing to the complex pathogenesis of COVID-19 anaemia. In this regard, during the peak of the COVID-19 outbreak in Northern Italy, we observed that 39% of the inpatients required transfusion support after a median of 15 days of hospitalisation. Red blood cell (RBC)-bound antibodies were detectable by direct antiglobulin test (DAT) in almost half of the patients with severe COVID-19, and their presence was associated with lower Hb levels and greater transfusion requirement2. In addition, we and others have recently demonstrated that the ABO blood group is one of the two loci associated with the susceptibility to develop COVID-19-induced respiratory failure with genome-wide significance. Among several possible explanations, interactions between neutralising antibodies against protein-linked N-glycans and RBC antigens have to be considered3.
In RBC disorders, analysis of the peripheral blood smear remains an important diagnostic tool4, but no information is yet available regarding RBC morphology in patients with COVID-19. Here we report on blood films examined from 20 patients with COVID-19-related anaemia who had been consecutively referred for pre-transfusion testing or ABO typing. Ten patients were treated in the intensive care unit (ICU) and ten in the sub-intensive care unit (SICU). All showed mild to severe anaemia (Hb concentration 7.2–10.5 g/dL), and four of them had a moderate transfusion requirement (1–4 packed RBC units during the month of observation). They were receiving treatments with several drugs2 and their median duration of hospital stay was 30 days (range 18–50). Overall, 7 of 20 (35%) were DAT positive.
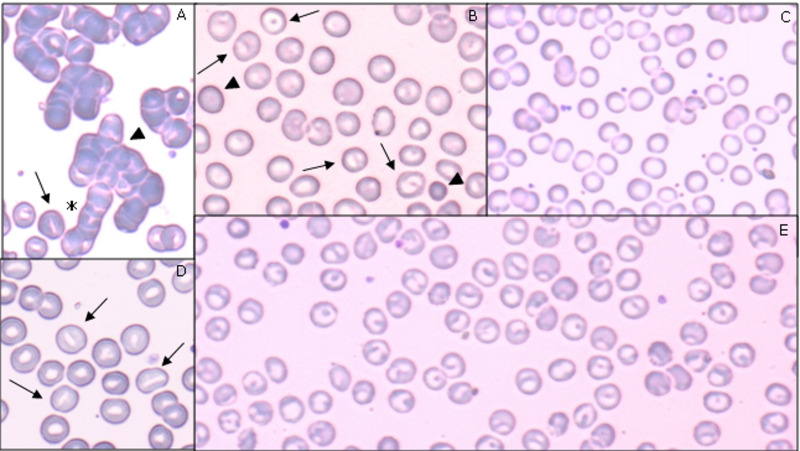
Figure 1 shows the blood films prepared from five representative patients. May-Grünwald Giemsa stains were analysed by means of light microscope. At least ten fields for every blood stain were evaluated in the area where red cell biconcavity was evident. Polychromasia and basophilic stippling were common, probably as a consequence of an increased reticulocyte count4. Huge rouleaux formations and autoagglutination were observed in 17 (85%) of the 20 patients studied. Rouleaux are generally attributed to an increase in cathodal proteins, such as immunoglobulins and fibrinogen, as it occurs during viral infections5. Spontaneous RBC agglutination depends on complement and/or antibody coating of RBCs, a common feature of autoimmune haemolytic anaemia6. Blood smears were characterised by several RBC shape abnormalities compatible with haemolytic mechanisms. Spherocytes (considered a common marker of haemolysis) were found in six patients (30%). Schistocytes were found in eight patients (40%), possibly as a consequence of membrane damage induced by fibrin strands occurring in vascular thrombosis with microangiopathy, which are a common feature in COVID-19 pneumonia7. Mushroom-shaped erythrocytes were seen in two cases (10%), as a possible expression of acquired membrane protein defects8.
Figure 1. Blood smear features of five patients with COVID-19-related anaemia.
(A) A 40-year old man, on mechanical ventilation, at 43 days of hospitalisation. Blood type was A positive, direct antiglobulin test (DAT) positive, haemoglobin (Hb) 7 g/dL, mean corpuscular volume (MCV) 92 fl, red cell distribution width (RDW) 15.9% at the time of investigation. In this field, autoagglutination (arrowhead) and rouleaux (asterisk) formations are visible. At the bottom of the panel, stomatocytes (arrows) are visible. 80× objective. (B) A 47-year old man, on mechanical ventilation after 32 days of hospitalisation; under treatment with steroids, anakinra, quinolones. Blood type was A positive, DAT negative, Hb 11.3 g/dL, MCV 89 fl, RDW 12.4% at the time of investigation. Spherocytes (arrowheads) and knizocytes (arrows) are visible. 100× objective. (C) A 76-year old woman, on mechanically assisted ventilation, after 36 days of hospitalisation. Blood type was A positive, DAT positive, Hb 8.3g/dL, MCV 81 fl, RDW 14.6% at the time of investigation. This field is widely occupied by cup-shaped erythrocytes. Cytoplasmic rim is intensely coloured, surrounding a wider than normal central area. 100× objective. (D) A 66-year old woman, on mechanically assisted ventilation, after 49 days of hospitalisation, under treatment with hydroxychloroquine and piperacillin. Blood type was O positive, DAT positive, Hb 7.7 g/dL, MCV 88 fl, RDW 13.4% at the time of investigation. Stomatocytes (arrows) are present in this field. 100× objective. (E) A 69-year old man, on mechanical ventilation after 40 days of hospitalisation, under treatment with hydroxychloroquine, lopinavir, ritonavir. Blood type was A positive, DAT negative, Hb 7.4 g/dL, MCV 91 fl, RDW 15.4% at the time of investigation. In this field, red cells are totally represented by knizocytes 100× objective.
Another common observation was the presence of high percentages of stomatocytes in 11 patients and knizocytes in 12 (55% and 60% of the cases, respectively). Stomatocytes are characterised by a mouth-shaped slit in the central biconcave area; they are an unusual finding outside inherited stomatocytosis (frequency 1:50,000). Knizocytes, with a doubled or tripled or variably shaped pale central ridge are rarely found in normal adults. Stomatocytes and knizocytes may be observed in patients with severe liver disease, or impaired lipid metabolism. In half of patients of the present series, they were interspersed with cup-shaped erythrocytes, characterised by a wider than normal central pallor surrounded by an intensely coloured rim. Actually, these three shapes (stomatocytes, knizocytes, and cup-shaped cells) are considered interchanging features of a continuum of metamorphic transformations leading to spherocyte shape9, and are characterised by the loss of elastic properties. Overall, these red cell elements were found in 70% of the blood smears, thereby representing the most typical morphological features associated with COVID-19-related anaemia. The aforementioned features were not linked to preparation artefacts, as they were not present in the blood film simultaneously prepared from ICU patients with anaemia but without COVID-19, and healthy individuals.
No significant differences were observed between ICU and non-ICU patients, or between DAT positive and negative patients, although the limited number of observations does not allow us to exclude the possibility that RBC morphological abnormalities are an expression of disease severity status or that such abnormalities could be related to the immune-mediated mechanisms described in COVID-19 patients (see Online Supplementary Content, Table SI).
Very few blood smears prepared from COVID-19 patients have been published so far. Almost all the RBC abnormalities described in the present series can be recognised in those included in three case reports10–12, which, however, focused on the morphology features of white cells during COVID-19. We had the chance to examine the samples of five patients after recovery from COVID-19, at least one month after hospital discharge. As Hb levels had returned to normal, the blood smear showed unremarkable RBC morphology ( Online Supplementary Content, Figure S1).
In summary, the analysis of blood smears obtained from patients with COVID-19-related anaemia showed several RBC shape abnormalities. The presence of a high frequency of stomatocytes and knizocytes, which are not frequently encountered on blood smears in other types of anaemia, were the most common finding. In line with recent studies, it could be hypothesised that RBC injury occurs as a consequence of immune-mediated mechanisms3, and/or physical cell damage due to COVID-19 microangiopathy7. The loss of RBC biconcavity and complement activation observed in COVID-19 may facilitate RBC stacking and spontaneous agglutination, and possibly contribute to the microvascular thrombosis typical of COVID-19.
Supplementary Information
Footnotes
FUNDING
This work was supported in part by grants to Dr. Luca Valenti and Dr. Daniele Prati from Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (Ricerca Corrente 2020 and COVID-19 biobank).
AUTHORSHIP CONTRIBUTIONS
AB, CB, LV, and DP wrote the first and subsequent drafts of the manuscript; AB, CB, ACM, and MM conducted the laboratory experiments; LV and DP supervised the study. All Authors contributed to the acquisition or analysis of data, critically revised the manuscript, approved the final version for publication, and agreed to be accountable for the results published.
DISCLOSURE OF CONFLICTS OF INTEREST
AB serves as Associate Editor of Blood Transfusion. The other Authors have no conflict of interest regarding the content of this paper.
REFERENCES
- 1.Stanworth S, New HV, Apelseth TO, et al. The impact of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7:e756–64. doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzuini A, Bianco C, Paccapelo C, et al. Red cell bound antibodies and transfusion requirements in hospitalized patients with COVID-19. Blood. 2020;136:766–8. doi: 10.1182/blood.2020006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain BJ. Diagnosis from the Blood Smear. N Engl J Med. 2005;353:498–507. doi: 10.1056/NEJMra043442. [DOI] [PubMed] [Google Scholar]
- 5.Abramson N. Rouleaux formation. Blood. 2006;107:4205. [PubMed] [Google Scholar]
- 6.Packman CH. The clinical pictures of autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42:317–24. doi: 10.1159/000440656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M, Verleden S, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesesve JF. Mushroom-shaped red blood cells in protein band-3 deficiency. Am J Hematol. 2011;86:694. doi: 10.1002/ajh.21962. [DOI] [PubMed] [Google Scholar]
- 9.Bessis M. Spherocytes and knizocytes. In: Bessis M, editor. Corpuscles. Springer; Berlin, Heidelberg: 1974. pp. 65–70. [Google Scholar]
- 10.Singh A, Sood N, Narang V, et al. Morphology of COVID-19–affected cells in peripheral blood film. BMJ Case Rep. 2020;13:e236117. doi: 10.1136/bcr-2020-236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra A, Dwyre DM, Schivo M, et al. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95:999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones J, Ireland R. Morphological changes in a case of SARS-CoV-2 infection. Blood. 2020;135:2324. doi: 10.1182/blood.2020006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.