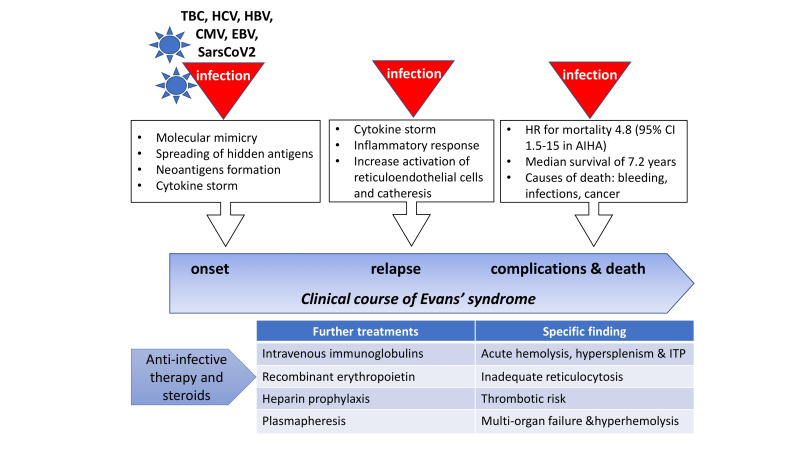
Evans syndrome (ES) is a rare condition characterised by the association of multiple autoimmune cytopenias, usually autoimmune haemolytic anaemia (AIHA) and immune thrombocytopenia (ITP), and, although rarely, autoimmune neutropenia. Up to 50% of ES cases may be secondary to a variety of conditions including infections, primary immunodeficiencies, particularly in children, systemic autoimmune diseases (such as systemic lupus erythematosus and rheumatoid arthritis), lymphoproliferative syndromes, and transplantation1,2. Infections may either contribute to the development of the autoantibodies, through molecular mimicry, spreading of hidden epitopes, and neoantigen formation, or trigger ES relapses along the natural history of the disease (Figure 1)3. The world-wide COVID-19 pandemic represents a further challenge for clinicians dealing with autoimmune cytopenias as SARS-CoV-2 infection can be a trigger for autoimmunity, and it is still not clear whether pneumonia may benefit of or be worsened by concurrent immunosuppressive treatments4. In this issue of Blood Transfusion, Demir et al. describe the case of a 22-year old male patient presenting with ES secondary to COVID-19 pneumonia, in April 2020, at the peak of contagions in Mediterranean countries5,6. Very severe warm type AIHA (haemoglobin [Hb] 3.9 g/dL, direct antiglobulin test [DAT] positive for IgG and strong complement activation) was diagnosed and managed with transfusions and steroids without response. The patient developed concurrent ITP and received plasmapheresis, followed by intravenous immunoglobulins that led to a progressive and eventually complete recovery. Importantly, prophylactic subcutaneous enoxaparin was given during hospitalisation, as long as platelet counts were above 50×109/L. As this case clearly illustrates, ES developing in an infected patient is a challenge at multiple levels (Figure 1):
Figure 1. Infections during the clinical course of Evans syndrome (ES).
Infectious triggers have an important role in ES development and its relapses through various mechanisms. ES patients may have a dismal survival, particularly secondary cases, and infections are one of the major causes of death. An infected patient with ES should promptly receive steroids along with anti-infective treatment. Other therapies are to be tailored on specific findings. Rituximab and splenectomy are well established second-line treatments for relapsing/refractory cases, after underlying secondary causes have been excluded/elucidated.
AIHA: autoimmune haemolytic anaemia; CI: confidential interval; CMV: cytomegalovirus, EBV: Epstein-Barr virus; HCV: hepatitis C virus HBV: hepatitis B virus; ITP: immune thrombocytopenia; HR: hazard risk; TBC: tuberculosis.
diagnostic, since the septic state per se (including COVID-19 pneumonia) may cause cytopenia and/or false positive autoimmunity tests7;
therapeutic, given that most therapies for treating ES are immunosuppressive and might worsen host defences;
prognostic, since infectious complications are an important cause of morbidity and mortality in patients with autoimmune cytopenias8.
Regarding diagnosis, ES is marked by the presence of at least two cytopenias of autoimmune origin in the absence of any underlying cause. It is therefore a diagnosis of exclusion and all the above-mentioned associations should be taken into account. Besides the classic workup (complete blood counts, vitamins and iron status, markers of haemolysis), DAT is fundamental for the diagnosis of AIHA, although it might be negative in 5–10% of cases3; at variance with this, lower sensitivity and specificity have been reported for anti-platelet and anti-neutrophil autoantibody testing, whose negativity does not exclude ES diagnosis. Finally, bone marrow (BM) evaluation and computed tomography scan are important to rule out underlying haematologic conditions, and Ig levels and medical history may suggest the presence of a congenital immunodeficiency1. Concerning ES secondary to infections (i.e., Parvovirus B19, hepatitis B and C, tuberculosis, etc.), the differential diagnosis may be problematic and could delay appropriate treatment. In fact, microbes may cause cytopenias through several mechanisms, including direct infection of BM precursors, inflammation and iron depletion, and enhancement of immune-mediated catheresis. These confounders also occur in SARS-CoV-2 infection. Other ES cases developing concomitantly to COVID-19 pneumonia have been reported9–11, and, in the pandemic setting, some symptoms related to a well-known disorder may be erroneously attributed to the emerging disease making differential diagnosis difficult11. In fact, SARS-COV-2 may also induce thrombocytopenia by favouring platelet aggregation in the lungs with microthrombi formation and consumption12,13. Furthermore, the erythrocyte membrane protein ankyrin-1 was found to share a 100% identity with the SARS-CoV-2 surface glycoprotein Spike14, suggesting a molecular mimicry mechanism inducing immune-mediated anaemia.
Concerning therapy, ES is treated according to the presenting cytopenia, usually with steroids 1 mg/kg/day. Rituximab and splenectomy are well established second-line treatments for relapsing/refractory cases, after underlying secondary causes have been excluded/elucidated. The case reported by Demir et al. points out that “most AIHA therapies are immunosuppressive and therefore potentially harmful during infections”. As a matter of fact, infections in ES may be the putative cause of disease onset/relapse15,16 but may also be favoured by ES therapy3. Besides steroids (that are also part of therapeutic protocols of the severe sepsis), high-dose immunoglobulins may be safely administered to ES patients during acute infections. Although more effective in ITP, their efficacy has been attested to be around 40% in AIHA; in this setting, their use is suggested in the highly haemolytic, potentially infected patient in whom steroids are ineffective and rituximab potentially harmful17. While the other reported ES cases concomitant to COVID-19 were milder and responded to steroids and intravenous immunoglobulin9–11, the patient reported by Demir et al. required plasma exchange. This procedure is, however, not always feasible and implies cardiovascular stress with risk of fluid imbalance; it can be considered in the acutely ill and younger patient as a “last treatment option”17. It is worth remembering that recombinant erythropoietin can be safely administered in patients with inadequate reticulocytosis with up to 70% responses even in the acute phase18. BM failure to compensate haemolysis may be observed in the presence of anti-erythroblast antibodies, in patients with underlying haematologic conditions (i.e., lymphoproliferative disorder, myelodysplasia), and when haemolysis is too acute to allow reticulocyte regeneration or the BM is “shocked” by the septic state7. Moreover, it has been shown that about one-third of chronic refractory AIHA cases may display features of BM dyserythropoiesis and fibrosis19, and most of them have inadequate reticulocytosis and endogenous erythropoietin levels18. Similar considerations may regard the use of thrombopoietin receptor agonists for ITP, although data in the ES setting are lacking. A final therapeutic challenge may be the higher transfusion requirement due to the blood cell consumption typical of the septic state; this adds to the risk of alloimmunisation of transfused patients3,17. Regarding anti-coagulant prophylaxis, thrombosis may complicate the clinical course of both ES and COVID-19 pneumonia. In the latter, an aberrant increase of D-dimers and prolonged coagulation times (the hallmark of the so-called “thromboinflammation”) are often observed and correlate with worse outcome20. In AIHA, about 15% of patients may experience a thrombotic episode that has been related to highly haemolytic disease (i.e., lactate dehydrogenase levels >1.5×ULN) and multi-treatment approaches (particularly splenectomy)3,8,17; similarly, ITP patients are considered at higher thromboembolic risk due to the increased fraction of “young platelets”. Thromboembolic prophylaxis is even more important when all three conditions (infection, AIHA and ITP) occur simultaneously, and is currently routinely instituted in patients admitted with COVID-19 pneumonia, provided platelet counts are adequate. For instance, the ES case described by Li et al. experienced thromboembolism11, while that described by Demir et al. received heparin prophylaxis5.
Finally, as regards prognosis, the described patient depicts the main clinical predictors of outcome in AIHA: anaemia severity at onset (particularly Hb<6 g/dL) is the major determinant of relapse and mortality risk; in a large retrospective study, each gram of reduction yielded a 7% greater risk of relapse (95% CI: 2–13; p<0.013)8. Increased hazard risks for relapse and mortality have also been reported for the presence of complement activation and for ITP association. Finally, infectious complications have been associated with an increase in mortality with an HR of 4.8 (95% CI: 1.5–15; p=0.007)8. In this context, a recent epidemiologic study from the Danish registry identified that adult patients with ES had a median survival of only 7.2 years, and this was markedly shorter in secondary cases (1.7 vs 10.9 years in primary cases)2. The prevailing causes of death were bleeding, infections, and haematologic cancer. As a matter of fact, the reported survival is not dissimilar to that of neoplastic conditions, like low-risk myelodysplastic syndromes, despite the markedly younger median age (58 vs 71 years) of ES patients.
In conclusion, ES during acute infections should be promptly managed balancing the aggressiveness of immunosuppression and the potential for BM stimulation in patients with inadequate reticulocytosis. Intravenous immunoglobulins and plasmapheresis are powerful “time-gaining” tools to be considered on a patient-by-patient strategy. The thrombotic risk should be taken into account and thromboprophylaxis instituted if platelet count is permissive. Finally, it is important to bear in mind the potential worst prognosis of infected patients with ES, particularly secondary cases.
Footnotes
The Author declares no conflict of interests.
Comment to DOI 10.2450/2020.0221-20 (BLT-19-085)
REFERENCES
- 1.Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167–72. doi: 10.1182/blood-2009-04-215368. [DOI] [PubMed] [Google Scholar]
- 2.Hansen DL, Möller S, Andersen K, et al. Evans syndrome in adults - incidence, prevalence, and survival in a nationwide cohort. Am J Hematol. 2019;94:1081–90. doi: 10.1002/ajh.25574. [DOI] [PubMed] [Google Scholar]
- 3.Barcellini W, Giannotta J, Fattizzo B. Autoimmune hemolytic anemia in adults: primary risk factors and diagnostic procedures. Expert Rev Hematol. 2020;13:585–97. doi: 10.1080/17474086.2020.1754791. [DOI] [PubMed] [Google Scholar]
- 4.Haberman R, Axelrad J, Chen A, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med. 2020;383:85–8. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir NA, Basturk A, Ural O, et al. A case of Evans syndrome secondary to COVID-19. Blood Transfusion. 2021;19:85–8. doi: 10.2450/2020.0221-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Word Health Organization [internet] Turkey: [Accessed on 4/10/2020.]. Available at: https://covid19.who.int/region/euro/country/tr. [Google Scholar]
- 7.Barcellini W, Fattizzo B. The changing landscape of autoimmune hemolytic anemia. Front Immunol. 2020;11:946. doi: 10.3389/fimmu.2020.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcellini W, Zaninoni A, Fattizzo B, et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hematol. 2018;93:E243–6. doi: 10.1002/ajh.25212. [DOI] [PubMed] [Google Scholar]
- 9.Zarza J, Von Horoch J, Aguayo N, Báez E. Evans syndrome associated with antiphospholipid antibodies in a patient with SARS-COV-2 infection. Hematol Transfus Cell Ther. 2020;42:309–12. doi: 10.1016/j.htct.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadlamudi G, Hong L, Keerthy M. Evans syndrome associated with pregnancy and COVID-19 infection. Case Rep Obstet Gynecol. 2020;2020 doi: 10.1155/2020/8862545. 8862545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Nguyen CB, Yeung Z, et al. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190:e59–61. doi: 10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angileri F, Légaré S, Marino Gammazza A, et al. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020;190:e92–3. doi: 10.1111/bjh.16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Masuya M, Katayama N, et al. Development of mixed-type autoimmune hemolytic anemia and Evans’ syndrome following chicken pox infection in a case of low-titer cold agglutinin disease. Int J Hematol. 2006;84:220–3. doi: 10.1532/IJH97.06046. [DOI] [PubMed] [Google Scholar]
- 16.Hyun DW, Kim DH, Jung JT, et al. A case of Epstein-Barr virus infection presented as Evans syndrome. Korean J Hematol. 1998;33:438–42. [Google Scholar]
- 17.Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. doi: 10.1016/j.blre.2019.100648. [DOI] [PubMed] [Google Scholar]
- 18.Fattizzo B, Michel M, Zaninoni A, et al. Efficacy of recombinant erythropoietin in autoimmune haemolytic anaemia: a multicentre international study. Haematologica. 2020 doi: 10.3324/haematol.2020.250522. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattizzo B, Zaninoni A, Gianelli U, et al. Prognostic impact of bone marrow fibrosis and dyserythropoiesis in autoimmune hemolytic anemia. Am J Hematol. 2018;93:E88–E91. doi: 10.1002/ajh.25020. [DOI] [PubMed] [Google Scholar]
- 20.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559–61. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]