Abstract
Background
Several articles reported the existence of an association between ABO blood groups and COVID-19 susceptibility. Group A and group O individuals showed a higher and lower risk, respectively, of becoming infected. No association was observed between ABO groups and mortality. To verify this association, we performed a retrospective study of two cohorts of patients with different demographic and clinical characteristics.
Material and methods
A total of 854 regular blood donors were recruited for convalescent plasma donation after recovering from a mild COVID-19 infection, and a group of 965 patients more severely affected who were transfused during hospitalisation were also included. We also investigated the potential role of the different risk factors on patient outcome and death. To eliminate the confounding effect of risk factors on mortality, a propensity score analysis was performed.
Results
Blood group A and blood group O COVID-19 blood donors showed a higher and lower risk, respectively, for acquiring COVID-19. In contrast, this association was not found in the group of patients transfused during hospitalisation, probably due to the great differences in demographic and clinical characteristics between the two groups. Regarding severity, age was one of the most significant risk factors. ABO blood groups were also seen to represent important risk factors for COVID-19 severity and mortality. Mortality risk in group A individuals was significantly higher than in group O individuals (OR: 1.75, 95% CI: 1.22–2.51).
Discussion
The association between the ABO blood groups and the susceptibility to acquire COVID-19 infection was confirmed in the group of blood donors. ABO blood groups were also associated to COVID-19 severity and mortality in the group of patients transfused during hospitalisation. Therefore, blood groups A and O are two important factors to be considered when evaluating the prognosis of patients with COVID-19.
Keywords: ABO blood group, COVID-19, disease susceptibility, mortality
INTRODUCTION
Coronavirus disease (COVID-19) represents a public health crisis of global proportions that causes substantial morbidity and a burden of mortality that has overwhelmed health systems worldwide. To date, Spain has registered the ninth highest number of confirmed cases and deaths from COVID-19 in the world1.
Since March 2020, several articles reported an association between the ABO blood group and COVID-19. These include papers by Zhao et al.2, Zietz et al.3, Zeng et al.4 Li et al.5 and Wu et al.6, all agreeing that group A individuals would have a higher risk of becoming infected, and group O individuals would have a lower risk. Epidemiological studies from the prior SARS-CoV-1 outbreak in Hong Kong showed that group O individuals also had a lower risk of infection during that outbreak7, but the rapid control of the epidemic and its low impact on the global population meant that this finding was not sufficiently debated or confirmed with additional studies.
Our current knowledge of how coronavirus invades our cells, as well as the results reported in some epidemiological and experimental studies, suggest that this relationship between the ABO blood group and the risk of acquiring the infection is highly plausible. In an experimental model, Guillon et al.8 found that anti-A antibodies specifically inhibited the adhesion of SARS-CoV S protein-expressing cells to ACE2-expressing cell lines. Therefore, they concluded that the lower susceptibility of blood group O and higher susceptibility of blood group A for COVID-19 could be linked to the presence of natural ABO antibodies, particularly anti-A antibody in the blood.
A recently published Genome-Wide Association Study (GWAS)9 has taken a further step by demonstrating that ABO polymorphism plays an inhibitory role in SARS-CoV-2 infection and is also implicated in COVID-19 progression. In order to verify the association between the ABO blood group and COVID-19 susceptibility and severity in our population, we performed a retrospective study of two cohorts of patients with different demographic and clinical characteristics. The first group, that had not previously been studied for this purpose, is made up of 854 regular blood donors who offered their convalescent plasma after recovering from a mild COVID-19 infection. The second group consists of 965 infected patients more severely affected who were transfused during their hospitalisation. The ABO blood group availability and the access to the most appropriate controls for each cohort led us to validate this relationship in both groups of patients.
MATERIALS AND METHODS
COVID-19 blood donors
The 854 blood donors included in this study were selected from a large group of people who responded to the call made by the Catalonia Blood and Tissue Bank (Banc de Sang i Teixits, BST) to obtain convalescent plasma from patients with COVID-19 infection. The main reason for this selection was that these people had already made blood donations before the pandemic, as recorded in our registry of blood donors in Catalonia. Most of them (95%) experienced a mild clinical course and did not require hospitalisation, but 5% of these donors presented a more severe clinical course of symptoms (pneumonia, respiratory failure solved with oxygen supplementation) requiring hospitalisation. COVID-19 infection was confirmed in all of them by a positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) test.
Demographic data and ABO blood group distribution from our cohort of infected blood donors were compared with those of a control group of 75,870 blood donors who donated blood in our organisation during the first four months of 2020. The ABO blood group distribution was also examined in a group of 34,770 donors who made their first blood donation during the first four months of 2020. This second group of donors was incorporated in order to assess the degree of variation in the distribution of ABO blood groups among first-time donors and regular blood donors.
COVID-19 patients who were transfused during hospitalisation
A cohort of 965 COVID-19 patients who were transfused in the course of their hospitalisation was also investigated. These patients were admitted to one of the 15 hospitals in Catalonia that participated in this study. The BST of Catalonia, in addition to recruiting blood donors and processing blood and blood components, is also responsible for the transfusion activity managed by hospital blood transfusion services. This transfusion model and this functional structure allow us to take part in all phases of the transfusion process from the donor to the transfused patient, and ensure easy access to all the information related to blood donors and transfused patients.
The ABO blood group distribution among this cohort of infected patients was compared with that of a control group of 52,584 uninfected patients who were transfused in the same Catalan hospitals during the years 2018 and 2019.
Statistical analysis
For each of the two cohorts of COVID-19 patients we performed a Pearson’s χ2 test to investigate whether ABO blood group distributions differ between these two cohorts and the corresponding control populations. In addition, we compared each blood group against all others using a 2×2 contingency table to determine effect sizes for each blood group itself. For the one-versus-rest blood group comparisons, we reported odds ratios (OR), p-values from Fisher’s exact test (two-sided), and the odds ratio confidence intervals.
Next, we investigated whether the ABO blood group distribution differed according to age, gender, and different comorbidities. By using a logistic regression analysis, we evaluated the potential role of the different risk factors on patient outcome and death, including ABO blood groups.
To prevent the confounding effect of risk factors on mortality, and to evaluate the exact role of the ABO blood groups in the death of patients, a propensity score analysis was performed10. This analysis consisted of matching a group A individual with a group O individual regarding the statistically significant clinical variables or those showing a marked trend. The matching window was a maximum of 0.01 propensity score. Likewise, by selecting the variables significantly associated with mortality or those that showed a marked trend, a prognostic score was built. The logistic regression coefficients of each of these variables associated with mortality were used to evaluate the sign and weight of each variable in the score. The main objective was to discriminate the exact role played by ABO blood groups in the score.
Statistical analyses were performed using the STATA software (version 14).
RESULTS
COVID-19 blood donors
Five-hundred and sixteen out of the 854 COVID-19 blood donors were women (60.5%) and 338 were men (39.5%). Median age was 45.0 years (range: 18–65 years; interquartile range: [IQR] 36.0–53.0) (Table I). As expected from their blood donor status, no comorbidity was identified in any of them. The ABO blood group distribution was 47.19%, 7.61%, 3.75% and 41.45% for groups A, B, AB, and O, respectively.
Table I.
Age, gender and ABO blood group distribution in the group of COVID-19 blood donors and in the group of COVID-19 patients who were transfused compared to those of their respective controls.
| Analysed patients | Control group | Analysed patients | Control group | |
|---|---|---|---|---|
| COVID-19 blood donors | Healthy blood donors from Catalonia | Infected patients who were transfused | Uninfected patients who were transfused | |
| N | 854 | 75,870 | 965 | 52,584 |
| Median age | 45.0 years (IQR 36.0–53.0) | 45.0 years (IQR 32.0–53.0) | 69.0 years (IQR 59.0–77.0) | 72.1 years (IQR 58.2–82.5) |
| Gender | ||||
| Women | 516 (60.5%) | 36,856 (48.5%) | 395 (40.93%) | 26,371 (50.15%) |
| Men | 338 (39.5%) | 39,014 (51.5%) | 570 (59.07%) | 26,213 (49.85%) |
| ABO distribution | ||||
| A | 47.19% | 42.02% | 45.60% | 44.11% |
| B | 7.61% | 7.52% | 9.53% | 9.52% |
| AB | 3.75% | 3.12% | 3.11% | 4% |
| O | 41.45% | 47.34% | 41.76% | 42.37% |
| p-value | 0.0052 | 0.4783 | ||
N: number; IQR: interquartile range. In bold statistically significant data.
Of the total 75,870 healthy blood donors used as control group, 36,856 were women (48.5%) and 39.014 were men (51.5%). Median age for this group was also 45.0 years (range: 18–65 years; IQR: 32.0–53.0) (Table I). The ABO blood group showed a distribution of 42.02%, 7.52%, 3.12% and 47.34% for A, B, AB, and O, respectively. As expected, the proportion of group O donors was higher than that usually found in the general population as well as in first-time donors due to the higher presence of this blood group among regular blood donors. The ABO blood group distribution of first-time donors from the Catalan registry of blood donors during the first four months of 2020 was: 42.03%, 9.65%, 3.77% and 44.55% for groups A, B, AB, and O, respectively. This distribution shows that the percentage of group O donors is clearly lower than that usually present in regular blood donors and likely very close to what we might find in the general population. As stated, the median age of the COVID-19 blood donors and the group of healthy blood donors used as control was the same: 45 years. However, the gender distribution was different in both groups. While in the group of blood donors in Catalonia the distribution was very even, among donors who became infected, and who later offered to donate convalescent plasma, a broader representation of female donors was observed.
The ABO blood group distribution in the COVID-19 blood donors was significantly different from that found in the control group (p=0.0052). Most striking, among COVID-19 donors, the ratio of blood group A to blood group O was the inverse of the ratio found among healthy regular blood donors in the control group (Table I). According to the results observed in this cohort of convalescent plasma donors previously infected with COVID-19, group A individuals were more represented than non-A individuals (OR: 1.23; 95% CI: 1.08–1.41; p=0.0024), while group O donors were under-represented compared to non-O donors (OR: 0.78; 95% CI: 9 [0.69–0.75]; p=0.0006) and the differences were very significant (Table II).
Table II.
Relationship between ABO blood group and the risk of acquiring a COVID-19 infection in the group of blood donors who have recovered from COVID-19 infection (n=854).
| Blood group | ||||
|---|---|---|---|---|
| A | B | AB | O | |
| N | 403 | 65 | 32 | 354 |
| % | 47.19 | 7.61 | 3.75 | 41.45 |
| OR | 1.23 | 1.012 | 1.209 | 0.787 |
| 95% CI | 1.08–1.41 | 0.79–1.31 | 0.85–1.73 | 0.69–0.90 |
| p-value | 0.0024 | 0.921 | 0.293 | 0.0006 |
N/n: number; OR: odds ratio; CI: confidence interval. In bold statistically significant data.
COVID-19 patients transfused during hospitalisation
The group of patients with COVID-19 who were transfused during hospitalisation only represented 7–10% of the total number of patients who were admitted for the infection in the time period from March 1st to June 15th, 2020, in the 15 Catalan hospitals who reported their data. Five-hundred and seventy out of the 965 COVID-19 patients were men (59.07%) and 395 were women (40.93%). The median age was 69.0 years (range: 1–102 years; IQR: 59.0–77.0) (Table I). Information was obtained on the existence of any underlying diseases in 897 of the 965 patients with the result that one or more comorbidities were present in 770 of them (85.84%). The comorbidities identified were: hypertension, diabetes mellitus, dyslipidaemia, obesity, heart disease, solid tumours, haematologic malignancies, chronic obstructive pulmonary disease, chronic renal disease, digestive disorders, chronic liver disease, neurological disorders, and autoimmune diseases. Some of these patients (n=149, 16.61%) are regularly transfused due to their underlying diseases and/or because of the immunosuppressive treatments prescribed for them. The comorbidities identified in this group of patients were: solid tumours, haematologic malignancies, chronic renal disease, digestive disorders, chronic liver disease, and autoimmune diseases. In the group of patients without comorbidities (n=127, 14.16%) or with comorbidities that are not usually accompanied by regular transfusion requirements (n=748, 69.23%), 50% of patients were admitted to the intensive care unit where a multifactorial anaemia (patients with extracorporeal membrane oxygenation, bleeding episodes, frequent blood sample extractions, anaemia associated to inflammation) required red blood cell transfusion. The remaining 50% of patients were also transfused for anaemia associated with inflammation and/or with bleeding episodes and/or with certain iatrogenic practices also related to frequent blood sample extractions.
The ABO blood group distribution in the 965 transfused COVID-19 patients was 45.60%, 9.53%, 3.11%, and 41.76% for groups A, B, AB, and O, respectively (Table I). The following ABO blood group distribution was observed in the group of 897 patients in whom the presence of comorbidities was investigated: 45.03%, 9.14%, 3.12%, and 42.69% for groups A, B, AB, and O, respectively. In the subgroup of patients with or without comorbidities who do not require regular blood transfusion, the ABO group distribution was: 44.38%, 9.50%, 3.21%, and 42.91% for groups A, B, AB, and O, respectively. Finally, in the subgroup of patients regularly transfused due to their underlying diseases and/or because of immunosuppressive treatments, the ABO distribution was: 48.32%, 7.38%, 2.69%, and 41.41% for groups A, B, AB, and O, respectively.
In the control group, 26,213 patients were men (49.85%) and 26,371 were women (50.15%). Median age was 72.1 years (range: 1–106 years; IQR: 58.2–82.5) (Table I). The ABO blood groups showed a distribution of 44.11%, 9.52%, 4%, and 42.37% for groups A, B, AB, and O, respectively (Table I). The distribution according to age and gender was similar between the set of analysed patients who were transfused and the reference population. The ABO blood group distribution in the COVID-19 patients was not significantly different from that found in the control group (Table I). However, group A was more represented in patients >60 years of age (p=0.015). We did not find significant differences in the ABO blood group distribution in relationship to the presence or absence of underlying comorbidities. When investigating whether the distribution of comorbidities varies across different ABO blood groups, only hypertension showed a significant association with blood group A, being present in a higher proportion in this group than in the control group (p=0.044) (Table III).
Table III.
Relationship between the ABO blood groups and comorbidities in COVID-19 patients transfused during hospitalisation (n=770)
| Comorbidities | ABO blood group | |
|---|---|---|
| χ2 | p | |
| Hypertension | 8.10 | 0.044 |
| Heart disease | 3.76 | 0.288 |
| Diabetes mellitus | 4.82 | 0.185 |
| Obesity | 1.08 | 0.782 |
| Dyslipidaemia | 3.17 | 0.366 |
| COPD | 1.20 | 0.751 |
| Chronic renal disease | 3.46 | 0.326 |
| Digestive disorder | 3.40 | 0.334 |
| Haematologic malignancy | 1.93 | 0.586 |
| Chronic liver disease | 1.25 | 0.739 |
| Neurological disorder | 1.65 | 0.647 |
| Autoimmune disease | 0.44 | 0.930 |
| Solid tumour | 4.55 | 0.207 |
n: number; COPD: chronic obstructive pulmonary disease. In bold statistically significant data.
Risk factors that can have an impact on COVID-19 severity and mortality
Age was confirmed as one of the most influential risk factors (Table IV). Specifically, we estimated that the risk of death increases by 1.5% each year (OR: 1.015, 95% CI: 1.005–1.025). By contrast, gender and comorbidities were not found to influence risk factors for death. However, heart disease showed a marked trend, although it did not reach statistical significance. More interestingly, logistic regression analysis showed that individuals with blood group A have a higher risk of death than those with group O (OR 1.39, 95% CI: 1.03–1.86). This association persisted when we compared the risk of individuals with blood group A with that of the rest of the ABO blood groups (OR: 1.35, 95% CI: 1.03–1.78). Conversely, group O individuals showed a lower risk of death than non-O blood group individuals (OR: 0.75, 95% CI: 0.56–0.99) (Table IV).
Table IV.
Relationship between age, gender, ABO blood group, comorbidities and mortality in COVID-19 patients transfused during hospitalisation (n=965)
| Analysed variables | Mortality | ||
|---|---|---|---|
| OR | 95% CI | p | |
| Age | 1.015 | 1.005–1.025 | 0.0026 |
| Gender | 0.88 | 0.67–1.16 | 0.397 |
| Group Avs O | 1.39 | 1.06–1.89 | 0.026 |
| Group Avs non-A | 1.35 | 1.03–1.78 | 0.029 |
| Group Ovs non-O | 0.75 | 0.56–0.99 | 0.044 |
| Any comorbidity | 1.37 | 0.90–2.08 | 0.142 |
| Hypertension | 1.14 | 0.86–1.52 | 0.346 |
| Heart disease | 1.36 | 0.98–1.88 | 0.067 |
| Diabetes mellitus | 0.84 | 0.61–1.16 | 0.305 |
| Obesity | 0.86 | 0.57–1.27 | 0.477 |
| Dyslipidaemia | 1.05 | 0.76–1.45 | 0.743 |
| COPD | 1.17 | 0.73–1.90 | 0.501 |
| Chronic renal disease | 1.07 | 0.71–1.61 | 0.716 |
| Digestive disorder | 1.62 | 0.77–3.38 | 0.205 |
| Haematologic malignancy | 1.45 | 0.89–2.34 | 0.131 |
| Chronic liver disease | 1.11 | 0.98–2.08 | 0.729 |
| Neurological disorder | 1.14 | 0.63–2.06 | 0.656 |
| Autoimmune disease | 1.34 | 0.57–3.15 | 0.494 |
| Solid tumour | 0.91 | 0.62–1.33 | 0.639 |
OR: odds ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease. In bold statistically significant data.
Propensity score analysis
In the propensity score analysis, patients in group A (n=271) were compared with those in group O (n=271) previously matched on the basis of age, gender, chronic obstructive pulmonary disease, heart disease, hypertension, diabetes mellitus, obesity, and whether comorbidities were or were not present (Table V). The analysis showed that the mortality risk in individuals in group A was much higher than in individuals in group O (OR: 1,75, 95% CI: 1.22–2.51).
Table V.
Propensity analysis score
| Blood group A |
Blood group O |
p | |
|---|---|---|---|
| N | 271 | 271 | |
| Median age (IQR) | 69.0 (60.0–77.0) | 70.0 (60.0–76.0) | 0.765 |
| Gender (M) | 161 | 161 | 1.000 |
| % | 59.4 | 59,4 | |
| COPD | 23 | 22 | 0.876 |
| % | 8.5 | 8.1 | |
| Heart disease | 69 | 68 | 0.921 |
| % | 25.5 | 25.1 | |
| Hypertension | 106 | 111 | 0.661 |
| % | 39.1 | 41.1 | |
| Diabetes mellitus | 74 | 72 | 0.847 |
| % | 27.3 | 26.6 | |
| Obesity | 34 | 36 | 0.798 |
| % | 12.5 | 13.3 | |
| Any comorbidity | 231 | 235 | 0.621 |
| % | 85.2 | 86.7 | |
| Mortality | 110 | 76 | 0 |
| % | 40,6 | 28 |
N: number; IQR: interquartile range; M: male; COPD: chronic obstructive pulmonary disease.
Prognostic score
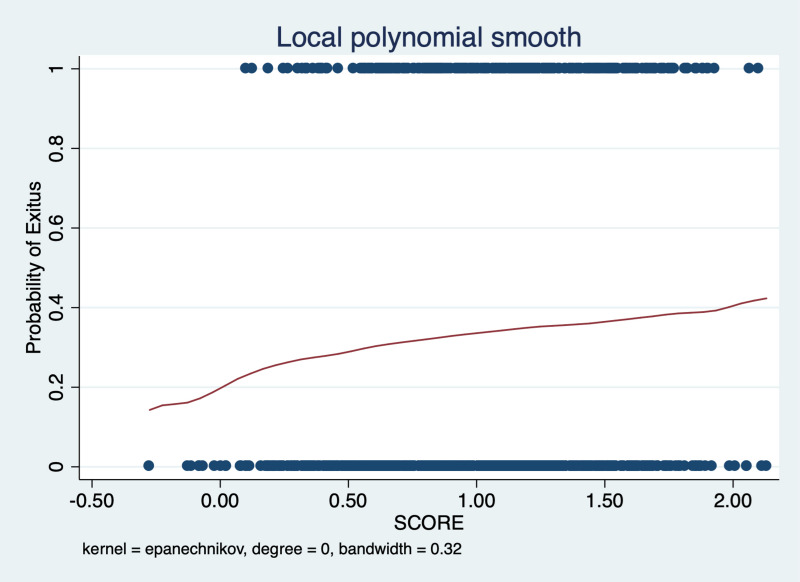
Finally, the variables that proved to be related to risk of mortality (age, blood group A, blood group O), or those showing a marked trend even though it had not reached statistical significance (gender, heart disease, hypertension, diabetes mellitus, and obesity) were selected to build a prognostic score. Considering the logistic regression coefficients of each variable regarding mortality, we observed that blood group A had a positive value and, conversely, blood group O had a negative value. These two opposite sign values would indicate that blood group A would be a risk factor related to mortality and, on the contrary, group O individuals would enjoy a certain protective effect. Moreover, these results were in agreement with those obtained in the propensity score analysis (Figure 1).
Figure 1. Prognostic score.
The prognostic score was designed considering age, gender, blood group A, blood group O, heart disease, hypertension, diabetes mellitus and obesity.
DISCUSSION
In this study, we confirmed the relationship between ABO blood groups and COVID-19 susceptibility in the group of blood donors infected by SARS-CoV-2. Blood group A donors had a significantly higher risk for acquiring COVID-19 compared with non-A blood groups and blood group O donors had a significantly lower risk for the infection compared with non-O blood groups. By contrast, we did not observe this relationship in the COVID-19 patients who were transfused during their hospitalisation. ABO blood group antigens are present not only on erythrocytes but also on epithelial cells of the respiratory and digestive tracts, and on endothelial cells underlying blood vessels which also have the ability to synthesise ABH carbohydrate epitopes. It has been hypothesised8 that the S protein of virions produced by either A or B individuals could be decorated with A or B carbohydrates epitopes, respectively. Natural anti-A or anti-B antibodies from blood group O, B, and A individuals could bind these epitopes on the S protein of viral particles and block its interaction with the ACE2 protein receptors in the host cell membrane, thereby preventing infection. As in transfusion11,12, the rules applying to this process would be the following: SARS-CoV-2 viruses produced in individuals from group A can express A antigens and infect individuals from groups A and AB without such antigen-antibody reactions. However, infection of these viruses to individuals in groups B or O who possess anti-A antibodies may be somehow hampered. Similarly, SARS-CoV-2 viruses that express B antigens can infect individuals from groups B or AB. However, infection in group A or O individuals possessing anti-B antibodies may be somehow limited. Group O individuals would benefit the most by having defences (anti-A and anti-B antibodies) against SARS-CoV-2 viruses expressing A or B antigens. This hypothesis was supported by the results obtained in an experimental model in which Chinese hamster ovary cells were engineered to express on cell surface S proteins carrying A glycan antigens8. The adhesion of those cells to Vero E6 cells expressing ACE2 was specifically inhibited by a mouse monoclonal anti-A antibody or human natural anti-A antibodies. From these observations, a mathematical model of viral transmission was constructed and the kinetics of the SARS epidemic was examined. The study estimated a substantial reduction in the infective capacity of the virus due to ABO polymorphism.
When the association of ABO blood group with susceptibility to COVID-19 was analysed from the perspective of ABO antibodies instead of ABO blood group antigens, the same conclusions were reached. Taking as reference the series of patients published by Zhao et al.2, it was observed that subjects with anti-A in serum (i.e. blood groups B and O) were significantly less represented in the COVID-19 group than those lacking anti-A whatever the blood group, whereas there was no significant difference vs circulating anti-B13. Furthermore, when they compared the supposed protective effect of anti-A from blood group O and from blood group B, they observed that individuals from blood group O were under-represented, whereas patients from group B, on the contrary, were over-represented, which means that anti-A from blood group O is more protective than anti-A from blood group B. This last observation was attributed to the fact that the predominant immunoglobulin isotype of anti-B/anti-A in the serum of individuals of blood groups A and B is IgM, but IgG in the serum of individuals of blood group O, as previously documented14.
The proper functioning of this defence mechanism against SARS-CoV-2 requires a strong immune system and adequate antibody production. One of the most recognised consequences of ageing is a decline in immune function. While elderly individuals are by no means immunodeficient, they often do not respond efficiently to novel or previously encountered antigens. Similarly, many disorders can weaken the immune system with the result that the individual is immunocompromised. Moreover, immunosuppressive treatments can also compromise the proper functioning of our defence mechanisms and make patients more vulnerable to infections15.
The different demographic and clinical characteristics of the two cohorts of patients investigated in the current study may partly explain the different results observed. The group of infected blood donors fulfilled the optimal conditions to defend themselves against the SARS-CoV-2 virus based on the antigens and antibodies involved in each case. This group was made up of younger people (median age: 45 years) without underlying diseases. This may explain why most of them (95%) only experienced a mild infection that did not require hospitalisation. Hospitalisation was only required in the remaining 5% who suffered from a more severe infection. By contrast, the group of COVID-19 patients who were transfused was older (median age: 69 years); all required hospitalisation, and one or more comorbidities were present in >85% of them. About 17% of these older patients were regularly transfused due to diseases and/or treatments that compromised their immune system. It is reasonable to assume that the role played by ABO antibodies against the virus in this subgroup of patients was probably not as efficient. However, we cannot exclude the possibility that a larger sample size including transfused and non-transfused patients, with fewer comorbidities and somewhat younger, could perhaps reveal differences in ABO group distribution compared to the control group. It should be remembered that our transfused patients only represent 7–10% of patients admitted to the same hospitals during the same period due to COVID-19.
Reference populations used to compare ABO distributions must be selected with care. In the paper by Zhao et al.2 and in other subsequent articles based on it, the origin and composition of the control group are not clearly defined. It can be inferred from the expression “normal individuals from Wuhan” that a group of regular blood donors was likely used as a reference population. This allowed them to find an association between the ABO blood group and susceptibility to COVID-19. However, this association was supported by the choice of a reference population in which the proportion of individuals in group O is greater than that of the general population. Indeed, it is well known that group O blood donors are recruited and loyalised with special interest, and they have an obviously bigger presence among regular blood donors.
In the current study, a group of regular blood donors was used as reference population to compare with our infected regular blood donors, but a group of uninfected transfused patients was used as reference population to compare with the cohort of infected patients who were transfused. This different strategy may also help to explain why in our cohort of transfused patients we were unable to demonstrate the relationship between the ABO blood group and COVID-19 susceptibility. Interestingly, when we compared ABO distributions in infected patients with regular blood donors we also observed a significant difference in the ABO blood group distribution (p=0.0023): blood group A was more common among infected patients, standing at the limit of statistical significance (OR: 1.13; 95% CI: 0.99–1.29; p=0.069), and group O was significantly less prevalent, showing the expected protective effect (OR: 0.82; 95% CI: 0.73–0.95; p=0.0057). Likewise, the comparison between regular blood donors and infected patients without comorbidities or with underlying conditions that are not usually accompanied by regular transfusion requirements maintained the significant difference found in the group of patients in whom comorbidities were investigated (p=0.0465). In contrast, the comparison between regular blood donors and patients infected with underlying conditions that weaken the immune system showed no significant difference in the distribution of ABO groups. These data reinforce the idea that the effectiveness of the role played by the antibodies of the ABO system in the virus entry is closely linked to the strength of the immune system present in each individual. Therefore, the demonstration of the relationship between the ABO blood group and the susceptibility to COVID-19 will depend, among other causes, on the sample size, demographic and clinical characteristics of the analysed patients, but also, and in a very decisive way, on the selection of the appropriate control group16–18.
According to the reported data, older people are at high risk of COVID-19, and over 90% of deaths occurred in those >60 years of age. Eighty percent of deaths occurred in individuals with at least one underlying comorbidity, in particular with cardiovascular disease/hypertension and diabetes, but also with a variety of other chronic underlying conditions. Male COVID-19 patients tend to have a worse prognosis than female patients19–21. When we investigated the risk factors that may influence COVID-19 severity and mortality in our transfused patients, age was confirmed as one of the most determining factors. More precisely, we estimated that for each passing year the risk of death increases by 1.5% compared to the previous year. Neither gender nor comorbidities were evidenced as risk factors for mortality in our COVID-19 patients. Heart disease showed a marked trend but did not reach statistical significance. The most interesting finding was that the ABO blood group in our patients represented an important risk factor for COVID-19 severity and mortality. Blood group A individuals had a higher risk of death than group O and, conversely, blood group O individuals had a lower risk of death than group A. This observation was confirmed when the confounding variables were eliminated and the propensity score analysis was performed, estimating that the risk of mortality in group A individuals was much higher than in group O individuals.
Finally, by combining the risk factors significantly associated with mortality (age, ABO blood group) with some others that show a certain trend (gender, heart disease, hypertension, diabetes mellitus and obesity) we built a prognostic score that can be useful to predict the risk of dying in COVID-19 hospitalised patients.
Several large collaborative efforts are currently underway to generate, share and analyse genetic data to understand the links between human genetic variation and COVID-19 susceptibility and severity, the most prominent of which is The COVID-19 Host Genetics Initiative22. There are several diseases the associations of which to the ABO polymorphism have been demonstrated not only by statistical analysis but also by GWAS. This was the case when it was shown that the A allele of the ABO gene was associated with a higher risk of developing cardiovascular diseases23. Therefore, it is feasible that patients with a blood group type A, especially those who have already been diagnosed with cardiovascular disease (particularly hypertension) are more likely to develop severe COVID-19 once infected. Viral infection may deregulate vascular tone and permeability, and induce cytokine storms and redox stress24. More recently, a new GWAS carried out in a collaborative effort by researchers from different European countries, including Spain, showed significant associations with SNPs on chromosome 3p21.31 and on 9q34.2 in patients requiring mechanical ventilation compared to those requiring oxygen supplementation9. The association on 9q34.2 was mapped at the ABO locus and a group-specific analysis showed a higher risk for blood group A (OR: 1.45; p=1.48×10−4) and a protective effect for group O (OR: 0.65; p=1.06×10−5). It is foreseeable that in the coming months we can expect to see new findings of this type that may better explain the influence of the ABO group not only on the susceptibility to infection, but also on the severity and mortality of COVID-19 patients. To elucidate the biochemical mechanisms responsible for these associations is not only of basic scientific interest but also of translational clinical importance.
CONCLUSIONS
In summary, the current study confirmed the existence of an association between the ABO blood group and the susceptibility to acquire the COVID-19 infection in a cohort of convalescent plasma donors not studied so far for this purpose. Group A individuals had a higher risk of viral infection and group O individuals had a lower susceptibility. This association was not observed in the group of infected patients who were transfused during their hospitalisation. However, an association between the ABO blood group and COVID-19 severity/mortality was observed in the cohort of transfused patients. The risk of mortality in COVID-19 patients in blood group A was significantly higher than that of patients in blood group O. Elderly male patients with blood group type A and underlying conditions, especially those with cardiovascular diseases (in particular hypertension), need to be quarantined and protected from COVID-19 infection or receive special medical care to prevent clinical deterioration and progression of severe illness.
ACKNOWLEDGEMENTS
I am grateful to Vicente Solivelles who took care of the database management; his assistance was critical to the result of this work. Josep Carbonell managed the data and controlled its accuracy. Jordi Carrión, David Miralles, Albert Ros, Tony Massi and Manel Gastó provided the most suitable data to be used as controls. Sandra Casals supported recruitment of study patients. Gloria Pliego participated in blood donor recruitment. The opinion and comments of Núria Nogués on the manuscript were very useful.
Footnotes
AUTHORSHIP CONTRIBUTIONS
EMD conceived the main idea and wrote the manuscript. JLLP performed the statistical analysis. RP, IR, GF, JG, AM, GE, LR, LM, NG, AP, AJ, AP, and GA collected the data and critically revised the manuscript. RV contacted the COVID-19 regular blood donors. SS, LLP, and EC critically revised the manuscript. All the Authors approved the manuscript final version and took responsibility for the integrity and accuracy of the data.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Centro de Coordinación de Alertas y Emergencias Sanitarias. Dirección General de Salud Pública. Ministerio de Sanidad. Situació Internacional. [Accessed on 09/08/2020]. Available at: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_202_COVID-19.pdf. [In Spanish.]
- 2.Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. 2020. [DOI]
- 3.Zietz M, Tatonetti NP. Testing the association between blood type and COVID-19 infection, intubation, and death. medRxiv. 2020 doi: 10.1101/2020.04.08.20058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng X, Fan H, Lu D, et al. Association between ABO blood groups and clinical outcome of coronavirus disease 2019: evidence from two cohorts. medRxiv. 2020. [DOI]
- 5.Li J, Wang X, Chen J, et al. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020;190:24–7. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta. 2020;509:220–1. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 8.Guillon P, Clément M, Sébille V, et al. Inhibition of the ninteraction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–93. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genome wide association study of severe Covid-19 with respiratory failure. NEJM. 2020;383:1522–34. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas L, Li F, Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA. 2020;323:466–7. doi: 10.1001/jama.2019.21558. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto F. ABO blood groups and SARS-CoV-2 infection. Research gate. [DOI]
- 12.Yamamoto F, Yamamoto M, Muñiz-Diaz E. Blood group ABO polymorphism inhibits SARS-CoV-2 infection and affects COVID-19 progression. Vox Sang. 2020 doi: 10.1111/vox.13004. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gérad C, Maggipinto G, Minon JM. COVID-19 and ABO blood group: another viewpoint. Br J Haematol. 2020;190:e93–4. doi: 10.1111/bjh.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stussi G, Huggel K, Lutz HU, et al. Isotype specific detection of ABO blood group antibodies using a novel flow cytometric method. Br J Haematol. 2005;130:954–63. doi: 10.1111/j.1365-2141.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 15.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–65. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzik S, Eliason K, Morris EB, et al. COVID-19 and ABO blood groups. Transfusion. 2020;60:1883–4. doi: 10.1111/trf.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latz CA, De Carlo C, Boitano L, et al. Blood types and outcomes in patients with COVID-19. Ann Hematol. 2020;99:2113–8. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaf RK, Al-Samkari H, Brenner SK, et al. ABO phenotype and death in critically ill patients with COVID-19. Br J Haematol. 2020;190:204–8. doi: 10.1111/bjh.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huan C, Wang Y, Xingwang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehra MR, Desai SS, Kuy S, et al. Cardiovascular disease, drug therapy and mortality in COVID-19. NEJM. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.The COVID-19 Host Genetic Initiative. The COVID-19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Human Genet. 2020;28:715–8. doi: 10.1038/s41431-020-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu O, Bayoumi N, Vickers MA, et al. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–9. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 24.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol. 2020;27:1436–7. doi: 10.1177/2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]