Abstract
The current study is to distinguish between osteochondrosis and osteochondritis, utilizing surface microscopy of individuals with documented pathology. Osteochondrosis is associated with smooth borders and gradient from edge to defect base, while osteochondritis and subchondral impaction fractures are associated with subsidence of the affected area of articular surface with irregular edges. The base of osteochondrosis is penetrated by multiple channels, smoothly perforate its surface, indistinguishable from unfused epiphyses, confirming their vascular nature. This study provides a technique for distinguishing osteochondrosis and osteochondritis and further documents of the value of epi-illumination microscopy in expanding our understanding of bone and joint disease.
Keywords: Subchondral impaction fractures, Bone modification, Vascular supply, Epiphyses, Epi-illumination microscopy
1. Introduction
1.1. Controversy
Osteochondrosis and osteochondritis, common causes of lameness, are terms often used interchangeably, but actually describe very different phenomena.1, 2, 3, 4, 5, 6 Osteochondrosis is the term applied to free cartilage bodies,7 related to specific joint defects.8 Ytrehus et al.9 (2004) and Olstad et al.10,11 suggested a role for vascular compromise during embryogenesis/development in osteochondrondrosis. This suggested role contrasts with König's12 application of the term osteochondritis dissecans to loose bodies resulting from subchondral knee fractures. Note use of the term “dissecans”, derives from the Latin term dissecare, which means “to separate”. The terminology becomes even more convoluted, as osteochondritis (at least of the knee) was once considered a form of avascular necrosis (AVN),13, 14, 15 with debate about the primary or secondary nature of the AVN.16 However, subsequent analysis has revealed that the pathology is actually that of subchondral fractures.2,5,6,16, 17, 18, 19, 20, 21, 22, 23 Subchondral fractures are found with repetitive impact injuries.3,23,24 This finding is in contrast to osteochondritis related to infection, a subject beyond the scope of the current analysis.25
1.2. Subchondral collapse
Collapse of subchondral bone is also a complication of AVN,15 but which is primary? AVN occurs in watershed regions where compromise of circulation restricts nutritional supply to the portion of bone served by that (those) vessel(s).26 The distribution of trauma-related (whether acute or repetitive) subchondral fractures is independent of vascular watersheds.6,27,28 Recognition that human knee “osteochondritis” distribution does not follow watershed vascular patterns led to revised perspectives.9,29 AVN is also a complication typically of corticosteroid use and decompression syndrome in humans.30,31 However, in the absence of those risk factors, AVN is not responsible for the damage in human knees.21
1.3. Vascular considerations
The character of the subchondral bone-cartilage interface has been subject of much discussion, with some authors describing macroscopic surface “porosity,”32 which is a claim that has subsequently disputed.33 Madry et al.15,p.420 noted it is the “cortical lamella directly underneath the radiologically discernible joint space” which is recognized macroscopically. Subchondral vasculature invades the calcified zone, but the tidemark is not penetrated.15,34,35 Moreover, Hoemann et al.36,p.2 stated epiphyseal bone expands “into the cartilage anlage until the interface forms a calcific layer that arrests vascular invasion”. Because osteochondrosis is perceived as failure of cartilage maturation, we hypothesize that vascular channels should be recognized, similar to those structures expected at the metaphyseal surface adjacent to unfused epiphyses.36
1.4. Goal of study
The current study was conducted: 1) to test the vascular channel hypothesis as to the characteristics of the surface of unfused epiphyses and to distinguish between the nature of osteochondrosis and osteochondritis/subchondral impaction fractures at a resolution several orders of magnitude greater than macroscopic or x-ray evaluation; and 2) to identify additional clues that would allow distinguishing between osteochondrosis and osteochondritis.
2. Material and methods
2.1. Sample selection for unfused epiphyses and tibial plateau collapse
All 23 individuals under age 17 with unfused distal tibial epiphyses, as well as adults with subchondral tibial plateau segmental collapse and other subchondral defects are selected for evaluation from the 2906 early 20th century individual human skeleton component of the Hamann-Todd (HT) Collection of the Cleveland Museum of Natural History (Cleveland, Ohio, USA).37 Individuals with tibial plateau fractures are identified from the HT database (L. Jellema, personal communication).
2.2. Sample selection for osteochondrosis
Phalanges with proximal articular surface defects, previously identified as caused by osteochondrosis,38 are selected from the Dinosaur Provincial Park hadrosaur collection of the Royal Tyrrell Museum of Palaeontology (TMP) (Drumheller, Alberta, Canada). The paleontological collections of the Carnegie Museum of Natural History (Pittsburgh, Pennsylvania, USA) and the Peabody Museum of Natural History at Yale University (New Haven, Connecticut, USA) are surveyed for phalangeal impact fractures.
2.3. Rationale and validation for trans-phylogenetic sample selection
As osteochondrosis and impact fractures are not catalogued in the HT collection and given the documented validity of trans-phylogenetic (e.g., mammal and reptiles, including dinosaurs) comparisons for diseases which affect bone,39, 40, 41 previously documented osteochondrosis cases38 in the TMP collection are chosen to provide an epidemiologically-derived sample. Utilization of a trans-phylogenetic source (i.e., dinosaurs) is valid, because of documentation that the character and skeletal distribution of affliction by a given disease are between human and veterinary collections (was trans-phylogenetically reliable) and, indeed, through geologic time in the fossil record.40,42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60
2.4. Criteria for recognition of pathology
Subchondral fractures are macroscopically recognized on the basis of focal irregular or linear depressions in articular surfaces. Focal defects are distinguished from erosions because of absence of new bone formation or presence of exposed unremodeled, underlying trabeculae (in the absence of taphonomic alterations).61 Impact fractures are recognized on the basis of tibial plateau collapse.23,24,62 Osteochondrosis is recognized on the basis of regular, smooth-bordered articular surface defects in absence of new bone formation.38,63, 64, 65
2.5. Examination technique
Epi-illumination microscopy is pursued on the surface of unfused distal tibial epiphyses, human tibial plateaus (both normal or containing subchondral defects), and dinosaur phalangeal defects using a white light-emitting diode (LED) (Dino-lite digital microscope (AM7915MZT, Dunwell Tech, Inc, 19803 Hamilton Ave. #200, Torrance, CA 90502) with polarizing optics to characterize the nature of macroscopically recognized cortical discontinuities.
3. Theory/calculation
Osteochondrosis and osteochondritis are very different phenomena which can be distinguished macroscopically on the basis of the characteristics of the edges of the lesions and microscopically (surface magnification) on the basis of presence or absence of vascular channels.
Beyond clarification of the differences between the phenomena, this study provides greater clarity in recognition of tibial plateau fractures.
4. Results
4.1. Defect border characteristics
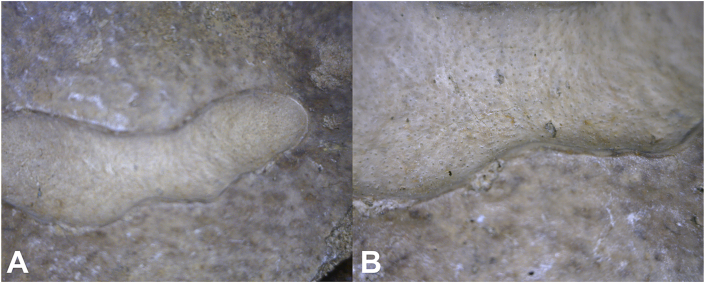
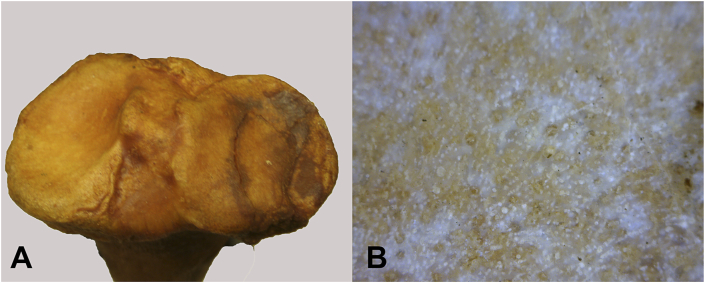
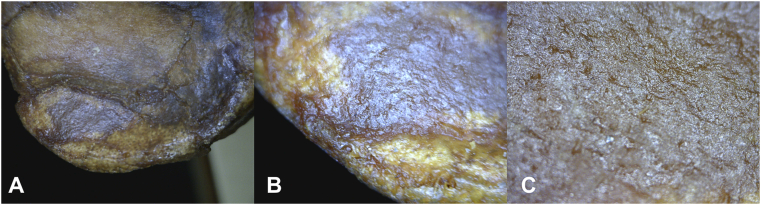
Defects are identified in: 1) 20 hadrosaur phalanges; 2) impact fractures in Diplodicus (sauropod) and Othnielosaurus (ornithischian dinosaur); and 3) osteochondritis-related surface defects in 10 humans. Use of epi-illumination microscopy reveals sharply defined borders in both osteochondrosis and osteochondritis. However, what distinguishes between osteochondrosis and osteochondritis is the nature of that border. Osteochondrosis in all so afflicted individuals is associated with a smooth gradient from the edge to the defect base (Fig. 1, Fig. 2), while lesions of osteochondritis and subchondral impaction fractures in all afflicted individuals is associated with “step off” subsidence of the affected area of articular surface (Fig. 3, Fig. 4). The border of osteochondrosis is smoothly elliptical in shape (Fig. 1, Fig. 2), while the edges of osteochondritis defects are irregular (Fig. 3, Fig. 4, Fig. 5).
Fig. 1.
Enface view of hadrosaur TMP 67/9/61 articular surface of proximal phalanx. Osteochondrosis is associated with a smooth gradient from the edge to the defect base. The borders of osteochondrosis are smoothly elliptical in shape. A. 20x. B. 200x.
Fig. 2.
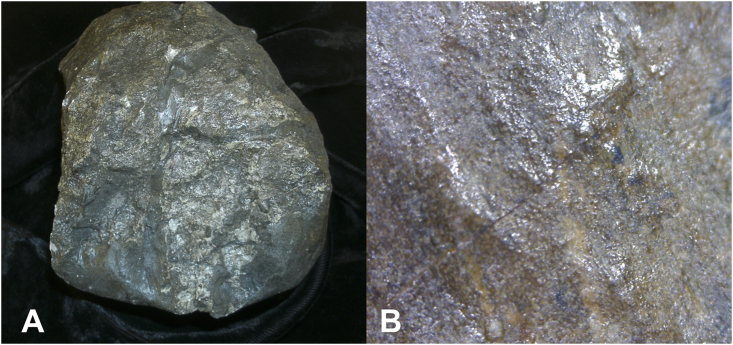
Enface view of HT 1204 osteochondrosis associated with the tibial plateau. A. Global view of tibial plateau. B. 200x. Multiple channels perforate exposed surface.
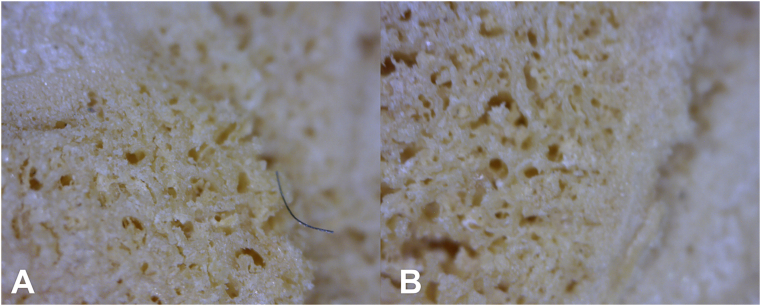
Fig. 3.
Enface view of HT 1149 tibial plateau. A. 20x. B. 50x. C. 200x. “Step off” subsidence of the affected area of articular surface in osteochondritis/subchondral fracture. The edges within the defect are irregular, and the base (visible articular surface) is indistinguishable from the appearance of unaffected adjacent bone.
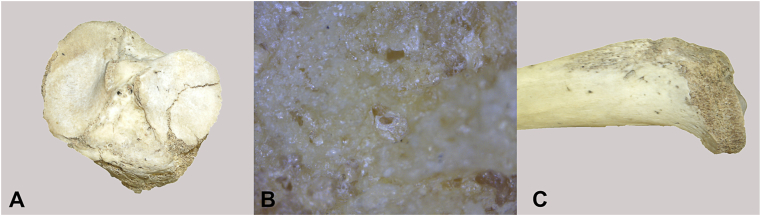
Fig. 4.
HT 1198 tibial plateau fracture. A. Enface view. B. 200x. Note area of exposure of underlying trabeculae. C. Medial view.
Fig. 5.
Enface view of distal surface of proximal Diplodicus CM 42699 phalanx. A. Global view. B. 200× magnified view. Note crack and irregular surface from impact fracture, but no vascular perforations.
4.2. Fractures and taphonomy
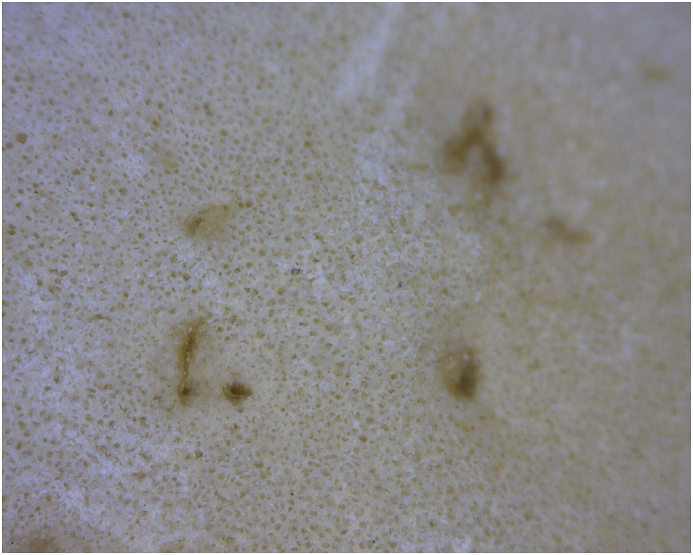
Taphonomic loss of medial metaphyseal surface in one individual clearly documents that the surface subsidence is due to a fracture (Fig. 4). The base (visible articular surface) of subchondral impaction fractures/osteochondritis is indistinguishable from the appearance of unaffected adjacent bone (Fig. 3, Fig. 4). There is no significant alteration of the articulating surface of the affected bone fragment, unless taphonomically-exposed (e.g., by drawer damage breaking off the most external portion of subchondral bone) (Fig. 6).
Fig. 6.
Enface view of HT 2141 tibial plateau. A. 50x. B. 200x. Taphonomically-exposed subchondral trabeculae.
4.3. Characteristics of lesion base
The base of osteochondrosis has a very different appearance (Fig. 1, Fig. 2). It is penetrated by multiple channels, which smoothly perforate its surface. No new bone formation is recognized. Compare these findings to the appearance of distal tibial epiphyses (Fig. 7) in 23 individuals ranging in age from 6 months to 13 years, with tibial lengths ranging from 57 to 261 mm.
Fig. 7.
Enface view of HT 2370 distal tibial epiphysis at 200× magnification. Multiple channels perforate exposed surface.
4.4. Epiphyses
The appearance of epiphyses at 200× magnification is independent of age or growth (measured by length). The microscopic appearance of epiphyseal surfaces remains consistent and indistinguishable from the noted appearance in the base of osteochondrosis defects.
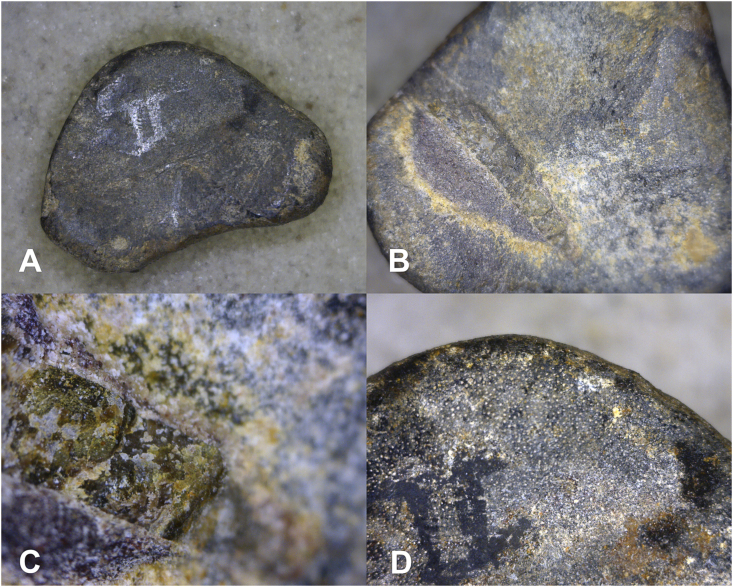
The caveat to microscopic recognition of osteochondrosis is the appearance of juvenile dinosaur bone. Smaller, less advanced dinosaurs (e.g., Othnielosaurus) are characterized by indeterminate growth, in contrast to determinate growth in larger more advanced species (e.g., Diplodicus).44 The reptilian equivalent of the mammalian epiphysis shares similar vascular penetration pattern (Fig. 8), which is a pattern that appears to be lost, or at least greatly diminished, in older individuals.
Fig. 8.
Enface view of proximal surface of Othneilosaurus phalanx YPM VP 001882. A 20x. B. affected area at 50 x. C. Affected area at 200x. D. Normal subchondral bone in juvenile dinosaur. Note vascular channels in contrast to B and C with subsided bone has lost vascular connections.
5. Discussion
Osteochondrosis and osteochondritis have characteristics which facilitate distinguishing between them macroscopically. Osteochondrosis is characterized as being elliptical with smooth borders, while the borders in osteochondritis are rough and irregular. It is the magnified view, however, that distinguishes between them and their pathophysiology clearly. Microscopic examination revealed that the base of osteochondrosis lesions has an appearance unique for articular surfaces. Because osteochondrosis is the result of failure of articular cartilage to calcify, the subchondral bone at that location focally retains its vascular continuity to overlying cartilage, which is normally lost with calcific maturation of overlying cartilage. This trait is indistinguishable from the appearance of epiphyseal bone-cartilage boundaries as manifest by epiphyseal junctions. Holmdahl and Ingelmark's66,p.157 perspective that “canal-like contacts between the articular cartilages and the medullary cavities of epiphyses” explains the “pores” seen on examination of both unfused epiphyses and the base of osteochondrosis lesions.
The articular surface of osteochondritis/subchondral fractures is indistinguishable from the appearance of normal bone. Part of the confusion in the literature may relate to a failure to recognize that the osteochondral junction is not the same as the tidemark separating calcified from non-calcified articular cartilage.15 Therefore, perforations in the osteochondral junction do not relate to the surface changes in bone in which soft tissues are no longer present.15,66, 67, 68 Vascular supply penetrates epiphyseal plates, which do not possess tidemarks related to joint cartilage.69 Thus, juvenile dinosaurs appear to preserve the penetrating vascular pattern that appears specific for osteochondrosis in mammals. However, the nature of the defect edge still appears to distinguish osteochondrosis from osteochondritis in those juvenile individuals with indeterminate growth. Interestingly, the subsided fracture (osteochondritic) region has lost its surface canals, which is a subject for future consideration.
6. Conclusions
Osteochondrosis and osteochondritis are very different phenomena which can be distinguished macroscopically on the basis of the characteristics of the edges of the lesions and microscopically (surface magnification) on the basis of presence or absence of vascular channels.
This study further provides documentation of the value of epi-illumination microscopy in expanding our understanding of bone and joint disease. Future study of effect of disease on the surface of subchondral bone will be of interest.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgements
Appreciation is expressed to Amy Henrici, Lyman Jellema, Darren Tanke and Gregory Watkins-Colwell for facilitating access to the collections they curate.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Langer F., Percy E.C. Osteochondritis dissecans and anomalous centres of ossification. A review of 80 lesions in 61 patients. Can J Surg. 1971;14(3):208–215. PubMed PMID 4996078. [PubMed] [Google Scholar]
- 2.Laverty S., Girard C. Pathogenesis of epiphyseal osteochondrosis. Vet J. 2013;197(1):3–12. doi: 10.1016/j.tvjl.2013.03.035. PubMed PMID 23647656. [DOI] [PubMed] [Google Scholar]
- 3.Norrdin R.W., Kawcak C.E., Capwell B.A., McIlwraith C.W. Subchondral bone failure in an equine model of overload arthrosis. Bone. 1998;22(2):133–139. doi: 10.1016/s8756-3282(97)00253-6. PubMed PMID 9477236. [DOI] [PubMed] [Google Scholar]
- 4.Pool R.R., Meagher D.M. Pathologic findings and pathogenesis of racetrack injuries. Vet Clin N Am Equine Pract. 1990;6(1):1–30. doi: 10.1016/s0749-0739(17)30555-2. PubMed PMID 2187565. [DOI] [PubMed] [Google Scholar]
- 5.Thomas H.W., Barnes L.G. The bone joint pathology osteochondrosis in extant and fossil marine mammals. Contrib Sci. 2015;523:1–35. Biostor, 214972 or BHL, 52211566. [Google Scholar]
- 6.Ytrehus B., Carlson C.S., Ekman S. Etiology and pathogenesis of osteochondrosis. Vet Pathol. 2007;44(4):429–448. doi: 10.1354/vp.44-4-429. PubMed PMID 17606505. [DOI] [PubMed] [Google Scholar]
- 7.Monro A. Billroth; Berlin, Germany: 1856. Microgeologie; p. 236. [Google Scholar]
- 8.Paget J. On the production of the loose bodies in joints. St Bartholomew’s Hosp Rep. 1870;6:1–4. [Google Scholar]
- 9.Ytrehus B., Ekman S., Carlson C.S., Teige J., Reinholt F.P. Focal changes in blood supply during normal epiphyseal growth are central in the pathogenesis of osteochondrosis in pigs. Bone. 2004;35(6):1294–1306. doi: 10.1016/j.bone.2004.08.016. PubMed PMID 15589210. [DOI] [PubMed] [Google Scholar]
- 10.Olstad K., Hendrickson E.H., Carlson C.S., Ekman S., Dolvik N.I. Transection of vessels in epiphyseal cartilage canals leads to osteochondrosis and osteochondrosis dissecans in the femoro–patellar joint of foals, a potential model of juvenile osteochondritis dissecans. Osteoarthritis Cartilage. 2013;21(5):730–738. doi: 10.1016/j.joca.2013.02.005. PubMed PMID 23428601. [DOI] [PubMed] [Google Scholar]
- 11.Olstad K., Ekman S., Carlson C.S. An update on the pathogenesis of osteochondrosis. Vet Pathol. 2015;42(5):785–802. doi: 10.1177/0300985815588778. PubMed PMID 26080832. [DOI] [PubMed] [Google Scholar]
- 12.König F. Ueber freie Körper in den Gelenken. Dtsch Z Chir. 1888;27:90–109. doi: 10.1007/BF02792135. [DOI] [Google Scholar]
- 13.Ahlbäck S., Bauer G.C., Hohne W.H. Spontaneous osteonecrosis of the knee. Arthritis Rheum. 1968;11(6):705–733. doi: 10.1002/art.1780110602. PubMed PMID 5700639. [DOI] [PubMed] [Google Scholar]
- 14.Imhof H., Breitenseher M., Kainberger F., Trattnig S. Degenerative joint disease, Cartilage or vascular disease? Skeletal Radiol. 1997;26(7):398–403. doi: 10.1007/s002560050254. PubMed PMI 9259096. [DOI] [PubMed] [Google Scholar]
- 15.Madry H., van Dijk C.N., Mueller-Gerb M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419–433. doi: 10.1007/s00167-010-1054-z. PubMed PMID 20119671. [DOI] [PubMed] [Google Scholar]
- 16.Schenck R.C., Jr., Goodnight J.M. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78(3):439–456. PubMed PMID 8613454. [PubMed] [Google Scholar]
- 17.Clanton T.O., DeLee J.C. Osteochondritis dissecans, History, pathophysiology and current treatment concepts. Clin Orthop Relat Res. 1982;167:50–64. PubMed PMID 6807595. [PubMed] [Google Scholar]
- 18.Dubois M.S., Morello S., Rayment K. Computed tomographic imaging of subchondral fatigue cracks in the distal end of the third metacarpal bone in the thoroughbred racehorse can predict crack micromotion in an ex–vivo model. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101230. PubMed PMID, 25077477; PMCID PMC4117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan M., Peng J., Qin L., Lu S. Experimental animal models of osteonecrosis. Rheumatol Int. 2011;31(8):983–994. doi: 10.1007/s00296-011-1819-9. PubMed PMID 21340568. [DOI] [PubMed] [Google Scholar]
- 20.Isaac D.I., Meyer E.G., Kopke K.S., Haut R.C. Chronic changes in the rabbit tibial plateau following blunt trauma to the tibiofemoral joint. J Biomech. 2010;43(9):1682–1688. doi: 10.1016/j.jbiomech.2010.03.001. PubMed PMID 20399435. [DOI] [PubMed] [Google Scholar]
- 21.Lotke P.A., Ecker M.L. Osteonecrosis of the knee. J Bone Joint Surg Am. 1988;70(3):470–473. PubMed PMID 3279040. [PubMed] [Google Scholar]
- 22.Yamamoto T., Bullough P.G. Spontaneous osteonecrosis of the knee. The result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858–866. doi: 10.2106/00004623-200006000-00013. PubMed PMID 10859106. [DOI] [PubMed] [Google Scholar]
- 23.Lacourt M., Gao C., Li A. Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthritis Cartilage. 2012;20:572–583. doi: 10.1016/j.joca.2012.02.004. 2012. [DOI] [PubMed] [Google Scholar]
- 24.Radin E.L., Parker H.G., Pugh J.W., Steinberg R.S., Paul I.L., Rose R.M. Response of joints to impact loading, III. Relationship between trabecular microfractures and cartilage degeneration. J Biomech. 1973;6(1):51–57. doi: 10.1016/0021-9290(73)90037-7. PubMed PMID 4693868. [DOI] [PubMed] [Google Scholar]
- 25.Baltimore R.S., Jenson H.B. Puncture wound osteochondritis of the foot caused by Pseudomonas maltophilia. Pediatr Infect Dis J. 1990;9(2):143–144. doi: 10.1097/00006454-199002000-00017. PubMed PMID 2314954. [DOI] [PubMed] [Google Scholar]
- 26.DiGiovanni C.W., Patel A., Calfee R., Nickisch F. Osteonecrosis in the foot. J Am Acad Orthop Surg. 2007;15(4):208–217. doi: 10.5435/00124635-200704000-00004. PubMed PMID 17426292. [DOI] [PubMed] [Google Scholar]
- 27.Martel G., Kiss S., Gilbert G. Differences in the vascular tree of the femoral trochlear growth cartilage in osteochondrosis–susceptible sites in foals revealed by SWI 3T MRI. J Orthop Res. 2016;34(9):1539–1546. doi: 10.1002/jor.23149. PubMed PMID 26740060. [DOI] [PubMed] [Google Scholar]
- 28.Turley S.M., Thambyah A., Riggs C.M., Firth E.C., Broom N.D. Microstructural changes in cartilage and bone related to repetitive overloading in an equine athlete model. J Anat. 2014;224(6):647–658. doi: 10.1111/joa.12177. PubMed PMID 24689513; PMCID, PMC4025892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olstad K., Ytrehus B., Ekman S., Carlson C.S., Dolvik N.I. Epiphyseal cartilage canal blood supply to the tarsus of foals and relationship to osteochondrosis. Equine Vet J. 2008;40(1):30–39. doi: 10.2746/042516407X239836. PubMed PMID 18083657. [DOI] [PubMed] [Google Scholar]
- 30.Aaron R.K., Voisinet A., Racine J., Ali Y., Feller E.R. Corticosteroid–associated avascular necrosis. Dose relationships and early diagnosis. Ann NY Acad Sci. 2011;1240:38–46. doi: 10.1111/j.1749-6632.2011.06218.x. PubMed PMID 22172038. [DOI] [PubMed] [Google Scholar]
- 31.Assouline–Dayan Y., Chang C., Greenspan A., Shoenfeld Y., Gershwin M.E. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32(2):94–124. PubMed PMID 12430099. [PubMed] [Google Scholar]
- 32.Rogers J., Waldron T. 1995. A field guide to joint disease in archaeology. New York, NY: John Wiley & Sons; 1995. ISBN 9780471955061; NLM ID 9442846. [Google Scholar]
- 33.Rothschild B.M. Porosity, A curiosity without diagnostic significance. Am J Phys Anthropol. 1997;104(4):529–533. doi: 10.1002/(SICI)1096-8644(199712)104:4<529::AID-AJPA7>3.0.CO;2-M. PubMed PMID 9453700. [DOI] [PubMed] [Google Scholar]
- 34.Bonde H.V., Talman M.L., Kofoed H. The area of the tidemark in osteoarthritis – a three–dimensional stereological study in 21 patients. APMIS. 2005;113(5):349–352. doi: 10.1111/j.1600-0463.2005.apm_113506.x. PubMed PMID 16011661. [DOI] [PubMed] [Google Scholar]
- 35.Bullough P.G., Yawitz P.S., Tafra L., Boskey A.L. Topographical variations in the morphology and biochemistry of adult canine tibial plateau articular cartilage. J Orthop Res. 1985;3(1):1–16. doi: 10.1002/jor.1100030101. PubMed PMID 3981289. [DOI] [PubMed] [Google Scholar]
- 36.Hoemann C.D., Lafantaisie–Favreau C.H., Lascau–Coman V., Chen G., Guzmán–Morales J. The cartilage–bone interface. J Knee Surg. 2012;25(2):85–97. doi: 10.1055/s-0032-1319782. PubMed PMID 22928426. [DOI] [PubMed] [Google Scholar]
- 37.Mensforth R.P., Latimer B.M. Hamann–Todd Collection aging studies, Osteoporosis fracture syndrome. Am J Phys Anthropol. 1989;80(4):461–479. doi: 10.1002/ajpa.1330800406. PubMed PMID 2603949. [DOI] [PubMed] [Google Scholar]
- 38.Rothschild B.M., Tanke D.H. Osteochondrosis in late cretaceous Hadrosauria, A manifestation of ontologic failure. In: Carpenter K., editor. Horns And Beaks, Ceratopsian And Ornithopod Dinosaurs. Bloomington. Indiana University Press; 2006. pp. 171–184. [Google Scholar]
- 39.Hershkovitz I., Rothschild B.M., Dutour O., Greenwald C. Clues to recognition of fungal origin of lytic skeletal lesions. Am J Phys Anthropol. 1998;106:47–60. doi: 10.1002/(SICI)1096-8644(199805)106:1%3C47::AID-AJPA4%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.Rothschild B.M., Martin L.D. Museum of Natural History Press; Albuquerque, NM: 2006. Skeletal Impact of Disease. [Google Scholar]
- 41.Rothschild B.M., Witzke B.J., Hershkovitz I. Metastatic cancer in the Jurassic. Lancet. 1999;354:398. doi: 10.1016/S0140-6736(99)01019-3. [DOI] [PubMed] [Google Scholar]
- 42.Albine A.M., Rothschild B.M., Carrillo-Briceño J.D., Neenan J.M. Spondyloarthropathy in vertebrae of the aquatic Cretaceous snake Lunaophis aquaticus, and its first recognition in modern snakes. Sci Nat-Heidelberg. 2018;5:1576. doi: 10.1007/s00114-018-1576-7. [DOI] [PubMed] [Google Scholar]
- 43.Rothschild B.M. Arthritis in Callithrix jacchus: calcium pyrophosphate deposition disease and spondyloarthropathy. J Med Primatol. 1993;22:313–316. doi: 10.1111/j.1600-0684.1993.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 44.Rothschild B.M. Spondyloarthropathy in the Jurassic. Lancet. 2002;360:1454. doi: 10.1016/S0140-6736(02)11471-1. [DOI] [PubMed] [Google Scholar]
- 45.Rothschild B.M., Rothschild C. No laughing matter: spondyloarthropathy in Hyaenidae. J Zoo Wildl Med. 1994;25:259–263. [Google Scholar]
- 46.Rothschild B.M., Rothschild C. Is there an epidemic/epizootic of spondyloarthropathy in baboons? J Med Primatol. 1996;25:69–70. doi: 10.1111/j.1600-0684.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 47.Rothschild B.M., Rothschild C. Spondyloarthropathy as a trans-mammalian phenomenon, reproducible in its manifestations across species lines. J Paleopath. 2000;11:103–104. [Google Scholar]
- 48.Rothschild B.M., Rühli F.J. Comparison of arthritis characteristics in lowland Gorilla gorilla and mountain Gorilla beringei. Am J Primatol. 2005;66:205–218. doi: 10.1002/ajp.20139. [DOI] [PubMed] [Google Scholar]
- 49.Rothschild B.M., Rühli F.J. Etiology of reactive arthritis in Pan paniscus, Pan troglodytes troglodytes and Pan schweinfurthii. Am J Primatol. 2005;66:219–231. doi: 10.1002/ajp.20140. [DOI] [PubMed] [Google Scholar]
- 50.Rothschild B.M., Woods R. Spondyloarthropathy as an old world phenomenon. Semin Arthritis Rheum. 1988;21:306–316. doi: 10.1016/0049-0172(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 51.Rothschild B.M., Woods R. Erosive arthritis and spondyloarthropathy in Old World primates. Am J Phys Anthropol. 1992;88:389–400. doi: 10.1002/ajpa.1330880310. [DOI] [PubMed] [Google Scholar]
- 52.Rothschild B.M., Woods R. Arthritis in non-human primates: osteoarthritis, calcium pyrophosphate deposition disease and spondyloarthropathy. Am J Primatol. 1992;27:53. [Google Scholar]
- 53.Rothschild B.M., Woods R. Arthritis in New World monkeys: osteoarthritis, calcium pyrophosphate deposition disease. Int J Primatol. 1993;4:61–78. doi: 10.1007/BF02196503. [DOI] [Google Scholar]
- 54.Rothschild B.M., Wang X.-M., Shoshani J. Spondyloarthropathy in proboscideans. J Zoo Wildl Med. 1994;25:360–366. [Google Scholar]
- 55.Rothschild B.M., Hong N., Turnquist J.E. Naturally occurring spondyloarthropathy in Cayo Santiago rhesus macaques. Clin Exp Rheumatol. 1997;15:45–51. [PubMed] [Google Scholar]
- 56.Rothschild B.M., Rothschild C., Woods R.J. Inflammatory arthritis in large cats: an expanded spectrum of spondyloarthropathy. J Zoo Wildl Med. 1998;29:279–284. [PubMed] [Google Scholar]
- 57.Rothschild B.M., Prothero D.R., Rothschild C. Origins of spondyloarthropathy in perissodactyla. Clin Exp Rheumatol. 2001;19:628–632. [PubMed] [Google Scholar]
- 58.Rothschild B.M., Rothschild C., Woods R.J. Inflammatory arthritis in canids: spondyloarthropathy. J Zoo Wildl Med. 2001;32:58–64. doi: 10.1638/1042-7260(2001)032[0058:IAICS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Rothschild B.M., Schultze H.-P., Peligrini R. Springer-Verlag; New York, NY: 2012. Herpetological Osteopathology: Annotated Bibliography of Amphibians and Reptiles. [DOI] [Google Scholar]
- 60.Rothschild B.M., Borkovic B., Tanke D.H. Occurrence of non-infectious spondyloarthropathy in a Late Cretaceous hadrosaur from southern Alberta, Canada. J Vertebr Paleontol. 2017;37:S185. [Google Scholar]
- 61.Rothschild B.M. Differential diagnostic perspectives provided by en face microscopic examination of articular surface defects. Clin Rheumatol. 2018;31(3):831–836. doi: 10.1007/s10067-018-4001-x. PubMed PMID 29399706. [DOI] [PubMed] [Google Scholar]
- 62.Norrdin R.W., Stover S.M. Subchondral bone failure in overload arthrosis, A scanning electron microscopic study in horses. J Musculoskelet Neuronal Interact. 2006;6(3):251–257. PubMed PMID 17142946. [PubMed] [Google Scholar]
- 63.Resnick D. fourth ed. WB Saunders; Philadelphia, PA: 2002. Diagnosis of Bone and Joint Disorders. 2002. [Google Scholar]
- 64.Thorp B.H., Ekman S., Jakowlew S.B., Goddard C. Porcine osteochondrosis, Deficiencies in transforming growth factor–β and insulin–like growth factor–I. Calcif Tissue Int. 1995;56(5):376–381. doi: 10.1007/BF00301606. PubMed PMID 7621345. [DOI] [PubMed] [Google Scholar]
- 65.Wegener K.M., Heje N.I. Dyschondroplasia (osteochondrosis) in articular–epiphyseal cartilage complexes of three calves from 24 to 103 days of age. Vet Pathol. 1992;29(6):562–563. doi: 10.1177/030098589202900618. PubMed PMID 1448908. [DOI] [PubMed] [Google Scholar]
- 66.Holmdahl D.E., Ingelmark B.E. The contact between the articular cartilage and the medullary cavities of the bone. Acta Orthop Scand. 1950;20(2):156–165. doi: 10.3109/17453675009043414. PubMed PMID 14868441. [DOI] [PubMed] [Google Scholar]
- 67.Li G., Yin J., Gao J. Subchondral bone in osteoarthritis, Insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405. PubMed PMID 24321104; PMCID 4061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lyons T.J., McClure S.F., Stoddart R.W., McClure J. The normal human chondro–osseous junctional region, Evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Muscoskel Disord. 2006;7:52. doi: 10.1186/1471-2474-7-52. PubMed PMID 16787529; PMCID, PMC1550228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKibbin B., Holdsworth F.W. The nutrition of immature joint cartilage in the lamb. J Bone Joint Surg Br. 1996;48(4):793–803. PubMed PMID 5953816. [PubMed] [Google Scholar]