Abstract
The management of oral and maxillofacial tissue defects caused by tumors, trauma, and congenital or acquired deformities has been a major challenge for surgeons over the last few decades. Autologous tissue transplantation, the gold standard of tissue reconstruction, is a valid method for repairing the oral and maxillofacial functions and aesthetics. However, several limitations hinder its clinical applications including complications of donor sites, limited tissue volume, and uncertain long-term outcomes. Adipose-derived mesenchymal stem cells (ADMSCs) widely exist in adipose tissue and can be easily obtained through liposuction. Like the bone marrow-derived mesenchymal stem cells (BMSCs), ADMSCs also have the multi-pluripotent potencies to differentiate into osteoblasts, chondrocytes, neurons, and myocytes. Therefore, the multilineage capacity of ADMSCs makes them valuable for cell-based medical therapies. In recent years, researchers have developed many candidates of ADMSCs-based biomaterial scaffolds to cater for the needs of oral and maxillofacial tissue engineering due to their superior performance. This review presents the advances and applications of ADMSCs-based biomaterial scaffolds, and explores their tissue engineering prospects in oral and maxillofacial reconstructions.
Keywords: Adipose-derived mesenchymal stem cell, Biomaterial scaffold, Oral and maxillofacial, Tissue engineering
Graphical abstract
Highlights
-
•
ADMSCs are more available than BMSCs and their multipotential differentiation ability is excellent.
-
•
ADMSCs and biomaterial scaffolds could complement each other.
-
•
ADMSCs-based biomaterial scaffolds have broad prospects in tissue engineering.
1. Introduction
Defects of the soft and hard tissues in the oral and maxillofacial region severely impair oral functions and cosmetic appearance, thereby causing several dilemmas in treatment [[1], [2], [3], [4]]. Over the past few decades, autographs have been considered as the gold standard for tissue reconstruction [2]. However, drawbacks such as the morbidity of the donor site and the limited number of transplanted tissues, have contributed to its limited clinical application [[3], [4], [5], [6], [7]]. This has led to numerous attempts such as allografts, xenografts, and synthetic substitutes, being investigated by researchers and clinicians. Nevertheless, none of these methods can solve the problem due to various limitations associated with the methods. Therefore, tissue engineering, which provides an alternative approach, has continued to draw the researchers’ attention.
Current tissue engineering strategy utilizes living cells, biomaterials, and appropriate biochemical, physical factors, and the combinations to create tissue-like structures. The ultimate goal of the method is to incorporate these tissue-like structures into the body in order to repair the damage or replace dysfunctional organs [2]. Early tissue engineering application involved the use of mesenchymal stem cells (MSCs) for tissue homeostasis and the regeneration in oral and maxillofacial regions due to their self-renewal ability and pluripotent differentiation [4]. Bone marrow-derived mesenchymal stem cells (BMSCs) are considered to be the most frequently studied MSCs in tissue engineering. However, their clinical application is limited by the low extraction yield and number of BMSCs, the need for in vitro culture expansion, sensitivity to senescence, cost, and the loss of proliferative and differentiation ability during cell expansion [8,9]. When compared with the BMSCs, evidence from both in vitro and in vivo studies have suggested that adipose-derived mesenchymal stem cells (ADMSCs) have the potential to differentiate into mesenchymal (such as adipocytes, osteocytes, and chondrocytes) and non-mesenchymal (such as neuron-like cells) cells [4,5]. With regards to the growth cycle of ADMSCs, despite their low proliferation and apoptosis rates in vitro, it is easy to obtain a high stem cell-to-volume ratio, low sensitivity to aging, and easy harvesting. Previous studies have reported that the content of ADMSCs is highly abundant in the stromal vascular fraction (SVF) [[10], [11], [12]]. In addition, ADMSCs within the SVF show high attachment rate to scaffold material, proliferate rapidly, and could differentiate into the osteogenic lineage [11]. In recent years, the application of biomaterial scaffolds based on ADMSCs has gradually become a hot topic.
The main aim of this review is to present and discuss the advancement of the application, vascularization, and tissue regeneration of ADMSCs in the oral and maxillofacial region. Moreover, we propose functional design and development of ADMSCs-based biomaterial scaffolds in oral and maxillofacial tissue engineering, which could lead to a feasible clinical application.
2. Adipose-derived mesenchymal stem cells
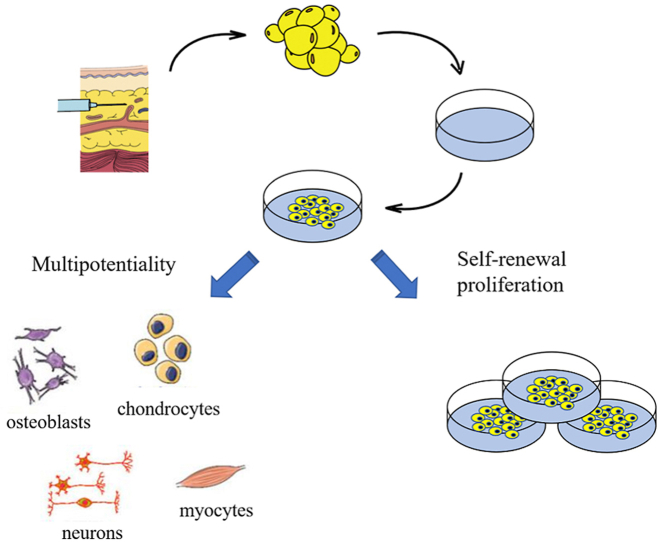
ADMSCs are multipotential stem cells which have an undifferentiated immunophenotype, with the potential for self-renewal and multipotency properties [[13], [14], [15], [16], [25]]. Therefore, ADMSCs can differentiate into several cell types including osteoblasts, chondrocytes, neurons, myocytes, endothelial cells, fat precursor cells, and myocardial cells [[17], [18], [19], [20]]. In addition, ADMSCs can secrete a large number of anti-inflammatory proteins in response to inflammatory reactions including tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-6, IL-10, and IL-1 receptor antagonist. These cells ADMSCs can also produce a variety of growth factors such as transforming growth factor-β1 (TGF-β1), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and stromal cell-derived factor-1 (SDF-1), which contribute to tissue remodeling, angiogenesis, and antiapoptotic events [[18], [19], [20], [21], [22]]. Moreover, ADMSCs could modulate immune response by preventing the immune cells from proliferating and functioning, such as T cells, B cells, macrophages, monocytes, and natural killer cells [21].
The study of ADMSCs in tissue engineering field has yielded promising results. In recent decades, Marta et al. [14] and Mario et al. [23] demonstrated that ADMSCs could be applied in musculoskeletal disorders and cartilage disorders such as osteoporosis, osteonecrosis, fractures, osteoarthritis, and cartilage lesions. On the other hand, Yang et al. [13] preclinically assessed the safety and efficacy of ADMSCs in vitro and in BALB/c-nu nude mice models. On this basis, this led to the application of ADMSCs to patients with knee osteoarthritis, where they improved the knee joint pain, function, and cartilage volume in the recipients. Meenakshi et al. [24] reported that ADMSCs could be used to treat what disorders, combat aging through dermatological rejuvenation and promote wound healing. Furthermore, Maryam et al. [26] showed regulatory effects of the ADMSCs on inflammatory responses and effective control over the immune response in treating colitis of a murine model. In the treatment of renal lesions, Wang et al. [15] reported that ADMSCs could restore the impaired cell regeneration and remodeling, which prevent further worsening of renal disease and even block or reverse renal fibrosis processes. In the oral and maxillofacial region, Mazzoni et al. [122] demonstrated the osteogenic differentiation potential of ADMSCs and applied ADMSCs/Hydroxylapatite‐collagen hybrid scaffold to patients who wanted to optimize facial esthetics. All the above mentioned studies indicate that ADMSCs, as pluripotent stem cells, has been widely used to address several medical issues and have so far yielded satisfactory therapeutic effects to an extent, especially in the oral and maxillofacial regions. The application of ADMSCs in the oral and maxillofacial regions would be introduced in detail as follow.
3. Biomaterial scaffolds combined with ADMSCs for oral and maxillofacial regeneration
Soft and hard tissue defects in the oral and maxillofacial region severely impair oral functions and cosmetic appearance, thereby causing several dilemmas in treatment [[27], [29]]. Tissue engineering is an alternative approach which has been the subject of growing interest from researchers due to the limitations of autologous tissue transplantation.
Although the function of ADMSCs has been widely proven, ADMSCs alone cannot achieve good results in tissue engineering [[30], [31], [32]]. Previous studies have reported that random cell migration reduces the concentration of cells at target site, thereby hindering the efficacy of cell-based tissue regeneration [31,32]. The other aspect of tissue engineering involves scaffolds, a cell attachment skeleton structure. Scaffold influences the effect of ADMSCs in tissue engineering based on their strength, porosity, biodegradability, biocompatibility, and support for cell adhesion, differentiation, proliferation, and growth [33]. A recent study reported that 3D structured scaffolds can be tailored with specific porosity, surfaces, and pore connectivity to optimize the cellular adhesion and migration, and nutrient transportation [34]. These 3D scaffolds could provide physical, chemical, and mechanical maintenance for extracellular matrix (ECM) formation in vitro, and control degradation, reabsorption, and metabolism in vivo [[36], [37], [38]]. Moreover, scaffolds with open and interconnected pores are conducive to the growth, proliferation, and migration of cells. They also promote tissue vascularization and the formation of new tissues to some extent. Scaffolds play an important role in tissue engineering because they are used as carriers for the transport, enrichment, and maintenance of ADMSCs [[33], [34], [35]]. Moreover, cells-based biomaterial scaffolds show a cooperative relationship and are currently the mainstream in tissue engineering. In this review, we summarized the biomaterial scaffolds that have been combined with ADMSCs in oral and maxillofacial regeneration (Table 1). In oral and maxillofacial regeneration, these biomaterials could be used independently with severe complications, while they are more widely used in the form of composite materials through specific integration. These composites are designed to achieve synergy by combining the corresponding properties. The application of bioactive materials which combined with ADMSCs in oral and maxillofacial regeneration would be introduced in detail as follow.
Table 1.
Target tissue, cells, scaffolds and types of materials in this review.
| Study, Year | Target Tissue | Cells | Material s of a Scaffold | Types of materials |
|---|---|---|---|---|
| Tobita et al., 2008 | Periodontal | ADMSCs | platelet-rich plasma (PRP) | Biologicals |
| Tobita et al., 2013 | Periodontal | ADMSCs | platelet-rich plasma (PRP) | Biologicals |
| Wu et al., 2016 | Periodontal | ADMSCs | Amniotic membrane (AM) | Biologicals |
| Requicha et al., 2014 | Periodontal | ADMSCs | The double-layer scaffolds were prepared from a blend of starch and poly (Ɛ-caprolactone) (SPCL). | Polymers |
| Sun et al., 2011 | Facial nerve | ADMSCs | Decellularized artery allograft | Biologicals |
| Ghoreishian et al., 2012 | Facial nerve | ADMSCs | Gore-Tex tube | Polymers |
| Watanabe et al., 2014 | Facial nerve | ADMSCs | A silicone tube with a type I collagen scaffold | Polymers |
| Han et al., 2015 | Adipose tissue | ADMSCs | Decellularized adipose tissue (DAT) bioscaffold | Biologicals |
| Venugopal et al., 2017 | Adipose tissue | ADMSCs | bovine type I collagen sponge | Polymers |
| Mauney et al., 2007 | Adipose tissue | ADMSCs | Aqueous (AB) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)- based silk fibroin 3-D scaffolds | Polymers |
| Lin et al., 2008 | Adipose tissue | ADMSCs | The scaffold was made of gelatin sponges, polyglycolic acid meshes, and polypropylene mesh. | Polymers |
| Probst et al., 2020 | Bone | ADMSCs | TCP-PLGA scaffolds | Bioceramic |
| Mesim et al., 2009 | Bone | ADMSCs | microvascular flap, beta-tricalcium phosphate and bone morphogenetic protein-2 | Bioceramic |
| Kang et al., 2011 | Bone | ADMSCs | a mixture of fibrin and hyaluronic acid (HA) and BMP-2 | Bioceramic |
| Lendeckel et al., 2004 | Bone | ADMSCs | autologous fibrin glue | Polymers |
3.1. Bioceramics
Bioceramics includes tri-calcium phosphate (TCP), hydroxyapatite (HA), and their composites. Besides, the combination of bioceramics and natural or synthetic polymers extensively exists. Generally, in the oral and maxillofacial region, bioceramics is commonly used in the engineering of bone tissues such as in orthopedic and oral and maxillofacial surgery [[36], [37], [38], [39]]. TCP is characterized by its biocompatibility, compressive mechanical property, and degradation when compared with other bioceramics, which makes it quite distinct for hard tissue regeneration [40]. However, pure bioceramics is brittle and the structure lacks interconnected pores, which are required for a better cell proliferation and migration. Thus, the application of pure bioceramics is relatively limited [40,41]. Nevertheless, bioceramic scaffolds containing natural or synthetic polymers are more desirable because their improved mechanics with osteoconductive properties are more conducive for oral and maxillofacial regeneration [41].
3.2. Non-collagenous proteins
Previous study reported that growth factor delivery (GFD) is important in mediating the micro-environment of scaffolds and subsequent cellular responses [42]. Growth factors (GFs) are soluble polypeptides that affect cellular function and bind to cell membrane receptors. Several studies have used osteo-inductive/-conductive factors (BMP, TGF-β, etc.) to guide cell differentiation and tissue formation in bone regenerative therapies [[43], [44], [45], [46], [47]]. Similarly, vascularization is critical for the sustainability of newly formed tissues, and it requires the addition of angiogenic GFs, such as platelet-derived growth factor (PDGF), VEGF and so on [48]. Different from injecting therapeutic factors into the defect site, scaffold-based GFD has several advantages including improved control over GF release kinetics and localization [[49], [50], [51]]. A major challenge for GFD is to release -GFs in a similar pattern to those during natural tissue repairing process or morphogenesis [52]. Therefore, scaffolds can be used for continuous or simultaneous spatiotemporal administration of multiple GFs based on the therapeutic time window when GF administration is most favorable for tissue regeneration. On the other hand, scaffolds can include a combination of multiple factors involved in tissue formation and angiogenesis [[53], [54], [55], [56]].
Platelet-rich plasma (PRP) has been used clinically since the 1970s. PRP is rich in secretory proteins and high levels of growth factors, thus, it promotes wound healing [100]. In addition, it releases several growth factors and facilitates the proliferation, migration, and differentiation of tissue regeneration cells [[93], [94], [101]]. Autologous PRP is the processed liquid fraction of autologous peripheral blood with a platelet concentration above the baseline [58]. According to Marx, the platelet concentrate is an effective tool for regulating wound healing and tissue regeneration, especially periodontal tissue engineering [57]. However, the studies investigating PRP in these contexts often show conflicting results, which most likely can be attributed to the lack of standardized preparation methods, particularly with regard to the platelet content and donor variation of PRP [121]. Ultimately, the use of PRP has to be tailored for the individual application.
3.3. Traditional membranes
The preferred treatment method involves using synthetic or tissue-derived membranes as barriers to guide the tissue/bone regeneration process with or without calcium phosphate bone graft materials [58]. The biomaterials can be divided into two categories; non-resorbable and resorbable, according to their degradation behavior [59]. Ideally, these biologicals should demonstrate biocompatibility to bind to the host without triggering an inflammatory response, as well as an appropriate degradation profile that not only matches the formation of new tissue, but more importantly allows the tissue to mature fully before the biologicals begin to degrade [[60], [61], [62]]. In addition, these biological agents must have sufficient initial strength and biological activity for clinical treatment and placement.
Several attempts have been made to develop a membrane with the optimum mechanical, degrading, and biological properties required to guide tissue regeneration with varying degrees of clinical success [63]. Despite significant progress being made, these attempts are only mentioned in the recently published work. Necessary functions such as antibacterial, anti-inflammatory, and cellular differentiation, and techniques such as additive manufacturing, are also required to complete the construction of specific biological agents. The progress of these biological agents is described below.
Infection determines the success or failure of tissue engineering to some extent, especially for oral and maxillofacial regeneration. The region of oral and maxillofacial has a complex network of blood vessels which is vulnerable to bacteria, thereby causing serious complications such as bacteremia. Therefore, many scholars have integrated antimicrobials with membranes for oral and maxillofacial regeneration. Furtos et al. [120] combined amoxicillin with nanocomposite polycaprolactone-based membranes for dental applications. Bottino et al. [39] incorporated zinc oxide (ZnO) nanoparticles into PCL-based nanofibrous membranes for periodontal regeneration. In addition, as a barrier, membranes could play a role in the delivery of substances and chemotactic migration of ADMSCs [39]. On this basis, many scholars have integrated membranes with other materials, such as bioactive glass and growth factors, to make composite materials for tissue engineering. Besides, there are multilayered membranes and multiphasic patient-specific scaffolds which could simulate tissue-specific structures with compositional and structural variation to remodel the structural organization or cellular and biochemical composition of native tissues for oral and maxillofacial regeneration.
3.4. Natural and synthetic polymers
To date, several researches have focused on the design of implantable scaffolds consisting of synthetic or natural polymers with predefined shapes and volumes [64]. Cellulose and its derivatives, alginates (such as polyanionic co-polysaccharides, agarose derivatives, etc.), chitosan, hyaluronate, fibrin, fibronectin, collagen, gelatine, and its derivatives are mainly used in the bone regeneration of natural polymeric materials [[65], [66], [67], [68], [69], [70]]. The biological agents can be added through gel formulation because natural scaffold materials are commonly used in gel-like phases [71]. Among them, collagen is the most widely used in tissue engineering. Collagen is the main structural protein in mammals which is widely distributed in the body, and is one of the main components of the ECM [72]. Collagen exhibits high tensile strength, which makes it an essential structural factor in providing mechanical strength to hard tissues [73]. In addition, it contains ligands that promote cell attachment and thus it influences cell migration and differentiation [74].
On the other hand, synthetic polymers are an attractive alternative due to the risks of immune rejection, clotting, or tissue hypertrophy, which are as a result of tissue-derived products. The molecular weight, molecular weight distribution, mechanical properties, and other physical properties can be customized. However, the major challenge is designing 3D architectures with clear porosity and customized surfaces to fit specific requirements for cell adhesion [75]. The synthetic scaffold materials for bone tissue engineering are mainly polyesters, with the most common being polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) [[76], [77], [78]].
Ceramic-polymer composites are synthesized by embedding ceramic particles into the polymer matrix and exposing them to the surface to enhance the bone conduction effect. The mechanical properties are mainly affected by the particle size of calcium phosphate and its distribution in the polymer matrix. One study reported that the stiffness of the composite increases as the particle size decreases [79]. Bioceramic scaffolds composed of biodegradable polymers such as poly (lactic acid-glycolic acid) (PLGA) have recently been developed with the overarching goal of improving the mechanical stability and tissue interaction [80]. In addition, recent studies have combined electrostatic spinning with melting to generate a hierarchical PCL/HR-TCP scaffold embedded with collagen nanofibers [80,81]. The scanning electron micrograph of the obtained structure indicated that there are evenly distributed polyacrylamide (PCS) particles in the polyacrylamide (PCL) struts, and the collagen nanofibers between the composite struts were well layered. Thus, the combination of collagen nanofibers and TCP provides a synergistic cell-related effect.
The polymers are usually biocompatible and most are bioresorbable. The development of injectable biomaterials such as hydrogels, particles, and microcarriers, which can provide more minimally invasive strategies for strengthening hard and soft tissues in the clinic, is also included in this application [84].
Recently, scholars have also made significant progress in the research of biomaterial scaffolds in order to adapt to the rapid development of tissue engineering. As mentioned above, numerous attempts such as mechanically-competent, tissue-specific, and multiphasic 3D scaffolds have been utilized in tissue engineering [39].
4. ADMSCs-based biomaterial scaffolds for oral and maxillofacial tissue engineering
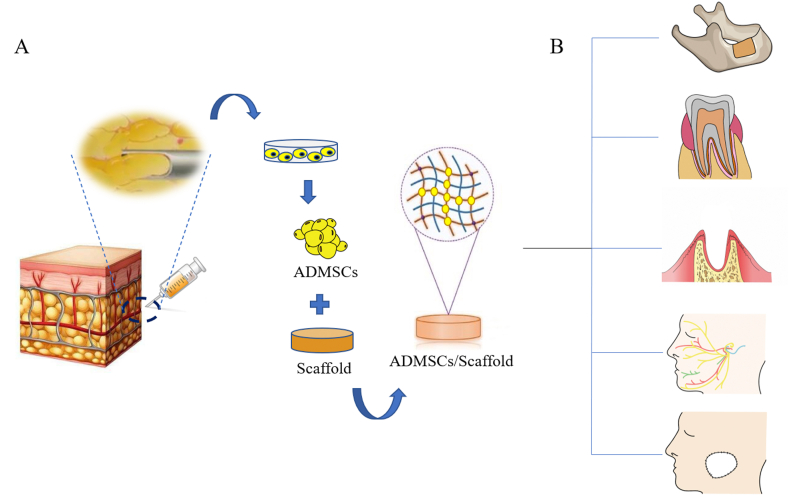
ADMSCs are characterized by their highly homogeneous (high purity) cell population which is highly proliferative. In addition, ADMSCs have a multilineage potential under appropriate in vitro and in vivo conditions to differentiate into different cell types including osteoblasts, chondrocytes, neurons, and adipocytes. The advantage of using ADMSCs in vivo for bone, periodontal tissue, tooth, peripheral nerve, and soft tissue regeneration has been documented in several animal models (Table 2). Therefore, the use of ADMSCs seems to be a promising cell source for oral and maxillofacial tissue engineering. Fig. 1 illustrates the isolation of ADMSCs from adipose tissue and the development of ADMSCs/biomaterial for repairing oral and maxillofacial soft and hard tissue defects.
Table 2.
ADMSCs-based biomaterial scaffolds for oral and maxillofacial tissue regeneration.
| Study, Year | Cell Source and Type | Biomaterial Scaffold | Donor and Host Animal | Site of Injury | Regeneration Advantage Conferred Over Vehicle |
|---|---|---|---|---|---|
| Tobita et al., 2008 | ASCs isolated from Wistar rats | platelet-rich plasma (PRP) | 48 10-week-old inbred male Wistar rats in two groups: controls and PRP groups | Periodontal tissue defects were created with a 1◊1◊1 mm defect, respectively. | PRP gels containing stem cells such as ASCs could be useful clinically for periodontal tissue regeneration. |
| Tobita et al., 2013 | ASCs isolated from inguinal fat pads of beagle dogs | platelet-rich plasma (PRP) | 9 or 10 months old beagle dogs (mean weight 8–10 kg) (n = 8) in three groups: ASC/PRP gel or PRP gel alone was implanted into the periodontal tissue defect in each dog bilaterally. Non-implantation sites were also prepared. | Class III periodontal tissue defects, in which the tissue defect penetrates to the root bifurcation of the tooth, were generated in eight dogs. | The advantages of PRP are as follows: (i) a simple procedure for gelation of PRP is available, (ii) various growth factors are present in PRP, (iii) PRP adheres strongly to the dental root and (iv) PRP is an autologous product, which eliminates concerns regarding immunogenic reactions and disease transmission. |
| Wu et al., 2016 | Human ADSCs were isolated from infrapatellar fat pad | Amniotic membrane (AM) | Male Sprague-Dawley rats (body weight 80–100 g) were divided into four groups, including control, ADSCs, AM, and ADSCs and AM coculture system groups. | Extract the right maxillary first molar (M1) and fill with newly formed bone 3 weeks after M1 extraction in terms of creating a two-wall intrabony defect (2.6 ◊2.0◊2.0 mm) | AM was used to reconstruct ocular surfaces or cover the wounds clinically. The strong elastic properties make it an ideal scaffold for surgical repair of a bone defect and tissue engineering. |
| Requicha et al., 2014 | ASCs were isolated from subcutaneous abdominal tissue of adult healthy dogs | The double-layer scaffolds were prepared from a blend of starch and poly (Ɛ-caprolactone) (SPCL). | Four groups: APCL membranes with non-patterned (SPCL-NP) or patterned (SPCL-P). SPCL wet-spun fiber mesh used non-functionalized with silanol groups (SPCL-WS) or functionalized with silanol groups (SPCL-WS-Si) | / | The wet-spun fiber mesh layer functionalized with silanol groups stimulated the osteogenic differentiation of ASCs, while the membrane layers enabled a good cell attachment and proliferation. |
| Sun et al., 2011 | autologous transdifferentiated ADSCs from visceral fat in rats | Decellularized artery allograft | 60 young female Sprague-Dawley rats (weighing 100–120 g) randomly divided into 6 groups: artery conduit, artery + ADSCs, artery + dADSCs, artery + SCs, nerve autografting, sham operation | under a surgical microscope the shaved facial skin was opened with a preauricular incision on the left side to expose the buccal branch of the facial nerve | This method achieved satisfactory regenerative outcomes approaching those achieved with SC-seeded artery conduits at week 8 |
| Ghoreishian et al., 2012 | undifferentiated MSCs extracted from autogenous adipose tissues from mongrel dogs | Gore-Tex tube | 7 mongrel dogs subjected to nerve bilateral transaction; in 4 animals the left nerve was repaired using ADSCs; in 3, stem cells were used on the right side | hair over zygomatic arches and preauricular area of the 2 sides was shaved; after prepping and draping, an incision 4 cm long was made over the midportion of the arch; the frontal branch of facial nerve was transected; gap size 7 mm | Addition of stem cells in GoreTex tube enhanced neural repair from a functional standpoint; for better functional and histologic results, differentiated SCs and other mediators may be warranted. |
| Watanabe et al., 2014 | ASCs were isolated from Lewis rats and were cultured into uASCs and dASCs. | A silicone tube with a type I collagen scaffold | Male syngeneic Lewis rats aged 8 weeks were divided into five groups: Undifferentiated ASCs (n = 16), dASCs (n = 16), SCs (n = 16) and collagen gel alone as a negative control (n = 16) were transplanted into the gap in the buccal branch of the left facial nerve. Autologous nerve graft transplantation (n = 13) was used | ||
| as a positive control. | A preauricular incision with a marginal mandibular extension was then made in the left side of the face. The buccal and marginal mandibular branches and parotid gland were exposed, and a gap of 7 mm was made in the buccal branch of the facial nerve under a microscope | ASCs have a therapeutic effect on axonal regeneration and could be used as an alternative and more abundant source than autologous SCs as a guidance conduit for bridging nerve gaps. | |||
| Han et al., 2015 | ASCs were isolated from male Wistar rats | Decellularized adipose tissue (DAT) bioscaffold | In each rat, one seeded and one unseeded DAT scaffold was carefully inserted and the incisions were closed with surgical staples. (n = 20) | Small incisions (~1 cm) were made on the back of each rat and two separate subcutaneous pockets were created below the panniculus carnosus using blunt ended forceps. | Adipose-derived ECM bioscaffolds provide an interesting model system for probing cellular interactions in the context of adipose tissue regeneration that may help to contribute new insight into the mechanisms of adipogenesis that could have broader implications in the fields of obesity and diabetes research. |
| Venugopal et al., 2017 | ASC's were isolated from the subcutaneous fat pad of male Sprague Dawley rats | bovine type I collagen sponge | Sprague Dawley rats (n = 6) of weight 190–210 g for a period of 21 days | Two separate small skin incisions on the dorsal side were making in each rat. | bovine type I collagen provides its physiochemical properties and cyto-compatibility |
| Mauney et al., 2007 | hMSCs and hASCs were obtained from male donors ≤25 years of age. | Aqueous (AB) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)- based silk fibroin 3-D scaffolds | Athymic nude male rats (RH-rnu, 300 g each) for a period of 4 weeks | Scaffolds were individually implanted into bilateral muscle pouches within the rectus abdominus muscles of athymic nude male rats (RH-rnu, 300 g each) and maintained there for 4 weeks. Nonseeded scaffolds served as negative controls and were treated similarly. | The slow degradation and mechanical integrity of silk scaffolds in comparison with other conventional biomaterials such as collagen and PLA, especially for long-term in vivo studies, suggest that silk fibroin-based scaffolds would be an efficient biomaterial for long-term adipose tissue growth and function. |
| Lin et al., 2008 | hAD-MSCs were isolated from abdominal subcutaneous adipose tissue excised from the transverse rectus abdominis myocutaneous flap for breast reconstruction | The scaffold was made of gelatin sponges, polyglycolic acid meshes, and polypropylene mesh. | Female immunedeficient mice (4 weeks of age). Group I (n = 2) was injected with MSCs only, Group II (n = 2) was implanted with scaffolds only, and Group III (n = 8) was implanted with scaffold-MSCs treated in adipogenic medium for 2 weeks. | Scaffolds were implanted individually into the subcutaneous pockets in the backs of female immune-deficient mice. | All of the successfully harvested scaffolds were filled with newly formed adipose tissue and had retained their predefined shape and dimensions. |
| Probst et al., 2020 | ASCs were isolated from pigs | TCP-PLGA scaffolds | Sixteen skeletally mature miniature pigs were divided into two groups: Control group and scaffolds seeded with osteogenic differentiated pADSCs (n = 8/group) | mandibular critical size defects | Tri-calcium phosphate (TCP) bioceramics are quite distinct for hard tissue regeneration due to their biocompatibility, degradation and new bone formation. |
| Mesim et al., 2009 | autologous ASCs | microvascular flap, beta-tricalcium phosphate and bone morphogenetic protein-2 | The patient underwent a hemimaxillectomy | maxilla | The homing capacity of the ASCs and the osteoconductivity of the betaTCP act synergistically towards producing a well-ossified construct |
| Kang et al., 2011 | Human ASCs were obtained by liposuction from informed and consenting patients | a mixture of fibrin and hyaluronic acid (HA) and BMP-2 | Six-week-old female athymic mice were divided into four groups: Undifferentiated ASCs were inoculated on uncoated scaffolds (group 1), fibrin/HA-coated scaffolds without BMP-2 (group 2), fibrin/HA-coated scaffolds injected once with BMP-2 (group 3), and BMP-2 -loaded fibrin/HA-coated scaffolds (group 4) (n = 6/group) | Transplanted into the dorsal subcutaneous sites of the mice | The fibrin/HA-coated and BMP-2-loaded scaffolds fabricated using MHDS would be useful for bone regeneration from undifferentiated ASCs |
| Lendeckel et al., 2004 | autologous ASCs | autologous fibrin glue | A 7-year-old girl suffering from widespread calvarial defects | Multi-fragment calvarial fractures with a 120 cm2 defect | It showed no inhibition of stem cell proliferation; it is highly biocompatible and biodegradable. Fibrin glue can also serve as a biological vehicle for cell transplantation |
Fig. 1.
Strategy for oral and maxillofacial regeneration based on ADMSCs from adipose tissue. The cells are grown in vivo by exposure to various factors and finally seeded onto the biomaterial scaffolds, which are implanted into the oral and maxillofacial soft and hard tissue defects. (A) Isolation of ADMSCs from adipose tissue and construct ADMSCs/biomaterial scaffolds which are prepared for implantation. (B) ADMSCs/biomaterial scaffolds are implanted into oral and maxillofacial soft and hard tissue defects.
4.1. ADMSCs-based biomaterial scaffolds in the treatment of cranio-jaw regeneration
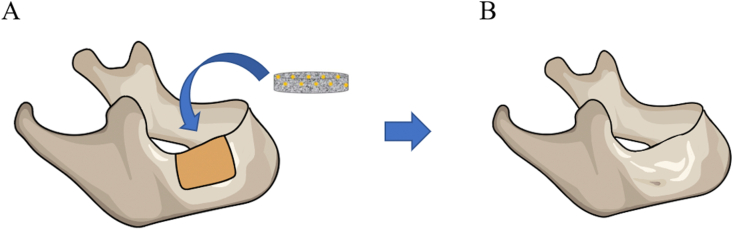
The basic requirement for tissue-engineered bone graft is the ability to integrate with the host organization, as well as provide and reconstruct bearing capacity. The ideal scaffold-stem cells compounds should simulate the induction of the natural bone structure or bone formation, and should include a variety of growth factors and cytokines in order to provide space for blood vessel formation. In addition, the transplantation of metabolites, degradation products, and harmless products, and the release of bioactive substances in the face of the host are shown in Fig. 2.
Fig. 2.
ADMSCs/biomaterial scaffold applying to jaw defect. (A) Schematic picture of the implantation of the ADMSCs/biomaterial scaffold in a jaw defect. (B) Repaired jaw tissue. ADMSCs in the construct regenerate jaw tissue at the implanted site.
Several studies have proved the in vitro osteogenic potential of ADMSCs in oral and maxillofacial regions [86]. Therefore, many laboratories have begun to seed osteogenic differentiated ADMSCs on different biomaterials and scaffolds in order to develop the potential for tissue-engineered bone repairs. Cowan et al. [49,50] investigated the in vivo osteogenic capacity of ADMSCs in the treatment of skull defects in rats. The obtained results indicated that the skull defects produced obvious intramembrane bone formation after two weeks through the implanted pure ADMSCs, apatite coating, and ADMSCs/PLGA scaffolds. Moreover, X-ray examination, histology, and live micromolecular imaging results indicated that the bone bridges were completely formed at 12 weeks. The ADMSCs/biological scaffolds have since been used by another laboratory for clinical applications. Lendeckel et al. [76] combined ADMSCs with autologous fibrin glue and used it in a 7-year-old girl suffering from extensive cranial defects to repair skull defects. The postoperative course was uneventful, and the obtained CT-scans revealed new bone formation and near-complete continuity of the skull three months after the reconstruction.
In addition to repairing skull defects, there are many instances where ADMSC/biological scaffolds have repaired maxillary and mandibular defects. Mesimaki et al. [74] completed maxillary reconstruction with a vascular flap using auto ADMSCs, TCP, and BMP-2. The donor flap had developed new bone structures and vasculature after eight months of follow-up, and was transplanted into the defect area. On the other hand, Mehrabani et al. [78] subsequently combined ADMSCs with fibrin glue scaffold and applied it to repair mandibular defects in rabbits. The obtained results indicated that the therapeutic effect of ADMSCs in conjunction with fibrin glue was similar to those of autologous bone graft, and the effect of ADMSCs/fibrin glue on bone formation was greater than that of fibrin glue alone. In addition, ADMSCs with TCP are widely used in bone regeneration to facilitate the formation of the bone extracellular matrix, thereby promoting reparative osteogenesis. Probst et al. [86] reported that ADMSCs seeding on ceramic and polymer composite scaffolds improves bone regeneration in large mandibular defects.
The oral and maxillofacial region is a complex system with the mutual attachment of hard and soft tissues. The displacement of the remaining jaw bone wound leads to facial deformities and dysfunction due to muscle contraction. As mentioned above, the ADMSCs used for the reconstruction of oral and maxillofacial hard tissue are usually combined with biomaterials possessing good mechanical properties such as bioceramics or bioceramics-collagen composites. Therefore, they can integrate ADMSCs with the host organization and fight with the pressure from muscles.
4.2. ADMSCs-based biomaterial scaffolds in the treatment of periodontal tissue regeneration
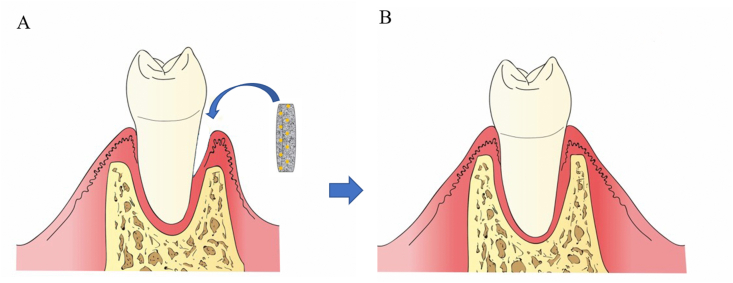
As the supporting tissue of teeth, the periodontal tissue is composed of the pericementum, alveolar bone, and gingiva. The gingival connective tissue is attached to the upper part of the alveolar bone, while the root of the tooth is connected to the alveolar bone through the periodontal ligament. Therefore, excessive growth of gingival fibroblasts can easily lead to defect of the periodontal tissue [45]. Traditional treatments for periodontal tissue regeneration include bone grafting, guided tissue regeneration, and the application of enamel matrix derivatives through basic research and clinical experience. However, it is difficult to completely reconstruct the periodontal tissue due to the limited regeneration ability once damaged [92]. Therefore, the application of ADMSCs-based biomaterial scaffolds for guided periodontal tissue regeneration is considered as the treatment of choice due to the excellent characteristics of ADMSCs (Fig. 3).
Fig. 3.
ADMSCs/biomaterial scaffold applying to periodontium. (A) Schematic picture of the implantation of the ADMSCs/biomaterial scaffold in a periodontal defect. (B) Repaired periodontal tissue. ADMSCs in the construct regenerate periodontal tissue at the implanted site.
The application of ADMSCs-based PRP composite material has been reported in periodontal tissue engineering. Many scholars have applied ADMSCs/PRP in vivo to demonstrate that PRP could be a promising component for tissue engineering. Tobita et al. [[83], [99],100] applied ADMSCs/PRP to rat and canine periodontal defect models and demonstrated the significance of PRP for periodontal tissue reconstruction. Pure PRP, non-implantation, and autologous ADMSCs-based PRP were respectively implanted into receptors with the defects of periodontal tissue. Histologic, immunohistochemical, and x-ray tests were then used to analyze tissue sections for the amount and scale of newly formed bone in the periodontal tissue defect region within one to two months of implantation. The obtained results indicated that the implantation of ADMSCs facilitated periodontal tissue regeneration and PRP prevented gingival invasion when compared with the control group. The biological effects of activated PRP may also influence periodontal tissue regeneration by ADMSCs because the ADMSCs-based PRP composite material could significantly enhance periodontal tissue regeneration. Furthermore, Kolaparthy et al. also applied ADMSCs/PRP to the rat periodontal defect model and yielded similar results [72].
In addition to the combination of growth factors and ADMSCs in periodontal regeneration, researchers have also applied ADMSCs in combination with other biomaterials in the last five years. One example is the amniotic membrane (AM), which is a biofilm that is the innermost layer of the fetal membrane surrounding the developing fetus. A previous study reported that the AM has good physicochemical and biological properties, and is rich in collagen type I-VII, elastin, laminin, and fibronectin [102]. Considering the excellent properties of AM, Wu et al. used an ADMSCs-based AM co-cultured system to study periodontal regeneration in vivo in rat periodontal defect models. The results obtained in the study demonstrated the potential of using stem cells in combination with appropriate scaffolds for periodontal disease in clinical periodontology [102]. In addition, Requicha et al. [67,68] combined a three-dimensional (3D) fiber mesh functionalized with membrane and silanol groups, both made of starch and poly-Ɛ-(caprolactone) (SPCL). The study further carried out corresponding in vitro experiments for the periodontal regeneration by co-culturing ADMSCs on the scaffold material. The scaffold consists of an osteoconductive wet-spun fiber mesh and a solvent casting membrane, which could further be enriched with ADMSCs. The main aim of combining the two was to achieve two main functions in the implantation of periodontal defect, thereby inducing the formation of new alveolar bones and guiding tissue regeneration as a barrier, respectively.
Beta-TCP is a bioceramic osteoinductive material which has been reported to induce osteogenic differentiation of ADMSCs. Sadeghi et al. seeded ADMSCs into beta-TCP and implanted the ADMSCs-based beta-TCP composite materials into the periodontal defects of Wistar rats with the goal of observing the regeneration of periodontal tissues in vivo [88]. This attempt also opens up new possibilities for periodontal tissue engineering.
Clinically, periodontal disease is still a major problem which causes many patients to lose teeth and related functions. The application of ADMSCs-based biomaterial materials in the clinical treatment of periodontal disease has a broad prospect and important significance.
4.3. ADMSCs-based biomaterial scaffolds in the treatment of dentine-pulp complex regeneration
Dental pulp necrosis which caused by infection, periodontal disease or trauma is treated conventionally by pulpectomy and root canal treatment (RCT). Although RCT is a currently available therapy, it carries the risk of reinfection and root fracture [114]. As an alternative method, the application of ADMSCs-based biomaterial scaffolds for guided dentine-pulp complex regeneration was considered the treatment of choice.
In the last decade, the research on tooth-derived stem cells (TDSCs), including dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), stem cells from human exfoliated deciduous teeth (SHEDs), dental follicle progenitor stem cells (DFPCs), and stem cells from apical papilla (SCAPs), is still the mainstream in this field [[115], [116], [117], [118]]. Due to the limitation of pulp tissue and in order to explore the feasibility of ADMSCs, Hung et al. [119] conducted a controlled trial between ADMSCs and DPSCs. Hung et al. implanted autologous ADMSCs and DPSCs with BMP-2 into adult rabbit extraction sockets, respectively, and both groups grew self-assembled new teeth which were living with nerves and vascular system. Besides, Ishizaka et al. [116] established an experimental dog model with whole pulp removal and transplanted autologous PDSCs, BMSCs and ADMSCs with stromal cell-derived factor (SDF)-1, respectively. Through the detection of the corresponding markers and genes, Ishizaka et al. demonstrated that ADMSCs could enhance matrix formation and induce pulp/dental regeneration.
As stated above, ADMSCs have ideal differentiation potential and could be an alternative cell sources for dental-pulp complex regeneration.
4.4. ADMSCs-based biomaterial scaffolds in the treatment of facial nerve regeneration
Clinically, the common causes of facial paralysis are inflammation, trauma, and facial nerve tumor. Facial nerve symptoms caused by facial paralysis can alter facial symmetry including loss of forehead lines, incomplete eyelid closure, shallow nasolabial groove, and angular mouth. Therefore, facial paralysis often causes a severe psychological blow to the patients. As a result, such conditions have serious consequences for the society and individuals, thereby requiring great efforts to try and understand the various factors which influence the damage, regeneration, and repair [54].
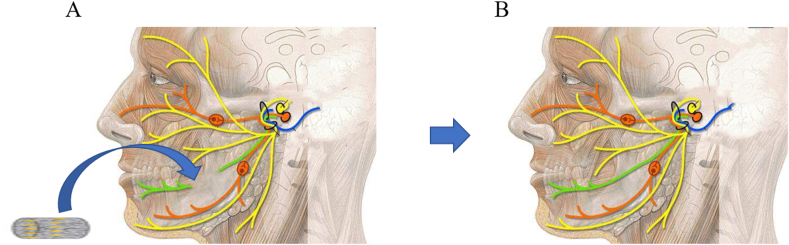
Peripheral nerve injury is a major challenge for nerve recovery, and the restoration of facial nerve function depends on the growth of new axons, myelin formation, and proper reconstruction of target organs. The myelin formation system of the peripheral nerve occurs when Schwann cells (SCs) spread their axons layer by layer, thereby ensuring a rapid conduction velocity. SCs are the main structural and functional cells of peripheral nerves, and play an important role in nerve regeneration and functional recovery after nerve injury [103,104]. Despite stem cells having matured as artificial nerve seed cells, some deficiencies have been reported including the limited source of autologous stem cells, the need for secondary surgery, and the immune rejection of stem cells. However, stem cells are still a promising choice for nerve regeneration. ADMSCs are pluripotent mesenchymal stem cells with the common characteristics of self-renewal and SC-like cell transformation, which can accelerate axonal regeneration and functional recovery [104,105]. ADMSCs may become better seed cells, which can transcend the limited use of SCs and provide a less invasive source of somatic cell transplantation to promote facial nerve regeneration. Fig. 4 displays the schematic diagram of facial nerve regeneration using ADMSCs/biomaterial scaffolds.
Fig. 4.
Clinical scenario for ADMSCs/biomaterial scaffold-based therapy for facial nerve injuries. (A) Schematic picture of the implantation of the ADMSCs/biomaterial scaffold in a facial nerve injury. (B) Repaired facial nerve. ADMSCs in the construct regenerate facial nerve injury at the implanted site.
Sun et al., examined the effects of a decellularized allogeneic artery conduit containing autologous transdifferentiated ADMSCs (dADMSCs) on an 8-mm facial nerve branch in a rat model [91]. The study also compared cultures by immunochemistry using GFAP and S-100 markers and confirmed that the trans-differentiated effectiveness of dAMDSCs was similar to that of SCs. The researchers segmented the abdominal artery of rats as an arterial catheter and wrapped it with dADMSCs to repair the facial nerve in vivo. The obtained results indicated that the peak amplitudes of the compound muscle action potential (CMAP) and nerve conduction velocity (NCV) values were significantly improved eight weeks after the operation when compared with the control group. Moreover, the amount of Fluorogold-labeled neurons were also significantly higher than those in the control group. The final results showed that dADMSCs-based biomaterial scaffolds can effectively promote facial nerve regeneration.
Although dADMSCs have been identified as SCs substitutes, Watanabe et al., distinguished the phenotypic and functional characteristics of undifferentiated ADMSCs (uADMSCs) from dADMSCs [70]. In addition, the silicone tubes of type I collagen scaffolds containing uADMSCs, dADMSCs, or SCs, respectively, were transplanted in vivo to study the effect of uADMSCs and dADMSCs as SC substitutes to repair the 7 mm gap in the facial nerve of rats. Their results suggest that uADMSCs and dADMSCs support axonal growth at the same level by optimizing SC phenotypic expression and cell transplantation into nerve channels. Moreover, their results support the idea that ADMSCs have a therapeutic effect on neuronal regeneration and may be a promising source of neuronal guided ducts, which are more abundant than autologous SCs to connect nerve gaps. Furthermore, biopolymer scaffolds with growth factors also provide the basis for facial nerve regeneration. Ghoreishian et al. used an uADMSCs-based expanded polytetrafluoroethylene tube encapsulated in alginate hydrogel to repair a 7 mm-gap facial nerve [57]. The obtained results indicated that the NCV and maximal amplitude of action potential were significantly improved in the experimental group than in the control group.
The above mentioned studies have shown that the features of ADMSCs are similar to those of SCs. Furthermore, the advancement of research has drawn further attention to the possibility of using polymer materials as biological scaffolds.
4.5. ADMSCs-based biomaterial scaffolds in the treatment of adipose tissue engineering
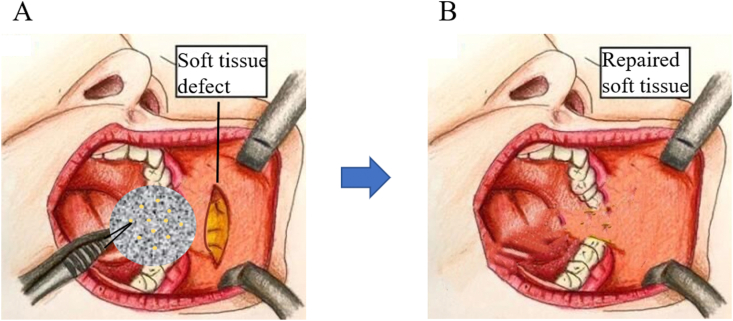
In the modern era of cosmetic and reconstructive surgery, tissue-engineered therapies for improving aesthetic appearance continue to be the focus of attention. Besides restoring normal tissue contours, augmentation therapy may affect a person's overall health. According to the American Society of Plastic Surgeons (ASPS) survey, millions of prosthetics and reconstructive operations are performed worldwide each year. The main focus of the operations is on tumor resection, laceration repair, and scar revision, which are the major challenges faced by plastic and reconstructive surgeons [98]. In recent years, facial reconstruction surgery has received significant attention and improvement in the field of augmentation therapy. In addition, facial reconstruction is a routine cosmetic surgery in China [99]. From the beginning, tissue engineering has focused on modifying traditional augmentation therapies using the concept of bioengineered structures [86] - the combination of cells and scaffolds that achieve a normal aesthetic. Currently, several prototypes of different tissues and organs have emerged in the field of biomedicine. However, recent research has focused on adipose tissue engineering (ATE) to repair soft tissue defects (Fig. 5).
Fig. 5.
Clinical scenario for ADMSCs/biomaterial scaffold-based therapy for oral and maxillofacial adipose tissue. (A) Schematic picture of the implantation of the ADMSCs/biomaterial scaffold in a soft tissue injury. (B) Repaired facial soft tissue. ADMSCs in the construct regenerate facial soft tissue at the implanted site.
In the development of engineered tissue construction, ADMSCs with proven multilineage differentiation potential are expected to be an ideal cell source. A previous study reported that the easy isolation, abundant stem cell population, and high differentiation potential of ADMSCs makes them to be an important candidate cell for adipose tissue engineering [95]. Meanwhile, a large number of synthetic and natural materials are being used for adipose tissue engineering, which can simultaneously perform regulated non-toxic degradation of host tissues occupying the tissue voids.
Collagen, a special ECM element, is a widely proven scaffold for ATE. Cells differentiating towards the adipogenic lineage secrete collagen, which is a marker of adipogenesis, and emphasizes the role of collagen in adipogenesis [88]. Therefore, the use of collagen as a scaffold is expected to promote cell anchoring [90] with the overarching goal of ensuring their differentiation in vitro and in vivo, while filling defective sites at the same time. Some researchers have studied the regeneration of adipose tissue by collagen scaffolds [97]. In situ regeneration of adipose tissue was achieved using various forms of collagen scaffolds. It is worth noting that the ADMSCs are multipotent and collagen type I is a natural extracellular matrix component. Because of this situation, Venugopal et al., attempted to apply ADMSCs-based type I collagen sponge, an established ATE scaffold, in vivo [69]. They observed the formation of the reticular meshwork of adipoid cells after 21 days of implantation of the constructed cell seed collagen into the rat dorsal muscle model, and histological characterization was performed using a variety of staining techniques. In addition, the retrieved samples showed fat formation in situ within 21 days.
Several studies have used decellularized biomaterial scaffolds in tissue engineering because of their potential to enhance the regeneration and repair of damaged tissues [[100], [101], [102], [103], [104], [105]]. Ideally, the decellularization process should be designed to gently extract cellular components while retaining the complex structure and composition of the extracellular matrix (ECM) [[106], [107], [108], [109], [110]]. These strategies can be used to create ready-made biomaterial scaffolds enriched with collagen and other ECM structural components, which can conduce to cell attachment and infiltration both in vivo and in vitro [[106], [107], [108]]. Han et al. [60] investigated the in vivo effects of ADMSCs-based decellularized adipose tissue (DAT) on adipose tissue engineering using an immunocompetent rat model. The obtained results indicated that the host significantly improved the ability of angiogenesis and adipogenesis through the implantation of ADMSCs/DAT. In addition, Pati et al. [82] constructed a composite that used DAT matrix bioink encapsulating ADMSCs to detect the expression of standard adipogenic genes and then applied it to mice in vivo. The study found that the printed DAT gels were more likely to express adipogenic genes than non-printed DAT gels. Similarly, adipose tissue formation and constructive tissue remodeling was observed in vivo. Furthermore, the decellularized tissue biomaterial scaffolds could also simulate the tissue-specific biochemical and biomechanical properties inherent in the cellular microenvironment, which are considered to be important mediators for various functions such as cell proliferation and differentiation [110,111].
5. Conclusion
Allogeneic ADMSCs based regeneration therapy may be the potential direction of future research because the ADMSCs have a low immunogenicity. For practical application, the osteogenic potential of ADMSCs decreases with age. On the other hand, the immunoregulation and angiogenesis characteristics of ADMSCs promote tumor cell growth, thereby making safety assessment a necessity.
Composite scaffolds should be the main direction of scaffold development in the future. It is worth noting that even modern methods such as inducing tissue regeneration, using bioactive proteins alone or loading bioactive proteins on scaffolds, and alternative materials with or without cells, are only moderately effective. Therefore, we must develop specific stem cells and innovative scaffolds to solve the above problems. In addition, clinical management and surgical techniques should be considered for potential inclusion in future oral and maxillofacial surgery.
Author contributions
Tong Liu was responsible for the design of the work, and undertook the implementation of the study and drafting of the manuscript; Huixu Xie and Xiaoyi Wang revised the manuscript and conducted final approval of the version to be published; Xun Pan and Zhangfan Ding contributed to quality assessment of the work and offered intellectual content to the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Xiaoyi Wang, Email: 1163953071@qq.com.
Huixu Xie, Email: aitian007@126.com.
References
- 1.Berebichez-Fridman R., Montero-Olvera P.R. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ. Med. J. 2018;18:e264–e277. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthiaume F., Maguire T.J., Yarmush M.L. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 3.Dave J.R., Tomar G.B. Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Crit. Rev. Biomed. Eng. 2018;46:429–468. doi: 10.1615/CritRevBiomedEng.2018027342. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler T., Rabe M., Mundkowski R.G., Oehmcke-Hecht S., Peters K. Adipose-derived mesenchymal stem cells release microvesicles with procoagulant activity. Int. J. Biochem. Cell Biol. 2018;100:49–53. doi: 10.1016/j.biocel.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto N., Honda Y., Momota Y., Tran S.D. Dedifferentiated Fat (DFAT) cells: a cell source for oral and maxillofacial tissue engineering. Oral Dis. 2018;24:1161–1167. doi: 10.1111/odi.12832. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudifar N., Doran P.M. Mesenchymal stem cells derived from human adipose tissue. Methods Mol. Biol. 2015;1340:53–64. doi: 10.1007/978-1-4939-2938-2_4. [DOI] [PubMed] [Google Scholar]
- 7.Tatullo M., Marrelli M., Paduano F. The regenerative medicine in oral and maxillofacial surgery: the most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med. Sci. 2015;12:72–77. doi: 10.7150/ijms.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheller E.L., Krebsbach P.H., Kohn D.H. Tissue engineering: state of the art in oral rehabilitation. J. Oral Rehabil. 2009;36:368–389. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu V., Helder M.N., Bravenboer N., Ten Bruggenkate C.M., Jin J., Klein-Nulend J., Schulten E. Bone tissue regeneration in the oral and maxillofacial region: a review on the application of stem cells and new strategies to improve vascularization. Stem Cell. Int. 2019;2019:6279721. doi: 10.1155/2019/6279721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merceron C., Vinatier C., Clouet J., Colliec-Jouault S., Weiss P., Guicheux J. Adipose-derived mesenchymal stem cells and biomaterials for cartilage tissue engineering. Joint Bone Spine. 2008;75:672–674. doi: 10.1016/j.jbspin.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Minteer D., Marra K.G., Rubin J.P. Adipose-derived mesenchymal stem cells: biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013;129:59–71. doi: 10.1007/10_2012_146. [DOI] [PubMed] [Google Scholar]
- 12.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 13.Song Y., Du H., Dai C., Zhang L., Li S., Hunter D.J., Lu L., Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen. Med. 2018;13:295–307. doi: 10.2217/rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Torrillas M., Rubio M., Damia E., Cuervo B., Del Romero A., Peláez P., Chicharro D., Miguel L., Sopena J.J. Adipose-derived mesenchymal stem cells: a promising tool in the treatment of musculoskeletal diseases. Int. J. Mol. Sci. 2019;20:1–22. doi: 10.3390/ijms20123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Sun D. Adipose-derived mesenchymal stem cells: a new tool for the treatment of renal fibrosis. Stem Cell. Dev. 2018;27:1406–1411. doi: 10.1089/scd.2017.0304. [DOI] [PubMed] [Google Scholar]
- 16.Barbanti Brodano G., Mazzoni E., Tognon M., Griffoni C., Manfrini M. Human mesenchymal stem cells and biomaterials interaction: a promising synergy to improve spine fusion. Eur. Spine J. 2012;21(Suppl 1):S3–S9. doi: 10.1007/s00586-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese G., Giuffrida R., Forte S., Fabbi C., Figallo E., Salvatorelli L., Memeo L., Parenti R., Gulisano M., Gulino R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017;7:7110. doi: 10.1038/s41598-017-07672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn A., Talovic M., Patel K., Patel A., Marcinczyk M., Garg K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019;37:1246–1262. doi: 10.1002/jor.24212. [DOI] [PubMed] [Google Scholar]
- 19.Huang R., Wang J., Chen H., Shi X., Wang X., Zhu Y., Tan Z. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater. Sci. 2019;7:4248–4259. doi: 10.1039/c9bm00939f. [DOI] [PubMed] [Google Scholar]
- 20.Banks J.M., Mozdzen L.C., Harley B.A., Bailey R.C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials. 2014;35:8951–8959. doi: 10.1016/j.biomaterials.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazimierczak P., Benko A., Nocun M., Przekora A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019;14:6615–6630. doi: 10.2147/IJN.S217245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marei N.H., El-Sherbiny I.M., Lotfy A., El-Badawy A., El-Badri N. Mesenchymal stem cells growth and proliferation enhancement using PLA vs PCL based nanofibrous scaffolds. Int. J. Biol. Macromol. 2016;93:9–19. doi: 10.1016/j.ijbiomac.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 23.Rivera-Izquierdo M., Cabeza L., Láinez-Ramos-Bossini A., Quesada R., Perazzoli G., Alvarez P., Prados J., Melguizo C. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expet Opin. Biol. Ther. 2019;19:233–248. doi: 10.1080/14712598.2019.1563069. [DOI] [PubMed] [Google Scholar]
- 24.Gaur M., Dobke M., Lunyak V.V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 2017;18:1–29. doi: 10.3390/ijms18010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y., Zhao L., Hantash B.M. Support of human adipose-derived mesenchymal stem cell multipotency by a poloxamer-octapeptide hybrid hydrogel. Biomaterials. 2010;31:5122–5130. doi: 10.1016/j.biomaterials.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Heidari M., Pouya S., Baghaei K., Aghdaei H.A., Namaki S., Zali M.R., Hashemi S.M. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J. Cell. Physiol. 2018;233:8754–8766. doi: 10.1002/jcp.26765. [DOI] [PubMed] [Google Scholar]
- 27.Schulze M., Tobiasch E. Artificial scaffolds and mesenchymal stem cells for hard tissues. Adv. Biochem. Eng. Biotechnol. 2012;126:153–194. doi: 10.1007/10_2011_115. [DOI] [PubMed] [Google Scholar]
- 29.Pilipchuk S.P., Plonka A.B., Monje A., Taut A.D., Lanis A., Kang B., Giannobile W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015;31:317–338. doi: 10.1016/j.dental.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff J., Farré-Guasch E., Sándor G.K., Gibbs S., Jager D.J., Forouzanfar T. Soft tissue augmentation techniques and materials used in the oral cavity: an overview. Implant Dent. 2016;25:427–434. doi: 10.1097/ID.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 31.Galindo S., Herreras J.M., López-Paniagua M., Rey E., de la Mata A., Plata-Cordero M., Calonge M., Nieto-Miguel T. Therapeutic effect of human adipose tissue-derived mesenchymal stem cells in experimental corneal failure due to limbal stem cell niche damage. Stem Cell. 2017;35:2160–2174. doi: 10.1002/stem.2672. [DOI] [PubMed] [Google Scholar]
- 32.Maria A.T., Maumus M., Le Quellec A., Jorgensen C., Noël D., Guilpain P. Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin. Rev. Allergy Immunol. 2017;52:234–259. doi: 10.1007/s12016-016-8552-9. [DOI] [PubMed] [Google Scholar]
- 33.Ivanovski S., Vaquette C., Gronthos S., Hutmacher D.W., Bartold P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014;93:1212–1221. doi: 10.1177/0022034514544301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Yan Z., Zhang H., Lu W., Liu S., Huang X., Luo H., Jin Y. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng. 2011;17:2981–2997. doi: 10.1089/ten.tea.2010.0707. [DOI] [PubMed] [Google Scholar]
- 35.Akita D., Kano K., Saito-Tamura Y., Mashimo T., Sato-Shionome M., Tsurumachi N., Yamanaka K., Kaneko T., Toriumi T., Arai Y., Tsukimura N., Matsumoto T., Ishigami T., Isokawa K., Honda M. Use of rat mature adipocyte-derived dedifferentiated fat cells as a cell source for periodontal tissue regeneration. Front. Physiol. 2016;7:50. doi: 10.3389/fphys.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S., Kai Z., Wang D., Tao L., Zhang P., Wang D., Liu D., Sun S., Zhong J. Allogenic chondrocyte/osteoblast-loaded β-tricalcium phosphate bioceramic scaffolds for articular cartilage defect treatment. Artif. Cells Nanomed. Biotechnol. 2019;47:1570–1576. doi: 10.1080/21691401.2019.1604534. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Li M., Kang E.T., Neoh K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016;32:46–56. doi: 10.1016/j.actbio.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Sugawara A., Sato S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Hum. Cell. 2014;27:12–21. doi: 10.1007/s13577-013-0075-6. [DOI] [PubMed] [Google Scholar]
- 39.Bottino M.C., Pankajakshan D., Nör J.E. Advanced scaffolds for dental pulp and periodontal regeneration. Dent. Clin. North Am. 2017;61:689–711. doi: 10.1016/j.cden.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano-Ruiz J.L., Gálvez-Martín P., López-Ruiz E., Suñer-Carbó J., Calpena-Campmany A.C., Marchal J.A., Clares-Naveros B. Design and evaluation of mesenchymal stem cells seeded chitosan/glycosaminoglycans quaternary hydrogel scaffolds for wound healing applications. Int. J. Pharm. 2019;570:118632. doi: 10.1016/j.ijpharm.2019.118632. [DOI] [PubMed] [Google Scholar]
- 41.Cheung H.K., Han T.T., Marecak D.M., Watkins J.F., Amsden B.G., Flynn L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914–1923. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 42.Mauney J.R., Nguyen T., Gillen K., Kirker-Head C., Gimble J.M., Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto W.R., Sarraf C.E. Culturing and differentiating human mesenchymal stem cells for biocompatible scaffolds in regenerative medicine. Methods Mol. Biol. 2012;806:407–426. doi: 10.1007/978-1-61779-367-7_27. [DOI] [PubMed] [Google Scholar]
- 44.Portron S., Soueidan A., Marsden A.C., Rakic M., Verner C., Weiss P., Badran Z., Struillou X. Periodontal regenerative medicine using mesenchymal stem cells and biomaterials: a systematic review of pre-clinical studies. Dent. Mater. J. 2019;38:867–883. doi: 10.4012/dmj.2018-315. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-Sánchez R., Brena-Molina A., Martínez-López V., Melgarejo-Ramírez Y., Tamay de Dios L., Gómez-García R., Reyes-Frías Mde L., Rodríguez-Rodríguez L., Garciadiego-Cázares D., Lugo-Martínez H., Ibarra C., Martínez-Pardo M.E., Velasquillo-Martínez C. Generation of two biological wound dressings as a potential delivery system of human adipose-derived mesenchymal stem cells. Am. Soc. Artif. Intern. Organs J. 2015;61:718–725. doi: 10.1097/MAT.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheets K.T., Bagó J.R., Hingtgen S.D. Delivery of cytotoxic mesenchymal stem cells with biodegradable scaffolds for treatment of postoperative brain cancer. Methods Mol. Biol. 2018;1831:49–58. doi: 10.1007/978-1-4939-8661-3_5. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y., Lee K., Kawazoe N., Yang Y., Chen G. PLGA-collagen-ECM hybrid scaffolds functionalized with biomimetic extracellular matrices secreted by mesenchymal stem cells during stepwise osteogenesis-co-adipogenesis. J. Mater. Chem. B. 2019;7:7195–7206. doi: 10.1039/c9tb01959f. [DOI] [PubMed] [Google Scholar]
- 48.Conejero J.A., Lee J.A., Parrett B.M., Terry M., Wear-Maggitti K., Grant R.T., Breitbart A.S. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast. Reconstr. Surg. 2006;117:857–863. doi: 10.1097/01.prs.0000204566.13979.c1. [DOI] [PubMed] [Google Scholar]
- 49.Cowan C.M., Shi Y.Y., Aalami O.O., Chou Y.F., Mari C., Thomas R., Quarto N., Contag C.H., Wu B., Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 50.Cowan C.M., Aalami O.O., Shi Y.Y., Chou Y.F., Mari C., Thomas R., Quarto N., Nacamuli R.P., Contag C.H., Wu B., Longaker M.T. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 51.Murata D., Fujimoto R., Nakayama K. Osteochondral regeneration using adipose tissue-derived mesenchymal stem cells. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudas J.R., Marra K.G., Cooper G.M., Penascino V.M., Mooney M.P., Jiang S., Rubin J.P., Losee J.E. The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann. Plast. Surg. 2006;56:543–548. doi: 10.1097/01.sap.0000210629.17727.bd. [DOI] [PubMed] [Google Scholar]
- 53.Mazzoni E., D'Agostino A., Iaquinta M.R., Bononi I., Trevisiol L., Rotondo J.C., Patergnani S., Giorgi C., Gunson M.J., Arnett G.W., Nocini P.F., Tognon M., Martini F. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl. Med. 2020;9:377–388. doi: 10.1002/sctm.19-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Euler de Souza Lucena E., Guzen F.P., Lopes de Paiva Cavalcanti J.R., Galvão Barboza C.A., Silva do Nascimento Júnior E., Cavalcante Jde S. Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J. Oral Maxillofac. Surg. 2014;72:1001–1012. doi: 10.1016/j.joms.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Faroni A., Terenghi G., Reid A.J. Adipose-derived stem cells and nerve regeneration: promises and pitfalls. Int. Rev. Neurobiol. 2013;108:121–136. doi: 10.1016/B978-0-12-410499-0.00005-8. [DOI] [PubMed] [Google Scholar]
- 56.Sheykhhasan M., Qomi R.T., Kalhor N., Mehdizadeh M., Ghiasi M. Evaluation of the ability of natural and synthetic scaffolds in providing an appropriate environment for growth and chondrogenic differentiation of adipose-derived mesenchymal stem cells. Indian J. Orthop. 2015;49:561–568. doi: 10.4103/0019-5413.164043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Ghoreishian M., Rezaei M., Beni B.H., Javanmard S.H., Attar B.M., Zalzali H. Facial nerve repair with Gore-Tex tube and adipose-derived stem cells: an animal study in dogs. J. Oral Maxillofac. Surg. 2013;71:577–587. doi: 10.1016/j.joms.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 58.Grottkau B.E., Lin Y. Osteogenesis of adipose-derived stem cells. Bone Res. 2013;1:133–145. doi: 10.4248/BR201302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halberstadt C., Austin C., Rowley J., Culberson C., Loebsack A., Wyatt S., Coleman S., Blacksten L., Burg K., Mooney D., Holder W., Jr. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Eng. 2002;8:309–319. doi: 10.1089/107632702753725067. [DOI] [PubMed] [Google Scholar]
- 60.Han T.T., Toutounji S., Amsden B.G., Flynn L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials. 2015;72:125–137. doi: 10.1016/j.biomaterials.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 61.Hattori H., Sato M., Masuoka K., Ishihara M., Kikuchi T., Matsui T., Takase B., Ishizuka T., Kikuchi M., Fujikawa K., Ishihara M. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2–12. doi: 10.1159/000081088. [DOI] [PubMed] [Google Scholar]
- 62.Hicok K.C., Du Laney T.V., Zhou Y.S., Halvorsen Y.D., Hitt D.C., Cooper L.F., Gimble J.M. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10:371–380. doi: 10.1089/107632704323061735. [DOI] [PubMed] [Google Scholar]
- 63.Justesen J., Pedersen S.B., Stenderup K., Kassem M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004;10:381–391. doi: 10.1089/107632704323061744. [DOI] [PubMed] [Google Scholar]
- 64.Kang S.W., Kim J.S., Park K.S., Cha B.H., Shim J.H., Kim J.Y., Cho D.W., Rhie J.W., Lee S.H. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48:298–306. doi: 10.1016/j.bone.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Khojasteh A., Hosseinpour S., Rad M.R., Alikhasi M. Buccal fat pad-derived stem cells in three-dimensional rehabilitation of large alveolar defects: a report of two cases. J. Oral Implantol. 2019;45:45–54. doi: 10.1563/aaid-joi-D-17-00215. [DOI] [PubMed] [Google Scholar]
- 66.Kimura Y., Ozeki M., Inamoto T., Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 67.Requicha J.F., Viegas C.A., Hede S., Leonor I.B., Reis R.L., Gomes M.E. Design and characterization of a biodegradable double-layer scaffold aimed at periodontal tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016;10:392–403. doi: 10.1002/term.1816. [DOI] [PubMed] [Google Scholar]
- 68.Requicha J.F., Viegas C.A., Muñoz F., Azevedo J.M., Leonor I.B., Reis R.L., Gomes M.E. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng. 2014;20:2483–2492. doi: 10.1089/ten.tea.2013.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venugopal B., Fernandez F.B., Harikrishnan V.S., John A. Post implantation fate of adipogenic induced mesenchymal stem cells on Type I collagen scaffold in a rat model, Journal of materials science. J. Mater. Sci. Mater. Med. 2017;28:28. doi: 10.1007/s10856-016-5838-7. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe Y., Sasaki R., Matsumine H., Yamato M., Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J. Tissue Eng. Regen. Med. 2017;11:362–374. doi: 10.1002/term.1919. [DOI] [PubMed] [Google Scholar]
- 71.Schoeller T., Lille S., Wechselberger G., Otto A., Mowlavi A., Piza-Katzer H. Histomorphologic and volumetric analysis of implanted autologous preadipocyte cultures suspended in fibrin glue: a potential new source for tissue augmentation. Aesthetic Plast. Surg. 2001;25:57–63. doi: 10.1007/s002660010096. [DOI] [PubMed] [Google Scholar]
- 72.Kolaparthy L.K., Sanivarapu S., Moogla S., Kutcham R.S. Adipose tissue - adequate, accessible regenerative material. Int. J. Stem. Cells. 2015;8:121–127. doi: 10.15283/ijsc.2015.8.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee S.J., Kang S.W., Do H.J., Han I., Shin D.A., Kim J.H., Lee S.H. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials. 2010;31:5652–5659. doi: 10.1016/j.biomaterials.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 74.Lemaitre M., Monsarrat P., Blasco-Baque V., Loubières P., Burcelin R., Casteilla L., Planat-Bénard V., Kémoun P. Periodontal tissue regeneration using syngeneic adipose-derived stromal cells in a mouse model. Stem Cells Transl. Med. 2017;6:656–665. doi: 10.5966/sctm.2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin S.D., Wang K.H., Kao A.P. Engineered adipose tissue of predefined shape and dimensions from human adipose-derived mesenchymal stem cells. Tissue Eng. 2008;14:571–581. doi: 10.1089/tea.2007.0192. [DOI] [PubMed] [Google Scholar]
- 76.Lendeckel S., Jödicke A., Christophis P., Heidinger K., Wolff J., Fraser J.K., Hedrick M.H., Berthold L., Howaldt H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J. Cranio-Maxillo-Fac. Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Mauney J.R., Nguyen T., Gillen K., Kirker-Head C., Gimble J.M., Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehrabani D., Khodakaram-Tafti A., Shaterzadeh-Yazdi H., Zamiri B., Omidi M. Comparison of the regenerative effect of adipose-derived stem cells, fibrin glue scaffold, and autologous bone graft in experimental mandibular defect in rabbit. Dent. Traumatol. 2018;34:413–420. doi: 10.1111/edt.12435. [DOI] [PubMed] [Google Scholar]
- 79.Mesimäki K., Lindroos B., Törnwall J., Mauno J., Lindqvist C., Kontio R., Miettinen S., Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Nuñez J., Vignoletti F., Caffesse R.G., Sanz M. Cellular therapy in periodontal regeneration. Periodontology. 2000;79:107–116. doi: 10.1111/prd.12250. [DOI] [PubMed] [Google Scholar]
- 81.Parker A.M., Katz A.J. Adipose-derived stem cells for the regeneration of damaged tissues. Expet Opin. Biol. Ther. 2006;6:567–578. doi: 10.1517/14712598.6.6.567. [DOI] [PubMed] [Google Scholar]
- 82.Pati F., Ha D.H., Jang J., Han H.H., Rhie J.W., Cho D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 83.Tobita M., Mizuno H. Periodontal disease and periodontal tissue regeneration, Curr. Stem Cell Res. Ther. 2010;5:168–174. doi: 10.2174/157488810791268672. [DOI] [PubMed] [Google Scholar]
- 84.Prichard H.L., Reichert W.M., Klitzman B. Adult adipose-derived stem cell attachment to biomaterials. Biomaterials. 2007;28:936–946. doi: 10.1016/j.biomaterials.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Probst F.A., Fliefel R., Burian E., Probst M., Eddicks M., Cornelsen M., Riedl C., Seitz H., Aszódi A., Schieker M., Otto S. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020;10:2062. doi: 10.1038/s41598-020-59038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sadeghi D., Nazarian H., Nojehdehian H. Adipose-derived stem cells combined with beta-tricalcium phosphate: a novel possible strategy for periodontal defects regeneration. Med. Hypotheses. 2014;82:54–56. doi: 10.1016/j.mehy.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Sari D.S., Maduratna E., Latief F.D.E., Nugraha A.P., Sudiana K., Rantam F.A. Osteogenic differentiation and biocompatibility of bovine teeth scaffold with rat adipose-derived mesenchymal stem cells. Eur. J. Dermatol. 2019;13:206–212. doi: 10.1055/s-0039-1694305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun F., Zhou K., Mi W.J., Qiu J.H. Repair of facial nerve defects with decellularized artery allografts containing autologous adipose-derived stem cells in a rat model. Neurosci. Lett. 2011;499:104–108. doi: 10.1016/j.neulet.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 92.Tassi S.A., Sergio N.Z., Misawa M.Y.O., Villar C.C. Efficacy of stem cells on periodontal regeneration: systematic review of pre-clinical studies. J. Periodontal. Res. 2017;52:793–812. doi: 10.1111/jre.12455. [DOI] [PubMed] [Google Scholar]
- 93.Sánchez-González D.J., Méndez-Bolaina E., Trejo-Bahena N.I. Platelet-rich plasma peptides: key for regeneration. Int. J. Pept. 2012;2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nurden A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 95.Chen X., Zhang T., Shi J., Xu P., Gu Z., Sandham A., Yang L., Ye Q. Notch1 signaling regulates the proliferation and self-renewal of human dental follicle cells by modulating the G1/S phase transition and telomerase activity. PloS One. 2013;8 doi: 10.1371/journal.pone.0069967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Z., Yin X., Ye Q., He W., Ge M., Zhou X., Hu J., Zou S. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J. Biomater. Appl. 2016;31:121–131. doi: 10.1177/0885328216637978. [DOI] [PubMed] [Google Scholar]
- 98.Yang S., Xu Y., Lin Q., Bai Y., Zan X., Ye Q. A bio-inspired, one-step but versatile coating onto various substrates with strong antibacterial and enhanced osteogenesis. Chem. Commun. (Camb.) 2019;55:2058–2061. doi: 10.1039/c8cc09986c. [DOI] [PubMed] [Google Scholar]
- 99.Tobita M., Uysal A.C., Ogawa R., Hyakusoku H., Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells, Tissue. Eng. Times Part A. 2008;14:945–953. doi: 10.1089/ten.tea.2007.0048. [DOI] [PubMed] [Google Scholar]
- 100.Tobita M., Uysal C.A., Guo X., Hyakusoku H., Mizuno H. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy. 2013;15:1517–1526. doi: 10.1016/j.jcyt.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Mamede A.C., Carvalho M.J., Abrantes A.M., Laranjo M., Maia C.J., Botelho M.F. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349:447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 102.Wu P.H., Chung H.Y., Wang J.H., Shih J.C., Kuo M.Y., Chang P.C., Huang Y.D., Wang P.C., Chang C.C. Amniotic membrane and adipose-derived stem cell co-culture system enhances bone regeneration in a rat periodontal defect model. J. Formos. Med. Assoc. 2016;115:186–194. doi: 10.1016/j.jfma.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Thesleff T., Lehtimäki K., Niskakangas T., Huovinen S., Mannerström B., Miettinen S., Seppänen-Kaijansinkko R., Öhman J. Cranioplasty with adipose-derived stem cells, beta-tricalcium phosphate granules and supporting mesh: six-year clinical follow-up results. Stem Cells Transl. Med. 2017;6:1576–1582. doi: 10.1002/sctm.16-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoon E., Dhar S., Chun D.E., Gharibjanian N.A., Evans G.R. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–627. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Q., Nguyen P.D., Shi S., Burrell J.C., Cullen D.K., Le A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018;8:6634. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zang X., He L., Zhao L., He Y., Xiao E., Zhang Y. Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor β-1-mediated gingival wound healing. Stem Cell Res. Ther. 2019;10:169. doi: 10.1186/s13287-019-1277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fansa H., Keilhoff G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol. Res. 2004;26:167–173. doi: 10.1179/016164104225013842. [DOI] [PubMed] [Google Scholar]
- 108.Schmalenberg K.E., Uhrich K.E. Micropatterned polymer substrates control alignment of proliferating Schwann cells to direct neuronal regeneration. Biomaterials. 2005;26:1423–1430. doi: 10.1016/j.biomaterials.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 109.di Summa P.G., Kingham P.J., Raffoul W., Wiberg M., Terenghi G., Kalbermatten D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthetic Surg. 2010;63:1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 110.Xie H., Gu Z., Li C., Franco C., Wang J., Li L., Meredith N., Ye Q., Wan C. A novel bioceramic scaffold integrating silk fibroin in calcium polyphosphate for bone tissue-engineering. Ceram. Int. 2016;42:2386–2392. [Google Scholar]
- 111.Mazigi O., Kannan M.B., Xu J., Choe H.C., Ye Q. Biocompatibility and degradation of a low elastic modulus Ti-35Nb-3Zr alloy: nanosurface engineering for enhanced degradation resistance. ACS Biomater. Sci. Eng. 2017;3:509–517. doi: 10.1021/acsbiomaterials.6b00563. [DOI] [PubMed] [Google Scholar]
- 114.Bakhtiar H., Mazidi S.A., Mohammadi Asl S., Ellini M.R., Moshiri A., Nekoofar M.H., Dummer P.M.H. The role of stem cell therapy in regeneration of dentine-pulp complex: a systematic review. Prog. Biomater. 2018;7:249–268. doi: 10.1007/s40204-018-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bossù M., Pacifici A., Carbone D., Tenore G., Ierardo G., Pacifici L., Polimeni A. Today prospects for tissue engineering therapeutic approach in dentistry. Sci. World J. 2014:151252. doi: 10.1155/2014/151252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ishizaka R., Iohara K., Murakami M., Fukuta O., Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–2118. doi: 10.1016/j.biomaterials.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 117.Zheng C., Chen J., Liu S., Jin Y. Stem cell-based bone and dental regeneration: a view of microenvironmental modulation. Int. J. Oral Sci. 2019;11:23. doi: 10.1038/s41368-019-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang G.T., Al-Habib M., Gauthier P. Challenges of stem cell-based pulp and dentin regeneration: a clinical perspective. Endod. Top. 2013;28:51–60. doi: 10.1111/etp.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hung C.N., Mar K., Chang H.C., Chiang Y.L., Hu H.Y., Lai C.C., Chu R.M., Ma C.M. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials. 2011;32:6995–7005. doi: 10.1016/j.biomaterials.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 120.Furtos G., Rivero G., Rapuntean S., Abraham G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:966–976. doi: 10.1002/jbm.b.33629. [DOI] [PubMed] [Google Scholar]
- 121.Lang S., Loibl M., Herrmann M. Platelet-rich plasma in tissue engineering: hype and hope. Eur. Surg. Res. 2018;59:265–275. doi: 10.1159/000492415. [DOI] [PubMed] [Google Scholar]
- 122.Mazzoni E., D'Agostino A., Iaquinta M.R., Bononi I., Trevisiol L., Rotondo J.C., Patergnani S., Giorgi C., Gunson M.J., Arnett G.W., Nocini P.F., Tognon M., Martini F. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl. Med. 2020;9:377–388. doi: 10.1002/sctm.19-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]