Abstract
We report a case of a 75 year old non-known cancer or organ transplant male with an unusual concurrent triple infection of Aspergillus, strongyloides stercoralis and herpes simplex virus in a bronchoalveolar lavage. He presented to an outside hospital with worsening respiratory distress and an open tracheostomy was performed due to concern he would not extubate. Following tracheostomy, there was concern for a possible esophageal perforation. A bronchoalveolar lavage (BAL) were performed and Strongyloides, herpes viral cytopathic changes and aspergillus microorganisms were identified. The patient subsequently expired following discharge.
Keywords: Strongyloides, Herpes simplex virus, Aspergillus, Bronchoalveolar lavage fluid
1. Case presentation
The patient is a 75 year old male with a history of tracheobronchomalacia, COPD, obstructive sleep apnea, atrial fibrillation, who presented to an outside hospital with worsening respiratory distress after a recent hospitalization for septic shock and pneumonia. He was treated with several courses of high dose steroids in the recent past. His hospital course was complicated for Citrobacter urosepsis, persistent atrial fibrillation, acute kidney injury and respiratory failure. The patient underwent an open tracheostomy because the surgical team believed he would not extubate. This was complicated by tracheal injury resulting in bilateral pneumothoraces, massive pneumomediastinum and subcutaneous emphysema. There was also a concern for esophageal perforation. He was subsequently transferred to our hospital for further management of what was later discovered to be a right lateral tracheal wall laceration and suspected esophageal injury. His arterial blood gas revealed a pH of 7.353 (normal, 7.35–7.45), a pO2 of 94.1 mmHg (normal, 83.0–108.0 mmHg) and a pCO2 of 71.3 mmHg (normal, 32.0–48.0 mmHg) and a HCO3 of 38.7 mmHg (normal, 22.0–26.0 mmHg). His WBC was normal 8.14 K/μL (normal, 3.73–10.10 K/μL) but increased later in his hospital course (11.93 K/μL) along with fevers. After an emergency bronchoscopy, there was concern for a tracheal occlusion from a tissue flap, and an endotracheal tube was placed through the tracheostomy site. A bronchial wash and a BAL were performed at the outside hospital and the slides were reviewed at our institution. Strongyloides, Aspergillus and herpes microorganisms were identified on BAL cytopathology. A review of outside hospital records revealed a positive Strongyloides IgG antibody and an absolute eosinophilia 0f 0.66 K/μL (normal. 0.00–0.48 K/μL). The patient was treated with vorizonazole per os (PO) for Aspergillus, ivermectin and albendazole (once a day treatment) PO for Stronglyoides and acyclovir (twice a day treatment) for herpes. BAL cultures were positive for Enterobacter and blood cultures were positive for Citrobacter. CT chest showed findings suspicious for aspiration pneumonia and findings compatible for inflammatory/infectious bronchiolitis. The patient's clinical course was complicated by persistent encephalopathy with minimal improvement, continued respiratory failure requiring ventilator support and acute kidney injury requiring hemodialysis. The patient was discharged according to his own will after a one month hospital stay and expired peacefully with his family.
2. Cytopathologic findings
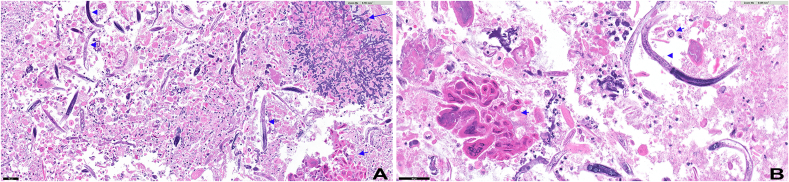
Our case showed a markedly necrotic background with numerous Strongyloides microorganisms, cells displaying herpes viral effect and abundant Aspergillus fungal organisms (Fig. 1). Herpes viral cytopathic effect such as multinucleation, chromatin margination and pale chromatin were identified (Fig. 4a and b). In addition, Strongyloides stercoralis organisms showed characteristic short buccal cavity and notched tail (Fig. 2, Fig. 3). Fungal organisms with morphologic features diagnostic of Aspergillus were also noted. These features included thin, septate, acute angle (45°) or dichotomous branching hyphae (Fig. 4a and b).
Fig. 1.
Low power representative image of bronchial wash. Multiple microorganisms were identified on the cell block with H&E stain including strongyloides (arrowhead), aspergillus (long blue arrow, bottom) and herpes simplex viral inclusions (short blue arrows). Bar = 50 um. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Fig. 4a Aspergillus showing acute angle branching with septated hyphae (long blue arrow) adjacent to cells displaying herpes viral cytopathic effect (short blue arrow) which include pale chromatin, multinucleation, and chromatin margination. Scattered strongyloides (arrowhead). Bar = 50 μm.
Fig. 4bHigh power shows herpes viral cytopathic effect (short blue arrow) and Strongyloides organisms (blue arrowhead). Bar = 50 μm.
Fig. 2.
Numerous strongyloides organisms are seen in a background of necrosis. Multiple microorganisms were identified on the cell block with H&E stain including strongyloides (arrowhead) and herpes simplex viral inclusions (short blue arrows). Bar = 50 um.
Fig. 3.
High power images of Strongyloides showing short buccal cavity and notched tail.
3. Discussion
Identification of Strongyloides, HSV and Aspergillus organisms in bronchoalveolar lavage specimens have been documented. To our knowledge, identification of these three organisms concurrently in a bronchial lavage in a non-immunosupressed patient has not been previously described in the literature.
Strongyloidiasis is caused by the nematode helminth Strongyloides stercoralis and is endemic in tropical and subtropical areas. In the United States, rates of infection are highest among residents of southeastern states and among individuals who have resided in endemic areas (including immigrants from tropical and subtropical regions, refugees, travelers and military personnel). The most common mode of transmission of strongyloidiasis is via skin contact via contaminated soil. The filiform larvae (the infective larval stage) of S. stercoralis is found in the soil or other materials contaminated with human stool. The filiform larvae penetrate the skin and migrate via the blood stream and lymphatics to the lungs, where they penetrate into the alveolar air sacs. The larvae then ascend the tracheobronchial tree and are swallowed. The larvae mature into adult worms that burrow into the mucosa of the duodenum and jejunum. Pathogenic adult females produce eggs from which noninfectious larvae (rhabditiform larvae) develop within the gastrointestinal tract. The rhabditiform larvae are then passed into the stool. A process called autoinfection can occur where the rhabditiform larvae become infective filariform larvae within the GI tract. Autoinfection is an important component of S. stercoralis life cycle, as it allows for infection to persist for decades. The filariform larvae evolved from eggs via parthenogenesis can penetrate the intestinal mucosa (internal autoinfection) or the perianal skin (external autoinfection) to compete the life cycle by migrating via the bloodstream and lymphatics to the lungs and then the intestine. In patients with a diminished cell mediated autoimmunity, hyperinfection with potentially fatal disseminated disease may develop as a consequence of autoinfection. The term “hyperinfection” is used to describe a massive increase in invasion of tissues by the larvae as a result of an increase in the autoinfection cycle. Detection of abundant larvae in the stool or sputum is indicative of hyperinfection. The term “dissemination” refers to the form of the disease in which worms are located in extraintestinal and extrapulmonary sites, sites outside the traditional life cycle. Mortality in disseminated Strongyloides is as high as 77% among compromised hosts. Thus, early detection of parasitic infection is important as disseminated Strongyloides is potentially fatal. Strongyloides is identified in sputum or bronchial material by its large size and is differentiated from other hookworms by its thin, notched tail and short, thick buccal cavity. The notching of the tail can be used to distinguish Strongyloides from hookworms, which have a pointed tail. S. stercoralis can be detected in BAL specimens by various staining methods including Gram, Gomori-Grocott methylamine silver (GMS), Pap and Diff Quik stains. The filariform larvae of Strongyloides are much longer and more slender than the rhabditoid larvae.
There have been a few cases reported in the literature of coinfection of Strongyloides with Aspergillus [[1], [2], [3], [4], [5]]; Wagonvoort et al., 1994). The underlying diseases in these patients included a patient with autoimmune pancreatitis who was treated with prednisone for six months and had a habit of eating inadequately cooked snails [2], a patient with Non Hodgkin lymphoma and COPD. These patients had reported pulmonary symptoms that ranged from hemoptysis, cough and pneumonia to progressive respiratory failure [4]. reported a case of an 82 year old Puerto Rican male
who had visited Puerto Rico four years prior to his illness. Interestingly [2], noted that a normal or reduced eosinophil count does not exclude a parasitic infection, as immunocompromised patients may present in this manner. A majority of these patients with co infection were immunosuppressed and were treated with a prolonged course of corticosteroids for underlying conditions (acute lymphoblastic leukemia, autoimmune pancreatitis). The cases of Strongyloides-Aspergillus coinfection arose in a steroid dependent patient with chronic obstructive pulmonary disease [5], an immunocompromised coal miner (Wagenvoort) and an immigrant from a developing country who had received prolonged courses of corticosteroids prior to admission [1]. A few patients presented with disseminated disease with stool exams showing Strongyloides and a skin biopsy showing intradermal Strongyloides.
Strongyloides pulmonary coinfection with other microorganisms have been documented. In a case report [6], reported a case of Strongyloides stercoralis coinfection with Pneumocystis jiroveci. Praharaj et al., 2014 documented pulmonary Strongyloides coinfection with Nocardia asteroides complex in a patient with autoimmune hemolytic anemia. Coinfection with Histoplasmosis (Kempe et al., 2014) has also been observed.
Strongyloides can result in a massive infection with multiplication of larvae and widespread hematogenous dissemination known as hyperinfection syndrome. Hyperinfection syndrome and disseminated infection occur in patients with impaired cell mediated immunity (patients receiving steroids or immunosuppressants, transplant patients or patients with human T cell lymphotrophic virus). Disseminated strongyloidiasis can involve organs outside the gut and the lungs, such as the liver, brain, heart and the urinary tract. Strongyloidiasis can occasionally be associated with translocation of gram negative bacteria across the gastrointestinal tract with the larvae serving as the carrier for the bacteria. Commonly associated gram negative bacteria include Escherichia coli and gram positive cocci such as Streptococcus bovis. In our case, the patient's BAL cultures were positive for Enterobacter and blood cultures were positive for Citrobacter.
In our case, both herpes virus and Aspergillus were identified along with Strongyloides. HSV-1 pneumonitis is very uncommon but can be seen in immunocompromised patients and may be due to direct extension from the upper airways secondary to tracheobronchitis. Cases have been reported in burn patients, surgical ICU patients, solid organ and hematopoietic cell transplant recipients, patients with malignancy, pregnant patients, and patients with HIV infection. HSV pneumonitis has been observed rarely in immunocompetent patients.
Sometimes it is hard to distinguish HSV colonization from HSV pneumonitis. As an example, although HSV detection from respiratory secretions in mechanically ventilated immunocompetent hosts has been reported, it is most often thought to represent viral contamination of the lower tract (eg, through instrumentation from the oropharynx or from local tracheobronchial viral reactivation without parenchymal involvement) rather than true bronchopneumonitis. However, true infection (rather than contamination) should be suspected in patients who have a clinical syndrome consistent with viral pneumonitis when diffuse ground glass opacities are seen on imaging, intranuclear inclusions are identified from cytologic examination of bronchoalveolar lavage fluid, and HSV-1 is isolated from culture.
Aspergillus species are ubiquitous in nature, and inhalation of infectious conidia is a frequent event. Tissue invasion is uncommon and occurs most frequently in the setting of immunosuppression associated with therapy for hematologic malignancies, hematopoietic cell transplantation, or solid organ transplantation [[7], [8], [9], [10]]. Although Aspergillus species are frequently inhaled into the airways, disease usually does not result because of effective conidial clearance. Culture isolation of Aspergillus species from the airway does not necessarily indicate disease because we inhale conidia constantly. Thus, the diagnosis of invasive aspergillosis is based upon both isolating the organism (or markers of the organism) and the probability that it is the cause of disease. The latter is a function of the host's risk factors for disease (eg, immune status) and the clinical presentation. A diagnosis of invasive aspergillosis is made by demonstrating hyphal elements invading tissues (from biopsy of any affected site, such as the lung or skin).
Aspergillus organisms observed in biopsy specimens are typically narrow (3–6 μm wide), septated hyaline hyphae with acute angle branching. However, several hyaline molds including Scedosporium spp and Fusarium spp have similar appearances to Aspergillus spp in histopathologic sections. The treatment of infections caused by these fungi may differ, so it is important to confirm genus and species by culture. Histopathologic examination can usually distinguish Aspergillus spp and the other fungi described above from the Mucorales, which appear as broad, nonseptate hyphae that exhibit right angle branching. Determining whether the fungus is a Mucorales is important because these molds are not susceptible to voriconazole, which is the treatment of choice for invasive aspergillosis. Our case showed the classic features of Aspergillus including the acute angle branching and septate hyphae.
In conclusion, we report the first case of a major triple infection consisting of Strongyloides, Aspergillus and herpes virus in a non-immunosuppressed patient. A heightened awareness of parasitic organisms, such as Strongyloides, in immunosuppressed patients with or without eosinophilia and hemoptysis from endemic regions is important. Early identification of parasitic organisms will result in prompt therapy and prevent disseminated Strongyloides, a potentially fatal complication. Due to its unique life cycle, Strongyloides is capable of infecting a host, until death ensues through a process of autoinfection, hyperinfection and dissemination. Physicians should think about the possibility of coinfection with additional microorganisms so appropriate additional therapies can be initiated.
Materials and methods
Bronchial wash and bronchoalveolar lavage specimens were processed at an outside hospital with cell block and H&E slide and the ThinPrep and cell blcok H&E slides were reviewed by the authors.
Declaration of competing interest
None.
Contributor Information
Terakeith Lertsburapa, Email: Terakeith.Lertsburapa@osumc.edu.
Rongqin Ren, Email: Rongqin.Ren@osumc.edu.
Rulong Shen, Email: Rulong.Shen@osumc.edu.
References
- 1.Shrestha Prakash. Hemoptysis in the immunocompromised patient: do not forget strongyloidiasis trop. Med. Infect. Dis. 2019;4(1):35. doi: 10.3390/tropicalmed4010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Jie. Coinfection of Strongyloides stercoralis and Aspergillus found in bronchoalveolar lavage fluid from a patient with stubborn pulmonary symptoms. J. Thorac. Dis. 2015;7(3):E43–E46. doi: 10.3978/j.issn.2072-1439.2014.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ting-ting Qu et al: A fatal strongyloides stercoralis hyperinfection syndrome in a patient with chronic kidney disease: a case report and literature review. Medicine 95(19):e3638; www.md-journal.comfile:///P:/strongyloides%20hyperinfection%20syndrome%20medi-95-e3638.pdf. [DOI] [PMC free article] [PubMed]
- 4.Bava A.J. A case report of pulmonary coinfection of Strongyloides stercoralis and Pneumocystis jiroveci. Asian Pac J Trop Biomed. 2011;1(4):334–336. doi: 10.1016/S2221-1691(11)60056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassalik Marco, Mönkemüller Klaus. Review: strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol. Hepatol. 2011;7(Issue 11):766–768. [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquemart C. Allergic bronchopulmonary aspergillosis associated with strongyloidiasis. Rev. Med. Liege. 2008;63:469–473. [PubMed] [Google Scholar]
- 7.Upadhyay D., Corbridge T., Jain M., Shah R. Pulmonary hyperinfection syndrome with S trongyloides stercoralis. Am. J. Med. 2001;110(2):167–169. doi: 10.1016/s0002-9343(01)00708-2. [DOI] [PubMed] [Google Scholar]
- 8.Tankanow & Eichenhorn: Disseminated strongyloides stercoralis and Aspergillus fumigatus presenting as diffuse interstitial pneumonitis in a steroid-dependent chronic obstructive pulmonary disease patient. Henry Ford Hosp. Med. J.—Vol 36, No 1, article 11 1988. [PubMed]
- 9.Bava A.J. A case report of pulmonary coinfection of Strongyloides stercoralis and Pneumocystis jiroveci. Asian Pacific J. Tropical Biomed. 2011:334–336. doi: 10.1016/S2221-1691(11)60056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pau Manisha. Clinico-epidemiological spectrum of strongyloidiasis in India: review of 166 cases. J. Fam. Med. Prim. Care. 2020;9(2):485–491. doi: 10.4103/jfmpc.jfmpc_1182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]