Abstract
Purpose
To describe the clinical course and microbial properties of the first two reported cases of nutritionally variant Streptococci (Granulicatella adiacens and Abiotrophia defectiva) endophthalmitis following intravitreal anti-vascular endothelial growth factor injection (IVI).
Observations
A 74 year-old female developed Granulicatella adiacens endophthalmitis following IVI. The patient underwent a pars plana vitrectomy and visual acuity recovered to 20/30 in six weeks. Similarly, an 88 year-old male developed Abiotrophia defectiva endophthalmitis after IVI. After a pars plana vitrectomy, the visual acuity recovered to 20/60 at five weeks.
Conclusions and Importance
Endophthalmitis due to Streptococcus species has traditionally resulted in uniformly poor visual outcomes. However, nutritionally variant Streptococci, now reclassified as Granulicatella and Abiotrophia species, appear to have a less aggressive clinical course and better visual acuity outcomes. To the authors’ knowledge, these are the first reports of nutritionally variant Streptococci following IVI related endophthalmitis.
Keywords: Endophthalmitis, Intravitreal injection, Anti-VEGF, Nutritionally variant streptococci, Granulicatella adiacens, Abiotrophia defectiva
1. Introduction
Infectious endophthalmitis following intravitreal anti-vascular endothelial growth factor injection (IVI) is a rare but potentially catastrophic complication. The reported incidence of endophthalmitis after IVI remains low, about 0.02% in retrospective reviews.1,2 Coagulase negative Staphylococcus and Streptococcus species are well recognized as the most common isolates, with generally poor visual outcomes associated with Streptococcus species.2, 3, 4 We report the first cases of nutritionally variant Streptococci (NVS) (Granulicatella adiacens and Abiotrophia defectiva) endophthalmitis after IVI injection. The visual acuity outcomes were better than expected for Streptococcus species.
2. Findings
See summarized findings in Table 1.
Table 1.
Clinical features of patients with nutritionally variant Streptococcus endophthalmitis following intravitreal anti-VEGF injection.
| Patient | Diagnosis | Causative Organism | Medication | Days to Presentation | Pre Injection VA | VA at Presentation | Final VA |
|---|---|---|---|---|---|---|---|
| 1a | AMD | Granulicatella adiacens | Aflibercept | 2 | 20/25 | HM | 20/30 |
| 2b | AMD | Abiotrophia defectiva | Aflibercept | 2 | 20/20 | LP | 20/60 |
Key: AMD = age related macular degeneration, VA = visual acuity, HM = hand motion, LP = light perception.
Patient 1 underwent vitreous tap/injection with intravitreal injection of vancomycin, ceftazidime, and triamcinolone acetonide at initial presentation, and pars plana vitrectomy with intravitreal triamcinolone acetonide 9 days after presentation.
Patient 2 underwent vitreous tap/injection with intravitreal injection of vancomycin and ceftazidime at initial presentation, and pars plana vitrectomy with intravitreal vancomycin and ceftazidime 6 days after presentation.
2.1. Case 1
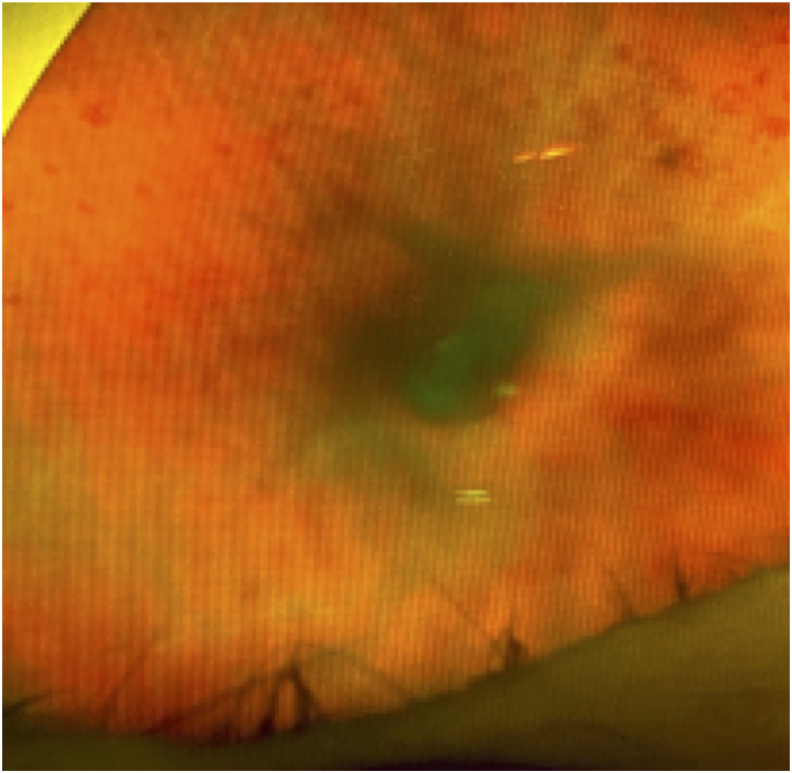
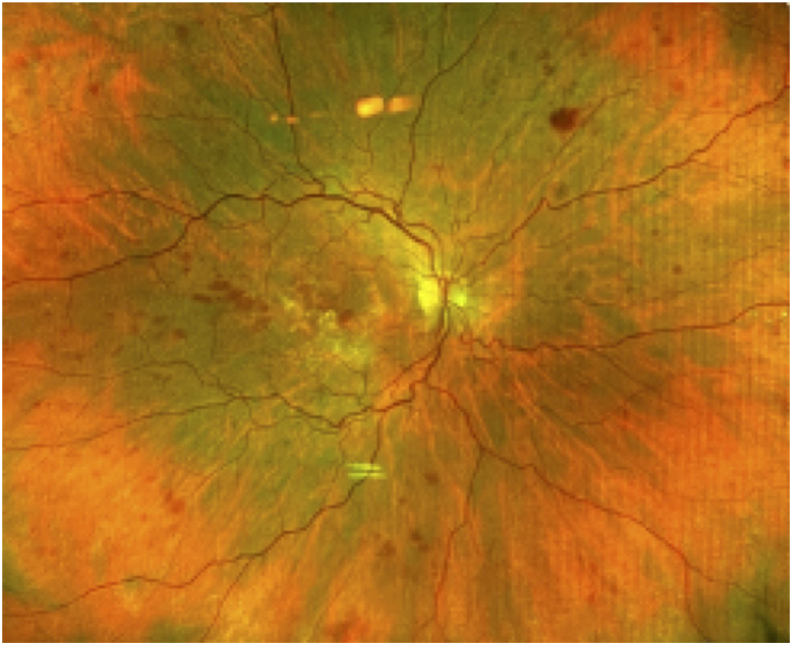
A healthy 74 year-old female with a history of neovascular age-related macular degeneration (AMD) who had undergone approximately 131 intravitreal anti-VEGF injections to both eyes received an intravitreal injection of aflibercept in the right eye for persistent subretinal fluid. During the injection, both the patient and physician wore a mask. The IVI injection technique consisted of inferotemporal subconjunctival lidocaine, followed by topical 5% povidone-iodine (PVI) eyelid scrubs and topical PVI to the conjunctiva fornix. After a lid speculum was placed, additional conjunctival PVI was applied, followed by a topical PVI-soaked pledget held on the injection site for 15 seconds prior to injection. The patient presented two days later with right eye pain, redness, and a decrease in vision. At presentation, best corrected visual acuity (BCVA) of the right eye was hand motion (decreased from 20/25) and examination revealed conjunctival injection, hypopyon, dense vitritis, and intraretinal hemorrhages (Fig. 1). The patient was diagnosed with endophthalmitis and underwent a vitreous tap and injection of intravitreal vancomycin (1 mg/0.1 mL), ceftazidime (2.25 mg/0.1 mL), and triamcinolone acetonide (0.40 mg/0.1 mL). No oral antibiotics were prescribed. Because of persistent vitreous opacities and inflammation, nine days after initial presentation the patient underwent pars plana vitrectomy with injection of triamcinolone. Six weeks after initial presentation, the BCVA improved to 20/30 with a marked reduction in intraretinal hemorrhages (Fig. 2). Final microbiology report of the original vitreous sample showed moderate growth of Granulicatella adiacens. Sensitivities were not performed.
Fig. 1.
Vitreous opacities and intraretinal hemorrhages on day of presentation with Granulicatella adiacens endophthalmitis after IVI.
Fig. 2.
Fundus photo 6 weeks after initial presentation demonstrating resolved vitritis following vitreous tap/inject of antibiotics and later vitrectomy for IVI endophthalmitis.
2.2. Case 2
An 88 year-old male with a history of neovascular AMD received an intravitreal injection of aflibercept in the left eye and presented two days later with pain, redness, and decrease in vision. The injection protocol consisted of the physician wearing a mask, application of topical proparacaine 0.5% drops, followed by instillation of PVI 5% and lidocaine 4% drops. The eyelids were swabbed with PVI 10% swabs. Supplemental lidocaine and PVI drops were applied and a 4% lidocaine-soaked cotton tip applicator was applied using pressure to the injection site on the sclera. A drop of topical moxifloxacin was applied immediately after the injection. A speculum was not used. At presentation, BCVA of the left eye was light perception (decreased from 20/20) and intraocular pressure was 13 mmHg. Examination showed conjunctival injection, corneal edema with keratic precipitates, hypopyon, fibrin, and dense vitritis with vitreous membranes on B-scan ultrasonography. The patient was diagnosed with endophthalmitis and underwent a vitreous tap and injection of intravitreal vancomycin (1 mg/0.1 mL) and ceftazidime (2.25 mg/0.1 mL). He was started on topical prednisolone every 2 hours and no oral antibiotics were prescribed. Due to non-improving status, 6 days after initial presentation the patient underwent a pars plana vitrectomy with repeat intravitreal vancomycin and ceftazidime that demonstrated vascular attenuation and widespread intraretinal hemorrhages. Five weeks after intravitreal treatment, the BCVA was 20/60. Eight months later at last follow up, the BCVA remained stable and the intraretinal hemorrhages were markedly improved, though not completely absent. Vitreous cultures from the original vitreous tap returned positive for Abiotrophia defectiva, susceptible to vancomycin (MIC < 0.50), ceftriaxone (MIC < 0.75), and cefuroxime (MIC < 0.50); and resistant to benzylpenicillin (MIC > 0.25), and levofloxacin. (MIC > 3).
3. Discussion
The first two cases of NVS endophthalmitis after IVI are described in this report. Infectious endophthalmitis following IVI is uncommon, with a rate of 0.038%–0.056% in large meta-analyses and approximately 0.02% in retrospective reviews.1, 2, 3,5 Coagulase negative Staphylococcus and Streptococcus species have been well recognized as the most common causative organisms, with poor outcomes in eyes infected with Streptococcus species.1, 2, 3,6 McCannel also reported higher rates of Streptococcus species endophthalmitis after IVI compared to other intraocular surgeries, hypothesizing that dispersion of aerosolized moisture droplets from the upper respiratory tract could result in contamination of the injection field.5
NVS have long been known to be normal inhabitants of the human oropharynx, urogenital, and gastrointestinal tracts, and are recognized causes of bacterial endocarditis. Based on genetic characteristics, the NVS were re-classified into two genera Granulicatella and Abiotrophia and four different species, two of which are Granulicatella adiacens and Abiotrophia defectiva.7 In the ophthalmic literature, Granulicatella adiacens has been implicated in chronic dacryocystitis and post-traumatic orbital abscess, while Abiotrophia defectiva has been reported in cases of infectious keratitis and in bleb-associated, Ozudex-associated, and post cataract extraction endophthalmitis.8, 9, 10, 11, 12, 13
To the authors’ knowledge, there have been no cases of NVS endophthalmitis reported after IVI. In the current literature, visual outcomes cases of Abiotrophia defectiva associated endophthalmitis have been poor with visual acuities generally worse than 20/100.8, 9 However, the two patients in this series both achieved visual acuities better than or equal to 20/60 at last follow up, suggesting that NVS may be less virulent than other Streptococcus species. It is possible that a pars plana vitrectomy played a role in the improved outcome in these two cases, such that early vitrectomy may be considered in NVS associated endophthalmitis.
4. Conclusions
In summary, the first two cases of NVS (Granulicatella adiacens and Abiotrophia defectiva) endophthalmitis following IVI are presented in this report. The authors propose dispersion of aerosolized NVS droplets from the oropharynx may have contaminated the injection field and that a firmly taped mask on both the patient and physician may reduce the risk of NVS associated endophthalmitis after IVI. Following standard diagnostic and clinical management, outcomes of NVS associated endophthalmitis may be better than expected for Streptococcus species.
Patient consent
Written consent was obtained to publish case details.
Funding
This study was supported in part by an unrestricted grant from Research to Prevent Blindness (New York, New York) and NIH Center Core Grant P30EY014801 (Bethesda, Maryland). The sponsor or funding organization had no role in the design or conduct of this study.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The following authors have no financial disclosures: RS, CS, DPR, HLB, JDS, DM, HWF.
References
- 1.Moshfeghi A.A., Rosenfeld P.J., Flynn H.W., Jr. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31(4):662–668. doi: 10.1097/IAE.0b013e31821067c4. [DOI] [PubMed] [Google Scholar]
- 2.Yannuzzi N.A., Gregori N.Z., Rosenfeld P.J. Endophthalmitis associated with intravitreal injections of anti-VEGF agents at a tertiary referral center: in-house and referred cases. Ophthalmic Surg Lasers Imaging Retina. 2018;49(5):313–319. doi: 10.3928/23258160-20180501-04. [DOI] [PubMed] [Google Scholar]
- 3.Fileta J.B., Scott I.U., Flynn H.W., Jr. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):143–149. doi: 10.3928/23258160-20140306-08. [DOI] [PubMed] [Google Scholar]
- 4.Xu K., Chin E.K., Bennett S.R. Endophthalmitis after intravitreal injection of vascular endothelial growth factor inhibitors: management and visual outcomes. Ophthalmology. 2018;125(8):1279–1286. doi: 10.1016/j.ophtha.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 5.McCannel C.A. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654–661. doi: 10.1097/IAE.0b013e31820a67e4. [DOI] [PubMed] [Google Scholar]
- 6.Chen E., Lin M.Y., Cox J., Brown D.M. Endophthalmitis after intravitreal injection: the importance of viridans streptococci. Retina. 2011;31(8):1525–1533. doi: 10.1097/IAE.0b013e318221594a. [DOI] [PubMed] [Google Scholar]
- 7.Alberti M.O., Hindler J.A., Humphries R.M. Antimicrobial susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegans. Antimicrob Agents Chemother. 2015;60(3):1411–1420. doi: 10.1128/AAC.02645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Inigo Y.J., Klein K., Reddy R.K. Case report of Abiotrophia defectiva endophthalmitis after repeated injections of dexamethasone intravitreal implant (ozurdex) Retin Cases Brief Rep. 2019 Aug 29 doi: 10.1097/ICB.0000000000000925. Epub ahead of print. PMID: 31479011. [DOI] [PubMed] [Google Scholar]
- 9.Horstkotte M.A., Dobinsky S., Rohde H. Abiotrophia defectiva endophthalmitis with retinal involvement and infiltrative keratitis: case report and review of the literature. Eur J Clin Microbiol Infect Dis. 2010;29(6):727–731. doi: 10.1007/s10096-010-0901-7. [DOI] [PubMed] [Google Scholar]
- 10.Hugo Lee M.H., Lawlor M., Lee A.J. Abiotrophia defectiva bleb-associated endophthalmitis confirmed with 16s ribosomal RNA sequencing. J Glaucoma. 2015;24(1):87–88. doi: 10.1097/IJG.0b013e3182953ad9. [DOI] [PubMed] [Google Scholar]
- 11.Ku C.A., Forcina B., LaSala P.R., Nguyen J. Granulicatella adiacens, an unusual causative agent in chronic dacryocystitis. J Ophthalmic Inflamm Infect. 2015;5:12. doi: 10.1186/s12348-015-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namdari H., Kintner K., Jackson B.A. Abiotrophia species as a cause of endophthalmitis following cataract extraction. J Clin Microbiol. 1999;37(5):1564–1566. doi: 10.1128/jcm.37.5.1564-1566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teo L., Looi A., Seah L.L. An unusual causative agent for an orbital abscess: Granulicatella adiacens. Orbit. 2011;30(3):162–164. doi: 10.3109/01676830.2011.569631. [DOI] [PubMed] [Google Scholar]