Abstract
Objective
To compare non-tuberculosis (non-TB)-cause mortality risk overall and cause-specific mortality risks within the immigrant population of British Columbia (BC) with and without TB diagnosis through time-dependent Cox regressions.
Methods
All people immigrating to BC during 1985–2015 (N = 1,030,873) were included with n = 2435 TB patients, and the remaining as non-TB controls. Outcomes were time-to-mortality for all non-TB causes, respiratory diseases, cardiovascular diseases, cancers, and injuries/poisonings, and were ascertained using ICD-coded vital statistics data. Cox regressions were used, with a time-varying exposure variable for TB diagnosis.
Results
The non-TB-cause mortality hazard ratio (HR) was 4.01 (95% CI 3.57–4.51) with covariate-adjusted HR of 1.69 (95% CI 1.50–1.91). Cause-specific covariate-adjusted mortality risk was elevated for respiratory diseases (aHR = 2.96; 95% CI 2.18–4.00), cardiovascular diseases (aHR = 1.63; 95% CI 1.32–2.02), cancers (aHR = 1.40; 95% CI 1.13–1.75), and injuries/poisonings (aHR = 1.85; 95% CI 1.25–2.72).
Conclusions
In any given year, if an immigrant to BC was diagnosed with TB, their risk of non-TB mortality was 69% higher than if they were not diagnosed with TB. Healthcare providers should consider multiple potential threats to the long-term health of TB patients during and after TB treatment. TB guidelines in high-income settings should address TB survivor health.
Electronic supplementary material
The online version of this article (10.17269/s41997-020-00345-y) contains supplementary material, which is available to authorized users.
Keywords: Post-tuberculosis, British Columbia, Canada, Mortality, Cohort studies, Administrative data, Survival analysis
Résumé
Objectif
Au moyen de régressions de Cox avec une covariable temporalisée, comparer le risque global de mortalité non due à la tuberculose et les risques de mortalité par cause au sein de la population immigrante de la Colombie-Britannique (C.-B.) avec et sans diagnostic de tuberculose.
Méthode
Toutes les personnes ayant immigré en C.-B. entre 1985 et 2015 (N = 1 030 873) ont été incluses, dont n = 2 435 patients tuberculeux, le reste étant des témoins non tuberculeux. Nos résultats incluaient le temps jusqu’à la mortalité de toute cause autre que la tuberculose, soit les maladies respiratoires, les maladies cardiovasculaires, les cancers et les blessures/empoisonnements, déterminé à l’aide des statistiques de l’état civil codées selon la CIM. Nous avons utilisé des régressions de Cox avec une variable d’exposition temporalisée pour le diagnostic de tuberculose.
Résultats
Le coefficient de danger (CD) de mortalité non due à la tuberculose était de 4,01 (IC de 95 % : 3,57-4,51) avec un CD ajusté selon la covariable de 1,69 (IC de 95 % : 1,50-1,91). Le risque de mortalité par cause ajusté selon la covariable était élevé pour : les maladies respiratoires (CDa = 2,96; IC de 95 % : 2,18-4,00), les maladies cardiovasculaires (CDa = 1,63; IC de 95 % : 1,32-2,02), les cancers (CDa = 1,40; IC de 95 % : 1,13-1,75) et les blessures/empoisonnements (CDa = 1,85; IC de 95 % : 1,25-2,72).
Conclusions
Chaque année, si une personne ayant immigré en C.-B. avait un diagnostic de tuberculose, son risque de mortalité non due à la tuberculose était supérieur de 69 % à celui d’une personne sans diagnostic de tuberculose. Les professionnels de santé devraient tenir compte des multiples menaces possibles à la santé à long terme de leurs patients tuberculeux pendant et après le traitement de la tuberculose. Les lignes directrices sur la tuberculose dans les milieux à revenu élevé devraient tenir compte de la santé des survivants de la tuberculose.
Mots-clés: Post-tuberculose, Colombie-Britannique, Canada, mortalité, études de cohortes, données administratives, analyse de survie
Introduction
In Canada, tuberculosis (TB) is primarily diagnosed among people who have immigrated from medium/high-TB-incidence countries (Ronald et al. 2018). In British Columbia (BC), a province of Canada, the percentage of incident TB occurring among people born outside of Canada (85%) is higher than that reported nationally (75%) (BC Centre for Disease Control 2018; Vachon et al. 2018). TB patients have a higher prevalence of alcohol and substance use (Simou et al. 2018), mental health disorders (Bender et al. 2012), diabetes (Workneh et al. 2017), HIV (Pettit et al. 2018), and exposure to poverty and related social determinants of health (Lix et al. 2018; Peltzer 2018; Rasanathan et al. 2011). While TB itself is potentially fatal, leading to 1.7 million deaths annually worldwide (World Health Organization (WHO) 2019), it may also act as a marker for increased risk of mortality from causes other than TB after successful completion of TB treatment. These factors may include social determinants of health for which Canadian TB programs have been criticized as neglecting among the Canadian immigrant population (Reitmanova and Gustafson 2012). To date, no Canadian study of post-tuberculosis mortality risk exists (Romanowski et al. 2019).
This study aimed to compare non-TB mortality risks between those diagnosed with TB and those not diagnosed with TB among persons immigrating to Canada and residing in BC using time-dependent Cox regressions. We studied the immigrant population of BC as this subpopulation makes up the vast majority of people diagnosed with TB in BC (Halverson et al. 2014). Moreover, there are important differences in TB epidemiology and in mortality risk between persons born outside of Canada and Canadian-born populations (Desmeules et al. 2005; Moniruzzaman 2010). We hypothesized higher mortality risk among immigrants diagnosed with TB compared with immigrants not diagnosed with TB (Romanowski et al. 2019).
Methods
Study design, population, setting, and analytic sample
This study used a retrospective cohort study design. Our study population was all persons born outside of Canada and immigrating to Canada from January 1, 1985 to December 31, 2012, and residing in BC from January 1, 1985 to December 31, 2015 (Appendix Figure 1). Data were derived from Population Data BC, a multiuniversity health data resource (Population Data BC 2019b). The Immigration, Refugee, and Citizenship Canada (IRCC) Permanent Residents database was used to include members of this population in our analytic sample (Immigration, Refugees and Citizenship Canada 2015; Ronald et al. 2016, 2018, 2019). Exclusion criteria included invalid dates for TB diagnosis or death (i.e., before January 1, 1985), diagnosis of TB without documented TB treatment completion (e.g., death during treatment), and missing value for any covariate (< 5% of cohort). Our primary outcome measure was post-TB treatment mortality from a non-TB cause. TB causes included ICD-9-CM codes 011-018 and ICD-10-CA codes A15-A19.
Mortality was ascertained from BC’s Vital Statistics Agency’s death certificates (BC Vital Statistics Agency 2018). Data on tuberculosis diagnosis and treatment were obtained from the BC Centre for Disease Control (BCCDC) TB Registry (BC Centre for Disease Control 2019; Ronald et al. 2018). Data on comorbidities were obtained through data from the hospital discharge abstracts database (DAD) and Medical Services Plan (MSP) Payment Information File for BC, and assembled using published algorithms (BC Ministry of Health 2018b; Canadian Institute for Health Information 2018; Manitoba Centre for Health Policy 2019; Quan et al. 2005). Data on demographic, geographic, and socio-economic factors were obtained from the IRCC Permanent Residents database, MSP Consolidation Files, and Canadian Census files (BC Ministry of Health 2018a; Immigration, Refugees and Citizenship Canada 2015; Ronald et al. 2018). This study was approved by the University of British Columbia’s Clinical Research Ethics Board (H16-00265). Data linkage was conducted using scrambled unique identifiers (Population Data BC 2019a).
Variables
Outcome
The primary outcome of interest was time from index date to date of non-TB-caused death measured in person-years. Secondary outcomes were time from index date to date of cause-specific death measured in person-years. The index date for this study was the date residency in BC was established, defined as (a) 90 days before MSP coverage start (n = 1,076,627; 99.45%) or (b) first healthcare contact in BC (n = 6008; 0.55%), whichever came first. The censoring date was defined as the first of death, discontinuation of MSP coverage, or December 31, 2015. Primary causes of death were coded by the ICD-9-CM or ICD-10-CA recorded on death certificates. We grouped the primary causes of death into the following categories: all non-TB causes (ICD-9-CM: all codes other than 011x-018x; ICD-10-CA: all codes other than A15x-A19x), cardiovascular diseases (ICD-9-CM: 39x-45x; ICD-10-CA: Ix), respiratory diseases (ICD-9-CM: 46x-51x; ICD-10-CA: Jx), cancers (ICD-9-CM: 14x-23x; ICD-10-CA: Cx, D1x, D2x, D3x, D4x), and injuries/poisonings (ICD-9-CM: Ex; ICD-10-CA: Sx, Tx, Vx, Wx, Xx, Yx).
Exposure
The exposure of interest was diagnosis with TB during residency in BC. BCCDC TB Registry diagnosis, treatment, and laboratory files were used to develop a TB definition based on Canadian TB Standards (Alvarez et al. 2014). Both clinically diagnosed and microbiologically confirmed cases were included in our TB definition (Ronald et al. 2018). Post-mortem TB diagnoses were removed from the TB exposure definition. We included people diagnosed with TB who had documented treatment completion to be consistent with the literature in this field (Romanowski et al. 2019) and included those without treatment completion in sensitivity analyses to test the robustness of our main analysis to this exclusion. Participants were classified as completing TB treatment when a value of “completed” was present in the BCCDC TB Registry treatment dataset’s “Reason Treatment Ended Code Description” variable. A participant would have been classified as “completed” in the TB Registry if they received an adequate regimen of anti-TB therapy as defined by a TB physician at the BCCDC. This would require at least six months of standard short course anti-TB therapy. We used a time-varying exposure variable for this analysis in which exposed time began at TB diagnosis date and continued until a censor or event date was reached. Unexposed time for those diagnosed with TB was time contributed from index date up to TB diagnosis date. We only considered the first diagnosis of TB; time thereafter was exposed time. The unit of time used in this analysis was “year”.
Cohort characteristics (covariates)
Cohort members were described in terms of age, sex, TB incidence rate per 100,000 population in country of origin (< 100, 100 to < 200, 200 to < 300, ≥ 300), neighbourhood income quintile, immigration class (economic, family, refugee, other), educational qualification (none/unknown, secondary or less, trade/diploma, or university degree), number of comorbidities in year prior to TB diagnosis or reference date (weighted Charlson Comorbidity Index: 17 conditions weighted from 1 to 6; continuous measure) (Charlson et al. 1987; Manitoba Centre for Health Policy 2019; Quan et al. 2005), and year of index (0: 1985 to 30: 2015; continuous measure), which is the year BC residency began.
Statistical analysis
We compared cohort characteristics between TB and non-TB groups by the use of standardized mean difference (SMD) (Zanuto 2006). We compared categorical cohort characteristics between people experiencing the event (non-TB mortality) and those censored using univariable Cox regressions of time-to-non-TB mortality.
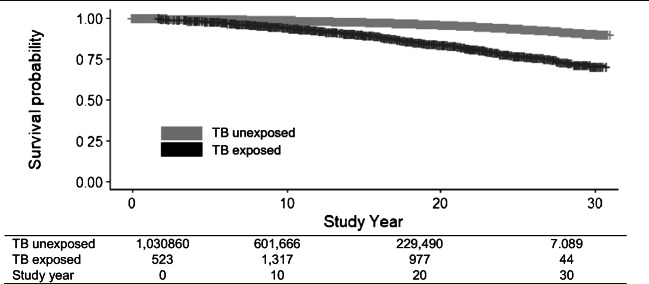
We plotted an extended Kaplan-Meier (KM) curve for non-TB mortality stratified by a time-varying indicator of TB diagnosis, using the date of residency as index date, and date of diagnosis as beginning of TB exposed time, to visually display the time-varying effect of TB diagnosis on non-TB mortality risk over the study period (Snapinn et al. 2005). We used time-dependent Cox regressions to compare time-to-death from date of residency between TB-exposed time (time from diagnosis with TB in BC until event or censoring date) and TB unexposed time (time from residency in BC up to TB diagnosis date for those who developed TB, plus time from residency in BC to event or censoring date for those not diagnosed with TB) through hazard ratios (HRs) for risk of non-TB mortality (Therneau and Grambsch 2000).
Unlike group-based Cox proportional hazards regression, which classifies people as exposed or unexposed for all person-time, and was used in a previous study of mortality among TB survivors in a low-incidence, high-income setting (Miller et al. 2015), time-dependent Cox regression compares person-time under exposure versus person-time under no exposure. The resulting HR estimates for TB diagnosis are interpretable as the ratio of the hazard of mortality for a person with a TB diagnosis versus that for a person without a TB diagnosis (but who may be diagnosed later) in any given year (Kleinbaum and Klein 2010). This modeling technique is superior to group-based analyses as it allows individuals to have a common index date (immigration to BC) and to contribute both exposed and unexposed time to the analysis (Therneau and Grambsch 2000), avoiding potential pitfalls due to immortal time bias (Lévesque et al. 2010; Suissa 2008; Zhou et al. 2005).
We conducted unadjusted time-dependent Cox regressions, then adjusted for age and sex, and then adjusted for covariates that were likely to confound the relationship between TB and mortality risk (Romanowski et al. 2019). When constructing the covariate-adjusted model, we created a series of directed acyclic graphs (DAGs) through an iterative process (Glymour 2006). After this DAG process, we assessed each covariate deemed valuable to our analysis based on association with the outcome through univariable Cox regressions at α = 0.50 for consideration in the multivariable model (main analysis) (Steyerberg 2019). When the full model was fit to the data, we used backward selection based on Akaike’s Information Criterion (AIC) to remove covariates, with decisions based on clinical and statistical considerations (Therneau and Grambsch 2000).
Sensitivity and subgroup analyses
A series of sensitivity analyses were performed. First, we included all persons diagnosed with TB, regardless of TB treatment outcome. Second, we required a minimum two-year observation window (alive and in BC) around the TB diagnosis or randomly selected reference date (one year before and one year after). Third, we restricted the main analysis to ages 18 to 60 years at index date and required treatment completion among those diagnosed with TB. Fourth, we required persons diagnosed with TB to have completed treatment and to have a minimum two-year observation window (alive and in BC) around the TB diagnosis or randomly selected reference date (one year before and one year after). Fifth, we switched from the Charlson Comorbidity Index to the Elixhauser Comorbidity Index, which contains 30 comorbidities, and was used to control for comorbidities instead (Elixhauser et al. 1998; Quan et al. 2005).
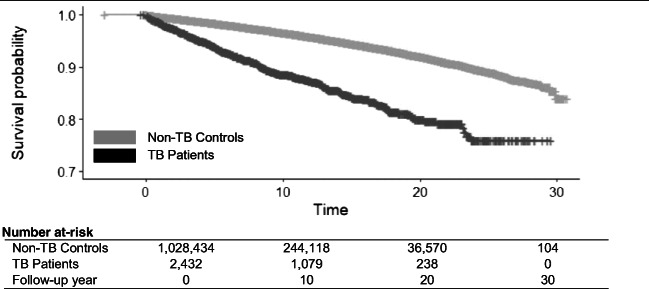
For subgroup analysis, we analyzed data for males and females separately, requiring treatment completion among those diagnosed with TB. We also conducted an effect modification analysis by immigration class. Finally, to examine the relationship between TB treatment completion and time-to-mortality from non-TB causes, we generated a group-based (standard) KM curve stratified by TB patients and non-TB controls using date of TB treatment completion and a randomly selected reference date, respectively, as index dates.
All analyses were conducted in Base SAS software v.9.4 and R v.3.4.4.
Results
Analytic sample
A total of 1,030,873 people immigrating to BC during 1985–2015 were included in our analytic sample (follow-up, 13.5 million person-years), with 2435 people diagnosed with TB in BC with documented treatment completion (Appendix Figure 1). The remaining 1,028,438 people were not diagnosed with TB in BC. A total of 26,376 deaths occurred, with 285 deaths among TB patients and 24,887 deaths among non-TB controls. A total of 51,762 people (4.8%) were excluded from the analytic sample (Appendix Figure 1).
Descriptive analysis
The study sample was evenly distributed by sex (Appendix Table 1). People diagnosed with TB were generally older, had higher levels of comorbidity, had lower socio-economic status, were more frequently born in countries with the highest TB incidence, and tended to have earlier index dates than those without TB. Substantial differences between TB patients and non-TB controls were observed for most cohort characteristics. The most substantial differences were in mean Charlson Comorbidity Index score, TB incidence rate in the country of origin, with a smaller difference in mean index year, immigration class, and age at index. There were proportionately fewer TB diagnosed persons in the highest income and highest education categories than non-TB diagnosed controls.
All measured cohort characteristics had significant association with the outcome of non-TB death in univariable Cox regressions (Table 1). Age and Charlson Comorbidity Index were most strongly associated with non-TB mortality risk. A higher proportion of persons who died immigrated under family class (64.6%) than those who were censored (Table 1).
Table 1.
Cohort characteristics among migrants to British Columbia, Canada, 1985–2015: comparison of people who died of non-TB causes (event) with those censored
| Event n (%) | Censored n (%) | Crude HRa | pa | |
|---|---|---|---|---|
| Total cohort (N) | 24,887 | 1,005,986 | ||
| Tuberculosis patients | 285 (1.1) | 2150 (0.2) | 3.06 | < 0.01 |
| Male | 13,389 (53.8) | 487,144 (48.4) | 1.28 | < 0.01 |
| Age at index in years (mean (SD)) | 57.92 (17.51) | 30.11 (16.14) | 1.10 | < 0.01 |
| Charlson Comorbidity Index (mean (SD))b | 0.81 (1.67) | 0.13 (0.50) | 1.52 | < 0.01 |
| Neighbourhood income quintile | < 0.01 | |||
| Highest 20% | 3413 (13.7) | 146,827 (14.6) | 1 (ref) | |
| Middle-high 20% | 3624 (14.6) | 144,031 (14.3) | 1.07 | |
| Middle 20% | 4534 (18.2) | 183,237 (18.2) | 1.04 | |
| Low-middle 20% | 6219 (25.0) | 230,383 (22.9) | 1.09 | |
| Lowest 20% | 7097 (28.5) | 301,508 (30.0) | 0.99 | |
| Educational qualification | < 0.01 | |||
| None/unknown | 3532 (14.2) | 129,308 (12.9) | 1 (ref) | |
| Secondary or less | 13,756 (55.3) | 427,478 (42.5) | 0.95 | |
| Trade/diploma | 4318 (17.4) | 184,562 (18.3) | 0.74 | |
| University degree | 3281 (13.2) | 264,638 (26.3) | 0.57 | |
| Immigration class | < 0.01 | |||
| Economic | 4897 (19.7) | 605,414 (60.2) | 1 (ref) | |
| Family | 16,072 (64.6) | 295,100 (29.3) | 5.27 | |
| Refugee | 1499 (6.0) | 79,550 (7.9) | 1.59 | |
| Other | 2419 (9.7) | 25,922 (2.6) | 8.18 | |
| TB incidence in country of birth | < 0.01 | |||
| < 100 per 100,000 population | 8699 (35.0) | 422,089 (42.0) | 1 (ref) | |
| 100 to < 200 per 100,000 population | 8815 (35.4) | 325,569 (32.4) | 0.99 | |
| 200 to < 300 per 100,000 population | 5322 (21.4) | 141,871 (14.1) | 1.22 | |
| 300+ per 100,000 population | 2051 (8.2) | 116,457 (11.6) | 0.69 | |
| Index year (mean (SD))c | 8.58 (5.88) | 14.85 (7.12) | 0.97 | < 0.01 |
HR, crude hazard ratio; p, p value; SD, standard deviation; TB, tuberculosis
aCalculated from univariable Cox proportional hazards regressions for association between time-to-non-TB mortality and each cohort characteristic. bWeighted Charlson comorbidity score calculated in the year prior to TB diagnosis or randomly selected reference date for non-TB controls using both physician claims data and hospital discharge abstracts data (all diagnosis fields).cIndex year is calculated as 0 = 1985 to 30 = 2015 (continuous) and represents the year residency in BC was established
Survival analysis
Unadjusted analyses indicated fourfold higher (HR = 4.01; 95% CI 3.57–4.51) mortality risk for TB patients compared with non-TB controls (Table 2). Age/sex-adjustments attenuated the HR to twofold (aHR = 1.95; 95% CI 1.74–2.20). Covariate-adjustment further attenuated the HR (aHR = 1.69; 95% CI 1.50–1.91), while remaining statistically significant. KM curves showed an increasing difference in survival over the study period between TB exposed and TB unexposed time (Fig. 1). In any given year from date of immigration to BC, if an immigrant to BC was diagnosed with TB, their risk of non-TB mortality was 69% higher than it would be if they were not diagnosed with TB: 196% higher for respiratory mortality, 63% higher for cardiovascular mortality, 40% higher for cancer mortality, and 85% higher for injury or poisoning mortality (Table 2).
Table 2.
Time-dependent Cox regression analyses of mortality risk among migrants to British Columbia, Canada, 1985–2015: tuberculosis (exposed) time compared with non-tuberculosis (unexposed) time
| Primary cause of mortality | Crude HR (95% CI) | Age/sex-adjusted HR (95% CI) | Covariate-adjusted HR (95% CI)a |
|---|---|---|---|
| All non-TB diseases | 4.01 (3.57–4.51) | 1.95 (1.74–2.20) | 1.69 (1.50–1.91) |
| Respiratory diseases | 8.51 (6.31–11.50) | 3.28 (2.43–4.43) | 2.96 (2.18–4.00) |
| Cardiovascular diseases | 4.26 (3.44–5.27) | 1.78 (1.44–2.20) | 1.63 (1.32–2.02) |
| Cancers | 3.30 (2.65–4.10) | 1.76 (1.41–2.19) | 1.40 (1.13–1.75) |
| Injuries and poisonings | 3.43 (2.33–5.06) | 2.28 (1.55–3.36) | 1.85 (1.25–2.72) |
CI, confidence interval; HR, hazard ratio; TB, tuberculosis
aCovariate-adjusted analyses included the following baseline variables: age, sex, neighbourhood income quintile, educational qualification, index year, TB incidence in country of birth, and weighted Charlson comorbidity score calculated in the year prior to TB diagnosis or randomly selected reference date for non-TB controls
Fig. 1.
Extended Kaplan-Meier survival curve from date of residency in British Columbia (BC) to date of death from a non-TB cause among migrants to BC, Canada, 1985–2015. TB tuberculosis
The analysis using KM plots with index time set to treatment completion date (or randomly selected reference date for non-TB controls) displayed immediate divergence in the probability of death between TB patients and non-TB controls at the end of TB treatment, among those successfully completing treatment (Fig. 2).
Fig. 2.
Kaplan-Meier survival curve calculated from date of TB treatment completion for TB patients and randomly selected reference date for non-TB controls: all non-TB causes of death among migrants to BC, Canada, 1985–2015. TB tuberculosis. Notes: time means person-years from TB treatment completion date (TB patients) or randomly selected reference date (non-TB controls) to event/censoring date
Sensitivity and subgroup analyses
Sex-specific subgroup analyses showed a higher overall HR for non-TB causes in males than in females, as well as for each cause of death, particularly for injury/poisoning (Appendix Table 2). Sensitivity analyses showed that the covariate-adjusted HR was 36% higher when those not completing TB treatment were included in the analysis (aHR = 2.05; Appendix Table 3). This difference was reduced when we required TB patients and non-TB controls to be alive 1 year pre-/post-diagnosis or reference date. In sensitivity analyses involving TB patients completing treatment, the covariate-adjusted HR did not differ substantially from the main analysis finding (Appendix Table 3). We found evidence of modification of the effect of TB diagnosis by immigration class. Immigrants under the refugee class experienced less of an effect on mortality risk due to a TB diagnosis (aHR = 1.4) compared with economic (aHR = 2.4), family (aHR = 2.8), or other (aHR = 2.5) immigration classes.
Discussion
This is the first Canadian study to examine long-term mortality risk among TB patients compared with non-TB controls. Moreover, this is the first study to examine mortality exclusively among migrants with and without TB. Across causes of death, we found a significantly increased risk of mortality among TB patients compared with people not diagnosed with TB. In addition, we noted a disproportionate number of respiratory deaths among TB patients with close to triple the respiratory mortality risk in any given year during follow-up. This discrepancy seems biologically plausible given that the majority of TB is respiratory, and may impact long-term pulmonary function (Allwood et al. 2019; Byrne et al. 2015). Moreover, smoking is more prevalent among people affected by TB and may contribute to higher mortality from respiratory diseases (Bates et al. 2007). Respiratory mortality is likely more directly attributable to pulmonary TB, while injury and poisoning mortality, which had the second-highest HR, is less clearly attributable to TB and may be mediated through the above-noted health determinants. Similarly, cancer mortality and cardiovascular mortality may be related to the prevalence of tobacco smoking among persons diagnosed with TB. However, we cannot rule out systemic inflammation as an additional driver of cardiovascular mortality in TB patients.
This is the first study to demonstrate effect modification of TB diagnosis on mortality hazard by immigration class. We demonstrated that people immigrating under the refugee classification did not experience as large an effect of TB on mortality risk as those immigrating under economic, family, or other classes. This finding reinforces the possibility that it is factors for which TB is a marker, rather than TB itself, that is driving the “effect” of TB on mortality. As a corollary, the higher aHR for a diagnosis of TB within economic, family, and other immigrant classes may also be interpreted as TB being a stronger marker of such factors for mortality risk, increasing the importance of post-TB treatment follow-up care among non-refugee immigrant classes.
Our findings signal that TB may function as either a marker or causal risk factor for various forms of morbidity and mortality (Blondal et al. 2013; Christensen et al. 2014; Datta and Evans 2019). Elevated prevalence of poverty, depression, alcohol, tobacco, and substance use among persons diagnosed with TB likely play roles in the relationship between TB and mortality risk (Bates et al. 2007; Rasanathan et al. 2011; Simou et al. 2018; Sweetland et al. 2017). Current TB care guidelines do not address long-term TB patient health, focusing entirely on successful TB treatment (Alvarez et al. 2014; Nahid et al. 2016). However, in HIV medicine, guidelines have embraced total patient health and involve complete patient histories, chronic disease screening, mental health supports, and other standards of care not generally applied in TB (Basham et al. 2019). Evidence of unmet post-TB treatment health needs that may best be flagged during TB care suggests a need for linkage to other services before TB treatment completion, with intervention research and guideline development also indicated (Wanner et al. 2018). As a low-barrier public health service, TB care reaches many populations that are generally underserved, making TB programs a potential hub for service integration among this often vulnerable patient population.
Several cohort studies have examined excess death in persons diagnosed with TB (Blondal et al. 2013; Christensen et al. 2014; Shuldiner et al. 2016). A recent systematic review by Romanowski et al. found a pooled standardized mortality ratio for persons treated for TB compared with the general population of 2.91 (95% CI 2.21–3.84) (Romanowski et al. 2019). A review by Byrne et al. of respiratory disease in TB patients compared with non-TB controls showed a pooled OR of 3.01 (95% CI 2.42–3.85). The studies included in the review by Romanowski et al. primarily used age and sex-specific rates to compute indirectly standardized mortality ratios or used age/sex-matched comparison groups. However, one study adjusted for covariates beyond age and sex (Miller et al. 2015), while another employed sibling controls (Christensen et al. 2014). Because we confined our analysis to persons born outside Canada (migrants), adjusted for more confounders, and used a more extended follow-up period, we cannot directly compare our results with any of the studies reviewed by Romanowski et al. However, our conclusions are similar.
Strengths and limitations
This study has important strengths, including use of a long follow-up period (almost 3 decades); virtually complete capture of the entire immigrant population of BC, including a large number of controls; a modern statistical modeling strategy, employing DAGs, extended Cox regressions to overcome potential immortal time bias; population-based, legislated, data sources for outcome, exposure, and covariate ascertainment; and multiple sensitivity and subgroup analyses.
As with any observational study, we can only demonstrate that TB is correlated with increased mortality risk and cannot make a causal interpretation. Moreover, observational administrative data are limited to variables collected in the provision of healthcare and other services. The relationship observed between TB and mortality risk is likely due, at least in part, to unmeasured risk factors for mortality. In this study, smoking was a key unmeasured potential source of confounding in the relationship between TB and time-to-mortality that we were unable to adjust for in this analysis. However, we did control for the Charlson comorbidity score, several conditions of which are strongly related to smoking, such as COPD. Our measure of neighbourhood income quintile may be subject to misclassification bias due to the use of an area-level measure for individuals, although results were robust to inclusion/exclusion of the income quintile variable. Generalization of the results of this study should be limited to immigrants within Canada but may have external validity in other high-income settings.
Future research
With an increasing number of people who have completed TB treatment worldwide, there is a growing concern about the long-term health outcomes of TB patients (Byrne et al. 2015; Huaman et al. 2015; World Health Organization (WHO) 2018). Prospective longitudinal research could provide a vital data source for future studies of long-term health outcomes, and would enable measurement of variables not recorded in administrative data, such as health behaviours. Such prospective research may help elucidate relationships between TB and long-term morbidity and mortality that would, in turn, provide evidence for public health interventions to improve long-term health outcomes for TB patients. Establishing longitudinal cohorts of TB patients would also provide opportunities to conduct qualitative and mixed methods research to develop interventions for improving TB survivor health.
Conclusions
Among immigrants to BC, a diagnosis of TB increases long-term risk of mortality from causes other than TB. This increased risk appears immediately post-TB treatment completion. Immigrants diagnosed with TB are also at increased risk of mortality from causes with limited biological relationship to TB, raising new questions about TB’s potential role as a marker for risk, in addition to being a potential causal risk factor. Prolonged follow-up post-TB treatment, and chronic disease prevention and management integrated with TB care, may provide avenues for improving post-TB survival and quality of life. The true cost-effectiveness of TB prevention activities is likely underestimated by not considering long-term morbidity and mortality among people surviving TB. We hope that our findings serve as a call to action for TB programs and care providers in high-income settings to address all potential threats to TB patients’ health.
Electronic supplementary material
(DOCX 38 kb)
Compliance with ethical standards
This study was approved by the University of British Columbia’s Clinical Research Ethics Board (H16-00265).
Conflict of interest
Over the last three years, MEK has received consulting fees from Biogen Inc. (unrelated to this manuscript). The other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allwood, B., van der Zalm, M., Makanda, G., Mortimer, K., Andre, F. S., Uzochukwu, E., et al. (2019). The long shadow post-tuberculosis. Lancet Infectious Diseases, 19(11), 1170–1171. 10.1016/S1473-3099(19)30564-X. [DOI] [PubMed]
- Alvarez, G. G., Archibald, C. P., Avendano, M., Behr, M., Christianson, S., Cook, V. J., et al. (2014). Canadian tuberculosis standards. In D. Menzies, E. Ellis, R. Long, M. Pai, & T. Wong (Eds.), Canadian Tuberculosis Standards (7th ed.). Ottawa: Public Health Agency of Canada.
- Basham, C. A., Romanowski, K., & Johnston, J. C. (2019). Life after tuberculosis: planning for health. Lancet Respiratory Medicine, 7(12), 1004–1006. 10.1016/S2213-2600(19)30371-6. [DOI] [PubMed]
- Bates, M. N., Khalakdina, A., Pai, M., Chang, L., Lessa, F., & Smith, K. R. (2007). Risk of tuberculosis from exposure to tobacco smoke. Archives of Internal Medicine, 167(4), 335-342. 10.1001/archinte.167.4.335. [DOI] [PubMed]
- BC Centre for Disease Control. (2018). TB in british columbia: annual surveillance report 2016. Vancouver: public health services authority. Retrieved May 29, 2020 from http://www.bccdc.ca/health-professionals/data-reports/tuberculosis-reports.
- BC Centre for Disease Control. (2019). BC provincial TB registry (BCCDC-iPHIS). Population data BC [publisher]. Data Extract. BCCDC (2019). Retrieved January 31, 2018 from www.popdata.bc.ca/data.
- BC Ministry of Health. (2018a). Consolidation file (MSP Registration & Premium Billing). Population Data BC [publisher]. Data Extract. MOH (2018). Retrieved January 31, 2018 from www.popdata.bc.ca/data.
- BC Ministry of Health. (2018b). Medical services plan (MSP) payment information file. Population Data BC [publisher]. Data Extract. MOH (2018). Retrieved January 31, 2018 from www.popdata.bc.ca/data..
- BC Vital Statistics Agency. (2018). Vital statistics deaths. V2. Population Data BC [publisher]. Data Extract BC Vital Statistics Agency (2018). Retrieved Jnauary 31, 2018 from www.popdata.bc.ca/data.
- Bender, A., Guruge, S., Hyman, I., & Janjua, M. (2012). Tuberculosis and common mental disorders: international lessons for Canadian immigrant health. Canadian Journal of Nursing Research, 44(4), 56–75. [PubMed]
- Blondal, K., Rahu, K., Altraja, A., Viiklepp, P., & Rahu, M. (2013). Overall and cause-specific mortality among patients with tuberculosis and multidrug-resistant tuberculosis. International Journal of Tuberculosis and Lung Disease, 17(7), 961–968. 10.1080/09720073.2015.11891863. [DOI] [PubMed]
- Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. International Journal of Infectious Diseases. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. (2018). Discharge abstract database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH (2018). Retrieved January 31, 2018 from http://www.popdata.bc.ca/ data.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Christensen ASH, Roed C, Andersen PH, Andersen ÅB, Obel N. Long-term mortality in patients with pulmonary and extrapulmonary tuberculosis: a Danish nationwide cohort study. Clinical Epidemiology. 2014;6:405–421. doi: 10.2147/CLEP.S65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, S., & Evans, C. A. (2019). Healthy survival after tuberculosis. Lancet Infectious Diseases, 19(10), 1045–1047. 10.1016/S1473-3099(19)30387-1. [DOI] [PubMed]
- Desmeules M, Gold J, Mcdermott S, Cao Z, Payne J, Lafrance B, et al. Disparities in mortality patterns among Canadian immigrants and refugees, 1980-1998: results of a national cohort study. Journal of Immigrant Health. 2005;7(4):221–232. doi: 10.1007/s10903-005-5118-y. [DOI] [PubMed] [Google Scholar]
- Elixhauser, A., Steiner, C., Harris, D. R., & Coffey, R. M. (1998). Comorbidity measures for use with administrative data. Medical Care, 36(1), 8-27. https://doi.org/10.1097/00005650-199801000-00004 [DOI] [PubMed]
- Glymour, M. (2006). Using causal diagrams to understand common problems in social epidemiology. In J. Oakes & J. Kaufman (Eds.), Methods in social epidemiology (1st ed., pp. 393–428). San Francisco: John Wiley & Sons, Inc..
- Halverson, J., Ellis, E., Gallant, V., & Archibald, C. P. (2014). Epidemiology of tuberculosis in Canada. In D. Menzies, E. Ellis, R. Long, M. Pai, & T. Wong (Eds.), Canadian tuberculosis standards (7th ed., pp. 7–23). Ottawa: Public Health Agency of Canada.
- Huaman, M. A., Henson, D., Ticona, E., Sterling, T. R., & Garvy, B. A. (2015). Tuberculosis and cardiovascular disease: linking the epidemics. Tropical Diseases, Travel Medicine and Vaccines, 1(1), 10. 10.1186/s40794-015-0014-5. [DOI] [PMC free article] [PubMed]
- Immigration Refugees and Citizenship Canada [creator]. (2015). Permanent resident database: population data BC [publisher]. Data Extract. IRCC (2015). Retrieved January 31, 2018 from www.popdata.bc.ca/ data.
- Kleinbaum, D. G., & Klein, M. (2010). Survival analysis: a self-learning text (3rd ed.). New York: Springer. https://doi.org/10.1007/978-1-4419-6646-9
- Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- Lix, L. M., Plourde, P. J., Larcombe, L., Kinew, K. A., Basham, C. A., Derksen, S., … McCulloch, S. (2018). Exploring tuberculosis treatment, management, and prevention in Manitoba’s administrative health data. Winnipeg: University of Manitoba. Retrieved January 28, 2019 from http://mchp-appserv.cpe.umanitoba.ca/reference/MBTB_Report_web.pdf
- Manitoba Centre for Health Policy. (2019). Concept: charlson comorbidity index. Retrieved September 17, 2019 from http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1098.
- Miller TL, Wilson FA, Pang JW, Beavers S, Hoger S, Sharnprapai S, et al. Mortality hazard and survival after tuberculosis treatment. American Journal of Public Health. 2015;105(5):930–937. doi: 10.2105/AJPH.2014.302431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman, A. (2010). Morbidity and mortality related to tuberculosis (TB) in British Columbia (BC), Canada. Vancouver: university of British Columbia. 10.14288/1.0071046.
- Nahid, P., Alipanah, N., Cattamanchi, A., Chaisson, L. H., Hopewell, P. C., Merrifield, C., et al. (2016). Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clinical Infectious Diseases, 63(7), 853–867. 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed]
- Peltzer K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. African Journal of Primary Health Care and Family Medicine. 2018;10(1):1–6. doi: 10.4102/phcfm.v10i1.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit AC, Giganti MJ, Ingle SM, May MT, Shepherd BE, Gill MJ, et al. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. Journal of the International AIDS Society. 2018;21(1):1–8. doi: 10.1002/jia2.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Data BC. (2019a). Data linkage. Vancouver: University of British Columbia. Retrieved November 5, 2019, from www.popdata.bc.ca/datalinkage
- Population Data BC. (2019b). Population data BC. Vancouver: University of British Columbia. Retrieved May 12, 2017, from www.popdata.bc.ca
- Quan H, Fong A, Burnand B, Saunders LD, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43(11):1130–1139. doi: 10.1016/j.aquaculture.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Rasanathan, K., Sivasankara Kurup, A., Jaramillo, E., Lonnroth, K., & Lönnroth, K. (2011). The social determinants of health: key to global tuberculosis control. International Journal of Tuberculosis and Lung Disease, 15(Suppl 2(6)), S30–S36. 10.5588/ijtld.10.0691. [DOI] [PubMed]
- Reitmanova S, Gustafson D. Rethinking immigrant tuberculosis control in Canada: from medical surveillance to tackling social determinants of health. Journal of Immigrant and Minority Health / Center for Minority Public Health. 2012;14(1):6–13. doi: 10.1007/s10903-011-9506-1. [DOI] [PubMed] [Google Scholar]
- Romanowski, K., Baumann, B., Basham, C. A., Ahmad Khan, F., Fox, G. J., & Johnston, J. C. (2019). Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infectious Diseases, 19(10), 1129–1137. 10.1016/S1473-3099(19)30309-3. [DOI] [PubMed]
- Ronald LA, Campbell JR, Balshaw RF, Roth DZ, Romanowski K, Marra F, et al. Predicting tuberculosis risk in the foreign-born population of British Columbia, Canada: study protocol for a retrospective population-based cohort study. BMJ Open. 2016;6(11):e013488. doi: 10.1136/bmjopen-2016-013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald LA, Campbell JR, Balshaw RF, Romanowski K, Roth DZ, Marra F, et al. Demographic predictors of active tuberculosis in people migrating to British Columbia, Canada: a retrospective cohort study. Canadian Medical Association Journal. 2018;190(8):E209–E216. doi: 10.1503/cmaj.170817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald, L. A., Campbell, J. R., Rose, C., Balshaw, R., Romanowski, K., Roth, D. Z., et al. (2019). Estimated impact of World Health Organization latent tuberculosis screening guidelines in a region with a low tuberculosis incidence: retrospective cohort study. Clinical Infectious Diseases, 69(12), 2101-2108. 10.1093/cid/ciz188. [DOI] [PMC free article] [PubMed]
- Shuldiner, J., Leventhal, A., Chemtob, D., & Mor, Z. (2016). Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. International Journal of Tuberculosis and Lung Disease, 20(1), 43–48. 10.5588/ijtld.14.0427. [DOI] [PubMed]
- Simou, E., Britton, J., & Leonardi-Bee, J. (2018). Alcohol consumption and risk of tuberculosis: a systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease, 22(11), 1277–1285. 10.5588/ijtld.18.0092. [DOI] [PubMed]
- Snapinn, S. M., Jiang, Q., & Iglewicz, B. (2005). Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. The American Statistician, 59(4), 301–307. 10.1198/000313005X70371.
- Steyerberg, E. W. (2019). Clinical prediction models: a practical approach to development, validation, and updating (2nd ed.). Switzerland: Springer Nature. 10.1007/978-3-030-16399-0.
- Suissa S. Immortal time bias in pharmacoepidemiology. American Journal of Epidemiology. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- Sweetland, A. C., Kritski, A., Oquendo, M. A., Sublette, M. E., Norcini Pala, A., Silva, L. R. B., et al. (2017). Addressing the tuberculosis-depression syndemic to end the tuberculosis epidemic. International Journal of Tuberculosis and Lung Disease, 21(8), 852–861. 10.5588/ijtld.16.0584. [DOI] [PMC free article] [PubMed]
- Therneau, T. M., & Grambsch, P. M. (2000). Modeling survival data: extending the Cox model. Berlin: Springer Science & Business Media. 10.1007/978-1-4757-3294-8.
- Vachon, J., Gallant, V., & Siu, W. (2018). Tuberculosis in Canada, 2016. Canadian Communicable Disease Report, 44(6), 75–81. 10.14745/ccdr.v44i03a01. [DOI] [PMC free article] [PubMed]
- Wanner A, Edwards M, Harries AD, Kirenga BJ, Chakaya J, Jones R, Van Kampen SC. International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Global Health. 2018;3(4):1–8. doi: 10.1136/bmjgh-2018-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workneh, M. H., Aksel Bjune, G., & Yimer, S. A. (2017). Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS ONE, 12(4), e0175925. 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed]
- World Health Organization (WHO). (2018). Global Tuberculosis Report 2018. Geneva: World Health Organization.
- World Health Organization (WHO). (2019). Global Tuberculosis Report 2019. Geneva: World Health Organization.
- Zanuto EL. A comparison of propensity score and linear regression analysis of complex survey data. Journal of Data Science. 2006;4:67–91. doi: 10.6339/JDS.2006.04(1).233. [DOI] [Google Scholar]
- Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. American Journal of Epidemiology. 2005;162(10):1016–1023. doi: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 38 kb)