Abstract
Background
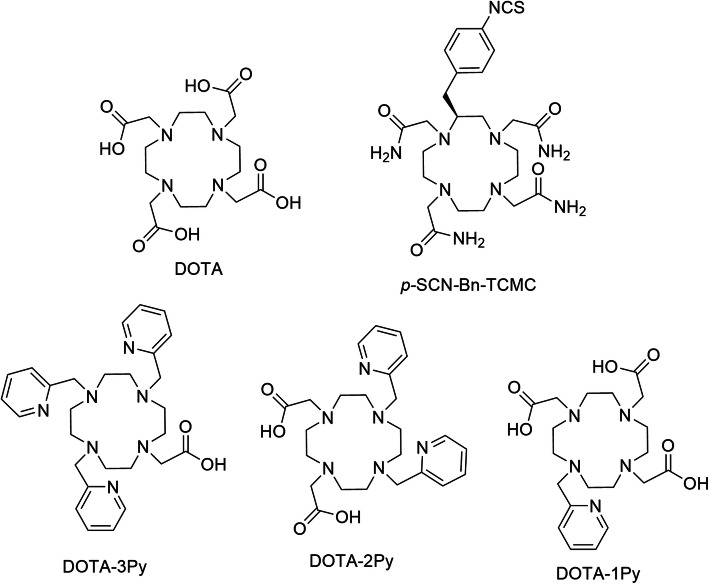
Lead-212 (212Pb, t1/2 = 10.6 h) and lead-203 (203Pb, t1/2 = 51.9 h) are an element-equivalent, or a matched theranostic radioisotope pair that show great potential for application in targeted radionuclide therapy (TRT) and single-photon emission computed tomography (SPECT), respectively. At TRIUMF we have produced both 203Pb and 212Pb using TRIUMF’s TR13 (13 MeV) and 500 MeV cyclotrons, and subsequently purified and evaluated both radioisotopes using a series of pyridine-modified DOTA analogues in comparison to the commercially available chelates DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and TCMC (1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane).
Results
Proton irradiation (12.8 MeV) of natural and enriched thallium-203 (203Tl) targets gave 203Pb saturation yields of 134 ± 25 and 483 ± 3 MBq/μA, respectively. Thorium-228 (228Th, t1/2 = 1.9 y), a by-product of 232Th proton spallation on TRIUMF’s main 500 MeV beamline (beamline 1A, BL1A), was recovered to build a 228Th/212Pb generator with the ability to deliver up to 9–10 MBq of 212Pb daily. Both lead isotopes were purified via solid phase extraction chromatography (Pb resin), and isolated in an acetate form ([203/212Pb]Pb(OAc)2) suitable for direct radiolabeling of chelators and bioconjugates. A series of cyclen-based chelators (herein referred to as DOTA-1Py, -2Py, and -3Py) along with established chelates DOTA and TCMC were evaluated for their ability to complex both 203Pb and 212Pb. All chelates incorporated 212Pb/203Pb efficiently, with higher radiolabeling yields observed for the 212Pb-complexes.
Conclusion
The production of 203Pb and 212Pb was established using TRIUMF 13 MeV and 500 MeV cyclotrons, respectively. Both production methods provided radiometals suitable for subsequent radiolabeling reactions using known and novel chelates. Furthermore, the novel chelate DOTA-3Py may be a good candidate for biomolecule conjugation and further theranostic 212Pb/203Pb studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41181-021-00121-4.
Keywords: Lead-212, Lead-203, Thorium-228 generator, Thallium-203, Theranostic, Cyclen, DOTA, Pyridyl, Chelators, Radiolabeling
Background
The fields of molecular imaging (MI) and targeted radionuclide therapy (TRT) rely on incorporating radioisotopes onto biomolecules that show high affinity for cancer cells in order to impart diagnostic and/or therapeutic information to health researchers and clinicians. Advances in understanding the molecular processes that define normal and aberrant cell behavior has led to the identification of an increasing number of biomolecular targets that can be exploited for targeted delivery of imaging and therapeutic agents specific to diseased cells. With targeted compound delivery, one can minimize ambiguous diagnostic outcomes and/or undesirable side effects during treatment by avoiding uptake or damage induced by off-target radiopharmaceutical accumulation (Kumar Bharti et al. 2012; Bono et al. 2003). MI relies on radionuclides which emit photons, either directly (such as in electron capture [EC] decay) or indirectly (such as in positron [β+] decay), while nuclides that emit cytocidal particles (such as beta [β−], alpha [α] particles, or Meitner-Auger electrons) can be used for TRT. Theranostic radiopharmaceuticals represent a combination of both MI and TRT isotopes onto a common biomolecule that can be used to both image and then treat disease, leading to a potent compound pairing that allows for visualization of the molecular processes underpinning disease and verifies cellular target presence for subsequent therapy (Yordanova et al. 2017; Rösch et al. 2017).
In general, a theranostic pair of radionuclides comprise of two chemically similar isotopes, one which can be used for imaging, and the other for therapy (Elgqvist et al. 2014). When the theranostic pair is composed of radionuclides of two different elements, the biodistribution of the radiopharmaceutical may differ and thus any quantitative dosimetric information predicted from the diagnostic imaging results may not be reflective of the therapeutic agent; this discrepancy can be minimized with matched theranostic pairs (Elgqvist et al. 2014). Matched theranostic pairs utilize different isotopes of the same element for diagnosis and therapy, giving rise to identical chemical species and thus biodistribution, which can give further insight on the suitability of the radiopharmaceutical for a patient being assessed or treated (Yordanova et al. 2017). Only the different half lives and their effect on biodistribution may need to be considered.
Lead-203 (203Pb, t1/2 = 51.9 h) and lead-212 (212Pb, t1/2 = 10.6 h) are an element-equivalent matched theranostic pair that have generated significant interest for use in theranostic radiopharmaceutical development (Máthé et al. 2016). 212Pb emits two β− particles and one α particle during its decay chain and can be used for therapy. 203Pb decays by electron capture to ground state thallium-203 (203Tl), followed by the emission of a gamma-photon (279 keV; 81%) that is compatible for single photon emission computed tomography (SPECT) imaging while the lack of radioactive daughter products simplifies dosimetry calculations (Horlock et al. 1975).
212Pb is a member of the uranium-232 (232U) and thorium-232 (232Th) decay chain, and is commonly produced by the decay of 228Th (t1/2 = 1.9 y) (Hassfjell 2001; Hassfjell and Hoff 1994; Zucchini and Friedman 1982) and radium-224 (224Ra, t1/2 = 3.64 days) (Mirzadeh 1998; Westrøm et al. 2017; Atcher et al. 1988; Bartoś et al. 2013; Milenic et al. 2015; Baidoo et al. 2013; Li et al. 2017) (Fig. 1). Many 228Th generators exploit the chemical or physical separation of the daughters 224Ra (Zucchini and Friedman 1982) and radon-220 (220Rn, t1/2 = 55.6 s) (Hassfjell 2001; Hassfjell and Hoff 1994) by using cation exchange columns (Zucchini and Friedman 1982) or chamber walls (Hassfjell and Hoff 1994), and glass bubblers (Hassfjell 2001), respectively. 212Pb has been collected using nitric (Hassfjell 2001) or hydrochloric (Zucchini and Friedman 1982) acid or water (Hassfjell and Hoff 1994) to give yields of 85–90%. However, many of the 228Th generators reported to date have difficulty providing practical quantities of 212Pb due to the radiolytic damage to the generator matrix material when higher levels of activity are included. To circumvent this, 224Ra generators have been used to produce 212Pb by separating 224Ra from 228Th on an anion exchange resin (Bartoś et al. 2013), followed by loading onto a cation (Mirzadeh 1998; Atcher et al. 1988; Bartoś et al. 2013; Milenic et al. 2015; Baidoo et al. 2013) exchange resin, actinide resin (Westrøm et al. 2017), or Pb-selective extraction resin (Li et al. 2017), from which 212Pb is eluted using HCl (Mirzadeh 1998; Westrøm et al. 2017; Atcher et al. 1988; Bartoś et al. 2013; Milenic et al. 2015; Baidoo et al. 2013) or a complexing agent (Li et al. 2017).
Fig. 1.
Decay scheme of 228Th to 212Pb and stable 208Pb
203Pb is a cyclotron produced isotope and can be prepared from charged particle (proton, deuteron, and alpha particle) bombardment of thallium (natural abundance 29.5% 203Tl, 70.5% 205Tl) (Horlock et al. 1975). At the end of bombardment, the thallium targets are dissolved in nitric acid (Horlock et al. 1975; Li et al. 2017; Henriksen and Hoff 1998; Garmestani et al. 2005) or a mix of nitric and hydrochloric acid (Máthé et al. 2016), before loading onto a Pb-selective extraction resin (Li et al. 2017; Henriksen and Hoff 1998; Garmestani et al. 2005), or anion exchange resin (Máthé et al. 2016). Alternatively, 203Pb is co-precipitated with Fe (OH)3 (Horlock et al. 1975) and eluted using complexing agents (Henriksen and Hoff 1998), dilute nitric acid (Li et al. 2017; Garmestani et al. 2005), or hydrochloric acid (Máthé et al. 2016). Many of the purification methods for both 212Pb and 203Pb produce large eluant volumes, of which the composition may be incompatible with radiolabeling, thus requiring evaporation and redissolution steps which can result in further reduction of yield or introduction of interfering stable impurities.
In this study, extraction chromatography and small volumes of radiolabeling-compatible complexing agents are used to elute cyclotron produced 203Pb and 228Th-generator produced 212Pb to shorten processing time and allow direct radiolabeling of the purified 203/212Pb isotopes. A novel 228Th/212Pb generator concept was applied using a 228Th generator stock solution obtained via 232Th spallation on TRIUMF’s 500 MeV cyclotron. The preparation of the 228Th stock allowed direct loading onto Pb-selective resin, which returned 228Th directly to a storage vessel followed by elution of absorbed 212Pb with ammonium acetate (NH4OAc). We present the production of the 203Pb and 212Pb theranostic pair using TRIUMF’s TR13 (13 MeV) and 500 MeV cyclotron, subsequent radiochemical purification and isotope characterization. The separated radioisotopes were evaluated alongside each other for radiolabeling a series of TRIUMF developed, pyridine based-DOTA analogues (Yang et al. 2021) along with commercial standards DOTA and TCMC (Fig. 2), and the stability of each radiometal-complex was evaluated in vitro.
Fig. 2.
Chemical structures of commercially available Pb-chelators DOTA, p-SCN-Bn-TCMC, and pyridine-based cyclen analogues DOTA-1Py, DOTA-2Py, DOTA-3Py radiolabeled herein
Methods
Materials and methods
All solvents and reagents were purchased from commercial suppliers (Sigma Aldrich, Fisher Scientific, VWR) and used as received, unless otherwise noted. Ultrapure nitric acid (Environmental Grade) was purchased from VWR and Ultrapure HCl (TraceSELECT), NaOH (99.99% trace metal grade), and ammonium acetate (ACS grade) were purchased from Fisher Scientific. Pb resin (Di-t-butylcyclohexano 18-crown-6, 100–150 μm particle size) was purchased from Eichrom Technologies (Lisle, IL). 1 mL polypropylene cartridges and 1/8″ polyethylene frits were purchased from United Chemical Technologies (Lewistown, PA). Natural Tl (99.99% metals basis) was purchased from Alfa Aesar (Tewksbury, MA) and enriched Tl (98.57 ± 0.05% 203Tl, 1.43 ± 0.05% 205Tl) was purchased from BWX Technologies (Vancouver, BC). Human serum and Dowex 1 × 8 chloride form anion exchange resin (100–200 mesh) was purchased from Sigma Aldrich (St. Louis, MO). DOTA and p-SCN-Bn-TCMC (referred to herein as TCMC) were purchased from Macrocyclics (Plano, TX). DOTA-1Py (2,2′,2″-(10-(pyridin-2-ylmethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl) triacetic acid), DOTA-2Py, (2,2′-(7,10-bis (pyridin-2-ylmethyl)-1,4,7,10-tetraazacyclododecane-1,4-diyl) diacetic acid) and DOTA-3Py (2-(4,7,10-tris (pyridin-2-ylmethyl)-1,4,7,10-tetraazacyclododecan-1-yl) acetic acid) were synthesized as previously described (Yang et al. 2021). Instant thin layer chromatography paper impregnated with silicic acid (iTLC-SA) was purchased from Agilent Technologies (Santa Clara, CA). Deionized water was prepared on site using a Millipore Direct-Q® 3UV water purification system. Nuclear magnetic resonance (NMR) spectra were obtained using MeOD, DMSO-d6, or D2O. Signals were measured relative to the signal of the solvent. NMR spectra were obtained using a Bruker 400 (400 MHz), Bruker 500 (500 MHz), or a Bruker 600 (600 MHz). Mass spectrometry was performed on an Agilent 6210 time-of-flight LC-MS spectrometer or an Advion expression LC-MS equipped with an electrospray source. All radioactivity measurements for the 203Pb purification from Tl targets and the 228Th/212Pb generator were performed using gamma ray spectroscopy on an N-type co-axial high purity germanium (HPGe) gamma spectrometer (Canberra Industries) calibrated with a 20 mL 152Eu and 133Ba source. Aliquots (5–100 μL) were removed and diluted to the 20 mL standard volume for measurement at a distance of at least 15 cm from the detector until the peak area uncertainties were below 5%; dead time was kept below 4%. Spectra were analyzed using the Genie 2000 software package (Version X, Canberra Industries) using the 279 keV and 401 keV gamma lines for 203Pb measurement, and 238 keV and 300 keV gamma lines for 212Pb measurement. To determine chemical purity of the 203Pb and 212Pb elutes, 1–1.5 mL aliquots were removed and analyzed by inductively coupled plasma mass spectrometry (ICP-MS) at Chalk River Laboratories. The High-Performance Liquid Chromatography (HPLC) system used for the analysis of 203Pb-labeled chelators consisted of an Agilent 1260 Infinity II Quaternary Pump, Agilent 1260 autosampler, Raytest Gabi Star NaI (Tl) radiation detector, Agilent 1260 variable wavelength detector, and Agilent 1260 analytical scale fraction collector with a Phenomenex Luna 5 μm C18 100 Å liquid chromatography analytical (250 × 4.6 mm – for serum stability studies, 100 × 4.6 mm – for radiolabeling) column. RadioTLC was performed using a BioScan System 200 Image Scanner.
Production and purification of 228Th nitrate
228Th is a by-product of the 232Th proton spallation used to produce 225Ac on TRIUMF’s main 500 MeV cyclotron (Robertson et al. 2018; Robertson et al. 2017; Robertson et al. 2020). 228Th is isolated from other elements during bulk thorium precipitation, as previously described (Robertson et al. 2020). To produce the 228Th generator stock solution, approximately 8 g of the ThCl4 salt was dissolved in 10 M HCl (200 mL) and loaded, via a peristaltic pump at 2 mL/min, onto a 10 mL Dowex 1 × 8 chloride form anion exchange resin column prepared as a slurry pre-conditioned with 10 M HCl (40 mL) prior to use. The column was washed with 10 M HCl (60 mL); 228Th did not adsorb to the column and thus was found in the load and wash fractions. Impurities were eluted from the column using 1 M HCl. The load and wash fractions were evaporated to dryness and exchanged three times with 10 M nitric acid before re-dissolving in 1 M HNO3 (40 mL) to produce the generator stock solution and the radionuclidic purity was assessed by gamma spectroscopy. All fractions were counted immediately after collection and were counted once again after 2 weeks to allow for the grow in of progeny (224Ra and 212Pb), which acted as a surrogate for measuring 228Th in gamma spectroscopy.
The 228Th/212Pb generator principle
The multi-component generator consists of: A peristaltic pump (Fig. 3a), running at 2 mL/min, to pump the 228Th generator stock solution (Fig. 3b) through an 80 mg Pb resin column (Fig. 3c), housed in a 1 mL polypropylene cartridge and preconditioned with MilliQ water (5 mL) followed by 1 M HNO3 (5 mL). The 228Th, 224Ra, and 212Bi are not retained on the column and are returned to the stock bottle via the pump using air to push the solution into a 50 mL storage loop. A syringe attached to a female luer fitting (Fig. 3d) was used to pass 1 M HNO3 (5 mL) through the Pb resin column to wash the column before eluting 212Pb with 1 M NH4OAc (pH 7) into a collection vial (Fig. 3e).
Fig. 3.
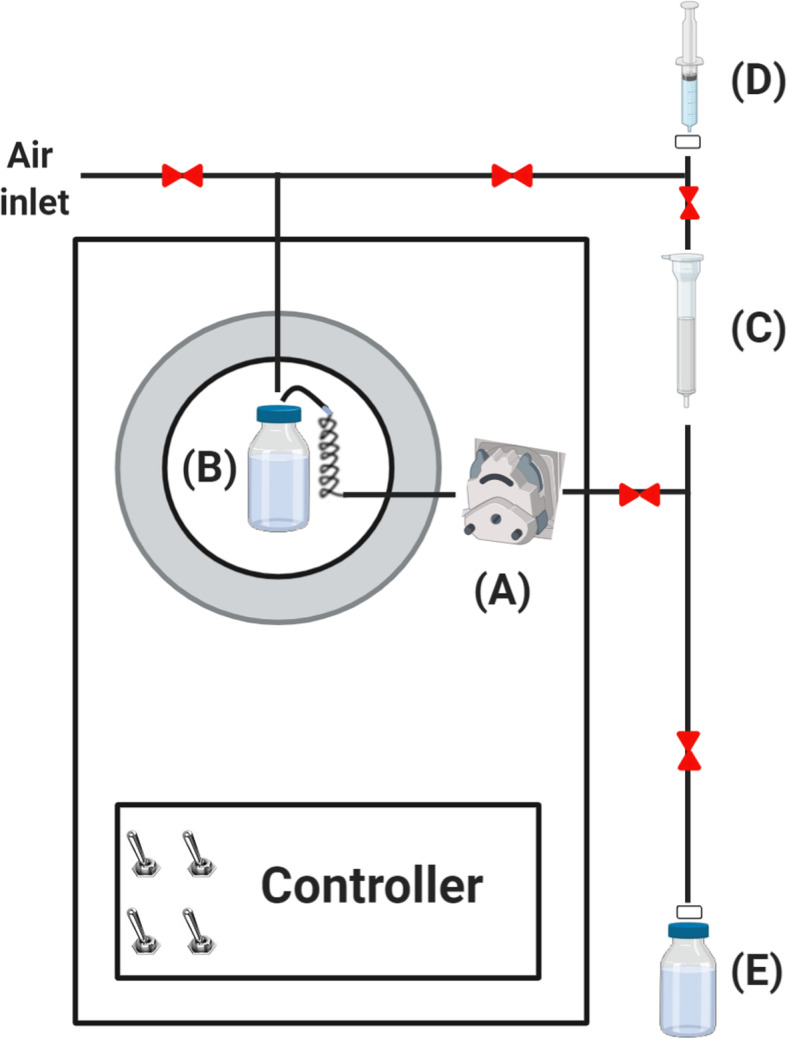
Schematic of the 228Th/212Pb generator. a Peristaltic pump. b Generator stock solution in lead shielded storage loop. c Pb resin column. d Syringe attached to a female luer fitting to control elution. e Collection vial for 212Pb(OAc)2. Image created with BioRender.com
Tl target production
A 1.6 mm thick, 35 mm diameter aluminum plate (6082 alloy) with a centered 10 mm diameter indent (0.25–0.30 mm depth) was used as a backing plate to hold the Tl. Natural or enriched 203Tl (320–330 mg) was pressed with a hydraulic press (Desktop Pellet Press, Across International, 4 MPa) into a 10 mm diameter pellet using a 10 mm die set and pressed into the aluminum backing indent (6 MPa). The target was then heated to 400 °C using a hotplate in a fume hood to allow for melting. Discolouration, indicative of thallium oxide formation was observed on the pellet surface. After cooling, the resultant thallium oxide was rinsed off the surface with deionized water. The structural integrity of the Al-Tl target was evaluated by scratch and drop testing from a height of 1.5 m onto a hard surface. The target was inspected for signs of physical damage before being vacuum sealed in a plastic envelope until irradiation.
Target irradiation
The Tl target plate was installed in a solid target holder (Zeisler et al. 2019) and 203Pb produced via the 203Tl (p,n) 203Pb nuclear reaction using TRIUMF’s TR13 cyclotron (Laxdal et al. 1994). The cross section of this reaction has a threshold energy of 8 MeV and a peak at 26.5 MeV (Azzam et al. 2014), making a small energy cyclotron suitable for production. The target plate was cooled via water (back) and helium (front) during irradiation. The 13 MeV protons were degraded to ~ 12.8 MeV using a 25 μm thick aluminum foil which separated the target system from the cyclotron vacuum. The backing plate thickness was chosen to completely stop the proton beam. Irradiation was performed at 8–9 μA for 2–4 h. After irradiation, the target was left in the target holder for 18–24 h to allow short-lived radionuclides produced during the irradiation [202mPb (t1/2 = 3.62 h)] to decay, reducing radiation exposure to personnel.
Purification of 203Pb
The target was dissolved in a beaker with 2 M HNO3 (20 mL) on a 125 °C hot plate after which the solution was allowed to cool to ambient temperature over 1–1.5 h. A 1 mL polypropylene cartridge was packed with Pb resin (60 mg) and was conditioned with MilliQ water (5 mL) followed by 2 M HNO3 (5 mL). The dissolved target solution was loaded onto the column by gravity and washed with 2 M HNO3 (5 mL) to remove any residual thallium. The 203Pb was eluted with 1 M NH4OAc (pH 7, 3 mL) at 0.5 mL/min. The yield and radionuclidic purity of 203Pb in the load, wash, and elute fractions were assessed using gamma spectroscopy. Chemical purity of the 203Pb elute was assessed using ICP-MS to identify any stable metal species that may compete with Pb during radiolabeling reactions.
Inductively coupled plasma mass spectrometry
Aliquots (1.5 mL; n = 3) of the 212Pb and 203Pb elutes were lyophilized to dryness and diluted to 10 mL using trace metal grade 1 M HNO3. The analysis utilized a multi-element standard1 for the measurement of common stable impurities which may interfere with radiolabeling. Stable impurities found in blank samples, which contained 10 mL of trace metal grade 1 M HNO3, were subtracted from the amount found in the elutes to quantify the impurities in the elutes.
Non-radioactive Pb(II) complexes
Chelates (DOTA-xPy, x = 1–3) were screened for their ability to complex non-radioactive Pb(II), with metal complex formation confirmed and characterized using ESI-MS and 1H NMR. Briefly, Pb(OAc)2 (1.2 equiv., 5–6 μL of 7.8 mg/mL in H2O), was added to 10–15 mg of the respective chelator (1 equiv., DOTA-1Py, DOTA-2Py, DOTA-3Py), and the pH adjusted to approximately pH 6 using HCl or NaOH. The reaction mixture was stirred up to 2 h at ambient temperature, after which the solvent was evaporated in vacuo to give a white solid. [Pb(DOTA-1Py)]− ESI−-MS: m/z found 642.1, calcd C20H28N5O6Pb (M−) 642.18. [Pb(DOTA-2Py)] ESI+-MS: m/z found 677.1, calcd C24H33N6O4Pb (M + H+) 677.23. [Pb(DOTA-3Py)]+ ESI+-MS: m/z found 710.3, calculated C28H36N7O2Pb (M+) 710.27. 1H NMR spectra of the Pb-complexes can be found in the Supporting Information.
212Pb and 203Pb radiolabeling studies
Chelators DOTA, TCMC, DOTA-1Py, DOTA-2Py, and DOTA-3Py were dissolved to give stock solutions (10− 3 M) in deionized water. Serial dilutions were used to prepare chelator solutions at 10− 4, 10− 5, and 10− 6 M in deionized water. A 10 μL aliquot of each chelator (or water as a negative control) was diluted with 1 M NH4OAc (pH 7, 80 μL). For 203Pb labeling studies, [203Pb]Pb(OAc)2 (50 kBq, 10 μL) was added and mixed to begin the radiolabeling reaction at ambient temperature. For 212Pb labeling studies, [212Pb]Pb(OAc)2 (23 kBq, 10 μL) was added to each reaction, all performed in triplicate. The iTLC plate system used for both 203Pb and 212Pb studies was iTLC-SA plates (2 cm × 10 cm, baseline at 1.5 cm) developed using EDTA (50 mM, pH 5.0). Under these conditions, the labeled Pb remained at the baseline (Rf = 0) and free 203Pb2+ and 212Pb2+ migrated with the solvent front (Rf ~ 1). Aliquots (10 μL) were removed from each reaction solution at 5, 30, and 60 min and analyzed via iTLC. For 212Pb, the plates were measured 24 h later to allow for the decay of free short-lived daughter products (212Bi, t1/2 = 60.55 min). 203Pb-complex formation with the DOTA-xPy ligands was further confirmed via analytical RP-HPLC (gradient: A, 0.1% TFA in water; B, 0.1% TFA in acetonitrile; 0–100% B over 20 min, 1 mL/min) by co-injection of pre-formed ‘stable’ Pb-complexes with the [203Pb]Pb-tracers and tracking both the UV and radioactive chromatograms (Figure S16).
Human serum stability studies
To prepare the 203Pb labeled complexes for human serum stability studies, an aliquot (10 μL) of the 10− 3 M chelator solution (or water as a negative control) was added to 1 M NH4OAc (pH 7, 80 μL) and [203Pb]Pb(OAc)2 (100–125 kBq, 10 μL) and the reaction was allowed to proceed for 1 h at ambient temperature. Prior to the start of the study, an aliquot (10 μL) was removed and analyzed via iTLC-SA and developed as per the method described in section above, to ensure quantitative radiolabeling. Human serum (100 μL) was added and mixed and the reactions were incubated at 37 °C for 72 h post serum addition. At 8, 24, 48, and 72 h time points, an aliquot (10 μL) of the reaction mixture was removed and spotted on iTLC-SA plates and developed. At 72 h, the tubes were removed from the incubator and acetonitrile (160 μL) was added to precipitate the serum proteins. The tubes were centrifuged at 14,000 rpm for 20 min and then the supernatant was removed. The supernatant was diluted with deionized water (1.3 mL) and analyzed using analytical Radio-RP-HPLC (gradient: A, 0.1% TFA in water; B, 0.1% TFA in acetonitrile; 0–100% B over 30 min, 1 mL/min). To determine the % stability of each lead complex, the area under the curves in the radioactivity trace was calculated. The retention time of unbound “free” Pb2+ was 4.3 min, [203Pb][Pb(DOTA)]2− was 7.9 min, [203Pb][Pb(TCMC)]2+ was 9.3 min, [203Pb][Pb(DOTA-1Py)]+ was 8.6 min, [203Pb][Pb(DOTA-2Py)] was 9.9 min, and [203Pb][Pb(DOTA-3Py)]− was 11.2 min.
Results
Isotope production
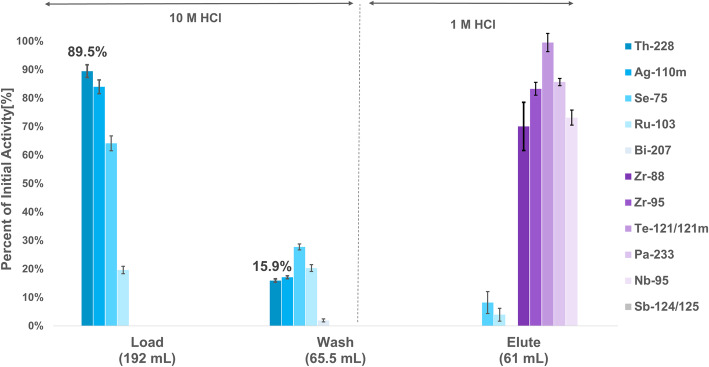
Initial isotopes present in the thorium precipitate included 228Th, 227Th (t1/2 = 18.7 d), 75Se (t1/2 = 119.8 d), 110mAg (t1/2 = 249.8 d), 103Ru (t1/2 = 39.2 d), 207Bi (t1/2 = 31.6 y), 88Zr (t1/2 = 83.4 d), 95Zr (t1/2 = 64.0 d), 95Nb (t1/2 = 35.0 d), 233Pa (t1/2 = 26.9 d), 121mTe (t1/2 = 19.1 d), 121Te (t1/2 = 164.2 d), 124Sb (t1/2 = 60.2 d), and 125Sb (t1/2 = 2.8 y) (Fig. 4). Further information on the activity of each isotope can be found in Table S3. 95Nb and 95Zr were removed via anion exchange, giving load and wash fractions for the generator stock solution containing 75Se,110mAg, 103Ru, 207Bi, and 227Th, in addition to 228Th.
Fig. 4.
Separation of 228Th from other isotopes on a 1 × 8 Dowex anion exchange column (10 mL)
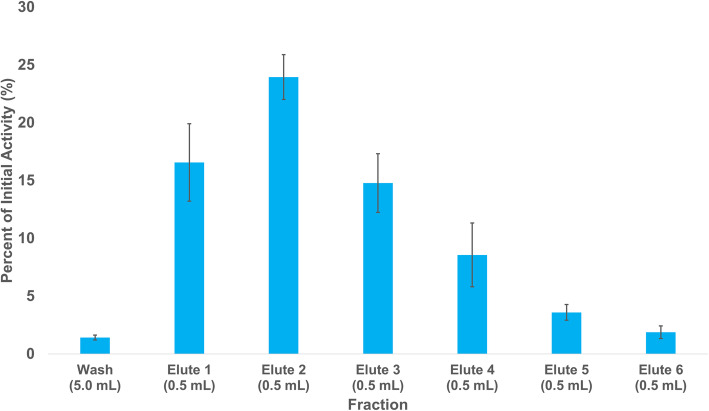
The purification of 212Pb from its parent isotope 228Th with a generator based on Pb-selective extraction resin produced product with > 99% radiochemical purity (Figure S1) with an average yield of 69.3 ± 4.4%. The initial radionuclide generator solution, which initially contained 9.780 ± 0.002 MBq of 228Th, was used to supply 212Pb for at least 2 years. The average elution profile is shown in Fig. 5.
Fig. 5.
Average elution profile for 228Th/212Pb separation from Pb resin using NH4OAc (1 M, pH 7) (n = 4)
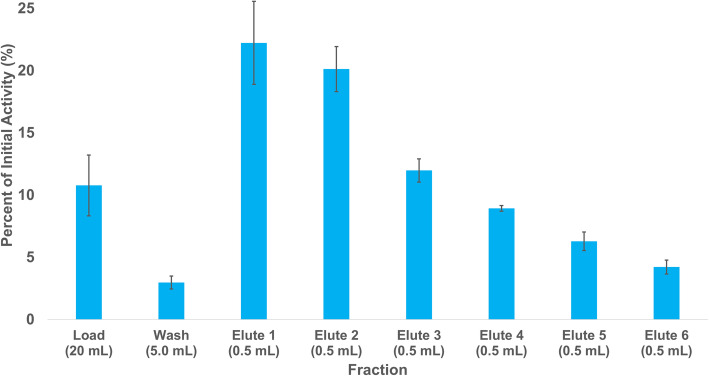
Radiochemically-pure 203Pb was produced and isolated from irradiated aluminum-backed thallium targets (Figure S2) with an average yield, decay corrected to the end of beam (EOB), of 73.8 ± 2.1% (n = 3) and all yields correspond to a calculated saturation yield of 134 ± 25 MBq/μA. Natural Tl targets irradiated for 2 h at 8 or 9 μA produced, on average as determined by gamma spectroscopy, 27.3 ± 4.7 MBq (n = 5) and 32.9 ± 2.7 MBq (n = 8) of 203Pb, respectively. Enriched 203Tl targets irradiated for 3.5 to 4 h at a current of 8 μA produced, as determined by gamma spectroscopy, 175.3 MBq and 201.9 MBq of 203Pb, respectively, corresponding to a calculated saturation yield of 483 ± 3 MBq/μA (n = 2). The elution profile of an average 203Pb separation is shown in Fig. 6.
Fig. 6.
Average elution profile for 203Pb purification from Pb resin using NH4OAc (1 M, pH 7) (n = 6)
Although both Pb products were observed to be radionuclidically pure, the elemental purity was assessed via ICP-MS to quantify any stable impurities that may have interfered with radiolabeling (Table 1). In the 203Pb product, moderate values of Tl were observed with 58,220 ± 35,392 ppb (175 ± 105 μg; n = 3) present in the entire elute. Although there was a ~ 1700 fold reduction in the mass of the Tl found in the elute compared to the mass of the Tl present initially in the target, an additional washing step may help to further reduce the mass found in the elute, which will be necessary for clinical applications. Tl, Ca, Ge, Ni, and Zn were not found to be at levels above the blank concentration in the 212Pb product, while Th and Ti were not detected in the 203Pb product. Mg, Al, Ca, Fe, Co, Ni, Cu, Zn, and Pb were found in low to modest amounts. Ca, Al, and stable Pb were of greatest concern as DOTA has a strong affinity for Ca and Al (Clarke and Martell 1991), while stable Pb can compete with radioactive Pb during the radiolabeling process as they are chemically identical and cannot be separated. In the 212Pb product, the concentration of stable Pb was lower (2.1 ± 2.0 ppb; 6.4 ± 6.0 ng) than in the 203Pb product (495 ± 218 ppb; 1.49 ± 0.66 μg); however, Mg and Ti concentrations were higher at 612 ± 226 ppb and 354 ± 168 ppb, respectively. ICP-MS results for additional elements can be found in Table S1.
Table 1.
Metal content in elute fractions in ppb (μg/L) determined by ICP-MS (n = 3)
| Isotope | Mg | Al | Ca | Ti | Fe | Co |
| 203Pb | 44 ± 14 | 168 ± 152 | 568 ± 263 | N.S. | 18 ± 11 | 0.3 ± 0.5 |
| 212Pb | 612 ± 226 | 22 ± 9 | N.S. | 354 ± 168 | N.S. | 26 ± 11 |
| Isotope | Ni | Cu | Zn | Tl | Pb | Th |
| 203Pb | 10 ± 11 | 3 ± 2 | 21 ± 4 | 58,220 ± 35,392 | 495 ± 218 | N.S. |
| 212Pb | N.S. | 3 ± 2 | N.S. | N.S. | 2 ± 2 | 24,352 ± 16,227 |
N.S. Not significant
Radiolabeling studies
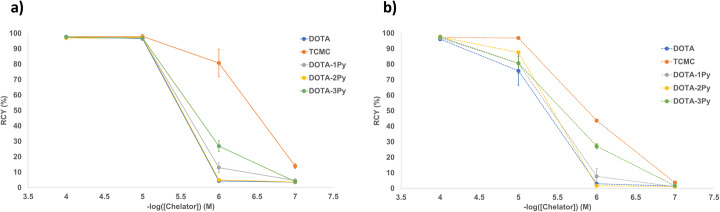
All radiolabeling reactions in this study were performed at room temperature and pH 7 in triplicate and the percent radiochemical yield (% RCY) is reported at the one-hour time point as determined by radio-iTLC. The gold standard for Pb2+ complexation, p-SCN-Bn-TCMC, had 203Pb-radiochemical yields of 97.2 ± 0.6%, 96.9 ± 0.6%, 43.7 ± 1.0%, and 3.7 ± 0.4% (n = 3), at concentrations of 10− 4 to 10− 7 M, respectively (Fig. 6). When the study was repeated with 212Pb, the RCYs were 97.8 ± 0.4%, 98.1 ± 0.5%, 80.8 ± 8.9%, and 13.9 ± 1.6% (n = 3), respectively. DOTA, although not the gold standard for Pb complexation but used in a number of 212Pb/203Pb preclinical studies (Bartoś et al. 2013; Li et al. 2017; Garmestani et al. 2005; Miao et al. 2005; Jianquan et al. 2017; Miao et al. 2008), was able to efficiently complex 203Pb with radiochemical yields of 96.1 ± 1.0%, 75.8 ± 9.4%, 3.0 ± 0.8%, and 1.5 ± 0.2% at concentrations of 10− 4 M to 10− 7 M, respectively. When the study was repeated with 212Pb, the RCYs were 97.6 ± 0.1%, 96.5 ± 0.8%, 4.3 ± 1.2%, and 3.6 ± 0.5%, respectively.
With 1–3 pyridine rings in place of the carboxylic acid groups found on DOTA, the chelators DOTA-1Py, DOTA-2Py, and DOTA-3Py were able to efficiently complex 203Pb at the 10− 4 M concentration (RCYs of 97.0 ± 0.3, 97.6 ± 0.3%, and 97.7 ± 0.5%, respectively). When repeated with 212Pb, all three of the chelators were able to complex 212Pb efficiently at not only 10− 4 M, but also at 10− 5 M. For both 203Pb and 212Pb, the RCYs reduced sequentially at lower concentrations of 10− 5 to 10− 7 M. DOTA-1Py had 203Pb-RCYs of 80.6 ± 6.6%, 7.7 ± 5.0%, and 1.1 ± 0.2% at concentrations of 10− 5 to 10− 7 M, respectively, and when repeated with 212Pb over concentrations of 10− 4 to 10− 7 M, the RCYs were 97.0 ± 0.9%, 96.8 ± 0.6%, 13.0 ± 3.1%, and 4.6 ± 1.1%, respectively. DOTA-2Py had 203Pb-RCYs of 87.6 ± 0.3%, 1.7 ± 0.6%, and 1.2 ± 0.3% at concentrations of 10− 5 to 10− 7 M, respectively. When repeated with 212Pb, at concentrations of 10− 4 to 10− 7 M, the RCYs were 96.9 ± 0.8%, 97.4 ± 0.6%, 5.0 ± 0.4%, and 3.7 ± 0.8%, respectively. DOTA-3Py had 203Pb-RCYs of 80.6 ± 6.6%, 27.2 ± 1.56%, and 1.7 ± 0.1% at concentrations of 10− 5 to 10− 7 M, respectively. When repeated with 212Pb, with concentrations of 10− 4 to 10− 7 M, the RCYs were 97.6 ± 0.7%, 97.0 ± 0.1%, 26.9 ± 3.6%, and 3.9 + 0.9%, respectively. The 212Pb and 203Pb radiolabeling results of these chelators are shown in Fig. 7. Metal complexation with the chelators was also confirmed by synthesizing non-radioactive Pb-complexes of all chelates and characterizing by mass spectrometry and 1H NMR spectroscopy. The distinct isotope distribution pattern for Pb (204Pb [1.4%], 216Pb [24.1%], 207Pb [22.1%], 208Pb [52.1%]) in the MS helped to confirm metal complexes (Figures S6, S7, S8, S9, S10).
Fig. 7.
Radiochemical yield (RCY, %) for a 212Pb and b 203Pb radiolabeling reactions at pH 7 and room temperature at 1 h at chelator concentrations of 10− 4 – 10− 7 M
Human serum stability studies
The stability of 203Pb complexes in human serum for [203Pb][Pb(TCMC)]2+, [203Pb][Pb(DOTA]2−, [203Pb][Pb(DOTA-1Py)]−, [203Pb][Pb(DOTA-2Py)], [203Pb][Pb(DOTA-3Py)]+ is shown in Table 2. The radio-HPLC traces of the human serum stability reactions are shown in Figure S14 in the supplementary data. All of the commercial and pyridine-based chelators were exceptionally stable (> 95% stability at 72 h), as shown by both iTLC and radio-HPLC (Figure S15).
Table 2.
In vitro stability of 203Pb-labeled chelator complexes at 37 °C in human serum (n = 3)
| 203Pb-complex % stable | Time point | |||
|---|---|---|---|---|
| 8 h | 24 h | 48 h | 72 h | |
| [203Pb][Pb(TCMC)]2+ | 98.0 ± 0.5% | 98.1 ± 0.2% | 98.2 ± 0.3% | 97.2 ± 0.7% |
| [203Pb][Pb(DOTA)]2− | 97.1 ± 0.7% | 97.3 ± 0.6% | 98.1 ± 0.2% | 97.4 ± 0.5% |
| [203Pb][Pb(DOTA-1Py)]− | 97.4 ± 0.4% | 97.7 ± 0.3% | 98.1 ± 0.4% | 97.5 ± 0.7% |
| [203Pb][Pb(DOTA-2Py)] | 97.7 ± 0.1% | 97.8 ± 0.1% | 97.8 ± 0.9% | 97.1 ± 0.2% |
| [203Pb][Pb(DOTA-3Py)]+ | 98.4 ± 0.3% | 98.0 ± 0.4% | 98.4 ± 0.1% | 97.7 ± 0.3% |
| Negative Control | 1.2 ± 0.3% | 1.4 ± 0.2% | 1.4 ± 0.2% | 1.5 ± 0.2% |
Discussion
203Pb and 212Pb produced at TRIUMF, either on the TR13 cyclotron or as a by-product of 500 MeV proton irradiation of 232Th, respectively, can be rapidly separated (< 3 h for 203Pb and < 1 h for 212Pb) with high radionuclidic purity (> 99%), moderate yield (73.8 ± 2.1% for 203Pb, 69.3 ± 4.4% for 212Pb), and a chemical purity that is suitable for pre-clinical screening of potential chelators to be used for theranostic purposes.
The direct elution of the Pb products into a radiolabeling compatible solution (1 M NH4OAc, pH 7) reduces the number of steps in the purification procedure and allows for rapid, immediate use of the product for potential clinical purposes, an advantage over methods that require the use of several columns or solution exchange, which can prolong the radiochemist’s exposure to radiation. The use of a single Pb-selective extraction resin allows for easy separation of 203Pb when the Tl target is dissolved in 2 M HNO3, Pb readily sorbs onto the resin while thallium passes through; thus there is no need for solution exchange to produce a column compatible loading solution, reducing the length of the procedure. In addition, when the nitric acid concentration is 2 M the capacity factor of Pb (k’Pb) is nearly 100 times greater than that of thallium (k’Tl) (Philip Horwitz et al. 1994). Due to the high capacity factor of Pb on this resin, a single 60 mg Pb resin column allowed for a 1700 fold reduction in Tl content, which is of importance for clinical purposes due to the high toxicity of Tl. As shown in Table 1, in the entire 3 mL elute 175 (+ 105) μg of thallium was found, and although the mass is below regulated toxicity levels (occupational limit of 0.1 mg/m3 = approx. 6.5 mg of thallium assuming an average body mass of 65 kg) (Kemnic and Coleman 2020), it would be ideal to further reduce Tl content to reduce potential radiolabeling interference.
During method development, we found that increasing the column mass from 30 to 60 mg reduced the percent of initial activity lost in the load from 40.3 + 1.0% to 20.5 + 0.5% (Figure S3). Further increasing the Pb-resin mass did not improve activity losses in the load fraction. The volume of the loading solution was then optimised, and it was found that reducing the loading volume from 30 to 20 mL reduced the loss of activity in the load fraction from 20.5 + 0.5% to 8.7 + 0.3% (Figure S4). Other investigators have loaded uncooled solutions onto columns (Li et al. 2017), however, in this study cooling the solution to ambient temperature prior to loading the column was found to be critical, as it was found that the average yield of eluted 203Pb dropped to 36.1 + 9.6% at elevated solution temperatures when compared to 73.8 + 2.1% cooled (Figure S5); we hypothesize that the reduction in yield in the former was likely due to damage caused to the resin’s structure upon exposure to the elevated temperature.
Previous animal studies have utilized approximately 6–7.5 MBq of 203Pb-labeled bioconjugate for imaging studies (Miao et al. 2008; Yang et al. 2019). In human studies by dos Santos and colleagues, 250–310 MBq of 203Pb-labeled bioconjugate were required for imaging and it was estimated that up to 750 MBq could be utilized in future dosimetry studies (dos Santos et al. 2019). With 201.9 MBq of 203Pb produced by irradiating enriched Tl at 8 μA for 4 h, it is reasonable to expect that with greater beam current and longer irradiation times that sufficient quantities of 203Pb can be produced to enable both preclinical and clinical studies.
Despite the use of high purity (99.99% metals basis) thallium, significant levels of stable Pb (1.49 + 0.66 μg) were found in the elute. At this level, experiments utilizing lower amounts of activity will need to carefully consider the impact of molar activity for radiolabeling and in vitro studies. Longer irradiation times and higher beam current will produce more 203Pb and thus further decrease this ratio. Radiolabeling results would be most improved if the source of the stable Pb was identified and the mass reduced. A potential source of stable Pb may be the thallium metal used for target manufacturing and future studies will evaluate different methods to increase molar activity.
Small (20 mCi) 224Ra generators are available, but their production is reliant on the extraction of 228Th from an aging stockpile of 232U (Nuclear Science Advisory Commitee : Isotopes Subcommmitee 2015). Due to the comparatively short half life of 224Ra (t1/2 = 3.63 d), a 224Ra/212Pb generator can only be used for 1–2 weeks, thus increasing costs of isotope production. 228Th/212Pb generators, however, could potentially be used for extended periods of time. Herein we have reported the production and isolation of 228Th as a by-product of the proton irradiation of 232Th on TRIUMF’s 500 MeV cyclotron and is isolated by peroxide-induced precipitation (Robertson et al. 2020). Previous generators used cation exchange columns and eluted 212Pb with water (Zucchini and Friedman 1982), which is not immediately radiolabeling compatible, while others utilized [228Th] barium stearate and collected 212Pb on glass walls (Hassfjell and Hoff 1994) and with bubblers (Hassfjell 2001), and although these generators were effective, scaled-up production would be challenging, unlike the generator introduced in these studies. Although there were radiochemical impurities in the final generator stock solution, as shown in Fig. 4, with the high selectivity of the Pb resin employed in the generator, none of these contaminants were observed in the elute fraction. The elute was also found to be low in stable Pb (6 + 6 ng in 3 mL), which resulted in higher radiolabeling yields, as observed in Fig. 6, for all chelators, thus further demonstrating the potential of the pyridine-based cyclen analogues (DOTA-xPy, x = 1–3) for chelation of Pb isotopes.
In previous animal studies, 0.2–7.4 MBq of 212Pb-labeled bioconjugates were used for biodistribution and therapy studies (Miao et al. 2005; Stallons et al. 2019). In human studies, dos Santos and colleagues predict the dose range for 212Pb-labeled bioconjugates to be 50 to 150 MBq (dos Santos et al. 2019). Upon assembly, the generator produced 9.780 + 0.002 MBq of 228Th and provided ~ 10 MBq of 212Pb on elution, capable of enabling both preclinical biodistribution and therapy studies. Although the current activity produced is not high enough for clinical applications, further scale up efforts are underway. The current purification procedure gives modest 212Pb yields of 69.3 + 4.4%, which may be due to the presence of approximately 8 g of 232Th present in the generator stock solution hindering the sorption of 212Pb to the column. The ideal resin mass used for the purification was 80 mg, as opposed to 60 mg for 203Pb, as it was found that larger masses did not increase the yield, but did increase the elute volume required to reach the same yield. The mass of Th found in the elute (24,352 ± 16,227 ppb, 73.1 + 48.6 μg) represents a separation factor of approximately 105 (see Table 1). Future work will examine process optimization that may further reduced Th burden.
All chelators showed quantitative RCYs (> 95%) at room temperature for both 203Pb and 212Pb at a concentration of 10− 4 M. However, at 10− 5 M the 212Pb radiolabeling yield was higher for all tested chelators and bioconjugates, demonstrating a positive effect of increased molar activity of the 212Pb compared to 203Pb. In order to avoid the accumulation of stable 208Pb, the terminus of the 212Pb decay chain, the generator was milked 24 h prior to radiolabeling tests and the next day’s elute was used for labeling immediately in order to minimize the grow in of the stable daughter. In addition to the commercial chelators, DOTA and p-SCN-Bn-TCMC, three pyridine-based cyclen ligands, DOTA-1Py, DOTA-2Py, and DOTA-3Py, were also screened for their ability to complex 203Pb/212Pb, as the softer pyridine (N-) donors were hypothesized to form stable metal-ligand coordinate bonds with the softer PbII ion (Price and Orvig 2014; Ramogida and Orvig 2013). At 10− 5 M and ambient temperature, it was observed that for the pyridine-based chelators, as the number of pyridine groups increased, so did the radiolabeling yield; this trend was observed for both 212Pb and 203Pb. With the greatest radiolabeling yield of the pyridine-based chelators and with high human serum stability (> 97% at 72 h), DOTA-3Py shows the greatest promise as a new Pb chelator and is a good candidate for incorporation into a bioconjugate for theranostic purposes.
Conclusion
Routine production of both members of the 212Pb/203Pb theranostic pair was established at TRIUMF. 228Th, a by-product of 232Th spallation on TRIUMF’s 500 MeV cyclotron, was used to produce a novel 228Th/212Pb generator and was combined with 203Pb production via thallium irradiation with 13 MeV protons. Both 203Pb and 212Pb were produced at quantities and purities (radionuclidic and chemical) acceptable for preclinical radiopharmaceutical screening. Increased irradiation times may lead to production at clinical quantities. Separation of the lead products was achieved using a Pb-selective extraction chromatographic resin in moderate yields (73.8 + 2.1% for 203Pb, 69.3 + 4.4% for 212Pb) in a form suitable for direct radiolabeling. The lead products were used to screen the radiolabeling ability and serum stability with commercially available (DOTA and p-SCN-Bn-TCMC) and pyridine-based DOTA derivative chelators (DOTA-1Py, DOTA-2Py, and DOTA-3Py). DOTA-1Py, −2Py, and -3Py all exhibited ability to complex 212Pb/203Pb at ambient temperature, with [212Pb/203Pb]Pb-DOTA-3Py showing the highest radiolabeling yield of the three. Further investigation of the PbII-coordination chemistry with these ligands as well as preparation of bioconjugates for in vivo studies are planned in the future. In conclusion, together these studies demonstrate the ability of TRIUMF to produce a theranostic pair that can be used for pre-screening potential radiopharmaceuticals at the pre-clinical level with the potential to increase production to the clinical level.
Supplementary Information
Additional file 1. Detailed ICP-MS results, activities of the components in the thorium precipitate solution, representative gamma spectra for 203Pb and 212Pb, elution profiles for radiochemical purification method development runs, MS and 1H NMR of Pb-complexes, representative radio-HPLC chromatograms of [203Pb]Pb-complexes and human serum stability studies, representative radio-iTLC chromatograms of 203Pb- and 212Pb-radiolabeling.
Acknowledgements
We would like to extend our gratitude to TRIUMF’s TR13 cyclotron operators, Linda Graham, Dave Prevost, Toni Epp, Ryley Morgan and Spencer Staiger. We also wish to thank Canadian Nuclear Laboratory for analyzing the ICP-MS samples.
Authors’ contributions
AKHR conceived the 228Th/212Pb generator and 203Pb production experiments. AKHR and BM developed and performed the 212Pb generator and 203Pb production and radiochemical purification experiments. BM performed all radiolabeling and stability studies and wrote the manuscript. HY and WF aided in isotope production and radiolabeling studies. CR provided oversight to the radiolabeling and metal complexation experiments and aided with manuscript preparation. CH assisted with the TR13 irradiation management. PS conceived, designed, and provided oversight to all aspects of the project. All authors read and approved the final manuscript.
Funding
Funding for this work was provided by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grants (CFR & PS). TRIUMF received funding via a contribution agreement with the National Research Council of Canada.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author(s) on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Na, Mg, Al, Ca, Ti, V, Cr, Mn, Fe, Ni, Co, Cu, Zn, Ga, Ge, As, Se, Rb, Sr, Zr, Nb, Mo, Ru, Rh, Pd, Ag, Cd, Sn, Te, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Tl, Pb, Th, Hg
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Brooke L. McNeil and Andrew K. H. Robertson contributed equally to this work.
Contributor Information
Caterina F. Ramogida, Email: cfr@sfu.ca
Paul Schaffer, Email: pschaffer@triumf.ca.
References
- Atcher RW, Friedman AM, Hines JJ. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int J Radiat Appl Instrum Part. 1988;39(4):283–286. doi: 10.1016/0883-2889(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Azzam A, Said SA, Al-abyad M. Evaluation of different production routes for the radio medical isotope 203Pb using TALYS 1.4 and EMPIRE 3.1 code calculations. Appl Radiat Isot. 2014;91:109–113. doi: 10.1016/j.apradiso.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212. Nucl Med Biol. 2013;40(5):592–599. doi: 10.1016/j.nucmedbio.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoś B, Lyczko K, Kasperek A, Krajewski S, Bilewicz A. Search of ligands suitable for 212Pb/212Bi in vivo generators. J Radioanal Nucl Chem. 2013;295(1):205–209. doi: 10.1007/s10967-012-2238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono J, Tolcher A, Rowinsky E. The future of cytotoxic therapy: selective cytotoxicity based on biology is the key. Breast Cancer Res. 2003;5(3):154–159. doi: 10.1186/bcr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke ET, Martell AE. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorg Chim Acta. 1991;190(1):37–46. doi: 10.1016/S0020-1693(00)80229-7. [DOI] [Google Scholar]
- dos Santos JC, Schäfer M, Bauder-Wüst U, Lehnert W, Leotta K, Morgenstern A, Kopka K, Haberkorn U, Mier W, Kratochwil C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: bringing “the lead” into PSMA-targeted alpha therapy? Eur J Nucl Med Mol Imaging. 2019:1–11. [DOI] [PMC free article] [PubMed]
- Elgqvist J, Frost S, Pouget J-P, Albertsson P. The potential and hurdles of targeted alpha therapy - clinical trials and beyond. Front Oncol. 2014;3:324. doi: 10.3389/fonc.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmestani K, Milenic DE, Brady ED, Plascjak PS, Brechbiel MW. Purification of cyclotron-produced 203Pb for labeling herceptin. Nucl Med Biol. 2005;32(3):301–305. doi: 10.1016/j.nucmedbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hassfjell S. A 212Pb generator based on a 228Th source. Appl Radiat Isot. 2001;55(4):433–439. doi: 10.1016/S0969-8043(00)00372-9. [DOI] [PubMed] [Google Scholar]
- Hassfjell SP, Hoff P. A generator for production of 212Pb and 212Bi. Appl Radiat Isot. 1994;45(10):1021–1025. doi: 10.1016/0969-8043(94)90170-8. [DOI] [Google Scholar]
- Henriksen G, Hoff P. Isolation of cyclotron produced 205Bi, 206Bi and 203Pb using a lead-selective extraction chromatographic resin. Appl Radiat Isot. 1998;49(4):357–359. doi: 10.1016/S0969-8043(97)00251-0. [DOI] [Google Scholar]
- Horlock P, Thakur M, Watson I. Cyclotron produced lead-203. Postgrad Med J. 1975;51:751–754. doi: 10.1136/pgmj.51.601.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianquan, Xu J, Hu L, Miao Y. A novel Pb-203-labeled DOTA-conjugated lactam bridge-cyclized alpha-MSH peptide for melanoma imaging. J Nucl Med. 2017;58(supplement 1):897. [Google Scholar]
- Kemnic TR, Coleman M. Thallium toxicity: StatPearls Publishing; 2020. [PubMed]
- Kumar Bharti S, Banerjee T, Brosh RM. Setting the stage for cohesion establishment by the replication fork. Cell Cycle. 2012;11:2114–2135. doi: 10.4161/cc.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxdal RE, Altman A, Kuo T. Beam measurements on a small commercial cyclotron. 1994. [Google Scholar]
- Li M, Zhang X, Quinn TP, Lee D, Liu D, Kunkel F, Zimmerman BE, McAlister D, Olewein K, Menda Y, Mirzadeh S, Copping R, Johnson F, Schultz M. Automated cassette-based production of high specific activity [203/212Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl Radiat Isot. 2017;127:52–60. doi: 10.1016/j.apradiso.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Máthé D, Szigeti K, Hegedűs N, Horváth I, Veres DS, Kovács B, Szűcs Z. Production and in vivo imaging of 203Pb as a surrogate isotope for in vivo 212Pb internal absorbed dose studies. Appl Radiat Isot. 2016;114:1–6. doi: 10.1016/j.apradiso.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, Quinn TP. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49(5):823–829. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman T, Quinn TP. Preclinical melanoma therapy via peptide-targeted α-radiation. Clin Cancer Res. 2005;11(15):5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- Milenic DE, Baidoo KE, Brechbiel MW. Bench to bedside: stability studies of GMP produced trastuzumab-TCMC in support of a clinical trial. Pharmaceuticals. 2015;8(3):435–454. doi: 10.3390/ph8030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh S. Generator-produced alpha-emitters. Appl Radiat Isot. 1998;49(4):345–349. doi: 10.1016/S0969-8043(97)00175-9. [DOI] [Google Scholar]
- Nuclear Science Advisory Commitee : Isotopes Subcommmitee. Meeting isotope needs and capturing opportunities for the future: Technical Report for the United States Department of Energy; 2015.
- Philip Horwitz E, Dietz ML, Rhoads S, Felinto C, Gale NH, Houghton J. A lead-selective extraction chromatographic resin and its application to the isolation of lead from geological samples. Anal Chim Acta. 1994;292(3):263–273. doi: 10.1016/0003-2670(94)00068-9. [DOI] [Google Scholar]
- Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43(1):260–290. doi: 10.1039/C3CS60304K. [DOI] [PubMed] [Google Scholar]
- Ramogida CF, Orvig C. Tumour targeting with radiometals for diagnosis and therapy. Chem Commun (Camb) 2013;49(42):4720–4739. doi: 10.1039/c3cc41554f. [DOI] [PubMed] [Google Scholar]
- Robertson AKH, Ladouceur K, Nozar M, Moskven L, Ramogida CF, D’Auria J, Sossi V, Schaffer P. Design and simulation of thorium target for Ac-225 production. AIP Conf Proc. 2017;1845(1):020019–1–020019–5. [Google Scholar]
- Robertson AKH, McNeil BL, Yang H, Gendron D, Perron R, Radchenko V, Zeisler S, Causey P, Schaffer P. 232Th-spallation-produced 225Ac with reduced 227Ac content. Inorg Chem. 2020;59(17):12156–12165. doi: 10.1021/acs.inorgchem.0c01081. [DOI] [PubMed] [Google Scholar]
- Robertson AKH, Ramogida CF, Schaffer P, Radchenko V. Development of 225Ac radiopharmaceuticals: TRIUMF perspectives and experiences. Curr Radiopharm. 2018;11(3):156–172. doi: 10.2174/1874471011666180416161908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösch F, Herzog H, Qaim SM. The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair 86Y and 90Y. Pharmaceuticals. 2017;10(2):1–28. doi: 10.3390/ph10020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallons TAR, Saidi A, Tworowska I, Delpassand ES, Torgue JJ. Preclinical investigation of 212Pb-DOTAMTATE for peptide receptor radionuclide therapy in a neuroendocrine tumor model. Mol Cancer Ther. 2019;18(5):1012–1021. doi: 10.1158/1535-7163.MCT-18-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrøm S, Generalov R, Bønsdorff TB, Larsen RH. Preparation of 212Pb-labeled monoclonal antibody using a novel 224Ra-based generator solution. Nucl Med Biol. 2017;51:1–9. doi: 10.1016/j.nucmedbio.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Yang H, Gao F, McNeil B, et al. Synthesis of DOTA-pyridine chelates for 64Cu coordination and radiolabeling of αMSH peptide. EJNMMI radiopharm. Chem. 2021;6:3. doi: 10.1186/s41181-020-00119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Xu J, Cheuy L, Gonzalez R, Fisher DR, Miao Y. Evaluation of a novel Pb-203-labeled lactam-cyclized alpha- melanocyte-stimulating hormone peptide for melanoma targeting. Mol Pharm. 2019;16(4):1694–1702. doi: 10.1021/acs.molpharmaceut.9b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova A, Eppard E, Kürpig S, Bundschuh RA, Schönberger S, Gonzalez-Carmona M, Feldmann G, Ahmadzadehfar H, Essler M. Theranostics in nuclear medicine practice. OncoTargets Therapy. 2017;10:4821–4828. doi: 10.2147/OTT.S140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisler S, Clarke B, Kumlin J, Hook B, Varah S, Hoehr C. A compact quick-release solid target system for the TRIUMF TR13 cyclotron. Instruments. 2019;3(1):16. doi: 10.3390/instruments3010016. [DOI] [Google Scholar]
- Zucchini GL, Friedman AM. Isotopic generator for 212Pb and 212Bi. Int J Nucl Med Biol. 1982;9:83–84. doi: 10.1016/0047-0740(82)90082-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Detailed ICP-MS results, activities of the components in the thorium precipitate solution, representative gamma spectra for 203Pb and 212Pb, elution profiles for radiochemical purification method development runs, MS and 1H NMR of Pb-complexes, representative radio-HPLC chromatograms of [203Pb]Pb-complexes and human serum stability studies, representative radio-iTLC chromatograms of 203Pb- and 212Pb-radiolabeling.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author(s) on reasonable request.