Abstract
The inhibition of the enzyme soluble epoxide hydrolase (sEH) has demonstrated clinical therapeutic effects in several peripheral inflammatory-related diseases, with 3 compounds in clinical trials. However, the role of this enzyme in the neuroinflammation process has been largely neglected. Herein, we disclose the pharmacological validation of sEH as a novel target for the treatment of Alzheimer’s disease (AD). Evaluation of cognitive impairment and pathological hallmarks were used in 2 models of age-related cognitive decline and AD using 3 structurally different and potent sEH inhibitors as chemical probes. sEH is upregulated in brains from AD patients. Our findings supported the beneficial effects of central sEH inhibition, regarding reducing cognitive impairment, neuroinflammation, tau hyperphosphorylation pathology, and the number of amyloid plaques. This study suggests that inhibition of inflammation in the brain by targeting sEH is a relevant therapeutic strategy for AD.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00854-1) contains supplementary material, which is available to authorized users.
Key Words: Soluble epoxide hydrolase, Inflammation, Tau, β-amyloid, Target engagement, Druggability
Introduction
Chronic inflammation is recognized as a key player in both the onset and progression of Alzheimer’s disease (AD) [1–3]. Indeed, 16% of the investment in ongoing clinical trials for AD is related to inflammation [4]. Neuroinflammation is intimately linked to the oxidative stress associated with AD [5, 6], controlling the interactions between the immune system and the nervous system [7, 8]. However, several antioxidant therapies and nonsteroidal anti-inflammatory drugs have failed in clinical trials. Therefore, it is of vital importance to expand the scope towards novel targets, preferably related to several pathophysiological pathways of the disease [9].
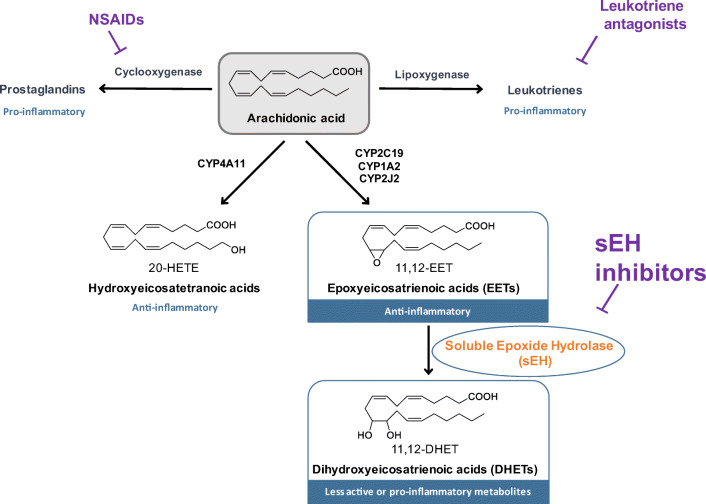
Epoxyeicosatrienoic acids (EETs) mediate vasodilatation, reduce inflammation, attenuate oxidative stress, and block the pathological endoplasmic reticulum (ER) stress response [10, 11]. The soluble epoxide hydrolase enzyme (sEH, EC 3.3.2.10, EPHX2), widely expressed in relatively high abundance in murine and human brains [12, 13], converts EETs and other epoxyfatty acids (EpFA) to their corresponding dihydroxyeicosatrienoic acids (DHETs), whereby diminishing, eliminating, or altering the beneficial effects of EETs [14] (Fig. 1).
Fig. 1.
The arachidonic acid cascade. The arachidonic acid (AA) cascade is a group of metabolic pathways in which AA and other polyunsaturated fatty acids are the central molecules. Metabolism via the cyclooxygenase (COX) and lipoxygenase (LOX) pathways gives rise to largely proinflammatory and proalgesic metabolites. Both pathways have been pharmaceutically targeted. CYP enzymes either hydroxylate or epoxidize AA leading to hydroxyeicosatetranoic acids (HETEs) or epoxyeicosatrienoic acids (EETs), respectively. The latter compounds, which are endowed with potent anti-inflammatory properties, are rapidly subjected to hydrolysis to their corresponding diols by the soluble epoxide hydrolase (sEH) enzyme. Inhibitors of sEH block this degradation and stabilize EET levels in vivo [14]. They also reduce the corresponding diols which have some inflammatory properties. Major CYPs that oxidize AA are listed in the figure, but many others make a contribution
Considering that several lines of evidence underline a broad involvement of signaling by EETs and other EpFA in the central nervous system (CNS) function and disease [15, 16] and that lack of sEH by genetic deletion improves the signs of AD in a mouse model [17], we hypothesized that brain-penetrant sEH inhibitors (sEHI) would stabilize EETs in the brain, resulting in a reduction of reactive oxygen species (ROS) and diminished neuroinflammation and neurodegeneration, leading to a positive outcome in AD. To this end, we studied the neuroprotectant role mediated by sEHI in 2 models ofL AD: the senescence-accelerated mouse prone 8 (SAMP8) and 5XFAD mice models. Because SAMP8, a paradigm of late-onset AD and cognitive impairment in age, is characterized by oxidative stress, neuroinflammation, tau hyperphosphorylation, and proamilodogenic APP processing, but lacks β-amyloid (βA) plaques [18–22], we studied plaque load and cognitive impairment in 5XFAD, a mouse model of early-onset AD, to unveil the effect of sEHI on this AD hallmark [23, 24].
Methods
Details of the experimental protocols, including chemicals, animals, novel object recognition test (NORT), biochemical and molecular methods, target engagement, drug properties characterization, and statistical analysis, are given in the Supplemental information.
Results
Changes in sEH Expression in the Hippocampus from AD Patients, SAMP8, and 5XFAD
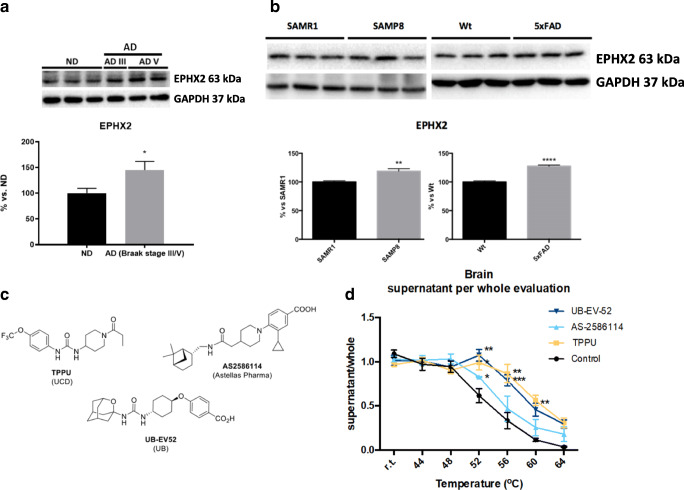
The key question was to determine whether sEH expression differs from healthy to pathological conditions. Results demonstrated that sEH levels were higher in AD patients’ brains (Braak III and V) in comparison with those of healthy individuals (Table S1 and Fig. 2a). Moreover, sEH expression was also elevated in SAMP8 and 5XFAD hippocampus in comparison with to their respective controls (Fig. 2b).
Fig. 2.
Soluble epoxide inhibition and its relevance in AD. a Immunoblot of sEH (EPHX2) of human brains from AD patients (Braak stage III-V). Groups were compared by Student t test (n = 4-7). *p < 0.05 vs. non-demented. b Immunoblot of sEH (EPHX2) in the hippocampus of SAMP8 mice (groups were compared by Student t test, n = 12-14, **p < 0.01 vs. SAMR1) and 5xFAD mice (groups were compared by Student t test, n = 12-14, ****p < 0.0001 vs. Wt). c Chemical structure of the sEH inhibitors employed. d CETSA experiments to monitor brain target engagement. Groups were compared by Student t test or 2-way ANOVA and post hoc Dunnett’s, n = 3 per group, *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control
On-Target Drug Inhibition of sEH
We evaluated 3 structurally different sEH inhibitors as chemical probes [25]: TPPU (UC1770, IC50 for human sEH = 3.7 nM) [26], AS-2586114 (IC50 for human sEH = 0.4 nM) [27], and UB-EV-52 (IC50 for human sEH = 9 nM) [28] (Fig. 2c). Previous pharmacokinetic data suggest that TPPU, a very well characterized sEH inhibitor, can enter into the brain [29]. It is known that AS-2586114 has a prolonged action in vivo and an ability to cross the blood–brain barrier (BBB) [30, 31]. UB-EV-52 is a new inhibitor somewhat related with previously reported adamantane-derived sEH inhibitors such as t-AUCB [32] and the clinically studied AR9281 (UC1153) [33]. To determine whether UB-EV-52 possesses drug-like characteristics, we performed in vitro ADMET assays. We found that UB-EV-52 has excellent solubility (> 100 μM at 37 °C in 5% DMSO: 95% PBS buffer) and good microsomal stability (Table S2), and does not inhibit drug-metabolizing cytochromes or the hERG channel (Table S3). Of relevance, some cytochromes are potential off-target effects of sEH (Fig. 1), because they are situated upstream in the arachidonic acid cascade. UB-EV-52 showed less than 5% inhibition of the studied cytochromes (CYP1A2, CYP2C9, CYP2C19, CYP3A4, and CYP2D6) at 10 μM (Table S3). As a preliminary assessment of brain permeability, UB-EV-52 was subjected to the parallel artificial membrane permeation assay-BBB (PAMPA-BBB), a well-established in vitro model of passive transcellular permeation [34]. UB-EV-52 was predicted to be able to cross the BBB (Table S4), which anticipates its ability to enter the brain. In order to characterize the toxicity of UB-EV-52, we evaluated cell viability in human neuroblastoma SH-SY5Y cells, using an MTT assay for cell metabolic activity and a propidium iodide stain assay for cell death. In both assays, UB-EV-52 showed no cytotoxicity at 1, 10, 50, and 100 μM (Table 1).
Table 1.
Cell viability in human neuroblastoma SH-SY5Y cells after 24 h exposure to UB-EV-52
| UB-EV-52 concentration | Metabolic activity (%) MTT (mean ± SEM)* |
Cell death (%) propidium iodide (mean ± SEM)* |
|---|---|---|
| 0 μM | 100.00 ± 3.72 | 0.00 ± 0.78 |
| 1 μM | 96.84 ± 5.01 | 1.47 ± 1.42 |
| 10 μM | 105.13 ± 4.75 | 0.95 ± 1.31 |
| 50 μM | 118.26 ± 8.09 | − 0.59 ± 1.34 |
| 100 μM | 120.43 ± 7.30 | 1.80 ± 0.97 |
*Results of 3 independent experiments performed in quadruplicated wells
To evaluate whether sEH is the direct binding target of the inhibitors in brain tissue, we performed an in vivo thermal shift assay (CETSA) [35]. The results showed a significant shift in the sEH melting curve in the hippocampus of CD-1 mice orally treated with TPPU, AS-2586114, and UB-EV-52, demonstrating in vivo compound-induced target stabilization, providing also evidence of central action (Fig. 2d).
To demonstrate that the tested compounds reduced sEH activity, we measured levels of regulatory lipid mediators in the cortex of treated and control SAMP8 mice. As shown in Fig. S1, the levels of proinflammatory lipid mediators in the cortex such as PGD2 and TXB2 are higher in the control group. At the same time, the anti-inflammatory epoxy fatty acids, including those from Ƴ-linoleic acid (EpODE) and other polyunsaturated fatty acids, are all higher in the treated groups, especially in the TPPU-treated group. These differences also verified the target engagement of inhibition of sEH in vivo.
Once demonstrated that the compounds tested were able to inhibit sEH at the brain level, we evaluated the pathological hallmarks and the cognitive impairment associated with AD in SAMP8 and 5XFAD.
sEH Inhibitors Reduce Biomarkers of Inflammation, Oxidative Stress, and Endoplasmic Reticulum Stress
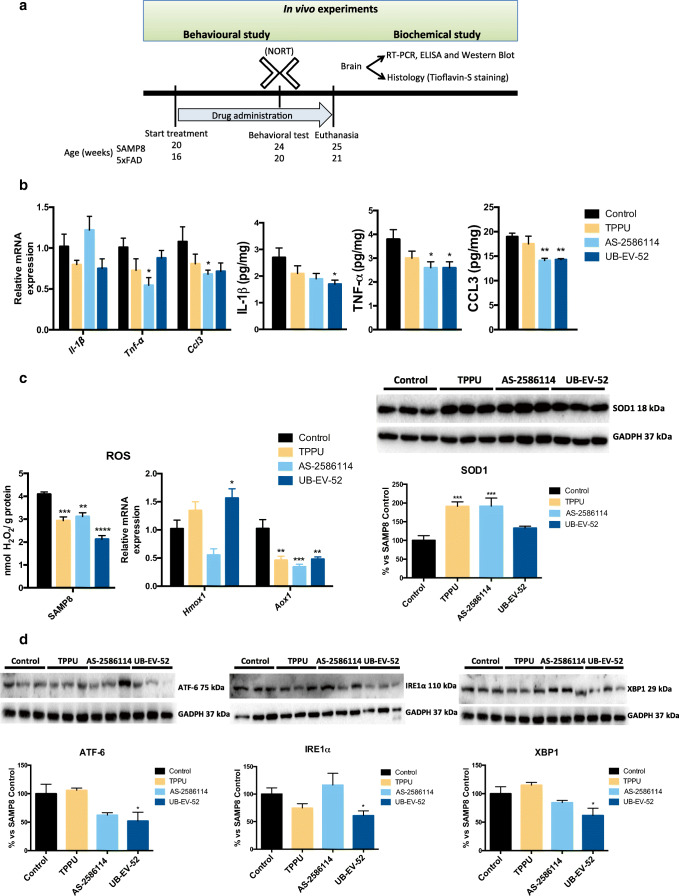
Indicators of brain neuroinflammation were determined after oral treatment with TPPU (5 mg/kg/day), AS-2586114 (7.5 mg/kg/day), and UB-EV-52 (5 mg/kg/day) (Fig. 3a and Fig. S2). The 3 inhibitors reduced gene expression and brain protein levels of the proinflammatory cytokines IL-1β (interleukin-β), CCL3 (C-C motif ligand 3), and, importantly, TNF-α (tumor necrosis factor-α) in SAMP8 (Fig. 3b) and in 5XFAD (data not shown). IL-1β is intimately involved in neuroinflammatory processes in the CNS [34], and its activity is thought to be closely tied to the process of memory consolidation [34, 35]. Chemokines play a critical role in phagocytic activity. Concretely, CCL3 is expressed in astrocytes and is described as a component of the inflammasome complex [36]. Of note, TNF-α not only is involved in AD-related brain neuroinflammation [37] but also contributes to amyloidogenesis via β-secretase regulation [38]. Additionally, these results suggest an involvement of the 2 main inflammasome-signaling pathways, NF-kβ and NLRP3 [39].
Fig. 3.
Role of sEH inhibitors in neurodegenerative biomarkers. a Scheme of experimental procedures in in vivo experiments. b Gene expression of neuroinflammatory markers (Il-1β, Tnf-α, and Ccl3) and protein levels of proinflammatory cytokines IL-1β, TNF-α, and CCL3 in the hippocampus of SAMP8 mice after treatment with sEH inhibitors. c Oxidative stress measured by hydrogen peroxide concentration in homogenates of the hippocampus. Representative gene expression of Hmox1 and Aox1 and representative Western blot and quantification of protein levels for (antioxidant enzyme) SOD1 in the hippocampus of SAMP8 mice after treatment with sEH inhibitors. d Representative Western blot and quantification of protein levels for ER stress markers ATF-6, IRE1α, and XBP1 in the hippocampus of SAMP8 mice after treatment with sEH inhibitors. Gene expression levels were determined by real-time PCR, cytokine protein levels by ELISA, and SOD1 by immunoblotting. Results are expressed as a mean ± SEM and were significantly different from the control group. Groups were compared by Student t test or by 1-way ANOVA and post hoc Dunnett’s, n = 4-6 per group, (*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001) vs. control. See partial correlations between selected variables in Fig. S2 and Table S7
To investigate the influence of the sEH inhibitors in the oxidative stress process, we determined the concentration of hydrogen peroxide in the brain of SAMP8 mice. The 3 inhibitors significantly reduce hydrogen peroxide (Fig. 3c). Moreover, determination of the brain oxidative machinery was addressed by evaluating the gene expression of Hmox1, Aox1, and protein levels of SOD1 (Fig. 3c). Hmox1 (antioxidant activity) [40] was significantly increased by UB-EV-52 and TPPU, but not by AS-2586114 (Fig. 3c). qPCR analysis also demonstrated that treated SAMP8 mice had lower Aox1 expression (Fig. 3c). Aox1 controls the production of hydrogen peroxide, and under certain conditions, can catalyze the formation of superoxide [41]. Furthermore, SOD1 (antioxidant activity) protein levels were significantly increased in all treated groups (Fig. 3c), indicating a reinforcement of the antioxidant response after treatment with sEHI [42]. By contrast, in 5XFAD, a model with reduced oxidative stress, no significant changes were determined in oxidative stress parameters evaluated (data not shown).
It is known that the ER stress plays a role in the pathogenesis of neurodegenerative diseases, including AD [43], and sEHI attenuate activation of the ER stress response [10]. Therefore, we measured the levels of the ER stress sensors ATF-6 and IRE1α (Fig. 3d). Especially, UB-EV-52 was able to reduce the levels of either protein. Furthermore, we evaluated XBP1, a major regulator of the unfolded protein response, which is induced by ER stress [44]. XBP1 was significantly reduced by UB-EV-52 and slightly decreased by AS-2586114, but not by TPPU (Fig. 3d). Altogether, these results suggest that the inhibition of sEH protects against oxidative stress and the associated ER stress in the brain.
sEH Inhibitors Modify the 2 Main Physiopathological Hallmarks of AD
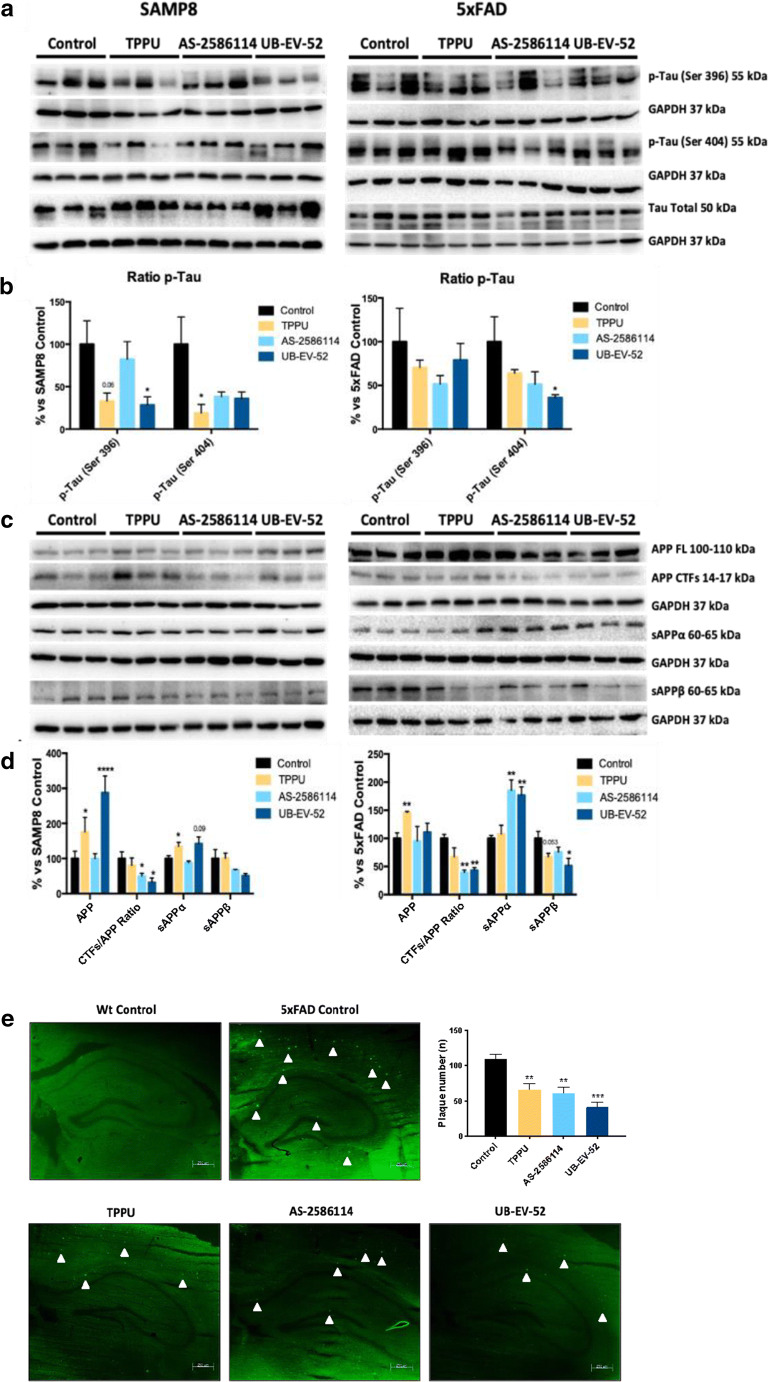
The brains of patients with AD contain 2 main physiopathological hallmarks: tangles of hyperphosphorylated tau protein and βA plaques. As mentioned, considering that SAMP8 has disturbances in tau hyperphosphorylation and APP processing but lacks βA plaques, 5XFAD was used to support the protective effect of sEHI in AD hallmarks studied. On the one hand, after oral treatment of SAMP8 and 5XFAD mice with TPPU (5 mg/kg/day), AS-2586114 (7.5 mg/kg/day), and UB-EV-52 (5 mg/kg/day), sEH inhibition provoked a reduction of the tau hyperphosphorylated species (Ser396 and Ser404), especially Ser404 (Fig. 4a, b), in agreement with the idea that oxidative stress can promote tau hyperphosphorylation and aggregation [45, 46]. On the other hand, we examined the ability of the sEHI to modify the amyloid processing cascade. Whereas the 5XFAD transgenic mouse model develops early aggressive hallmarks of amyloid burden and cognitive loss [47], SAMP8 is characterized by an abnormal amyloid precursor protein (APP) processing, with a misbalance to the amyloidogenic pathway. Importantly, C-terminal fragment (CTF) levels are strongly implicated in neurodegeneration and the cognitive decline process in SAMP8 [21, 22]. We observed a substantial decrease in the ratio of CTFs/APP protein levels in both mice models after treatment with sEHI (Fig. 4c, d). In addition, an increase of the sAPPα and a decrease of sAPPβ supported that sEHI are able to shift the APP processing towards the nonamyloidogenic pathway, thereby reducing the probability of increasing βA aggregation. Finally, treatment of 5XFAD mice with sEHI had a strong effect in reducing the number of βA plaques stained with thioflavin-S (by an average of 40%) (Fig. 4e), indicating the prevention of amyloid burden in a model characterized by βA plaques formation at early ages such as 2 months.
Fig. 4.
AD hallmarks in both SAMP8 and 5xFAD mice models after treatment with sEH inhibitors. a and b Representative Western blot and quantifications for p-Tau Ser396 and p-Tau Ser404. c and d Representative Western blot and quantifications for CTFs/APP ratio, sAPPα, and sAPPβ. e Histological images and quantification of amyloid plaques stained with Thioflavin-S in Wt and 5xFAD. Values in bar graphs are adjusted to 100% for a protein of the control group from each strain. Results are expressed as a mean ± SEM and were significantly different from the control group. Groups were compared by Student t test or by 1-way ANOVA and post hoc Dunnett’s, n = 12 per group (*significant at p < 0.05, **significant at p < 0.01, ***significant at p < 0.001, and ****significant at p < 0.0001). See partial correlations between selected variables in Fig. S2, Fig. S3, Table S7, and Table S8
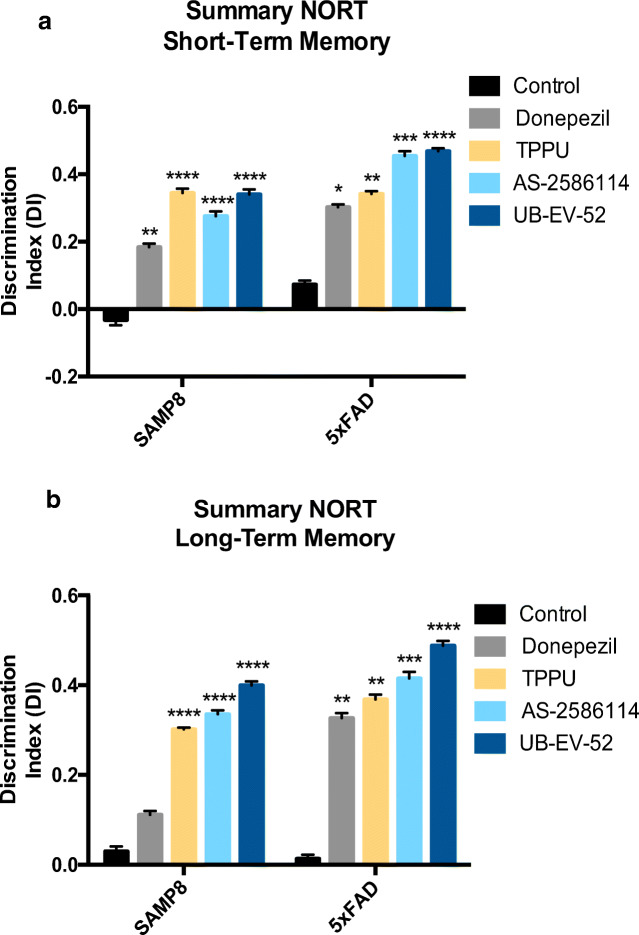
sEH Inhibitors Reduce Cognitive Impairment
To demonstrate the efficacy on the cognitive decline of the sEH inhibitors, we performed a NORT to obtain a measure of cognition for short- and long-term memory. Treatment of both murine models with the 3 sEH inhibitors drastically increased the discrimination index (DI). The significant increase indicates clear preservation of both memories (Fig. 5a, b). In both models, we add a comparator arm of mice treated with donepezil, which is a standard of care in the treatment of AD. As expected, donepezil treatment (5 mg/kg/day) also shifted the DI to values significantly higher than zero. Remarkably, in all the conditions, the sEH inhibitors reduced cognitive decline better than donepezil.
Fig. 5.
Characterization of the effect of sEH inhibitors and donepezil on cognitive status in both SAMP8 and 5xFAD mice models. a Short-term memory evaluation after 2 h acquisition trial by discrimination index and b long-term memory evaluation after 24 h acquisition trial by discrimination index after exposure to novel objects. Results are expressed as a mean ± SEM and were significantly different from the control group. Groups were compared by Student t test or by 1-way ANOVA and post hoc Dunnett’s, n = 12 per group (*significant at p < 0.05, **significant at p < 0.01, ***significant at p < 0.001, and ****significant at p < 0.0001). See partial correlations between selected variables in Fig. S3, Fig. S4, Table S7, and Table S8
Discussion
Our results suggest that the pharmacological stabilization of EETs in the brain has the potential to address multiple etiologies and physiopathological processes of AD, i.e., neuroinflammation, ER stress, and oxidative stress, increasing the chances of success of future therapies based on sEH inhibition. Although the decisive role of sEH inhibition in multiple inflammation-related diseases has been studied, only a few investigations have been conducted about its crucial role in the neuroinflammation process [15, 16, 30]. Besides, the question of if neuroinflammation is the malicious driver or “just” a consequence still represents an important conundrum in the AD field. Our findings reinforce the idea that neuroinflammation might drive the pathogenic process in AD. A partial correlation calculation has demonstrated that the anti-inflammatory effects of sEH inhibitors correspond with changes in AD hallmarks, slowing the progression of the disease and pushing up the cognitive capabilities in the studied animal models (Fig. S3-S4 and Table S5-S6).
A characteristic feature of acute inflammatory processes is a general increase in the levels of classic proinflammatory eicosanoids (prostaglandins, leukotrienes, and thromboxanes). In neurodegenerative diseases, there is a basal, chronic, and silent inflammation that is more related to a disbalance in proinflammatory and anti-inflammatory cytokines, such as those studied IL-1β [36], CCL3 [49], and TNF-α, as well as by acting on different mechanisms implied in neurodegeneration (e.g., increase of the oxidative stress, increase in the glutamate pathway, among others). Importantly in our landscape, βA activates inflammasomes that, in turn, mediate IL-1β maturation in microglial cells [47]. This allowed us to anticipate different biological and therapeutic outcomes for sEH inhibition than for the COX and LOX pathway inhibition.
Two structurally different sEH inhibitors have been proven to be safe in human clinical trials for other peripheral indications (AR9281 for hypertension and GSK2256294 for diabetes mellitus, chronic pulmonary obstructive disease, and subarachnoid hemorrhage) [26, 50]. A third inhibitor, EC5026, is in a human safety trial on a clinical path to chronic pain. This fact, undoubtedly, accelerates the development of new sEHI for the treatment of AD and avoids uncertainties about the possibility of angiogenic effects when inhibiting sEH. Of relevance, for this study, we have employed 3 structurally different sEH inhibitors, supporting the hypothesis that the biological outcomes observed are not because of off-target effects related to a particular inhibitor.
In summary, we have demonstrated that sEH levels are altered in AD mouse models and, more importantly, in the brain of AD patients. We have further shown that the inhibition of sEH has a plethora of beneficial central effects, such as reducing inflammation, ER stress, oxidative stress markers, p-tau pathology, and the amyloid burden. Consequently, sEHI improves the functional efficacy endpoint for cognitive status in neurodegeneration and AD animal models.
The anti-inflammatory effect of sEHI has been demonstrated in different pathologies [16, 17, 30]. Nevertheless, considering the results obtained in this work, we cannot rule out the beneficial effects of inhibition of sEH by regulating the processes of proteostasis and OS. Those effects should be implicated in the β-amyloid plaque removal and tau hyperphosphorylation reduction. Based on the results presented in this work, we firmly believe that inhibitors of sEH could represent an entirely new stand-alone treatment for the treatment of AD. However, herein, we do not demonstrate, because is it beyond the scope of this study, if inhibition of sEH could also represent an add-on therapy together with more symptomatic-like drugs, i.e., donepezil.
Electronic supplementary material
(PDF 1.12 kb)
(PDF 515 kb)
Acknowledgments
This study was supported by the Ministerio de Economía, Industria y Competitividad (Agencia Estatal de Investigación, AEI) and Fondo Europeo de Desarrollo Regional (MINECO-FEDER) (Projects SAF2017-82771, SAF2016-77703, Fundació La Caixa (project CI18-00002) and SAF2015-68749) and Generalitat de Catalunya (2017 SGR 106). S.C. and E.P. thank the Universitat de Barcelona and the Institute of Biomedicine of the Universitat de Barcelona (IBUB), respectively, for PhD grants. R.L. and D.P.-I. thank the Ministerio de Educación, Cultura y Deporte for PhD grants (FPU program). We would also like to thank Xunta de Galicia (ED431C 2018/21) and Ministerio de Economía, Industria y Competitividad (Innopharma Project) and Fondo Europeo de Desarrollo Regional (MINECO-FEDER). This study was also supported, in part, by grants from the National Institute of Environmental Health Sciences (NIEHS), the RIVER Award, NIEHS/R35 ES030443, and NIEHS Superfund Research Program P42 ES004699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Highlights
• sEH levels are increased in the AD human brain and in murine models.
• Inhibition of sEH reduces oxidative stress and inflammation in murine AD models.
• AD hallmarks in AD mice models are reduced after treatment with sEH inhibitors.
• sEH inhibitors improve cognition in AD mice models.
• sEH can be proposed as a new pharmacological target for AD therapy.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Griñán-Ferré, Email: christian.grinan@ub.edu.
Santiago Vázquez, Email: svazquez@ub.edu.
Mercè Pallàs, Email: pallas@ub.edu.
Carles Galdeano, Email: cgaldeano@ub.edu.
References
- 1.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 2012;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 2.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimer’s Dementia. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Fu W-Y, Wang X, Ip NY. Targeting neuroinflammation as a therapeutic strategy for Alzheimer’s disease: mechanisms, drug candidates, and new opportunities. ACS Chem. Neurosci. 2019;10:872–879. doi: 10.1021/acschemneuro.8b00402. [DOI] [PubMed] [Google Scholar]
- 4.Kodamullil AT, Zekri F, Sood M, et al. Tracing investment in drug development for Alzheimer disease. Nat. Rev. Drug Discovery. 2017;16:819. doi: 10.1038/nrd.2017.169. [DOI] [PubMed] [Google Scholar]
- 5.Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discovery. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 9.Makin S. The amyloid hypothesis on trial. Nature. 2018;559:S4–S7. doi: 10.1038/d41586-018-05719-4. [DOI] [PubMed] [Google Scholar]
- 10.Inceoglu B, Bettaieb A, Haj FG, Gomes AV, Hammock BD. Modulation of mitochondrial dysfunction and endoplasmic reticulum stress are key mechanisms for the wide-ranging actions of epoxy fatty acids and soluble epoxide hydrolase inhibitors. Prostaglandins Other Lipid Mediators. 2017;133:68–78. doi: 10.1016/j.prostaglandins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Davis CM, Alkayed NJ. P450 eicosanoids and reactive oxygen species interplay in brain injury and neuroprotection. Antioxid. Redox Signaling. 2018;28:987–1007. doi: 10.1089/ars.2017.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163:646–661. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Sura P, Sura R, EnayetAllah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J. Histochem. Cytochem. 2007;56:551–559. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Luo G, Zhang LF, Geng HX. Neuroprotective effects of epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediators. 2018;138:9–14. doi: 10.1016/j.prostaglandins.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol. Ther. 2017;180:62–76. doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HT, Lee KI, Chen CH, Lee TS. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation. 2019;16:267. doi: 10.1186/s12974-019-1635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley JE, Farr SA, Flood JF. Antibody to amyloid β protein alleviates impaired acquisition, retention, and memory processing in SAMP8 mice. Neurobiol. Learn. Mem. 2002;78:125–138. doi: 10.1006/nlme.2001.4047. [DOI] [PubMed] [Google Scholar]
- 19.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 20.Akiguchi I, Pallàs M, Budka H, et al. SAMP8 mice as a neuropathological model of accelerated aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology. 2017;37:293–05. doi: 10.1111/neup.12373. [DOI] [PubMed] [Google Scholar]
- 21.Pallàs M. Senescence-accelerated mice P8: a tool to study brain aging and Alzheimer’s disease in a mouse model. ISRN Cell Biol. 2012;2012:917167. [Google Scholar]
- 22.Griñan-Ferré C, Palomera-Ávalos V, Puigoriol-Illamola D, et al. Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp. Gerontol. 2016;80:57–69. doi: 10.1016/j.exger.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Oakley H, Cole SL, Logan S, et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5xFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrowsmith CH, Audia JE, Austin C, et al. The promise and peril of chemical probes. Nat. Chem. Biol. 2015;11:536–541. doi: 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose TE, Morisseau C, Liu JY, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics and reduction of inflammatory pain. J. Med. Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.M. Miura, I. Sato, H. Kiyohara, et al. Cyclic amino compound, or salt thereof. JP2011016743 (2011).
- 28.S. Vázquez, E. Valverde, R. Leiva, M. Vázquez-Carrera, S. Codony, Analogs of adamantylureas as soluble epoxide hydrolase inhibitors. WO 2017017048 (2017).
- 29.Ostermann AI, Herbers J, Willenberg I, et al. Oral treatment of rodents with soluble epoxide hydrolase inhibitor 1-(1-propanoylpiperidin-4-yl-)-3-[4-(trifluoromethoxy)phenyl]urea (TPPU): resulting drug levels and modulation of oxylipin pattern. Prostaglandins Other Lipid Mediators. 2015;121:131–137. doi: 10.1016/j.prostaglandins.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taguchi N, Nakayama S, Tanaka M. Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice. Neurosci. Res. 2016;111:56–63. doi: 10.1016/j.neures.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Ma M, Ren Q, Fujita Y, Ishima T, Zhang JC, Hashimoto K. Effects of AS2586114, a soluble epoxide hydrolase inhibitor, on hyperlocomotion and prepulse inhibition deficits in mice after administration of phencyclidine. Pharmacol. Biochem. Behav. 2013;110:98–103. doi: 10.1016/j.pbb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J. Med. Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, Whitcomb E, MacIntyre V, et al. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J. Clin. Pharmacol. 2013;52:319–328. doi: 10.1177/0091270010397049. [DOI] [PubMed] [Google Scholar]
- 34.Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003;38:223–232. doi: 10.1016/s0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 35.Martínez Molina R, Jafari M, Ignatushchenko M, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science. 2013;341:84–87. doi: 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 36.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JH, Johnson RE, O’Banion MK. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, H.O.Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7778–83. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andjelkovic AV, Kerkovich D, Shanley J, Pulliam L, Pachter JS. Expression of binding sites for beta chemokines on human astrocytes. Glia. 1999;28:225–35. [PubMed] [Google Scholar]
- 39.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 40.Si Z, Wang X, Zhang Z, et al. Heme oxygenase 1 induces tau oligomer formation and synapse aberrations in hippocampal neurons. J. Alzheimers Dis. 2018;65:409–419. doi: 10.3233/JAD-180451. [DOI] [PubMed] [Google Scholar]
- 41.Wright RM, Weigel LK, Varella-Garcia M, Vaitaitis G, Repine JE. Molecular cloning, refined chromosomal mapping and structural analysis of the human gene encoding aldehyde oxidase (AOX1), a candidate for the ALS2 gene. Redox Rep. 2016;3:135–144. doi: 10.1080/13510002.1997.11747101. [DOI] [PubMed] [Google Scholar]
- 42.Murakami K, Murata N, Noda Y, et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid β protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017;13:477–491. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 44.Corona C, Pensalfini A, Frazzini V, Sensi SL. New therapeutic targets in Alzheimer’s disease: brain deregulation of calcium and zinc. Cell Death Dis. 2011;2:e176. doi: 10.1038/cddis.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girard SD, Baranger K, Gauthier C, Jacquet M, et al. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer's disease. J. Alzheimers Dis. 2013;33:781–796. doi: 10.3233/JAD-2012-120982. [DOI] [PubMed] [Google Scholar]
- 47.J .Couturier, I.C. Stancu, O. Schakman et al. Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J Neuroinflammation. 27, 13-20 (2016). [DOI] [PMC free article] [PubMed]
- 48.Lazaar AL, Yang L, Boardley RL, Goyal NS, et al. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br. J. Clin. Pharmacol. 2016;81:971–979. doi: 10.1111/bcp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.H. Braak, E. Braak, in Cerebral Cortex, Neurodegenerative and age-related changes in structure and function of cerebral cortex, A. Peters, J. H. Morrison, Eds. (Kluwer Academic/Plenum Publisher, New York, 1999), vol. 14, pp. 475–512.
- 50.J Bhattacharya S, Maelicke A, Montag D. Nasal application of the galantamine pro-drug Memogain slows down plaque deposition and ameliorates behavior in 5X Familial Alzheimer’s disease mice. Alzheimers Dis. 46,123-36(2015) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.12 kb)
(PDF 515 kb)