Abstract
Early descriptions of subtypes of Parkinson’s disease (PD) are dominated by the approach of predetermined groups. Experts defined, from clinical observation, groups based on clinical or demographic features that appeared to divide PD into clinically distinct subsets. Common bases on which to define subtypes have been motor phenotype (tremor dominant vs akinetic-rigid or postural instability gait disorder types), age, nonmotor dominant symptoms, and genetic forms. Recently, data-driven approaches have been used to define PD subtypes, taking an unbiased statistical approach to the identification of PD subgroups. The vast majority of data-driven subtyping has been done based on clinical features. Biomarker-based subtyping is an emerging but still quite undeveloped field. Not all of the subtyping methods have established therapeutic implications. This may not be surprising given that they were born largely from clinical observations of phenotype and not in observations regarding treatment response or biological hypotheses. The next frontier for subtypes research as it applies to personalized medicine in PD is the development of genotype-specific therapies. Therapies for GBA-PD and LRRK2-PD are already under development. This review discusses each of the major subtyping systems/methods in terms of its applicability to therapy in PD, and the opportunities and challenges designing clinical trials to develop the evidence base for personalized medicine based on subtypes.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00894-7) contains supplementary material, which is available to authorized users.
Key Words: Parkinson’s disease, subtypes, therapy
PD Subtypes: a Brief History
The heterogeneity of Parkinson’s disease (PD) has long been recognized, even in the early description by James Parkinson [1]. The idea that this heterogeneity is dividable into discrete subtypes is also not new, with proposals that PD is not one but many diseases dating back decades [2, 3]. Early descriptions of subtypes of PD are dominated by the approach of pre-determined groups. Experts defined, from clinical observation, groups based on clinical or demographic features that appeared to divide PD into clinically distinct subsets. These early investigations into PD subtypes focused mainly on motor features of the disease and divided PD patients into some variation of tremor dominant and akinetic-rigid or postural instability gait disorder (PIGD) phenotypes [4]. Explorations of motor subtypes identified differences based on the propensity to develop cognitive impairment and rate of accumulation of motor disability, both more likely in the akinetic-rigid or PIGD forms [4, 5]. Cross-sectional differences were also seen in other symptoms such as olfactory impairment [6] and age at onset [7]. Later, pathological differences between the motor subtypes were demonstrated [8].
Another common basis on which to subdivide patients has been age at onset, with a more rapid accumulation of motor and cognitive disability in those with older onset [9, 10]. Other methods for defining subtypes have been based on sex [11, 12], or predominant nonmotor symptoms (depressed vs not depressed, cognitive profile, autonomic, sleep disturbance) [13–15]. There has been minimal subtyping based on biomarkers to date, with the exception of genetic subtypes of PD. Many studies have examined the unique features of individuals with PD carrying different genetic variants, particularly the two most common, leucine-rich repeat kinase 2 (LRRK2) and glucocerebrosidase (GBA) mutations [16–20].
Recently, hypothesis-free, data-driven approaches have been used to define PD subtypes. Data-driven subtyping takes an unbiased statistical approach to the identification of subgroups. The vast majority of data-driven subtyping has been done based on clinical features. These analyses allow a multidimensional exploration of the data and the placement of individuals into clusters based on the co-occurrence of features. The first data-driven subtyping study defined three subtypes described as motor only, motor and executive dysfunction progressing to global cognitive impairment, and older onset characterized by rapid motor and cognitive progression [21]. Since that first data-driven subtyping study, a number of similar studies have followed, producing different combinations and numbers of subtypes (although common variables included in the cluster descriptors are age, rate of progression, motor and cognitive impairment) [22]. The differences between the resulting subtypes likely reflect differences in the cohort characteristics and the input variables used to define the clusters.
There are several possible goals of dividing PD into subtypes. Broadly, subtyping may be used to guide clinical care or research. In research, it may provide more homogeneous groups within which to investigate pathophysiology of disease, or to guide the design of clinical trials. In clinical care, subtypes may assist in counseling regarding prognosis, should the subtypes have different rates of progression of symptoms, or may guide treatment if subtypes are associated with response to therapy. Given the broad spectrum of symptoms and the variable response and tolerability of current symptomatic treatments between patients, there is great interest in the applicability of described subtypes to advance personalized medicine in PD. Not all of the subtyping methods mentioned above have established therapeutic implications. This may not be surprising given that they were born from purely clinical observations of phenotype and not in observations regarding treatment response or biological hypotheses. We will discuss each of the major subtyping systems/methods in terms of its applicability to therapy in PD, and the opportunities and challenges designing clinical trials to develop the evidence base for personalized medicine based on subtypes.
Therapy of PD Subtypes
Subtypes Based on Age
Age has been considered a sole criterion for pre-defined PD subtypes. Late-onset PD (frequently defined as above age 70) is associated with a higher prevalence of tremor [23–25], greater axial involvement [26], and a faster motor progression [27, 28]. In contrast, early-onset PD (diagnosis before the age of 45) has been associated with a higher prevalence of dystonia [24] and a lower risk of dementia [28]. A lesser motor response to dopaminergic treatment [29] and greater risk of psychosis with levodopa/carbidopa treatment [25] have been found in late-onset PD, and the early-onset PD group has a higher incidence of levodopa-associated dyskinesia [25, 30], but not of motor fluctuations [30]. Recently, age at diagnosis was associated with increased risk for death in a data-driven PD subtypes study [31].
These differences have therapeutic implications and age is considered in the clinical management of PD (for summary, see Table 1) although some have pointed out that the research conducted on the role of age in the clinical presentation in PD may be marred by methodological issues related with small sample size, sampling bias, and the variable cutoff adopted for age in the different studies [27]. Older patients are likely to have poorer tolerability to some symptomatic pharmacological options such as dopamine agonists and anti-cholinergics, and levodopa is more frequently selected as first line therapy in this age group [26]. The higher incidence of motor complications and lesser cognitive problems in early-onset PD favors more frequent use of dopaminergic agents other than levodopa to delay the onset of motor complications [32]. Older age is considered a relative contra-indication for deep brain stimulation (DBS). At the time of DBS, older age (> 70) is associated with postoperative delirium [33] and has a lesser benefit to quality of life [34] than when performed in younger patients. Along the same lines, the clinical characteristics of PD subtypes defined by age at the time of diagnosis may be confounded by the presence of brain co-pathology, an aspect rarely evaluated in PD subtypes studies pre-defined by age. For example, Alzheimer’s disease (AD) or cerebrovascular disease is associated with features found in older onset PD such as dementia [35, 36], a lesser responsiveness to dopaminergic treatment and postural instability [37]. Consequently, diagnostic tests to assess the presence of these conditions deserve special attention to better inform prognosis and anticipate associated symptoms.
Table 1.
Personalized medicine approaches by PD subtype
| Subtype | Therapeutic strategy | |
|---|---|---|
| Age | Young onset |
• Monitor for emergence of motor fluctuations and dyskinesias • Consider dopamine agonists to delay motor complications |
| Older onset |
• Caution when using dopamine agonists, anti-cholinergics, or deep brain stimulation • Consider the role of co-pathology (e.g., Alzheimer’s disease or cerebrovascular disease), if atypical cognitive or motor features appear |
|
| Motor | Tremor dominant | • May consider anti-cholinergic drugs, clozapine, or deep brain stimulation for levodopa-refractory tremor affecting quality of life |
| Akinetic-rigid |
• Early referral to physical therapy to reduce fear of falling and prevent falls • Consider GPi as a deep brain stimulation target (vs. STN) for significant gait impairment |
|
| Nonmotor | Cholinergic: Early cognitive decline and falls |
• Consider intervention with cholinesterase inhibitors, e.g., rivastigmine (E) • Avoid anti-cholinergics from the onset or drugs with anti-cholinergic potential as well as dopamine agonists, unless specifically required • Early bone health screening to ensure optimal management of osteoporosis due to higher risk of falls and fractures • Physiotherapy and exercise programs focused on gait and falls prevention |
| Noradrenergic: dysautonomia, esp. delayed gastric emptying and constipation |
• Monitor for postural hypotension, treat as required, e.g., droxidopa (E) • Monitor for constipation, treat as required, e.g., polyethylene glycol (E), lubiprostone (E), probiotics (LE) • Screen for delayed gastric emptying. • Consider use of nonoral dopaminergic therapies • Screen and counsel for RBD • Lifestyle advice for peripheral sympathetic dysfunction such as peripheral pain and thermoregulatory function |
|
| Serotonergic: somnolence, fatigue |
• Avoid dopamine agonists with a D3 activity (e.g., pramipexole, ropinirole) • Consider proactive recognition and treatment of fatigue, e.g., rasagiline (E) • Evaluate and treat sleep-disordered breathing (continuous positive airway pressure, E), if present • Lifestyle advice regarding driving and other hazardous occupations, in which sleepiness could be a problem |
|
| Genetic subtypes | GBA-PD |
• Avoid use of anti-cholinergics and dopamine agonists in cognitive impairment • Cautious use of deep brain stimulation given poorer outcomes |
| LRRK2-PD | • As in idiopathic PD | |
E = efficacious; LE = likely efficacious (according to the International Parkinson’s and Movement Disorders Society Evidence-Based Medicine Review [55])
Motor Subtypes
Patients with PD have been typically divided into those with tremor and those with predominant axial symptoms, the latter being labeled the postural instability and gait dysfunction (PIGD) or akinetic-rigid type. Among the motor features of PD tremor has particular implications in management (Table 1). Tremor may not respond significantly to dopaminergic treatments in a significant proportion of patients [38, 39]. In clinical practice, there are therapeutic options considered specifically for an anti-tremor effect when it is refractory to dopaminergic agents. Anti-cholinergics are a classic example, though the level of evidence is low, and its current use limited due to the risk of cognitive impairment [40]. Clozapine, an atypical neuroleptic, has been shown to improve tremor in PD [41]. Despite the need for white blood cell count monitoring due to the risk of agranulocytosis, clozapine may be considered in clinical practice for cases of severe tremor refractory to dopaminergic treatment when other options such as DBS or lesion therapy are not available. Finally, tremor is the single motor feature in PD that may improve with DBS regardless of being responsive or not to dopaminergic treatment [42]. It should be remembered that commonly patients with more severe tremor (score ≥ 3 in tremor items of the MDS-UPDRS [43] or the preceding UPDRS) [44] have been excluded from clinical trials, limiting the transferability of efficacy results of motor symptomatic therapies into clinical practice for this group of patients.
The presence of axial features in the PIGD motor subtype has relevant therapeutic implications, as these symptoms may become less responsive to pharmacotherapy with disease progression [29]. These patients are at particular risk of recurrent falls, a major landmark in the natural history of PD that, together with dementia, are associated with loss of autonomy and institutionalization [45]. Physical therapy referral for falls prevention strategies is recommended in this population [46]. In those with gait impairment that is responsive to dopaminergic treatment, GPI-DBS may be preferred over STN-DBS in cases of significant gait impairment pre-DBS [43, 44, 47]. As outlined earlier, the motor PD subtype may have prognostic value, with a PIGD phenotype being associated with worse prognosis in terms of cognitive impairment and motor disability [4, 5]. The application of this information in clinical trials of disease-modifying therapies is greatly limited by recent observations that the individual patient can transit between motor PD subtypes (particularly from the tremor dominant form to other forms) both earlier in the disease [48, 49] and at more advanced stages [50, 51].
Nonmotor Subtypes
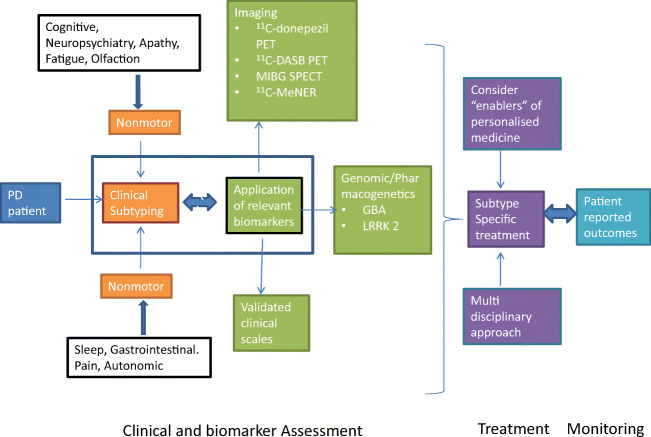
PD is a syndromic condition with great heterogeneity of specific nonmotor symptoms (NMS) presentations. NMS-dominant subtypes are evident from cluster analysis based on clinical phenotype studies [52, 53]. This concept can form a basis for delivery of personalized treatment strategies, a relatively new concept in PD (Fig. 1) [54]. This includes emerging personalized genomic-based therapeutic strategies specifically targeting GBA and LRRK2 mutation positive PD or nonmanifesting carriers in which nonmotor symptom dominant clinical phenotypes are emerging [53].
Fig. 1.
Integration of subtyping (endophenotyping) into personalized medicine in Parkinson’s disease, adapted from [119]. Patients with Parkinson’s disease can be clinically subtyped anchored on the dominant nonmotor symptoms (NMS) evident in the denovo or early motor stage using specific biomarkers (imaging or genetic) where available. The resulting subtype can then be clinically managed using a subtype-specific treatment approach which incorporates several strands of personalized medicine and multi-disciplinary input as well as monitoring with patient related outcome measures (PRO)
Cluster analysis-based subtypes reveal either nonmotor dominant phenotype or a mixed motor and nonmotor presentation [13]. For personalized treatment, and specifically if one is to follow the recently published International Parkinson and Movement Disorders Society evidence-based guidelines for management of NMS in PD then clinical subtypes are highly relevant [55]. Subtypes such as Park Cognitive, Park Pain, Park Apathy, and Park Sleep are postulated to be associated with the impairment of specific neurotransmitter system(s): cholinergic, noradrenergic, serotonergic, and mixed dopaminergic [13, 53, 56]. Individual studies anchored on a specific NMS have focused on exploring specific biomarkers underpinned by a specific neurotransmitter. The neurotransmitter-based subtyping concept brings together clinical subtyping (as in multiple sclerosis, progressive supranuclear palsy or multiple system atrophy subtypes), coupled with biomarkers (neutrotransmitter based nuclear/PET imaging) where available. Personalized strategies for the neurotransmitter underpinned subtypes relevant to clinical practice have been proposed for adoption in some healthcare systems such as the United Kingdom’s National Institute for Clinical Excellence (NICE). Examples are provided below and summarized in Table 1.
The cholinergic or cognitive dysfunction dominated presentations are possibly the best characterized and are more frequent in late-onset PD patients who show early cognitive deficit on screening [52]. Up to 40% of such patients can progress to early dementia and may have concurrent gait related issues such as freezing and falls in the ON state [57]. Positron emission tomography (PET) studies (e.g., with donepezil PET) suggest cholinergic involvement of the cortical and gastrointestinal tract possibly from the prodromal stage [58, 59]. For these patients, a personalized management package should include:
Early counseling and advanced planning concerning a high probability of cognitive impairment proceeding to dementia including advice regarding employment, lifestyle, and pension plans.
Consideration of use of cholinesterase inhibitors, along with dopamine replacement therapy as clinically dictated.
Avoiding anti-cholinergics from the onset or drugs with anti-cholinergic potential as well as dopamine agonists.
Early bone health screening to ensure optimal management of osteoporosis as there is higher risk of falls and fractures.
Physiotherapy and exercise programs focused on gait and falls prevention.
PET studies using the serotonin transporter ligand 11C-DASB supports the existence of a serotonergic subtype dominated by somnolence, sudden onset of sleep resembling narcolepsy as well as central fatigue [60, 61]. In patients with fatigue, as compared to either healthy controls or age, motor stage and dopamine uptake-matched nondepressed, nonsomnolent and nonfatigued PD, there is selective reduction of serotonergic uptake in the limbic striatum and right insula [60]. Other studies have found a serotonergic uptake deficiency in the raphe area in somnolent PD only [61]. MRI studies examining cortical thickness and subcortical nuclei volume have linked both fatigue and somnolence to thalamic atrophy as well [62]. There may also be susceptibility to levodopa-induced dyskinesias [63]. This subgroup needs specific personalized strategies, including:
Avoidance of dopamine D3 receptor active agents such as pramipexole and ropinirole.
Considering proactive recognition and treatment of fatigue based on the MDS recommendations [55].
Lifestyle advice regarding driving and other hazardous occupations in which sleepiness could be a problem.
The posited noradrenergic subtype is dominated by focal or generalized dysautonomia [64]. The burden of the dysautonomia appears to be largely borne by the gastrointestinal system, namely with delayed gastric emptying (DGE) and constipation. Studies focused on nonbrain imaging confirm widespread gut, cardiac, and peripheral noradrenergic dysfunction [65]. In a study of PD with and without REM sleep behavior disorder (RBD) and using 11C-MeNER (a noradrenergic ligand) PET imaging, reduced binding correlated with EEG slowing, cognitive performance, and orthostatic hypotension in those with RBD supporting the concept of RBD being a clinical marker for the noradrenergic subtype usually dominated by dysautonomia [66]. Peripheral noradrenergic involvement can be further characterized by abnormalities on cardiac MIBG SPECT scans showing noradrenergic denervation in PD. Cognitive impairment can also co-exist suggesting a clinical overlap between the noradrenergic and cholinergic subtypes. There is also pathophysiological overlap as cholinergic and glutamatergic mechanisms within the sublaterodorsal nucleus of the brainstem postulated as being involved in the control of sleep paralysis during REM sleep [52, 65, 67]. A pragmatic personalized medicine approach should include:
Early screening and management of symptomatic postural hypotension.
Screening and awareness of DGE with earlier use of nonoral dopaminergic therapies and use of prokinetic agents (e.g., domperidone).
Screening for the emergence of REM behavior disorder [55].
Early monitoring and management of symptoms related to peripheral sympathetic dysfunction such as peripheral pain and thermoregulatory dysfunction.
For the most common presentation of a range of NMS and parkinsonism, the management approach needs to focus on the most intrusive NMS as judged by the patient and carer. The MDS recommendation on evidence-based guideline for NMS in PD is useful [55].
Despite the above recommendations, there are substantial knowledge gaps regarding the treatment of NMS-based subtypes. The management options for NMS in PD are scarce and warrant more clinical research to address the current gaps in care. Studies of neurotransmitter-based subtypes have been cross-sectional so far and based on individual studies which have linked neurotransmitter specific central or peripheral biomarkers. It is conceivable that, if followed longitudinally, the subtypes might converge substantially. From cross-sectional analyses, however, it appears that the differences are more obvious in de novo and early motor stages of PD. As mentioned above, there is considerable instability of motor subtypes requiring shifting therapeutic approaches over time and the same may be true for nonmotor features. It is a common evolution within individuals that early disease is dominated by levodopa-responsive tremor or bradykinesia, whereas later disease is dominated by levodopa-resistant postural instability with falls and other levodopa-resistant features such as urinary dysfunction, orthostatic hypotension, and cognitive impairment requiring entirely different therapeutic strategies.
A separate strand that is emerging is the genetic basis for personalized NMS treatment in PD endophenotypes underpinned by LRRK2 and GBA mutations [68]. Preliminary evidence suggests a link of cognitive dysfunction and a higher NMS burden in GBA mutation positive PD as well as carriers [69–71]. LRRK2 mutation carriers (G2019S mutation) appear to have a greater propensity for a range of sleep dysfunction [68]. Management strategies are discussed in the following section.
Genetic Subtypes
The existence of genetic forms of PD has a potential impact on therapeutics. Causative genes or genetic risk factors represent an intuitive target for disease-modifying therapies in PD that are potentially generalizable to a broader group of patients. We illustrate these aspects with GBA- and LRRK2-related PD, in which there is active therapeutic development.
GBA mutations are the most common genetic risk factor for PD, with 7 to 10% of PD patients carrying a GBA mutation [72, 73]. The prevalence is higher (estimated to be 17.6%) in the Ashkenazi Jewish population [74]. PD patients carrying a pathogenic GBA mutation have an earlier onset of symptoms, and the PIGD phenotype is more prevalent compared with sporadic PD [17, 75]. Nonmotor symptoms such as depression or anxiety [76] and cognitive impairment [77] are more frequent, though significant cognitive impairment is not generally present at clinical presentation [17]. Faster progression [75, 78, 79] and reduced survival [75, 80] have also been reported. The type of pathogenic GBA mutation is associated with the severity of PD phenotype, namely, with the progression of cognitive impairment [69, 80].
LRRK2 mutations are the most common monogenic form of PD [81]. The most common pathogenic LRRK2 mutation, G2019S, corresponds to 1% of sporadic PD and 4% of familial PD [82]. There is a higher prevalence in some populations like North African Arabs [83] and Ashkenazi Jews [84]. In LRRK2-PD, the clinical phenotype largely overlaps with sporadic PD, although with some differentiating features such as an earlier onset of symptoms [82], more frequent lower extremity involvement at onset and a PIGD subtype with increased risk of falls [85], lack of cognitive impairment [20], and lower prevalence of hyposmia and RBD [18, 86]. Overall, LRRK2-PD is associated with a slower decline in the motor UPDRS [20, 82, 87] and longer survival compared with idiopathic PD [88].
The current management of GBA- and LRRK2-related forms of PD does not differ from that of idiopathic PD with the exception of vigilance for symptoms that have a higher frequency in this subtype (Table 1). Some treatments may be associated with different outcomes though LRRK2-PD has been associated with better motor outcomes after DBS [89] though others found that LRRK2 gene status did not predict outcome in DBS [90]. Patients with a GBA mutation have worse post-surgical cognitive and functional performance [91]. In addition, the greater cognitive decline in GBA-associated PD discourages the use of anti-cholinergic drugs or dopaminergic treatments other than levodopa that can lead to delirium or psychosis in those with cognitive impairment.
Aside from symptomatic therapy, genetically determined forms of PD are perhaps the best models available to explore the concept of disease modification through the development of therapies that attempt to mitigate the pathogenic effects of the gene mutation in the brain and delay the progression of PD. LRRK2-PD and GBA-PD are two examples with ongoing therapeutic development. In the context of disease-modifying therapeutic development, substrate reduction and the increase of GCase activity are being investigated in GBA-PD to rescue the enzymatic defect associated with a GBA mutation and potentially slow disease progression [92]. Restoration of GCase activity has been approached using small chaperone molecules that facilitate the transit to the lysosome of GCase and increase enzyme activity, direct enzyme activation, or gene replacement using an adeno-associated virus vector-9 (AAV9) [92]. Ambroxol is a mucolytic drug repurposed for GBA-PD for its function as a small molecule chaperone. A phase II study reported an encouraging increase in CSF β-GCase enzyme activity although being safe and well-tolerated in PD with and without GBA mutation [93]. An activator of GCase (LTI-291) has been tested in a phase 1b trial conducted in patients with a GBA mutation and showed dose-dependent brain penetration without safety concerns. The AAV9-based gene therapy for GBA-PD is currently under evaluation with a phase 1/2 clinical trial assessing the safety of intra-cisternal administration of PR001A in patients with moderate to severe PD and at least one pathogenic GBA1 mutation (NCT04127578). The substrate reduction approach targets lipid biosynthesis to prevent the accumulation of glucosylceramide. A glucosylceramide synthase inhibitor, venglustat, was initially tested in a phase I study that showed target engagement with a favorable safety and tolerability profile [94]. A phase II clinical trial is ongoing (NCT02906020).
In LRRK2-PD, the observation that some pathogenic LRRK2 mutations increase the kinase activity led to the development of novel therapeutic interventions [95]. The mutated LRRK2 pathway is associated with increased formation of synuclein inclusion in cultured neurons [96]. Its downregulation with LRRK2 antisense oligonucleotides (ASOs), for example, reduces fibril-induced α-synuclein inclusions and increases the number of tyrosine hydroxylase neurons [97], strengthening the evidence for potential disease-modifying effects of a therapy targeting LRRK2 function. One barrier to the use of inhibitors of LRRK2 is the systemic expression of LRRK2 in the immune system, lung, and kidney with potential for poor tolerability and unacceptable safety. Currently, small molecule LRRK2 inhibitors are in pre-clinical development. These compounds were designed to have brain permeability, greater enzymatic selectivity, and a degree of inhibitory activity that is predicted to allow for significant biological activity and mitigate potential systemic adverse events [92]. Two LRRK2 inhibitors, DNL201 and DNL151, are already in a clinical phase of development. The compound DNL201 seems to be well tolerated, as reported by the developing pharmaceutical company, with greater than 50% inhibition of LRRK2 kinase activity in PD patients [98]. A recent phase 1b trial demonstrated target engagement in healthy volunteers with DNL151 and a phase 1b in LRRK2-related PD and PD without a known gene mutation is ongoing (NCT04056689). One of these compounds is planned to advance to phase 2/3 trials in the future [98]. A genetic-based approach targeting mRNA is also being pursued in a phase 1 study evaluating safety, tolerability, and pharmacokinetics of intrathecal injection of an ASO for the mRNA of the LRRK2 gene (BIIB094), regardless of LRRK2 mutation status (NCT03976349).
In the future, it is possible that genetic target-specific therapies may be developed in other monogenic forms of PD and potentially in the broader population of sporadic PD. Recent meta-analyses of GWAS studies suggest that the heritable component of PD is about 20% [99]. Some of the clinical trials targeting GBA or LRRK2 are already recruiting patients with PD without a gene mutation, which is supported by data showing that patients not carrying mutations in either gene can present with enzymatic dysfunction of GCase [100] or LRRK2 [101]. Interestingly, LRRK2-PD patients carrying a G2019S mutation may have increased GCase activity [100], and it is thus conceivable that targeting multiple genes involved in PD may be of wider applicability as a disease-modifying approach in other genetic subtypes or even idiopathic PD.
Data-Driven Subtypes
Although currently there are no therapies based on data-driven subtypes, it is conceivable that the information about data-driven subtypes may be used to inform clinical care and better inform patients about prognosis in the future. Clinical PD subtypes defined using data-driven techniques have demonstrated prognostic value with differentiated progression rates between groups [102, 103] and differing risk for adverse disease milestones [31]. Despite these data, there are barriers to using this information in clinical care. In general, there is a lack of validation efforts in most data-driven PD subtyping systems (personal communication), which limits its use in other populations beyond the original study. Another important barrier derives from data-driven subtypes being defined based on multi-factor group characteristics and consequently, there is a need to identify post hoc criteria that guide the allocation of individuals to a given subtype. Ideally, an algorithm for classifying individual patients should be highly specific and sensitive, easy to administer and use readily available data to permit its future application in clinical practice. The development of algorithms to assign membership to a specific PD subtype at the individual level is very sparse in data-driven PD subtyping and warrants more attention. So far, results show modest specificity and sensitivity [103] that would make them of limited applicability as a classification tool in clinical practice.
Subtypes Based on Biomarkers
A biomarker-based definition of subtypes is appealing for a personalized approach to treatment in PD due to its assumed relevance to underlying pathophysiology. Therapeutic applications of biomarkers include the identification of groups of patients in a PD population that share a molecular or biochemical feature related to a specific disease mechanism, which in turn informs a particular therapeutic approach.
The field of biomarker-based subtypes is developing and the potential application in relation to nonmotor phenotyping has been previously discussed, particularly with respect to PET-based imaging studies. These biomarkers have been used to explore the biological basis of data-driven clinical PD subtypes [103, 104]. This has been a “top-down” clinical to biomarker approach to understanding the pathophysiology of NMS. If instead we take a bottom-up approach starting from the biomarker itself to define subtypes, we may come closer to defining groups that are therapeutically relevant. From the descriptions of PD subtypes discussed above, it is clear that the current (mostly) clinically defined PD subtypes have overlapping features. For example, the presence of dystonia in an autosomal recessive form of genetic PD such as PARK-Parkin [105] was equally reported in PD subtypes pre-defined by the age of onset of PD. As in the parable of “The Blind Men and an Elephant,” the reported PD subtypes seem to be one piece of many pieces of the PD puzzle [106], blind to the core disease mechanisms in PD that may better explain heterogeneity. The use of biomarker-defined PD subtypes based on systems biology may be a solution to overcome the current redundancy [106] and may have great applicability to personalized medicine.
Therapeutic Development Based on PD Subtypes
Knowledge about PD subtypes can be used to inform the development of interventions and various aspects of clinical trial design, although this opportunity has not been exploited significantly to date. We will use the cases of GBA and LRRK2-PD to illustrate the advantages and challenges of developing a therapy based on a genetically determined PD subtype. Although LRRK2 and GBA mutations are the most common known genetic forms of PD, they remain rare in the PD population, and this presents a significant challenge for participant recruitment in a trial of a gene-targeted intervention. A multinational, multicenter trial would require extensive genetic testing screening pre-recruitment, which is not routinely performed in most clinical centers. Ongoing efforts to overcome this barrier to successfully conducting future disease-modifying trials include use of recruitment algorithms [107] to identify eligible individuals regardless of being followed at a clinical trials unit involved in PD trial, thus expanding its recruitment pool. It is also possible that the acceptability of genetic testing may change as more clinical trials of target-specific therapies are conducted in the future [108].
Knowledge about rates of clinical progression of GBA- and LRRK2-PD can be used to inform clinical trial design: slower or faster progression rates reported in GBA- or LRRK2-PD can be used to determine the duration of a clinical trial and inform power calculations for outcomes such as the start of a dopaminergic treatment, onset of motor complications, or dementia. Additionally, more homogeneous progression in some genetically defined PD subtypes could improve trial efficiency [109].
The future development of reliable and accessible assays to measure the enzymatic activity of proteins associated with a genetically determined PD subtypes, such as the case of GCase and LRRK2, can support a more rational development of a target-specific intervention. For example, results of these assays can help to identify the interventions and the corresponding doses with the best combination of target engagement, therapeutic biological effect, and safety. Further, patients with the most significant enzymatic changes related to a given gene mutation may benefit the most from a corresponding targeted therapy. This feature could be used, in turn, as an enrichment strategy or inclusion criterion in clinical trials to increase the yield of positive results, and in the future as a basis for personalized treatment decisions in clinical practice.
In PD, the use of disease-modifying therapies in asymptomatic mutation carriers is thought likely to yield a larger therapeutic effect compared with clinical PD when the neurodegenerative process has progressed to cause clinical symptoms [110]. A significant barrier to treatment at the pre-symptomatic stage is that both LRKK2-PD [82, 111, 112] and GBA-associated PD [113, 114] have variable and generally low penetrance, making it currently impossible to determine if and when these individuals will develop PD [115]. Predictive analytics may prove to be instrumental in the identification of asymptomatic carriers at higher risk of incident PD in a timeline suitable for a clinical trial. For example, studies have included genetic, clinical, and environmental exposure data to predict incident PD [116, 117] or the likelihood of achieving a disease milestone such as cognitive impairment [118].
The Future of Subtype-Specific Therapeutics
Subtypes described to date have not been derived with the direct intent of guiding therapy. Therapeutic implications have been investigated post hoc and to date have not had a major influence on choice of therapy with the possible exception of age at onset and some nonmotor subtypes. The field of pharmacogenomics is rapidly expanding, relating genetic variants to response to specific drugs, but is not to date applied in routine clinical practice in PD and response to treatment alone has not to date been considered to define a PD subtype.
At this point, the science of PD subtypes is evolving, in directions more biologically based and presumably with more direct therapeutic implications. The next frontier is the development of genotype-specific therapies. Uncharted territories are therapies based on data-driven subtypes and biomarker-based subtypes.
There has been relatively little formal research on therapies by subtypes, which must be done to establish an evidence base to guide treatment recommendations that are tailored to a particular patient. To be adopted into clinical practice subtyping must be practical, based on parameters easily measured in clinical practice and provide an easily applied classification system. To date, the field of data-driven subtypes has not emphasized this aspect, which would facilitate translation into clinical practice, although this is changing. Recently, several data-driven classifications have been applied on an individual level [31, 103] but this has yet to be applied to therapy.
The increasing availability of genetic testing will facilitate the integration of genetically based therapies into clinical practice. Several large research-based efforts are underway to offer genotyping to PD patients without exclusions (NCT04057794 and NCT03866603). As genotype-specific therapies are developed, genotyping will have to become more available through health insurers in order to enable the widespread application of genetically based personalized medicine. In the future, gene-based profiling of patients may be more impactful by multiple genetic variants, multiple disease pathways, and corresponding available therapies for the individual patient. At present, however, known PD-related variants are found in a small minority of individuals with PD. Therefore, genetically determined PD subtypes based on “monogenic” PD may not apply to a large proportion of patients with PD. If PD subtypes are to have a greater influence on therapy, more must be known about the relationship between the response of individuals to treatments and other subtype-defining characteristics such as symptoms or demographic factors. There is much data in existing clinical trial datasets to provide preliminary data on this issues that can then be tested prospectively. Such efforts will help to move the field toward the goal of personalized medicine.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Electronic Supplementary Material
(PDF 1224 kb)
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parkinson J. An essay on the shaking palsy. 1817. The Journal of neuropsychiatry and clinical neurosciences. 2002;14(2):223–36. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Fahn S. The history of parkinsonism. Movement Disorders. 1989;4(S1):S2–S10. doi: 10.1002/mds.870040502. [DOI] [PubMed] [Google Scholar]
- 3.Weiner WJ. There is no Parkinson disease. Archives of neurology. 2008;65(6):705–8. doi: 10.1001/archneur.65.6.705. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 5.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognostic implications. Neurology. 1985;35(4):522–6. doi: 10.1212/wnl.35.4.522. [DOI] [PubMed] [Google Scholar]
- 6.Stern MB, Doty RL, Dotti M, Corcoran P, Crawford D, McKeown DA, et al. Olfactory function in Parkinson’s disease subtypes. Neurology. 1994;44(2):266–8. doi: 10.1212/wnl.44.2.266. [DOI] [PubMed] [Google Scholar]
- 7.Korchounov A, Schipper HI, Preobrazhenskaya IS, Kessler KR, Yakhno NN, Korchounov A, et al. Differences in age at onset and familial aggregation between clinical types of idiopathic Parkinson’s disease. Movement Disorders. 2004;19(9):1059–64. doi: 10.1002/mds.20061. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA. Post mortem studies in Parkinson’s disease--is it possible to detect brain areas for specific symptoms? Journal of Neural Transmission Supplementum. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- 9.Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. Effect of age at onset on progression and mortality in Parkinson’s disease. Neurology. 1989;39(9):1187–90. doi: 10.1212/wnl.39.9.1187. [DOI] [PubMed] [Google Scholar]
- 10.Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Parkinson disease phenotype. Neurology. 2016;86(15):1400–7. doi: 10.1212/WNL.0000000000002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerri S, Mus L, Blandini F. Parkinson’s Disease in Women and Men: What’s the Difference? J Parkinsons Dis. 2019;9(3):501–15. doi: 10.3233/JPD-191683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819–24. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016;31(8):1095–102. doi: 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 14.Brown RG, Landau S, Hindle JV, Playfer J, Samuel M, Wilson KC, et al. Depression and anxiety related subtypes in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(7):803–9. doi: 10.1136/jnnp.2010.213652. [DOI] [PubMed] [Google Scholar]
- 15.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16(3):177–80. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler A, Bregman N, Gurevich T, Shiner T, Dror Y, Zmira O, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45–9. doi: 10.1016/j.parkreldis.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Malek N, Weil RS, Bresner C, Lawton MA, Grosset KA, Tan M, et al. Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J Neurol Neurosurg Psychiatry. 2018;89(7):702–9. doi: 10.1136/jnnp-2017-317348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marras C, Schule B, Munhoz RP, Rogaeva E, Langston JW, Kasten M, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology. 2011;77(4):325–33. doi: 10.1212/WNL.0b013e318227042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marras C, Alcalay RN, Caspell-Garcia C, Coffey C, Chan P, Duda JE, et al. Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson’s disease. Mov Disord. 2016;31(8):1192–202. doi: 10.1002/mds.26614. [DOI] [PubMed] [Google Scholar]
- 20.Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966–71. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham JM, Sagar HJ. A data-driven approach to the study of heterogeneity in idiopathic Parkinson’s disease: identification of three distinct subtypes. Mov Disord. 1999;14(1):10–20. doi: 10.1002/1531-8257(199901)14:1<10::aid-mds1005>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Mestre TA, Eberly S, Tanner C, Grimes D, Lang AE, Oakes D, et al. Reproducibility of data-driven Parkinson’s disease subtypes for clinical research. Parkinsonism Relat Disord. 2018. [DOI] [PubMed]
- 23.Mehanna R, Moore S, Hou JG, Sarwar AI, Lai EC. Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(5):530–4. doi: 10.1016/j.parkreldis.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Wickremaratchi MM, Knipe MD, Sastry BS, Morgan E, Jones A, Salmon R, et al. The motor phenotype of Parkinson’s disease in relation to age at onset. Mov Disord. 2011;26(3):457–63. doi: 10.1002/mds.23469. [DOI] [PubMed] [Google Scholar]
- 25.Friedman A. Old-onset Parkinson’s disease compared with young-onset disease: clinical differences and similarities. Acta Neurol Scand. 1994;89(4):258–61. doi: 10.1111/j.1600-0404.1994.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 26.Diederich NJ, Moore CG, Leurgans SE, Chmura TA, Goetz CG. Parkinson disease with old-age onset: a comparative study with subjects with middle-age onset. Archives of neurology. 2003;60(4):529–33. doi: 10.1001/archneur.60.4.529. [DOI] [PubMed] [Google Scholar]
- 27.Goetz CG, Tanner CM, Stebbins GT, Buchman AS. Risk factors for progression in Parkinson’s disease. Neurology. 1988;38(12):1841–4. doi: 10.1212/wnl.38.12.1841. [DOI] [PubMed] [Google Scholar]
- 28.Wickremaratchi MM, Ben-Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson’s disease. European Journal of Neurology. 2009;16(4):450–6. doi: 10.1111/j.1468-1331.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 29.Gomez Arevalo G, Jorge R, Garcia S, Scipioni O, Gershanik O. Clinical and pharmacological differences in early- versus late-onset Parkinson’s disease. Mov Disord. 1997;12(3):277–84. doi: 10.1002/mds.870120303. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Ruiz PJ, Del Val J, Fernandez IM, Herranz A. What factors influence motor complications in Parkinson disease?: a 10-year prospective study. Clin Neuropharmacol. 2012;35(1):1–5. doi: 10.1097/WNF.0b013e31823dec73. [DOI] [PubMed] [Google Scholar]
- 31.De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019;76(4):470–9. doi: 10.1001/jamaneurol.2018.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. The New England journal of medicine. 2000;342(20):1484–91. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Tani N, Maruo T, Oshino S, Hosomi K, Saitoh Y, et al. Risk Factors for Postoperative Delirium After Deep Brain Stimulation Surgery for Parkinson Disease. World Neurosurg. 2018;114:e518–e23. doi: 10.1016/j.wneu.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Dafsari HS, Reker P, Stalinski L, Silverdale M, Rizos A, Ashkan K, et al. Quality of life outcome after subthalamic stimulation in Parkinson’s disease depends on age. Mov Disord. 2018;33(1):99–107. doi: 10.1002/mds.27222. [DOI] [PubMed] [Google Scholar]
- 35.Howlett DR, Whitfield D, Johnson M, Attems J, O'Brien JT, Aarsland D, et al. Regional Multiple Pathology Scores Are Associated with Cognitive Decline in Lewy Body Dementias. Brain Pathol. 2015;25(4):401–8. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vesely B, Rektor I. The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: A critical review of the literature. Parkinsonism Relat Disord. 2016;22(Suppl 1):S166–70. doi: 10.1016/j.parkreldis.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Vesely B, Antonini A, Rektor I. The contribution of white matter lesions to Parkinson’s disease motor and gait symptoms: a critical review of the literature. J Neural Transm (Vienna). 2016;123(3):241–50. doi: 10.1007/s00702-015-1470-9. [DOI] [PubMed] [Google Scholar]
- 38.Koller WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson’s disease. Clin Neuropharmacol. 1989;12(4):293–7. doi: 10.1097/00002826-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sung YH, Chung SJ, Kim SR, Lee MC. Factors predicting response to dopaminergic treatment for resting tremor of Parkinson’s disease. Mov Disord. 2008;23(1):137–40. doi: 10.1002/mds.21793. [DOI] [PubMed] [Google Scholar]
- 40.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003(2):CD003735. [DOI] [PMC free article] [PubMed]
- 41.Bonuccelli U, Ceravolo R, Salvetti S, D'Avino C, Del Dotto P, Rossi G, et al. Clozapine in Parkinson’s disease tremor. Effects of acute and chronic administration. Neurology. 1997;49(6):1587–90. doi: 10.1212/wnl.49.6.1587. [DOI] [PubMed] [Google Scholar]
- 42.Savica R, Matsumoto JY, Josephs KA, Ahlskog JE, Stead M, Lee KH, et al. Deep brain stimulation in benign tremulous parkinsonism. Archives of neurology. 2011;68(8):1033–6. doi: 10.1001/archneurol.2011.160. [DOI] [PubMed] [Google Scholar]
- 43.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Movement Disorders. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 44.Fahn S, Elton R, Committee. MotUD. The Unified Parkinson’s Disease Rating Scale. In: Fahn S MC, Calne DB, Goldstein M, editor. Recent Developments in Parkinson’s Disease. 2. Florham Park: Macmillan Health Care Information; 1987. p. 153-63.
- 45.Bjornestad A, Tysnes OB, Larsen JP, Alves G. Loss of independence in early Parkinson disease: A 5-year population-based incident cohort study. Neurology. 2016;87(15):1599–606. doi: 10.1212/WNL.0000000000003213. [DOI] [PubMed] [Google Scholar]
- 46.Factor SA, Bennett A, Hohler AD, Wang D, Miyasaki JM. Quality improvement in neurology: Parkinson disease update quality measurement set: Executive summary. Neurology. 2016;86(24):2278–83. doi: 10.1212/WNL.0000000000002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz M, Luciano MS, Carlson K, Luo P, Marks WJ, Jr, Larson PS, et al. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Annals of neurology. 2015;77(4):710–9. doi: 10.1002/ana.24374. [DOI] [PubMed] [Google Scholar]
- 48.Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K, et al. How stable are Parkinson’s disease subtypes in de novo patients: Analysis of the PPMI cohort? Parkinsonism Relat Disord. 2016;28:62–7. doi: 10.1016/j.parkreldis.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Eisinger RS, Hess CW, Martinez-Ramirez D, Almeida L, Foote KD, Okun MS, et al. Motor subtype changes in early Parkinson’s disease. Parkinsonism Relat Disord. 2017;43:67–72. doi: 10.1016/j.parkreldis.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo L, Andrews H, Alcalay RN, Poyraz FC, Boehme AK, Goldman JG, et al. Motor phenotype classification in moderate to advanced PD in BioFIND study. Parkinsonism Relat Disord. 2019;65:178–83. doi: 10.1016/j.parkreldis.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain : a journal of neurology. 2009;132(Pt 11):2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 52.Titova N, Chaudhuri KR. Personalized medicine in Parkinson’s disease: Time to be precise. Mov Disord. 2017;32(8):1147–54. doi: 10.1002/mds.27027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(7):435–50. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 54.Titova N, Chaudhuri KR. Nonmotor Parkinson’s and Future Directions. Int Rev Neurobiol. 2017;134:1493–505. doi: 10.1016/bs.irn.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 55.Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord. 2019;34(2):180–98. doi: 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord. 2016;22(Suppl 1):S41–6. doi: 10.1016/j.parkreldis.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13(4):217–31. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gjerloff T, Fedorova T, Knudsen K, Munk OL, Nahimi A, Jacobsen S, et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain : a journal of neurology. 2015;138(Pt 3):653–63. doi: 10.1093/brain/awu369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fedorova TD, Seidelin LB, Knudsen K, Schacht AC, Geday J, Pavese N, et al. Decreased intestinal acetylcholinesterase in early Parkinson disease: An (11)C-donepezil PET study. Neurology. 2017;88(8):775–81. doi: 10.1212/WNL.0000000000003633. [DOI] [PubMed] [Google Scholar]
- 60.Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain : a journal of neurology. 2010;133(11):3434–43. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- 61.Wilson H, Giordano B, Turkheimer FE, Chaudhuri KR, Politis M. Serotonergic dysregulation is linked to sleep problems in Parkinson’s disease. Neuroimage Clin. 2018;18:630–7. doi: 10.1016/j.nicl.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niccolini F, Wilson H, Giordano B, Diamantopoulos K, Pagano G, Chaudhuri KR, et al. Sleep disturbances and gastrointestinal dysfunction are associated with thalamic atrophy in Parkinson’s disease. BMC neuroscience. 2019;20(1):55. doi: 10.1186/s12868-019-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagano G, Niccolini F, Politis M. The serotonergic system in Parkinson’s patients with dyskinesia: evidence from imaging studies. J Neural Transm (Vienna). 2018;125(8):1217–23. doi: 10.1007/s00702-017-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Espay AJ, LeWitt PA, Kaufmann H. Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord. 2014;29(14):1710–9. doi: 10.1002/mds.26048. [DOI] [PubMed] [Google Scholar]
- 65.Borghammer P, Knudsen K, Fedorova TD, Brooks DJ. Imaging Parkinson’s disease below the neck. NPJ Parkinsons Dis. 2017;3:15. doi: 10.1038/s41531-017-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommerauer M, Fedorova TD, Hansen AK, Knudsen K, Otto M, Jeppesen J, et al. Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain : a journal of neurology. 2018;141(2):496–504. doi: 10.1093/brain/awx348. [DOI] [PubMed] [Google Scholar]
- 67.Torontali ZA, Grace KP, Horner RL, Peever JH. Cholinergic involvement in control of REM sleep paralysis. The Journal of physiology. 2014;592(7):1425–6. doi: 10.1113/jphysiol.2014.271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasten M, Marras C, Klein C. Nonmotor Signs in Genetic Forms of Parkinson’s Disease. Int Rev Neurobiol. 2017;133:129–78. doi: 10.1016/bs.irn.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 69.Liu G, Boot B, Locascio JJ, Jansen IE, Winder-Rhodes S, Eberly S, et al. Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Annals of neurology. 2016;80(5):674–85. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, et al. GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov Disord. 2016;31(1):95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schapira AHV, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proceedings of the National Academy of Sciences. 2013;110(9):3214. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark LN, Ross BM, Wang Y, Mejia-Santana H, Harris J, Louis ED, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69(12):1270–7. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. The New England journal of medicine. 2009;361(17):1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–83. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 75.Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, et al. GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407–11. doi: 10.1002/mds.26071. [DOI] [PubMed] [Google Scholar]
- 76.Swan M, Doan N, Ortega RA, Barrett M, Nichols W, Ozelius L, et al. Neuropsychiatric characteristics of GBA-associated Parkinson disease. J Neurol Sci. 2016;370:63–9. doi: 10.1016/j.jns.2016.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18):1434–40. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain : a journal of neurology. 2013;136(Pt 2):392–9. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- 79.Davis MY, Johnson CO, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, et al. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA Neurol. 2016;73(10):1217–24. doi: 10.1001/jamaneurol.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Annals of neurology. 2016;80(5):662–73. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- 81.Tolosa E, Vila M, Klein C, Rascol O. LRRK2 in Parkinson disease: challenges of clinical trials. Nat Rev Neurol. 2020;16(2):97–107. doi: 10.1038/s41582-019-0301-2. [DOI] [PubMed] [Google Scholar]
- 82.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–90. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, et al. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. The New England journal of medicine. 2006;354:422–3. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 84.Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. The New England journal of medicine. 2006;354:424–5. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 85.Mirelman A, Heman T, Yasinovsky K, Thaler A, Gurevich T, Marder K, et al. Fall risk and gait in Parkinson’s disease: the role of the LRRK2 G2019S mutation. Mov Disord. 2013;28(12):1683–90. doi: 10.1002/mds.25587. [DOI] [PubMed] [Google Scholar]
- 86.Pont-Sunyer C, Iranzo A, Gaig C, Fernandez-Arcos A, Vilas D, Valldeoriola F, et al. Sleep Disorders in Parkinsonian and Nonparkinsonian LRRK2 Mutation Carriers. PloS one. 2015;10(7):e0132368. doi: 10.1371/journal.pone.0132368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saunders-Pullman R, Mirelman A, Alcalay RN, Wang C, Ortega RA, Raymond D, et al. Progression in the LRRK2-Asssociated Parkinson Disease Population. JAMA Neurol. 2018;75(3):312–9. doi: 10.1001/jamaneurol.2017.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thaler A, Kozlovski T, Gurevich T, Bar-Shira A, Gana-Weisz M, Orr-Urtreger A, et al. Survival rates among Parkinson’s disease patients who carry mutations in the LRRK2 and GBA genes. Mov Disord. 2018;33(10):1656–60. doi: 10.1002/mds.27490. [DOI] [PubMed] [Google Scholar]
- 89.Sayad M, Zouambia M, Chaouch M, Ferrat F, Nebbal M, Bendini M, et al. Greater improvement in LRRK2 G2019S patients undergoing Subthalamic Nucleus Deep Brain Stimulation compared to non-mutation carriers. BMC neuroscience. 2016;17:6. doi: 10.1186/s12868-016-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenbaum L, Israeli-Korn SD, Cohen OS, Elincx-Benizri S, Yahalom G, Kozlova E, et al. The LRRK2 G2019S mutation status does not affect the outcome of subthalamic stimulation in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):1053–6. doi: 10.1016/j.parkreldis.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Artusi CA, Dwivedi AK, Romagnolo A, Pal G, Kauffman M, Mata I, et al. Association of Subthalamic Deep Brain Stimulation With Motor, Functional, and Pharmacologic Outcomes in Patients With Monogenic Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2(2):e187800. doi: 10.1001/jamanetworkopen.2018.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider SA, Alcalay RN. Precision medicine in Parkinson’s disease: emerging treatments for genetic Parkinson’s disease. J Neurol. 2020;267(3):860–9. doi: 10.1007/s00415-020-09705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullin S, Smith L, Lee K, D'Souza G, Woodgate P, Elflein J, et al. Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed]
- 94.Peterschmitt M, Gasser T, Isaacson S. Safety, tolerability and pharmacokinetics of oral venglustat in Parkinson disease patients with a GBA mutation. Mol Genet Metabol Rep. 2019;126.
- 95.Sardi SP, Simuni T. New Era in disease modification in Parkinson’s disease: Review of genetically targeted therapeutics. Parkinsonism Relat Disord. 2019;59:32–8. doi: 10.1016/j.parkreldis.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 96.Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ, et al. G2019S-LRRK2 Expression Augments alpha-Synuclein Sequestration into Inclusions in Neurons. J Neurosci. 2016;36(28):7415–27. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao HT, John N, Delic V, Ikeda-Lee K, Kim A, Weihofen A, et al. LRRK2 Antisense Oligonucleotides Ameliorate alpha-Synuclein Inclusion Formation in a Parkinson’s Disease Mouse Model. Mol Ther Nucleic Acids. 2017;8:508–19. doi: 10.1016/j.omtn.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denali Therapeutics Announces Broad Pipeline Progress Including Positive Results From Its LRRK2 Program for Parkinson’s Disease 2019 [Available from: https://www.biospace.com/article/releases/denali-therapeutics-announces-broad-pipeline-progress-including-positive-results-from-its-lrrk2-program-for-parkinson-s-disease/] Accessed 30 July 2020
- 99.Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo SH, et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain : a journal of neurology. 2015;138(Pt 9):2648–58. doi: 10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Maio R, Hoffman EK, Rocha EM, Keeney MT, Sanders LH, De Miranda BR, et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci Transl Med. 2018;10(451):eaar5429. doi: 10.1126/scitranslmed.aar5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lawton M, Ben-Shlomo Y, May MT, Baig F, Barber TR, Klein JC, et al. Developing and validating Parkinson’s disease subtypes and their motor and cognitive progression. J Neurol Neurosurg Psychiatry. 2018;89(12):1279–87. doi: 10.1136/jnnp-2018-318337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain : a journal of neurology. 2017;140(7):1959–76. doi: 10.1093/brain/awx118. [DOI] [PubMed] [Google Scholar]
- 104.Lawton M, Baig F, Toulson G, Morovat A, Evetts SG, Ben-Shlomo Y, et al. Blood biomarkers with Parkinson’s disease clusters and prognosis: The oxford discovery cohort. Mov Disord. 2020;35(2):279–87. doi: 10.1002/mds.27888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. The New England journal of medicine. 2000;342(21):1560–7. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 106.Espay AJ, Lang AE. Parkinson Diseases in the 2020s and Beyond: Replacing Clinico-Pathologic Convergence With Systems Biology Divergence. J Parkinsons Dis. 2018;8(s1):S59–s64. doi: 10.3233/JPD-181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foroud T, Smith D, Jackson J, Verbrugge J, Halter C, Wetherill L, et al. Novel recruitment strategy to enrich for LRRK2 mutation carriers. Mol Genet Genomic Med. 2015;3(5):404–12. doi: 10.1002/mgg3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupte M, Alcalay RN, Mejia-Santana H, Raymond D, Saunders-Pullman R, Roos E, et al. Interest in genetic testing in Ashkenazi Jewish Parkinson’s disease patients and their unaffected relatives. J Genet Couns. 2015;24(2):238–46. doi: 10.1007/s10897-014-9756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahamadi M, Conrado DJ, Macha S, Sinha V, Stone J, Burton J, et al. Development of a Disease Progression Model for Leucine-Rich Repeat Kinase 2 in Parkinson’s Disease to Inform Clinical Trial Designs. Clin Pharmacol Ther. 2020;107(3):553–62. doi: 10.1002/cpt.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Annals of neurology. 2010;67(6):715–25. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee AJ, Wang Y, Alcalay RN, Mejia-Santana H, Saunders-Pullman R, Bressman S, et al. Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord. 2017;32(10):1432–8. doi: 10.1002/mds.27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Latourelle JC, Sun M, Lew MF, Suchowersky O, Klein C, Golbe LI, et al. The Gly2019Ser mutation in LRRK2 is not fully penetrant in familial Parkinson’s disease: the GenePD study. BMC Med. 2008;6:32. doi: 10.1186/1741-7015-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2013;15(2):146–9. doi: 10.1038/gim.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, et al. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012;78(6):417–20. doi: 10.1212/WNL.0b013e318245f476. [DOI] [PubMed] [Google Scholar]
- 115.Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):637–48. doi: 10.1016/S1474-4422(16)00060-0. [DOI] [PubMed] [Google Scholar]
- 116.Fereshtehnejad SM, Montplaisir JY, Pelletier A, Gagnon JF, Berg D, Postuma RB. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov Disord. 2017;32(6):865–73. doi: 10.1002/mds.26989. [DOI] [PubMed] [Google Scholar]
- 117.Schlossmacher MG, Tomlinson JJ, Santos G, Shutinoski B, Brown EG, Manuel D, et al. Modelling idiopathic Parkinson disease as a complex illness can inform incidence rate in healthy adults: the PR EDIGT score. Eur J Neurosci. 2017;45(1):175–91. doi: 10.1111/ejn.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu G, Locascio JJ, Corvol JC, Boot B, Liao Z, Page K, et al. Prediction of cognition in Parkinson’s disease with a clinical-genetic score: a longitudinal analysis of nine cohorts. Lancet Neurol. 2017;16(8):620–9. doi: 10.1016/S1474-4422(17)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Titova N, Qamar MA, Chaudhuri KR. The Nonmotor Features of Parkinson’s Disease. Int Rev Neurobiol. 2017;132:33–54. doi: 10.1016/bs.irn.2017.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)