Abstract
Dementia poses major health challenges worldwide, yet current treatments are faced with issues of efficacy and toxicity. Deep brain stimulation (DBS) is a promising non-pharmacological treatment for dementia, but most DBS studies use young healthy animals, which may not be aetiologically relevant. In this study, we used an aged rat model in which cognitive decline occurs through a natural ageing process. We used a Morris water maze (MWM) to determine the effects of prelimbic cortex (PrL) DBS on memory in aged rats. To investigate the underlying mechanisms of the effects of DBS, we carried out microarray, quantitative PCR analysis, and mass spectrometry to detect gene expression and neurotransmitter changes in the hippocampus. We showed PrL DBS improved the performance in MWM, with related distinct patterns of gene expression involving G protein–coupled receptor pathways. We further found neurotransmitter changes in the dorsal hippocampus, which corroborated and extended the microarray findings. Our results suggest that non-neurogenesis pathways play roles in the effects of DBS. Further studies are needed to investigate the effects of DBS on memory beyond neurogenesis and to consider the highlighted pathways suggested by our data.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00913-7) contains supplementary material, which is available to authorized users.
Keywords: Deep brain stimulation, dementia, memory, microarray, prelimbic cortex, hippocampus.
Introduction
The ageing worldwide population has propelled dementia to become a major health challenge, as age is a major risk factor. An estimated 35.6 million people suffer from dementia and the number is expected to more than triple by 2050 [1, 2]. Current therapies focus on pharmacological interventions that treat symptoms rather than actually slowing down or stopping neuronal damage [3]. Furthermore, the effects of these drug interventions tend to wear off over time, and issues of toxicity and the lack of significant therapeutic effects have resulted in high rates of treatment failure [4, 5].
Deep brain stimulation (DBS) is a promising non-pharmacological treatment for dementia, and initial evidence demonstrates it can offer cognitive enhancements [6]. Although many animal studies have shown that DBS has the ability to enhance memory [7–10], a major limitation in most of these studies is that they used young healthy animals, which may not be aetiologically relevant. Furthermore, the molecular mechanisms behind these enhancements are still relatively unknown. In this study, we used an aged animal model, in which cognitive decline occurs through a natural ageing process. The 2-year-old rats used in our study are comparable to 60-year-old humans [11], which should yield more representative and relevant data [7]. We implanted DBS electrodes bilaterally into the prelimbic cortex (PrL), a structure known to be an effective target for DBS for memory improvement [7]. We subjected the rats to a chronic protocol of PrL DBS and evaluated the effects on hippocampal-dependent memory using Morris water maze (MWM). We found DBS induced significant memory improvements in the aged rats. We then attempted to unravel some of the mechanisms underlying the memory enhancements using microarrays, which can harness advancements in omics technologies and computational power, to probe key molecular signalling mechanisms mediating the observed effects [12]. Microarray analysis revealed distinct patterns of changes in gene expression in the hippocampus, with G protein–coupled receptors (GPCRs) being a key focus. Based on our microarray data, we further investigated changes in neurotransmitters and metabolites in the dorsal hippocampus, which we previously described as a potential mechanism by which DBS can enhance memories [13]. Changes in monoamines corroborated our microarray data, which partly explained some of the mechanisms behind the observed effects. Overall, we found PrL DBS induced memory improvements in aged rats associated with distinct patterns of change in the dorsal hippocampus.
Methods and Materials
Subjects
The study was approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR) of the University of Hong Kong (Ref.: 18-220). Male Sprague-Dawley rats (n = 40) were individually housed in standard cages with food and water available ad libitum until 23 months of age. Out of 40 animals, 12 died between 23 and 24 months of age. Three animals in the DBS group were excluded due to electrode misplacement. The final number of rats used in experiments was 25 (DBS n = 12, sham n = 13). Details of the electrode placements in the animals (including the three with electrode misplacements) can be found in Supplementary Figure 1. Healthy adult male Sprague-Dawley rats (3 months of age, n = 10) were also kept in similar conditions and used as the young animal control group. The environmental conditions were maintained at a temperature of 25 ± 1 °C and humidity of 60–65% under a reversed 12-h/12-h light/dark cycle.
Surgical and Deep Brain Stimulation Procedures
The surgery and DBS were performed as previously described [7, 14]. In brief, rats were implanted with DBS electrodes bilaterally in the prelimbic cortex (PrL). The stimulation electrodes consisted of an inner platinum-iridium wire core and gold-plated cannula (Synergy Engineering Pte Ltd., Singapore; Technomed, Beek, the Netherlands). The animals were initially anesthetised with 5% v/v isoflurane mixed with oxygen until loss of righting reflex. Rats were then mounted onto a stereotaxic frame (Leica Biosystems, Nussloch, Germany) and maintained with 2.5% v/v isoflurane via a nose cone. A midline incision was made to expose the skull and a sagittal suture was used to align the skull along the anterior-posterior axis in the stereotaxic frame. Paxinos and Watson Rat Brain Atlas was used as a guide for the stereotaxic implantation of electrodes (Bregma + 3.0 mm anterior-posterior; ± 0.6 mm mediolateral; and − 3.6 mm dorsoventral) [15]. The electrode construct was anchored to the rat skull with stainless steel screws and dental acrylic (Paladur, Heraeus Kulzer GmbH, Hanau, Germany). After recovery for 2 weeks, rats were subjected to daily 1 h of stimulation (100 Hz, 200 μA, and 100-μs pulse width) for 28 days using a digital stimulator (Model 3800 MultiStim: 8-Channel Stimulator; A-M Systems, Carlsborg, USA) with two stimulus isolators (Model 3820; A-M Systems). Wires were tethered to the animals only during stimulation. Stimulations were performed in the home cage during the dark cycle (behaviour tests were done at the same time of day) and animals were allowed to move freely. Animals in the sham group were subjected to the same conditions, but without electrical stimulation.
Behavioural Protocols
The behavioural protocols were based on our previously published data [7]. Prior to MWM, animals underwent an open field test (OFT) to control for any locomotor and anxiety differences. Briefly, individual animals were placed in the middle of the open field (100 × 100 × 40 cm made of black plexiglass) under a low-light setting and allowed to explore for 300 s. The behaviour of each animal was video-recorded and the distance travelled and duration of time spent in the different zones was analysed using Anymaze 5.0. The MWM test was conducted on week 4 (days 22–28). Animals (PrL DBS, n = 12; sham, n = 13) received 30 min of electrical stimulation (or sham stimulation) prior to MWM test during 8:00–14:00 h (Fig. 1A). After each testing, rats were again stimulated for another 30 min during 14:00–19:00 h for a total of 1 h of stimulation per day based on our previous experiments [7]. The MWM was conducted in a dimly lit room with distinct peripheral cues (specific symbols like a cross printed in black on A4-sized paper) placed on the walls of the room 50 cm above the pool and roughly 10 cm from the pool. The MWM consisted of a black circular polyethylene pool (128-cm diameter and 60 cm high) filled with water (50 cm deep). A circular platform (10-cm diameter) was placed at the North quadrant of the pool 25 cm from the wall, and submerged to about 1 cm below the water surface and hidden from the animal’s view. The water temperature was maintained between 23 and 25 °C. The water maze spatial learning test took place over a period of 7 days. The first 6 days represented the training phase and consisted of four trials per day (training trial 1 min, inter-trial interval 1 min) to allow rats to learn the location of the hidden platform. Rats were placed in the pool in a different starting quadrant for each trial to avoid learning left or right navigation. Each trial began with the rat placed in the pool facing a sidewall and ended when the rat found the platform. If the rats failed to locate the platform in 60 s, then they were gently guided to the platform and left on the platform for 15 s before being returned to their home cage. Data were analysed as an average of four trials a day. On day 7, 24 h after the last training phase, rats were subjected to a probe trial to assess remote memory. The probe trial was performed for 60 s with the escape platform removed. The time spent in the peripheral and target quadrants was analysed using Anymaze 5.0 tracking software.
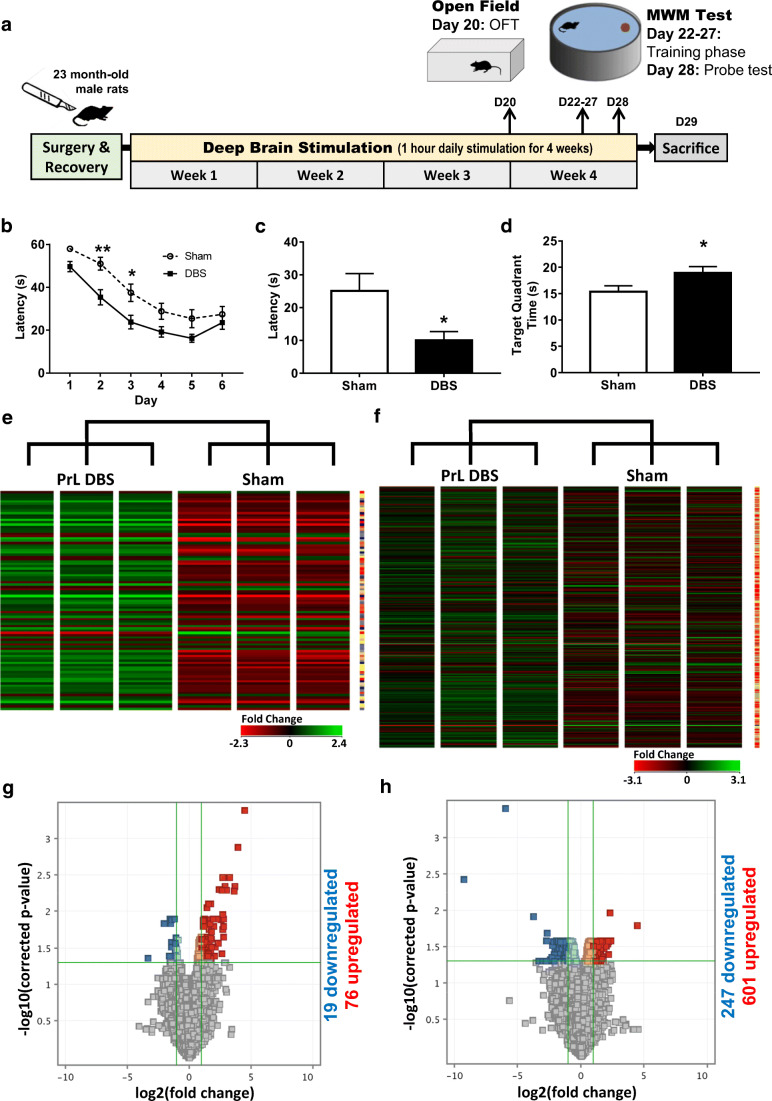
Fig. 1.
DNA microarray analysis of PrL DBS-enhanced spatial memory in aged animals. Rats underwent Morris water maze after receiving chronic DBS or sham DBS (A). (B) A significant improvement in the acquisition of spatial learning; (C, D) improvements in the probe trial in both latency to reach the imaginary platform and time spent in the target quadrant. Data are presented as mean ± SEM. (E, F) Hierarchical clustergrams of intensity values of genes with at least 2-fold change in PrL DBS-treated rat dorsal (E) and ventral (F) hippocampus. Volcano plot of threshold changes > 2-fold with a 0.05 corrected p value showing 95 entities (19 downregulated and 76 upregulated) and 848 entities (247 downregulated and 601 upregulated) in the dHPC (G) and vHPC (F), respectively. *p < 0.05
Microarray
On day 29, animals were stimulated for 1 h and immediately decapitated by guillotine performed by an experienced animal handler. Anaesthetic was not used to avoid changes to the mRNA expression profile. Similarly, NaCl perfusion was not done to ensure accurate evaluation of transient changes in neurotransmitters. The brains were immediately removed, frozen in liquid nitrogen, and stored at − 80 °C until sectioning in a cryostat. The dorsal hippocampus (dHPC) (Bregma − 3.14 to − 3.80 mm; 4 × 100 μm) and ventral hippocampus (vHPC) (Bregma − 4.80 to − 5.30 mm; 4 × 100 μm) were dissected out in a cryostat (Leica CM3050S, Nussloch GmbH, Germany) according to the anatomical regions based on the Paxinos and Watson Rat Brain Atlas. Sections were stored at − 80 °C until use in the microarray gene expression study. The RNA quality of each sample was analysed using an Agilent 2100 Bioanalyzer to measure the RNA integrity number (RIN). All samples were confirmed to be RIN 8 and above before proceeding for the gene expression experiment. The concentration of all RNA samples was normalised to 25 ng for Cyanine-3 (Cy3) labelling using a One-Color Low Input Quick Amp Labelling Kit (Agilent, Valencia, Ca) according to the manufacturer’s instructions.
The amplified Cy3-labelled cRNA samples were purified using an RNeasy Mini Kit (Qiagen, Valencia, CA). Dye incorporation and cRNA yield were checked on a NanoVue™ Plus Spectrophotometer (GE Healthcare, UK). Next, 0.825 μg Cy3-labelled cRNA (specific activity > 6 pmol Cy3 per μg cRNA) was fragmented at 60 °C for 30 min in a reaction volume of 25 μL containing 25 × Agilent fragmentation buffer and 10 × Agilent blocking agent according to the manufacturer’s instructions.
After fragmentation, 25 μL of the mixture was combined with 25 μL of 2 × Hi-RPM Hybridisation buffer and immediately hybridised using Agilent SurePrint G3 Rat Gene Expression v2 8 × 60K (Design ID: 074036) for 17 h at 65 °C in a rotating Agilent microarray hybridisation oven. After hybridisation, microarrays were washed with GE Wash buffer 1 (Agilent) for 1 min at room temperature and then with GE Wash buffer 2 (Agilent) for 1 min at 37 °C. Slides were immediately scanned using an Agilent SureScan Microarray Scanner (G4900DA) at a resolution of 3 μm and a wavelength of 532 nm (Cy3) using the extended dynamic range (10–100%) setting. Normalised intensities were extracted using the Agilent Feature Extraction Software using the protocol “GE1_1200_Jun14” and all data files were obtained in .txt format.
Visualisation and functional analysis of Gene Ontology (GO) was performed using GOplot package for R [16] with ‘GOBubble’, ‘GOChord’, and ‘GOHeat’ functions. Redundant terms were removed using the ‘reduce_overlap’ function set at 0.75.
Real-Time PCR
Immediately after the final DBS, rats were sacrificed and their brains were extracted and frozen in liquid nitrogen. The dorsal hippocampus (dHPC) (Bregma − 3.14 to − 3.80 mm; 4 × 100 μm) and ventral hippocampus (vHPC) (Bregma − 4.80 to − 5.30 mm; 2 × 100 μm) were dissected out in a cryostat (Leica CM3050S, Nussloch GmbH, Germany) according to the anatomical regions in the Paxinos and Watson Rat Brain Atlas. Sections were stored at − 80 °C until use. Total RNA was extracted using TRIzol reagent (Molecular Research Center Inc., Ohio, USA) and reverse transcribed using PrimeScript™ RT reagent kit with gDNA eraser (Takara Bio USA, California, USA). The cDNA products were stored at − 20 °C until use.
Real-time PCR was performed on a StepOne™ Real-Time PCR System (ThermoFisher Scientific, Massachusetts, USA). Reactions were performed in triplicate in MicroAmp 96-well plates with standard conditions (50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 10 s and 60 °C for 30 s), and SYBR Green fluorescence (Applied Biosystems, Life Technologies, Warrington, UK) was detected after each cycle. A melt curve from 60 to 95 °C with a step increase of + 5 °C was plotted at the end of the cycling stage to evaluate the amplification products. Data were analysed using StepOne™ Real-Time PCR software. All primers used have been previously published [17–20] and amplification efficiency was reassessed as previously described [21]. Hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as the internal control. Relative gene expression analysis was performed using the 2−ΔΔCT method, and DBS animals were normalised to sham animals as previously described [21, 22]. A list of primer sequences and details of the real-time PCR experiments can be found in Supplementary Table 3.
Mass Spectrometry
Tissue homogenisation and metabolite extraction was performed in 1.5 mL of methanol/MilliQ water (80%, v/v) with 0.1 mg norvaline as the internal standard. Tissue was homogenised on ice by 10 cycles of sonication at 10 μm for 20-s and 10-s pause time. Next, 750 μL of MilliQ water was added and the tube was vortexed for 30 s, and then 1200 μL of chloroform was added before vortexing again. After agitating for 15 min, the sample was centrifuged for 5 min at 10,000g. The polar phase was isolated and the dried residue was redissolved and derivatised for 2 h at 37 °C in 40 μL methoxylamine hydrochloride (30 mg/mL in pyridine), followed by trimethylsilylation for 1 h at 37 °C in 70 μL MSTFA with 1% TMCS. A 0.2-μL sample was analysed by GC-MS and the remaining sample was dried under vacuum.
The GC/MS spectra were acquired in SCAN and MRM mode on an Agilent 7890B GC—Agilent 7010 Triple Quadrapole Mass Spectrometer system (Agilent, CA, USA). The sample was separated in an Agilent DB-5MS capillary column (30 m × 0.25 mm ID, 0.25-μm film thickness) with a constant flow rate of 1 mL/min. The GC oven program started at 60 °C (holding time 1 min) and increased by 10 °C/min to 120 °C, then by 3 °C/min to 150 °C, followed by 10 °C/min to 200 °C, and finally by 30 °C/min to 280 °C (hold 5 min). Inlet temperature and transfer line temperature were 250 °C and 280 °C, respectively. Characteristic quantifier and qualifier transitions were monitored in MRM mode during the run. Mass spectra from m/z 50–500 were acquired in SCAN mode.
Data analysis was performed using the Agilent MassHunter Workstation Quantitative Analysis Software. Linear calibration curves for each analyte were generated by plotting the peak area ratio of the external/internal standard against the standard at different concentration levels. Analytes were confirmed by comparing the retention time and ratio of characteristic transitions between the sample and standard.
Dopamine (DA), serotonin (5-HT), γ-aminobutyric acid (GABA), glutamic acid (Glu), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindole acetic acid (5-HIAA) were analysed with norvaline as the internal standard. Due to the low amounts of neurotransmitters, samples were pooled (two samples pooled into one run, and all samples pooled and run in triplicates in another run). As we were interested in the differences in the neurotransmitter levels between the DBS and sham groups, the levels were normalised to the sham results.
Statistics
For behavioural tests, latency data (acquisition) was analysed using repeated measures analysis of variance (ANOVA). The probe trial was measured as the time spent in the target quadrant and latency to first entry onto the imaginary platform, which was analysed using independent sample t tests. For qPCR and mass spectrometry data, statistical analysis was performed using GraphPad Prism 7.00 using multiple t tests with Holm-Sidak Correction. The level of significance used for all analyses was p < 0.05.
Results
Aged Animals Show a Decline in Spatial Learning and Memory
To test if there were memory deficits in aged animals compared to healthy young animals, young rats were subjected to MWM and the results were compared with the aged rats in the sham group. Two-way repeated measures ANOVA revealed an effect of time (F(5,105) = 64.29, p < 0.0001) and DBS (F(1,21) = 49.38, p < 0.0001), but no interaction effects (F(5,105) = 1.78, p = 0.12). Sidak post hoc test revealed a significant effect on all days (ps < 0.005) (Supp. Fig. 2A). t test further revealed a significant difference in the latency to reach the imaginary platform (t(21)=2.97, p = 0.007; Supp. Fig. 2B) and a decrease in the time spent in the target quadrant (t(21) = 6.64, p < 0.0001; Supp. Fig. 2C) in aged rats compared to healthy young rats, indicating spatial memory deficits in aged animals.
PrL DBS Improves Spatial Learning and Memory
To investigate memory improvements due to PrL DBS, rats were implanted with electrodes bilaterally and treated with chronic DBS before undergoing MWM. Two-way repeated measures ANOVA revealed an effect of time (F(5,115) = 66.75, p < 0.0001) and DBS (F(1,23) = 8.64, p = 0.007), but no interaction (F(5,115) = 1.72, p = 0.13). Sidak post hoc test revealed a significant effect on days 2 and 3 (ps < 0.05) (Fig. 1B). t test further revealed a significant difference in the latency to reach the imaginary platform (t(23) = 2.52, p = 0.019; Fig. 1C) and an increase in the time spent in the target quadrant (t(23) = 2.13, p = 0.044; Fig. 1D) in PrL DBS aged rats compared to the sham group, indicating spatial memory enhancement in DBS animals. In order to control for locomotor and anxiety effects, animals underwent an OFT prior to MWM. t test of the distance travelled showed no significant difference between groups (t(23) = 0.10 p = 0.92; Supp. Fig. 3A). However, there was a significant increase in time spent in centre area (t(23) = 6.22 p < 0.0001; Supp. Fig. 3B) in DBS aged rats compared to the sham group.
Microarray Data Suggests Involvement of G Protein–Coupled Receptors
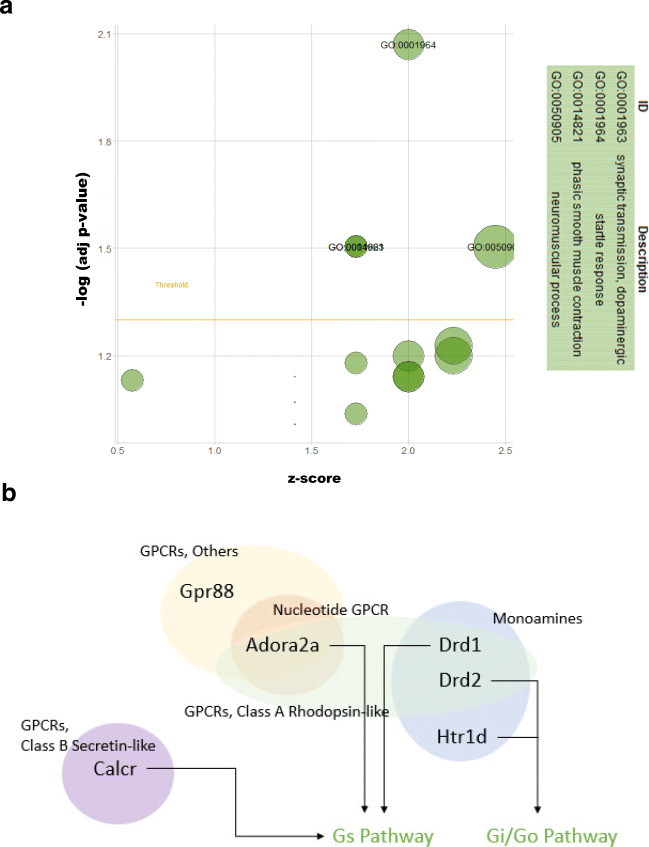
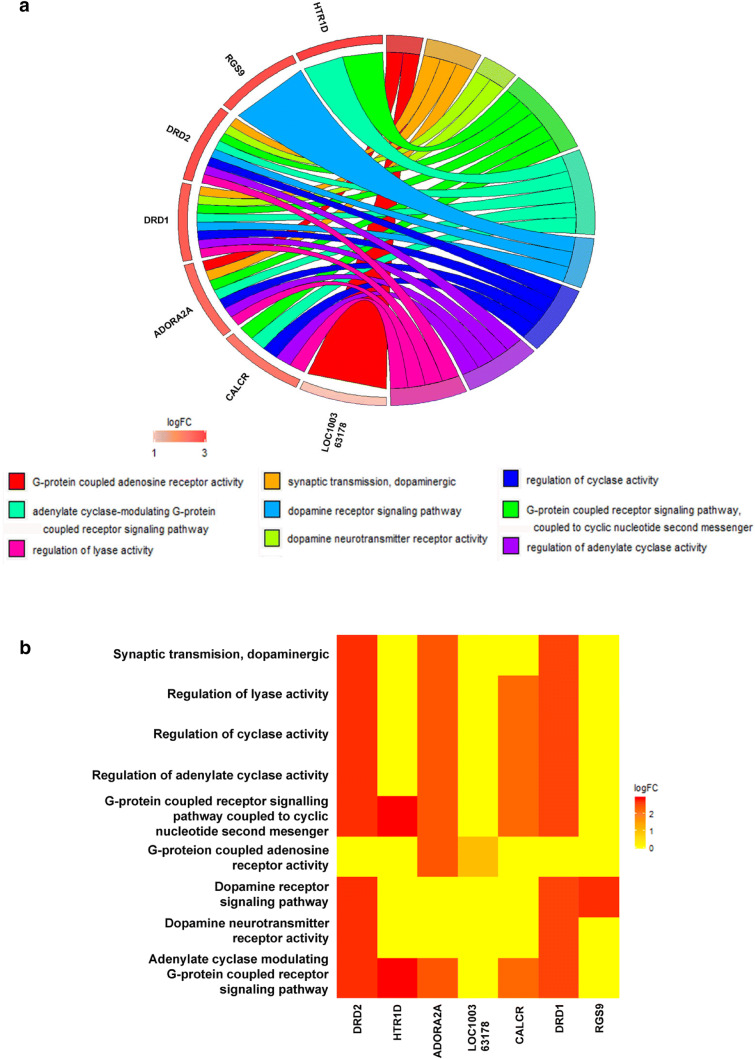
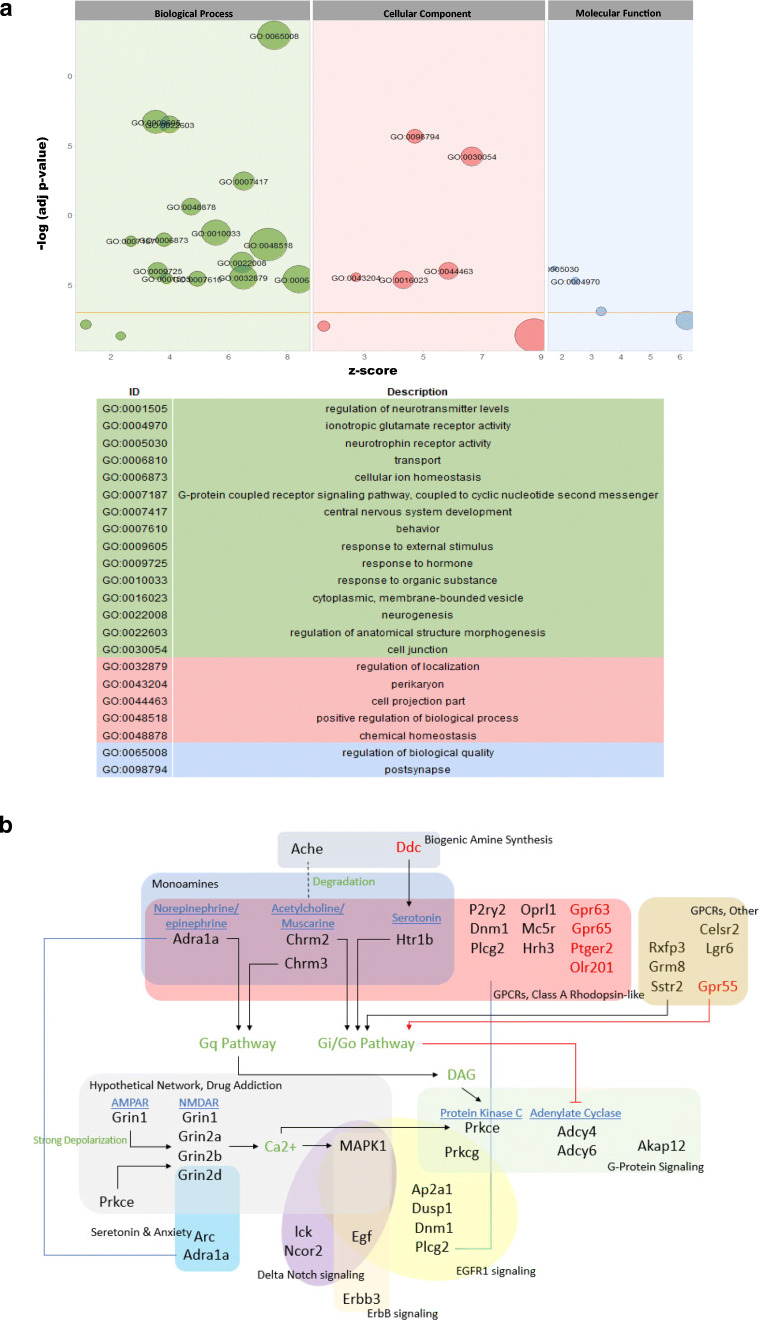
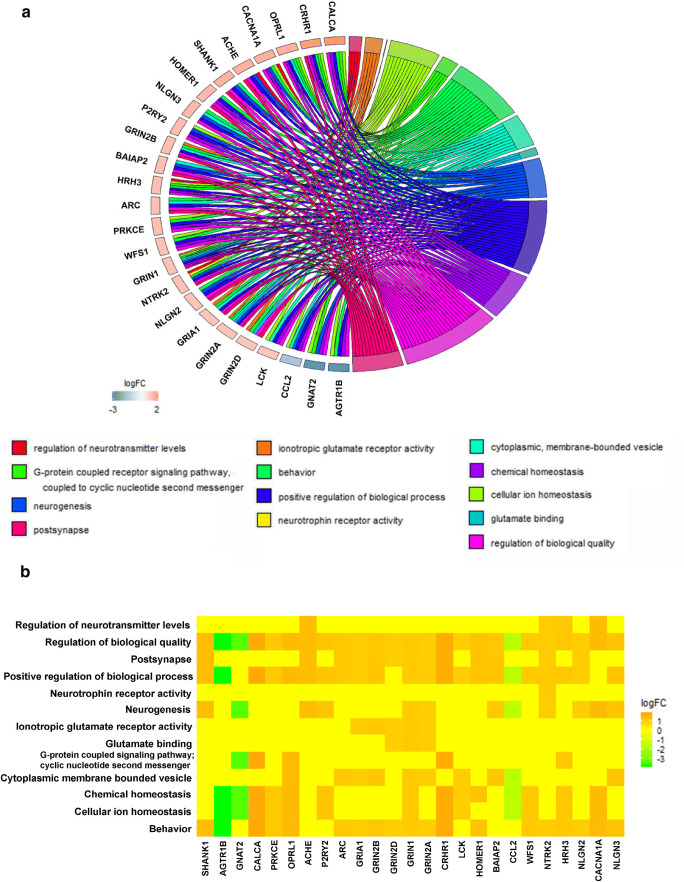
To unravel the mechanisms of the observed effects in aged rats, the dorsal and ventral hippocampus were micro-dissected separately for microarray analysis. Using a threshold of a 2-fold change and a 0.05 corrected p value, we found 95 entities (19 downregulated and 76 upregulated) in the dHPC and 848 entities (247 downregulated and 601 upregulated) in the vHPC (Fig. 1E–H). Gene Ontology (GO) analysis revealed 17 and 199 GO changes in the dHPC and vHPC, respectively. Visualisation and functional analysis of GO was performed using GOplot package for R [16]. Bubble plot of dHPC showed (among others) an upregulation of genes related to dopaminergic synaptic transmission (Fig. 2A). Pathway analysis of memory-related pathways further revealed a central role of downstream G protein pathways, particularly monoamines (Fig. 2B, Supplementary Table 1). Chord plot and heatmap of genes further showed a central role of Drd1, Drd2, and Adora2 (Fig. 3A, B). Because of the large amount of gene/GO changes in the vHPC, the number of GO terms was reduced using the “reduce_overlap” function. The bubble plot revealed changes (among others) in neurogenesis and glutamate transmission activity (Fig. 4A). Functional pathways analysis showed a central role of glutamate pathways in addition to the involvement of downstream G protein pathways (Fig. 4B, Supplementary Table 2). Relevant significant processes detected by the bubble plot were plotted in a chord plot, which showed a minimum of five connections to the central genes involved (Fig. 5A). Likewise, we showed the involvement of glutamate-related (particularly the NMDA receptors) genes (Gria1, Grin2B, Grin2D, Grin1, Grin2A) and neurogenesis genes (upregulated: Shank1, Ache, P2RY2, Grin1, Grin2A, BAIAP2, NTRK2, HRH3, CACNA1A, NLGN3; downregulated: GNAT2, CCL2) (Fig. 5B).
Fig. 2.
PrL DBS elicits functional changes in the dHPC. Bubble plot of dHPC (A) shows significant differences in the Gene Ontology (GO). Functional pathway analysis showed central roles of downstream G protein pathways (B)
Fig. 3.
PrL DBS elicits distinct patterns of dorsal hippocampal gene expression. Chord plot (A) and heatmap (B) of dHPC shows central roles of Drd1, Drd2, and Adora2
Fig. 4.
PrL DBS elicits functional changes in the vHPC. Bubble plot of vHPC (A) shows significant differences in the Gene Ontology (GO). Functional pathway analysis showed central roles of downstream G protein pathways and heavy involvement of glutamate pathways in the vHPC (B)
Fig. 5.
PrL DBS elicits distinct patterns of ventral hippocampal gene expression. Chord plot (A) and heatmap (B) of vHPC show major roles of glutamate-related (in particular NMDA receptors) genes and neurogenesis genes
qPCR Validation Suggests a Major Role of Monoamines in the dHPC
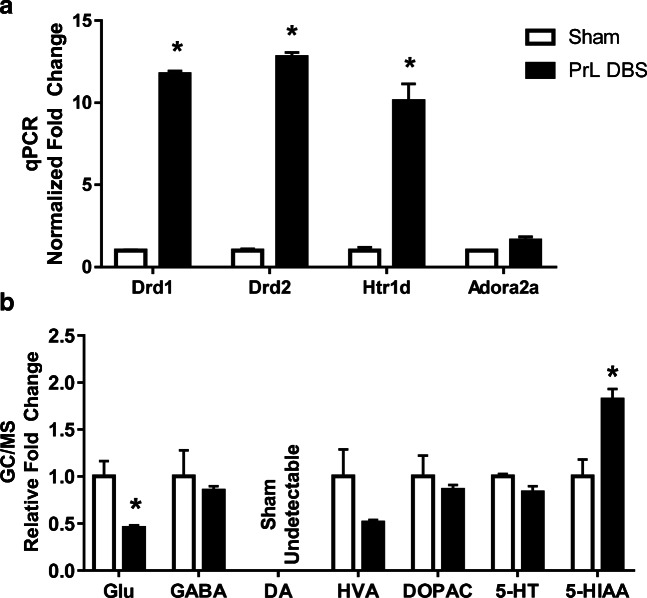
In order to validate the microarray data, real-time qPCR was performed on the major markers (Supp. Table 1) highlighted in the above analysis in the dHPC. t tests with Holm-Sidak correction revealed significant increases in Drd1, Drd2, and Htr1d (lowest t = 8.68, all ps < 0.05) (Fig. 6A), but not Adora2a (t(2)=2.88, p = 0.10) (Fig. 6A).
Fig. 6.
PrL DBS modulates changes of monoamines and their receptors in the dHPC. (A) qPCR validation of key genes in dHPC tissue showing a remarkable upregulation of Drd1, Drd2, and Htr1d. (B) GC/MS measurement of neurotransmitter levels showed downregulation of Glu and HVA, and significant upregulation of 5-HIAA. Dopamine was detectable in DBS groups, but undetectable in the sham group. *p < 0.05
PrL DBS Induces Changes in Neurotransmitter Levels in the dHPC
In order to investigate the effects of chronic DBS on neurotransmitters, GC/MS was performed on dHPC slices targeting Glu, GABA, HVA, DOPAC, DA, 5-HIAA, and 5-HT. Samples were pooled randomly in pairs. All targets were within the linear ranges of the standard curves, except for DA in the sham group. Multiple t test with Holm-Sidak corrections of the relative fold changes showed there were significantly lower levels of Glu (t(4.8) = 3.50, p = 0.006), significantly higher levels of 5-HIAA (t(4.0) = 5.27, p < 0.001), and no changes in the levels of GABA, HVA, DOPAC, and 5-HT (highest t = 0.66, all ps > 0.05) (Fig. 6B).
Discussion
The ability of DBS to improve memory has long been thought to be mediated through neurogenesis [7–9, 23]. Besides our previous study that used middle-aged animals [7], a major limitation of other previous studies was their use of young animals, which have been shown to have differences in hippocampal neurogenesis compared to aged animals [24]. In this study, we further extended our previous results in middle-aged animals to aged animals, which showed PrL DBS was also effective in improving memory in aged rats. We further investigated the mechanisms behind this effect, which suggested the involvement of alternative mechanisms besides neurogenesis.
We first confirmed that our aged animal model had declined memory function in the Morris water maze. This memory deficit in naturally aged rats is important, considering a major limitation of the aged animal model is that they might not spontaneously develop AD like pathologies [25]. Similar to our previous study using middle-aged animals [7], we found that memory improvements were accompanied by an anxiolytic effect as seen by less time spent in centre zone of the open field test. Although we cannot rule out the possibility that the enhanced performance was a result of lowered stress, we believe the memory improvements due to PrL DBS were accompanied by anxiolytic effects via the mechanisms previously discussed [7].
The stimulation parameters (chronic high-frequency stimulation) used in this study were based on our previous experiments [7]. We previously showed chronic DBS could enhance memory in middle-aged animals [7], and hypothesised that the effects of chronic DBS on memory circuitry would enhance rather than disrupt memories [13, 26]. Furthermore, the use of high-frequency stimulation rather than low-frequency stimulation was based on our previous data that suggested possible anxiogenic effects (decreased time spent in the centre zone of the OFT) with low-frequency ventromedial prefrontal cortex (vmPFC) stimulation [14]. We carried out a longer 6-day MWM protocol to allow both DBS and sham animals to achieve a similar level of learning before the probe trial, which should minimise differences in the carry-over effects from the acquisition of learning. Our results showed an improvement in both acquisition and retrieval of learning in animals that underwent PrL DBS. It should be noted that there appeared to be an increase in latency in the PrL DBS group on the last day of MWM acquisition, which might indicate overlearning. Nevertheless, our results suggest an overall improvement in both acquisition and retrieval of spatial memory. Another possible mechanism is an enhancement during consolidation, as DBS was continued for 30 mins after the behavioural testing. However, we do not think this is the case. Indeed, DBS during consolidation likely disrupts memory, as we have suggested both experimentally [27] and in a simulated model of memory [26], and have previously discussed in-depth [13]. Therefore, the observed effects are more likely due to chronic DBS. Regardless, a limitation of our current experiments is its open-loop nature, which makes subtle distinctions difficult to analyse. Using a closed-loop system that is dynamically optimised based on the underlying states of the brain together with machine learning–based signals associated with memory could allow for more controlled and predictable responses [28, 29], and allow us to better understand the underlying mechanisms of the observed effects.
Our microarray results in aged animals suggested a major role of neurotransmission (particularly GPCRs) in the effects of chronic PrL DBS. This is in agreement with several studies that also suggest the effects of DBS on memory are mediated through mechanisms other than neurogenesis [27, 30–32]. We targeted the PrL for DBS based on our previous study on middle-aged animals, which resulted in cognitive improvement [7]. We have previously argued that the PrL is a prime target for the modulation of memory [13, 33]. The large number of observed gene changes in the vHPC is perhaps unsurprising due to the direct monosynaptic projection from the vHPC to the vmPFC, which plays a crucial role in spatial memory [34], and thus backpropagation of signals could be one possible mechanism. However, it should be noted that more prominent changes were seen in monoamine (particularly dopamine) receptors in the dHPC, which we have similarly argued is a major mechanism of memory modulation in DBS [13]. Furthermore, the prominent changes in dHPC corroborates our findings on the improvements seen in MWM. The dHPC is more heavily involved in spatial learning, whereas the vHPC is involved in anxiety-like behaviour [35]. Given that DBS was administered directly before culling, we cannot exclude the possibility that the observed changes are due to acute rather than chronic DBS. However, we previously showed that acute PrL DBS after a spatial memory task did not induce changes in dopamine receptors in the dHPC, but rather caused a decrease in various receptors in the vHPC [27]. We therefore believe that the distinct pattern of gene expressions seen in the dHPC are due to (at least in part) chronic DBS. Further work is required to elucidate the precise mechanism of the major changes in the dHPC induced by chronic DBS.
Our finding that Htr1d (5HT receptor), D1 and D2 receptors in the dHPC were upregulated with DBS is perhaps unsurprising, given the role of both serotonin and dopamine in memory and their decrease with age (briefly reviewed by Peters [36]). Partly in line with this, Hamani et al. [37] used microdialysis to show that vmPFC DBS increased serotonin levels in the dHPC. Furthermore, increased Htr1d was also shown to be associated with learning in MWM [38]. To the best of our knowledge, our study is the first to report an increase in dopaminergic transmission in the hippocampus after PrL DBS. Our previous study using acute PrL DBS did not show changes to either receptors or the dopamine metabolite DOPAC in the dHPC [27]. However, this might be due to the use of young animals and different study parameters such as a shorter stimulation. Notably, the aged brain has reduced amounts of both D1 and D2 receptors [39, 40], and DBS induces upregulation to “normalise” D1/D2 receptors, which might be the mechanism by which PrL DBS enhances memory in aged animals. Regulator of G protein signalling 9 (RGS9) showed similar upregulation, which might suggest a response to the upregulation of D2 receptors [41], although it should be noted that the central nervous system splice variant RGS9-2 is almost exclusively located in the striatum [42], and upregulation here might not be associated with the observed behavioural changes. Interestingly, and contrary to expectation, Adora2a (adenosine A2a receptor) was upregulated. Its overexpression has been shown to be associated with ageing and age-related memory impairment [43, 44]. However, given that Adora2a and D2Rs are closely linked, the increase in Adora2a might be a facilitative modulator to D2R [45]. This result needs to be interpreted with caution, as the follow-up qPCR did not show significant changes in Adora2a levels. Although Calcr (calcitonin receptor) was upregulated, in situ and immunohistochemistry studies have shown that the hippocampus does not contain calcitonin receptors or its mRNA [46, 47]. Nevertheless, we cannot fully rule out the possibility that Calcr has a role in the observed effects. Curiously, microinjection of salmon calcitonin (a strong agonist) in the hippocampus was shown to affect feeding behaviour, but how it exerts its effects is still unknown [47].
The gene changes in vHPC (lower fold change), while more complex, appeared to be less pronounced than that in the dHPC. This is in stark contrast to acute PrL DBS, in which the vHPC, as opposed to the dHPC, appears to be the locus of the effects [27]. This further highlights the different effects between chronic and acute stimulation, which need to be considered when analysing the results. Although it was difficult to get a complete “picture” of the effects in the vHPC due to the large numbers of genes both up- and downregulated, certain patterns could be seen in the functional analysis (Fig. 4B). There was upregulation of a remarkable number of glutamatergic receptor genes and genes related to GPCRs. This was further confirmed by the chord plot and heatmap analysis, which showed changes in ionotropic glutamate receptor activity, glutamate binding, and GPCR pathways. The involvement of neurogenesis was also implicated, as various related markers such as TrkB and Egf were upregulated. Furthermore, the functional analysis showed changes possibly due other downstream effects. We suggest there is an interplay among changes in neurotransmission, GPCR pathways, and neurogenesis, although more work is needed to fully understand the precise mechanisms and their contribution to the observed effects.
Based on the findings from the microarray analysis, we decided to further analyse the changes in the dHPC. We performed qPCR validation and GC/MS to investigate the pronounced changes in the dHPC in relation to the enhanced spatial memory [35]. The choice to use qPCR instead of more direct protein quantitation methods to validate the results of the microarray was based on the fact that the effects appeared to be driven by GPCRs, and antibodies against GPCRs can be notoriously unreliable [48]. In addition, D1 and D2 receptors (also heavily involved) both have strikingly similar protein structures, making their accurate quantification difficult [49–51]. Furthermore, staining these receptors has proven to be problematic and several studies have reported contradictory results [50–53]. However, we recognise the limitations of measuring mRNA levels, as they may not correlate with the corresponding protein levels [54], but given the difficulties in measuring D1 and d2 receptors at the protein level, qPCR appears to be a more reasonable quantification method. Given the opposing roles of D1 and D2 receptors in GPCR transmission, further consideration of the phosphorylated states of downstream markers might prove useful in delineating the exact mechanisms involved. Regardless, to overcome this difficulty, and to extend our results, we followed up with these results by using GC/MS to measure changes in neurotransmitter levels.
Based on our microarray data and our previous discussions of how DBS could modulate memories through neurotransmission changes [13], we next conducted GC/MS on dHPC samples to determine the levels of various neurotransmitters and their metabolites. Unlike Hamani et al. [37], we did not observe an increase in 5-HT in the dHPC, but found its metabolite 5-HIAA was significantly increased. An increase in metabolised 5-HT might explain why 5-HT was not upregulated. Importantly, as animals were not perfused before their brains were extracted, 5-HT in the blood could affect the results; thus, any conclusions should be made cautiously. Furthermore, in the above study, the use of microdialysis exclusively measures extracellular 5-HT, whereas GC/MS used in our study measures total 5-HT. Although DA was undetectable in the sham group, it was detectable in small amounts in the dHPC of the DBS group, suggesting that DA was indeed increased by DBS. The dopaminergic system is known to decline with age and is a major mechanistic candidate for age-related cognitive decline [55, 56], which could explain the undetectable amounts DA in the sham group. Our results therefore suggest a potential mechanism of how DBS enhances memory in aged animals through the restoration of dopaminergic systems. Aside from monoamine changes, we also found significantly lower amounts of glutamate, which might be due to the actions of 5-HT and DA, as 5-HT has been shown to reduce glutamatergic synaptic transmission in the hippocampus [57, 58] and inhibit local glutamate release [59]. Similarly, increased dopamine was associated with lower glutamatergic plasticity in the hippocampus [60]. The lower glutamate levels, however, appeared to contradict the behavioural data, which showed improved memory function and presumably increased LTP. Overall, the effects of chronic PrL DBS on memory appear to involve complex interplay between various neurotransmitters and their receptors leading to increased memory function. However, much more work is needed to fully understand these effects. Furthermore, we cannot exclude the effects on non-neuronal sources of neurotransmitters such as in glia [61, 62].
In this study, we showed the potential of DBS in enhancing memory in aged animals. We anticipate that DBS technology could eventually be applied in aged-related disorders such as Alzheimer’s disease. Using a more aetiologically relevant model (aged animals) and harnessing high-throughput omics techniques such as microarray, we highlighted major molecular mechanisms related to the observed effects. We showed that mechanisms beyond neurogenesis might underlie the memory enhancement effects of DBS. Overall, it is likely that a complex interplay between changes in neurogenesis and non-neurogenesis genes/proteins contribute to the effects of DBS. Although we focused on the major changes in dopamine transmission in dHPC, the observed changes in glutamate regulation and serotonin metabolism could also be contributing factors. This study has only begun to uncover some of the mechanisms of how DBS enhances memories in aged animals and the findings are preliminary in nature; they provide a first step and a foundation for further research to uncover the mechanisms of the effects of DBS. Therefore, this paper serves as a call for more studies on the effects of DBS on memory that look beyond neurogenesis and to investigate the pathways highlighted in our data. We have made our data freely available (10.17632/d5xc8ns3vg.1) for other researchers to guide them in their studies, with the hope of validating and translating DBS as a memory enhancing technology for clinical applications.
Electronic Supplementary Material
(DOCX 35 kb)
(PDF 210 kb)
(PDF 1224 kb)
Acknowledgements
The scientific work was funded by grants from the Hong Kong Research Grants Council (RGC-ECS 27104616), and the University of Hong Kong Seed Funding awarded to LWL. We would like to thank the Proteomics and Metabolomics Core Facility (HKU), and Junhao Koh and Jose Perucho for their valuable input.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shawn Zheng Kai Tan, Email: shawntanzk@hku.hk.
Joveen Neoh, Email: joveen@neogenix.org.
Andrew John Lawrence, Email: andrew.lawrence@florey.edu.au.
Ed Xuekui Wu, Email: ewu@eee.hku.hk.
Lee Wei Lim, Email: drlimleewei@gmail.com.
References
- 1.World Health Organization. Dementia cases set to triple by 2050 but still largely ignored [Internet]. 2012. Available from: http://www.who.int/mediacentre/news/releases/2012/dementia_20120411/en/
- 2.Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement [Internet]. 2015;12(4):88. Available from: 10.1016/j.jalz.2016.03.001 [DOI] [PubMed]
- 3.Qaseem A, Snow V, Cross JT, Jr, Forciea MA, Hopkins R., Jr Current Pharmacologic Treatment of Dementia : A Clinical Practice Guideline from the American College of Physicians and the American. Ann Intern Med. 2008;148:370–8. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 4.Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer’s disease? Biol Psychiatry. 2010;68(10):876–8. Available from: 10.1016/j.biopsych.2010.09.020 [DOI] [PubMed]
- 5.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temel Y, Hescham SA, Jahanshahi A, Janssen MLF, Tan SKH, van Overbeeke JJ, et al. Neuromodulation in Psychiatric Disorders. Vol. 107, International Review of Neurobiology. 2012. 283–314 p. [DOI] [PubMed]
- 7.Liu A, Jain N, Vyas A, Lim LW. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. Elife. 2015;4:e04803. [DOI] [PMC free article] [PubMed]
- 8.Stone SSD, Teixeira CM, De Vito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of Entorhinal Cortex Promotes Adult Neurogenesis and Facilitates Spatial Memory. J Neurosci. 2011;31(38):13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone. Exp Neurol. 2011;232(1):100–4. doi: 10.1016/j.expneurol.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase i trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol. 2010;68(4):521–34. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- 11.Sengupta P. The laboratory rat: Relating its age with human’s. Int J Prev Med. 2013;4(6):624–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Geerts H, Hofmann-Apitius M, Anastasio TJ. Knowledge-driven computational modeling in Alzheimer’s disease research: Current state and future trends. Alzheimer’s Dement. 2017;13(11):1292–302. Available from: 10.1016/j.jalz.2017.08.011 [DOI] [PubMed]
- 13.Tan SZK, Fung M, Koh J, Chan Y, Lim LW. The Paradoxical Effect of Deep Brain Stimulation on Memory. Aging Dis. 2020;11(1):179–90. doi: 10.14336/AD.2019.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry. 2015;5(3):e535. doi: 10.1038/tp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sixth Edition by. Acad Press. 2006;170:547612.
- 16.Walter W, Sánchez-Cabo F, Ricote M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31(17):2912–4. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- 17.Dick ALW, Pooters T, Gibbs S, Giles E, Qama A, Lawrence AJ, et al. NMDA receptor binding is reduced within mesocorticolimbic regions following chronic inhalation of toluene in adolescent rats. Brain Res. 2015;1624:239–52. doi: 10.1016/j.brainres.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Tian B, Wang XL, Huang Y, Chen LH, Cheng RX, Zhou FM, et al. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci Rep. 2016;6:1–17. doi: 10.1038/srep36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madeira MH, Ortin-Martinez A, Nadal-Nícolas F, Ambrósio AF, Vidal-Sanz M, Agudo-Barriuso M, et al. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep. 2016;6:1–13. doi: 10.1038/srep27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covacu R, Arvidsson L, Andersson A, Khademi M, Erlandsson-Harris H, Harris RA, et al. TLR activation induces TNF-alpha production from adult neural stem/progenitor cells. J Immunol. 2009;182(11):6889–95. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg [Internet]. 2008;108(1):132–8. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan SZK, Zhao RC, Chakrabarti S, Stambler I, Jin K, Lim LW. Interdisciplinary Research in Alzheimer ’ s Disease and the Roles International Societies Can Play. Aging Dis. 2021;12(1):eprint ahead. [DOI] [PMC free article] [PubMed]
- 26.Tan SZK, Du R, Perucho JAU, Chopra SS, Vardhanabhuti V, Lim LW. Dropout in Neural Networks Simulates the Paradoxical Effects of Deep Brain Stimulation on Memory. Front. Aging Neurosci. 2020 (In press) [DOI] [PMC free article] [PubMed]
- 27.Tan SZK, Poon CH, Chan Y-S, Lim LW. Deep Brain Stimulation of the Ventromedial Prefrontal Cortex Disrupts Consolidation of Fear Memories. bioRxiv [Internet]. 2019 Jan 1;537514. Available from: http://biorxiv.org/content/early/2019/02/03/537514.abstract
- 28.Zanos S. Closed-loop neuromodulation in physiological and translational research. Cold Spring Harb Perspect Med. 2019;9(11):1–18. doi: 10.1101/cshperspect.a034314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song D, Robinson BS, Hampson RE, Marmarelis VZ, Deadwyler SA, Berger TW. Sparse Large-Scale Nonlinear Dynamical Modeling of Human Hippocampus for Memory Prostheses. IEEE Trans Neural Syst Rehabil Eng. 2018;26(2):272–80. doi: 10.1109/TNSRE.2016.2604423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hescham S, Temel Y, Schipper S, Lagiere M, Schönfeld L-M, Blokland A, et al. Fornix deep brain stimulation induced long-term spatial memory independent of hippocampal neurogenesis. Brain Struct Funct [Internet]. 2016; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26832921 [DOI] [PMC free article] [PubMed]
- 31.Hescham S, Jahanshahi A, Schweimer JV, Mitchell SN, Carter G, Blokland A, et al. Fornix deep brain stimulation enhances acetylcholine levels in the hippocampus. Brain Struct Funct. 2015;221(8):1–6. doi: 10.1007/s00429-015-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchanan RJ, Darrow DP, Meier KT, Robinson J, Schiehser DM, Glahn DC, et al. Changes in GABA and glutamate concentrations during memory tasks in patients with Parkinson’s disease undergoing DBS surgery. Front Hum Neurosci. 2014;8(March):81. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3945932&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 33.Tan SZK, Sheng V, Chan Y-S, Lim LW. Eternal Sunshine of the Neuromodulated Mind: Altering Fear Memories Through Neuromodulation. Exp Neurol. 2019;314:9–19. doi: 10.1016/j.expneurol.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Spellman T, Rigotti M, Ahmari SE, Fusi S, Joseph A, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522(7556):309–14. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential Control of Learning and Anxiety Along the Dorsoventral Axis of the Dentate Gyrus. Neuron. 2013;77(5):955–68. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters R. Ageing and the brain. Postgr Med J. 2006;82:84–8. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-Like Effects of Medial Prefrontal Cortex Deep Brain Stimulation in Rats. Biol Psychiatry. 2010;67(2):117–24. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Cavallaro S. Genomic analysis of serotonin receptors in learning and memory. Behav Brain Res. 2008;195(1):2–6. doi: 10.1016/j.bbr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Antonini A, Leenders Klaus L. Dopamine D2 Receptors in Normal Human Brain: Effect of Age Measured by Positron Emission Tomography (PET) and [11C] -Raclopride. Ann N Y Acad Sci. 1993;695:81–5. doi: 10.1111/j.1749-6632.1993.tb23033.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Chan GLY, Holden JE, Dobko T, Mak E, Schulzer M, et al. Age-dependent decline of dopamine D1 receptors in human brain: A PET study. Synapse. 1998;30(1):56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, et al. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A. 2004;101(46):16339–44. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: Region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17(20):8024–37. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Temido-Ferreira M, Ferreira DG, Batalha VL, Marques-Morgado I, Coelho JE, Pereira P, et al. Age-related shift in LTD is dependent on neuronal adenosine A 2A receptors interplay with mGluR5 and NMDA receptors. Mol Psychiatry. 2018;1–25. Available from:10.1038/s41380-018-0110-9 [DOI] [PMC free article] [PubMed]
- 44.Giménez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camón L, Wassholm M, et al. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem. 2007;87(1):42–56. doi: 10.1016/j.nlm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, et al. The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A2AR)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci. 2001;98(4):1970–5. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheward WJ, Lutz EM, Harmar AJ. The expression of the calcitonin receptor gene in the brain and pituitary gland of the rat. Neurosci Lett. 1994;181(1–2):31–4. doi: 10.1016/0304-3940(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 47.Becskei C, Riediger T, Zünd D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030(2):221–33. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379(4):385–8. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- 49.Hutchings CJ, Koglin M, Marshall FH. Therapeutic antibodies directed at G protein-coupled receptors. MAbs. 2010;2(6):594–606. doi: 10.4161/mabs.2.6.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90(19):8861–5. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zbukvic IC, Park CHJ, Ganella DE, Lawrence AJ, Kim JH. Prefrontal Dopaminergic Mechanisms of Extinction in Adolescence Compared to Adulthood in Rats. Front Behav Neurosci. 2017;11:32. doi: 10.3389/fnbeh.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14(1):88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariano MA, Fisher RS, Smyk-Randall E, Sibley DR, Levine MS. D2 dopamine receptor distribution in the rodent CNS using anti-peptide antisera. Brain Res. 1993;609(1–2):71–80. doi: 10.1016/0006-8993(93)90857-j. [DOI] [PubMed] [Google Scholar]
- 54.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2013;13(4):227–32. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev. 2010;34(5):670–7. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Res. 1995;701(1–2):249–54. doi: 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- 58.Mlinar B, Falsini C, Corradetti R. Pharmacological characterization of 5-HT 1B receptor-mediated inhibition of local excitatory synaptic transmission in the CA1 region of rat hippocampus. Br J Pharmacol. 2003;138(1):71–80. doi: 10.1038/sj.bjp.0705026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aït Amara D, Segu L, Naïli S, Buhot MC. Serotonin 1B receptor regulation after dorsal subiculum deafferentation. Brain Res Bull. 1995;38(1):17–23. doi: 10.1016/0361-9230(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 60.Neddens J, Vullhorst D, Paredes D, Buonanno A. Neuregulin links dopaminergic and glutamatergic neurotransmission to control hippocampal synaptic plasticity. Commun Integr Biol. 2009;2(3):261–4. doi: 10.4161/cib.2.3.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler B, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA. 1999;96:8733–8. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De-Miguel FF, Leon-Pinzon C, Noguez P, Mendez B. Serotonin release from the neuronal cell body and its long-lasting effects on the nervous system. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140196. doi: 10.1098/rstb.2014.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 35 kb)
(PDF 210 kb)
(PDF 1224 kb)