Abstract
Objectives
Home environmental exposures are a primary source of asthma exacerbation. There is a gap in decision support models that efficiently aggregate the home exposure assessment scores for focused and tailored interventions. Three development methods of a home environment allergen exposure scale for persons with asthma (weighted by dimension reduction, unweighted, precision biomarker-based) were compared, and racial disparity tested.
Methods
Baseline measures from a longitudinal cohort of 187 older adults with asthma were analyzed using humidity and particulate matter sensors, allergy testing, and a home environment checklist. Weights for the dimension reduction scale were obtained from factor analysis, applied for loadings > 0.35. Scales were tested in linear regression models with asthma control and asthma quality of life outcomes. Racial disparities were tested using t tests. Scale performance was tested using unadjusted regression analyses with asthma control and asthma quality of life outcomes, separately.
Results
The 7-item empirically weighted scale demonstrated best performance with asthma control associations (F = 4.65, p = 0.03, R2 = .02) and quality of life (F = 6.45, p = 0.01, R2 = .03) as follows: evidence of roach/mice, dust, mold, tobacco smoke exposure, properly venting bathroom fan, self-report of roach/mice/rats, and access to a HEPA filter vacuum. Pets indoors loaded on a separate scale. Racial differences were observed (t = − 3.09, p = 0.004).
Conclusion
The Home Environment Allergen Exposure Scale scores were associated with racial disparities. Replicating these methods in populations residing in high-risk/low-income housing may generate a clinically meaningful, tailored assessment of asthma triggers. Further consideration for variables that address allergic reactivity and biomarker results is indicated to enhance the potential for a precision prevention score.
Electronic supplementary material
The online version of this article (10.17269/s41997-020-00335-0) contains supplementary material, which is available to authorized users.
Keywords: Asthma, Allergy and immunology, Home care services, Environment, Adult, Aged
Résumé
Objectifs
Les expositions environnementales à domicile sont une source principale d’exacerbation de l’asthme. Il existe une lacune dans les modèles de soutien à la décision qui regroupent efficacement les scores d’évaluation de l’exposition à domicile pour des interventions ciblées et adaptées. Trois méthodes de développement d’une échelle d’exposition aux allergènes de l’environnement domestique pour les personnes atteints d’asthme (pondérée par réduction de dimension, non pondérée, basée sur un biomarqueur de précision) ont été comparées et la disparité raciale testée.
Méthodes
Les mesures de base d’une cohorte longitudinale de 187 personnes âgées asthmatiques ont été analysées à l’aide de capteurs d’humidité et de particules, de tests d’allergie et d’une liste de contrôle de l’environnement domestique. Les poids pour l’échelle de réduction des dimensions ont été obtenus à partir de l’analyse factorielle, appliquée aux charges > 0,35. Les échelles ont été testées dans des modèles de régression linéaire avec contrôle de l’asthme et résultats de la qualité de vie avec asthme. Les disparités raciales ont été testées à l’aide de tests t. La performance de l’échelle a été testée à l’aide d’analyses de régression non pondérées avec contrôle de l’asthme et résultats de la qualité de vie avec asthme, séparément.
Résultats
L’échelle pondérée empiriquement en 7 éléments a démontré les meilleures performances avec les associations de contrôle de l’asthme (F = 4,65, p = 0,03, R2 = 0,02) et la qualité de vie (F = 6,45, p = 0,01, R2 = 0,03) comme suit : preuve d’exposition aux cafards/souris, à la poussière, à la moisissure, à la fumée de tabac, ventilateur de salle de bain correctement aéré, auto-déclaration des cafards/souris/rats et accès à un aspirateur à filtre HEPA. Les animaux de compagnie à l'intérieur ont été mesurés sur une échelle séparée. Des différences raciales ont été observées (t = -3,09, p = 0,004).
Conclusion
Les scores de l’échelle d’exposition aux allergènes de l’environnement domestique étaient associés à des disparités raciales. La reproduction de ces méthodes dans des populations résidant dans des logements à haut risque/à faible revenu peut générer une évaluation cliniquement significative et adaptée des déclencheurs de l’asthme. Un examen plus approfondi des variables qui traitent de la réactivité allergique et des résultats des biomarqueurs est indiqué pour améliorer le potentiel d’un score de prévention de précision.
Mots-clés: Asthme, allergie et immunologie, services de soins domestiques, environnement, adulte, âgé
Introduction
Asthma affects about 8% of the Canadian and US population over the age of 64 years (Centers for Disease Control and Prevention 2017; Statistics Canada 2014). Home environment exposures can increase the risk of asthma development and exacerbation (Krieger et al. 2015; Wang et al. 2017), particularly for older adults (Baptist and Busse 2018; Skloot et al. 2016). Over 75% of older adults with asthma have at least one positive eosinophilic biomarker related to dust mites, mold spores, or pet dander (Huss et al. 2001). Interventions to reduce home environment allergen exposures, such as mattress and pillow encasements, have limited uptake among older adults with asthma (Shedd et al. 2007). When asthma-specific quality of life has been measured, older adults demonstrate worse scores in the environmental domain than working-aged adults (Kannan et al. 2015). While there is substantial research for children and low-income adults with asthma, there is a gap on the home environment management of asthma specific for older adults (Reddy et al. 2017; Gomez et al. 2017).
The Symptom Science Model for Environmental Health was the driving framework for this research (Castner 2019; Castner et al. 2019). The model posits that environmental exposures increase complex symptoms and are the causal factor (environmental endotype) for specific phenotypes of disease development and exacerbation. The focus of this study is development of a composite measure of home environment allergen exposures. The environmental endotype is conceptualized as blood immunoglobulin E (IgE)-mediated inflammation triggered by specific home environment allergen exposures. Asthma control and asthma-specific quality of life are valid and reliable measures incorporating complex asthma symptoms (Juniper et al. 1999; Nathan et al. 2004). The specific eosinophilic asthma phenotype is characterized by the presence of eosinophils in the blood, sputum, or airway with the clinical diagnoses of asthma (Carr et al. 2018). We contribute to biomarker discovery by developing a novel precision prevention scale that matches specific allergic biomarkers to home environment exposure variables. To clarify, we utilize the broad concept of precision that encompasses personalized prevention and does not narrowly focus on pharmaco-genomic therapeutics alone (Carlsten et al. 2014).
African American/Black (AA/B) individuals are substantially over-represented in those with asthma (Bhan et al. 2015), a disparity that persists after controlling for income and urban dwelling (New York State Department of Health 2013). Non-Hispanic AA/Bs demonstrated higher exposure to roach and mouse allergens, while other races/ethnicities demonstrated higher exposures to rat, cat, dog, alternaria mold, and dust mite allergens (Salo et al. 2018). Race was not identified as a causal factor in asthma emergencies, after controlling for environmental exposures, community features, and socio-economic differences (Fitzpatrick et al. 2019). Thus, environment assessment tools are essential to address a root cause of racial disparity.
While several home environment assessment instruments are available, there are no decision support models that efficiently aggregate the multiple home exposure assessment items for tailored interventions. Furthermore, there is a gap in assessment tools that incorporate both home environment assessments and common allergy biomarkers: skin prick testing (SPT) or specific IgE. Thus, the objectives of this study were to (1) develop and compare home environment allergen exposure scales using three different methods (unweighted, empirically weighted, and precision biomarker-based); (2) test the three home allergen exposure scales’ associations with both asthma control and asthma-specific quality of life; and (3) test for associations of the home allergen exposure scores with racial disparities.
Methods
This study is a secondary analysis of baseline measures of a longitudinal cohort of 187 older adults with asthma collected from 2015 to 2018. Institutional Review Board approval was obtained prior to data analysis. Three composite scales were calculated: allergen exposure composite (unweighted, theory-based), weighted allergen exposure score (weighted through dimension reduction), and precision allergen exposure score (includes biomarker).
Data
The study protocol is published elsewhere (Cavallazzi et al. 2018; Polivka et al. 2018). Briefly, included participants were ≥ 60 years old, had a diagnosis of asthma confirmed with spirometry, were non-smokers or had ≤ 20 packs/year smoking history, had no co-morbid lung diseases, and were not residing in a nursing home. While current smokers were excluded, participants living with a smoker were not.
The Home Environment Checklist (HEC) is an adapted home inspection and interview-assisted questionnaire (Krieger et al. 2015). Data on temperature and humidity were gathered for 24-h using Data Logger (Lascar Electronics, Whiteparish, England) and fine particulate matter (0.5–2.5 μm), or PM2.5, was collected using the Dylos 1700 (Dylos Corporation, Riverside, CA). PM2.5 readings are obtained every minute and averaged for an overall 24-h exposure. Inspections were completed by one of two expert data collectors. Training was conducted until 100% inter-rater reliability was achieved.
Both SPT and allergen-specific IgE measures were composed of 14 airborne allergens common to pulmonology and immunology clinical practice in the Louisville, KY, USA, region: dust mite farina, dust mite pteronyssinus, dog dander, cat dander, cockroach, alternaria mold, aspergillus mold, maple/box elder, bluegrass/June grass, Bermuda grass, ragweed, English plantain, lambs quarters, and oak mix. SPT results were obtained from medical records if performed ≤ 5 years prior to baseline enrollment. Otherwise, when not contra-indicated, SPT was performed using the Duotip-Test device (Lincoln Diagnostics, Decatur, IL) following standard SPT procedures. The test was interpreted as positive if a skin wheal of > 3 mm diameter, compared with negative control, was observed 15 min after the testing was applied. Blood samples were obtained to conduct allergen-specific and total IgE testing, and analyzed using Quest Diagnostics™ (Cincinnati, OH) or Phadia Immunology Reference Laboratory (PiRL, Portage, MI) standard assays. Tests were considered positive if total IgE was > 114 kU/L or allergen-specific IgE was ≥ 0.35 kUA/L. Both cat and total IgE had ceiling scores of > 100 kUA/L and > 5000 kU/L, respectively, that applied to this study (n = 1, Supplemental Figure).
Variables
Three self-report items from the interview-assisted HEC were used to calculate variables for this analysis. First, the variable “no HEPA vacuum” was calculated if the participant did not endorse a working vacuum, or did not endorse with HEPA filter by answering “No/Do not know” to the HEC checklist item, “Does the vacuum have a special air filter, such as a HEPA filter, to keep dust in the vacuum?” Second, the variable “furry/feathered pet allowed inside” was scored positively if the participant indicated “Yes” to “Do you have any pets, such as dogs, cats, rabbits, birds, hamsters/gerbils/other rodents or others?” and “Does it/Do they come inside?” Third, the variable “pest/rodent” was considered positive if the participant endorsed “Yes” to either “To the best of your knowledge, do you have cockroaches in your home now?” or “Have you had any problems with mice or rats in your home?”
Five home inspection variables are included in this study. First, for “Dust,” up to four rooms (bedroom, living room, kitchen, and basement) were inspected for the level of dust in the room which represents greater than “slight” amounts on a scale of 0 = none, 1 = slight, 2 = moderate, and 3 = heavy. If the home did not contain the corresponding HEC room (e.g., no basement), the item was left blank. The inspector’s dust level score was matched with the participant’s answer to “Where do you spend the majority of time in the house?” If the participant indicated “Other” or did not specify what room s/he spent the most time, the average, non-missing score for all rooms greater than or equal to 1.5 was used to score the dust variable as positive.
The second home inspection variable was “evidence of mold, leaks, or moisture,” derived from 39 items on the HEC checklist. If any of the following were observed in any room, the variable was scored as positive: mold odours, carpet damp to touch, visible evidence of water damage, condensation, water leaks/drips, mold, crawlspace noted by inspector as wet or damp. The third home inspection variable, evidence of pests/rodents, was scored as present if any one of 15 related inspection items was endorsed. The items addressed the bedroom, living room, kitchen, bathroom, and basement evidence of “Cockroaches (including eggs, feces, insects),” “Rodents (rats, mice),” or “Any cracks or holes in structure to allow entrance by mice?” Inspectors also used ultraviolet light to search for signs of rodent infestation as rodent urine was fluorescent, if detected. The fourth home inspection variable, evidence of tobacco, was considered positive if any of 10 items were endorsed by the inspector. Although current smokers were excluded from this study, participants were asked if anyone smoked in the home. There were two items for visual/odour inspections of each of the five rooms, indicating if tobacco odours were present or evidence of “Cigarette butts, ashtrays with ashes.” The last home inspection variable, poor bathroom ventilation, was scored as positive if the inspector either indicated that the bathroom fan was absent, failed a tissue test, or the fan did not ventilate outside. Objective measurement with a handheld anemometer (Hold Peak HP-866B, Zhuhai, China) was used to confirm ventilation inspection (equipment was obtained and utilized for approximately 50% of the inspections). Two objective measures, PM2.5 and relative humidity, were collected using sensors left in the home for 24 h. We utilized the 24-h maximum value for PM2.5 and 24-h average for relative humidity.
Table 1 contains logic and scoring for the 10 self-report, inspection, and objective variables used to construct the unweighted allergen exposure composite derived from expert priority order of HEC items (authors B.P. and R.B.) with common measurements and significant findings from recent environmental exposure and asthma studies (Fitzpatrick et al. 2019; Takaro et al. 2015). These values were also utilized in dimension reduction procedures to test the empirically derived weighted allergen exposure score. Online Supplemental Table 1 includes the scoring logic for the Precision Allergen Exposure Score that incorporated biomarkers, self-report, inspection, and objective measures. Environment risk was matched to each allergen: specific pet exposures indoors for cat/dog; evidence of cockroaches for German cockroach; evidence of mold, moisture, and humidity for alternaria and aspergillus; and dust/particulates for dust mite, grass, pollens, and tree sources. For example, if the SPT or IgE was positive for dog dander and the participant endorses a pet dog, a score of 1 is added. If the SPT or IgE was positive for dog dander, but the participant does not have a dog, no additional score is added.
Table 1.
Logic for unweighted allergen exposure composite
| Self-report | Inspection | Objective measures |
|---|---|---|
| No working vacuum with HEPA filter = 1 | Level of dust in room where participant spends the most time (none/slight = 0; moderate/heavy = 1) | Decile of max particulates scaled to 0–1 |
| Endorses any inside, furry pet = 1 | Anywhere in home: Any of the following—mold odour, carpet dampness, water damage, condensation, water leaks/drips present = 1 | Decile of average relative humidity scaled to 0–1 |
| Endorses any pest/rodent = 1 | Anywhere in home: any evidence of cockroach, rodent, or crack/holes that would allow mice = 1 | |
| Anywhere in home: any evidence of cigarette butts, ashtrays with ashes, or tobacco odour = 1 | ||
| Bathroom fan is not working or does not vent to outside = 1 |
Scores listed on table represent the value assigned to the variable before factor analysis
Race was dichotomized into two categories due to the small numbers of participants who did not indicate they were Caucasian or AA/B. AA/B individuals were considered in one category, and Caucasian/Other in the second. Asthma control was measured using the validated 5-item Asthma Control Test (ACT) questionnaire (Nathan et al. 2004). The score is interpreted as very poorly controlled (≤ 15), not well controlled (16–19), and well controlled (≥ 20). Quality of life was measured using the validated Asthma-Specific Mini Quality of Life Questionnaire (QOL) (Juniper et al. 1999).
Statistical methods
Factor analysis of the unrotated polychoric correlation matrix was conducted to construct the weighted allergen exposure score and applied if factor loadings were > 0.35. The weights were selected after an iterative process of exploring 1–4 factors in principal components analysis of the tetrachoric/polychoric serial matrices that included all possible factors from the composite allergy score and variables composing the score below. The developed scales were then tested in linear regression models with the same-time ACT category (well controlled, not well controlled, and very poorly controlled) and mini-asthma QOL outcomes. Racial disparities were tested using visualization of descriptive statistics and t tests.
Results
Participant characteristics in the longitudinal cohort are described elsewhere (Polivka et al. 2018). Briefly, 74% (N = 138) of participants were female, with a median of 66 years (IQR = 8 years). Twenty percent (N = 37) endorsed AA/B race. Of these, 5 participants indicated an additional racial identity (Native American/Alaskan Native, Asian, Caucasian/White, or a combination) to AA/B. Seventy-nine percent (N = 147) participants endorsed Caucasian/White race. Of these, 4 participants indicated an additional racial identity (Native American/Alaskan Native or other) to Caucasian/White. Less than 2% of participants (N = 3) listed other multiracial identity with no additional information or race. AA/B participants in this study were more likely to rent their home compared with Caucasian/Whites (58% vs. 11%) with 79% of AA/B renters living in public housing.
Descriptive statistics for each variable used are listed in Table 2. Figure 1 depicts a count of participants with each of the self-report and inspection home allergen exposure assessments. The correlations for each variable considered in the scales are listed in Supplemental Tables 2, 3, and 4.
Table 2.
Descriptive statistics of home allergen exposure variables and outcomes (N = 187)
| Variable | N | % |
|---|---|---|
| No HEPA vacuum | 102 | 55.55% |
| Furry/feathered pet allowed inside | 85 | 45.45% |
| Pest/rodent (self-report) | 50 | 26.74% |
| Dust | 54 | 28.88% |
| Evidence of mold, leaks, or moisture | 72 | 38.50% |
| Evidence of pests/rodents (inspection) | 14 | 7.49% |
| Evidence of tobacco (inspection) | 8 | 4.28% |
| Poor bathroom ventilation | 122 | 65.24% |
| Fine particulates per m3 (median, IQR) | 1455 | 672–3469 |
| Relative humidity, 24-h average in % (median, IQR) | 52.5 | 43.6–57.0 |
| Total IgE, high | 73 | 39.04% |
| Dust mite farina positive | ||
| IgE | 45 | 24.06% |
| SPT | 42 | 22.46% |
| Dust mite pteronyssinus positive | ||
| IgE | 42 | 22.46% |
| SPT | 40 | 21.39% |
| Dog dander positive | ||
| IgE | 42 | 22.46% |
| SPT | 24 | 12.83% |
| Cat dander positive | ||
| IgE | 61 | 32.62% |
| SPT | 44 | 23.53% |
| Cockroach positive | ||
| IgE | 31 | 16.58% |
| SPT | 31 | 16.58% |
| Alternaria mold positive | ||
| IgE | 25 | 13.37% |
| SPT | 20 | 10.70% |
| Aspergillus mold positive | ||
| IgE | 20 | 10.70% |
| SPT | 17 | 9.09% |
| Maple/box elder positive | ||
| IgE | 20 | 14.97% |
| SPT | 30 | 16.04% |
| Bluegrass/June grass positive | ||
| IgE | 39 | 20.86% |
| SPT | 33 | 17.65% |
| Bermuda grass positive | ||
| IgE | 30 | 16.04% |
| SPT | 26 | 13.90% |
| Ragweed positive | ||
| IgE | 30 | 16.04% |
| SPT | 33 | 17.65% |
| English plantain positive | ||
| IgE | 20 | 10.70% |
| SPT | 24 | 12.83% |
| Lambs quarters positive | ||
| IgE | 21 | 11.23% |
| SPT | 15 | 8.02% |
| Oak mix positive | ||
| IgE | 19 | 10.16% |
| SPT | 20 | 10.70% |
| Asthma control test (mean, SD) | 18.2 | 4.24 |
| Very poorly controlled | 52 | 27.81% |
| Not well controlled | 53 | 28.34% |
| Well controlled | 82 | 43.85% |
| Asthma Quality of Life Score (mean, SD) | 4.9 | 1.26 |
N = 4 participants with missing data for IgE results and N = 73 participants with missing data for SPT results
IQR, interquartile range; IgE, immunoglobulin E; SPT, skin prick testing
These depict either median/IQR or Mean/SD
Fig. 1.
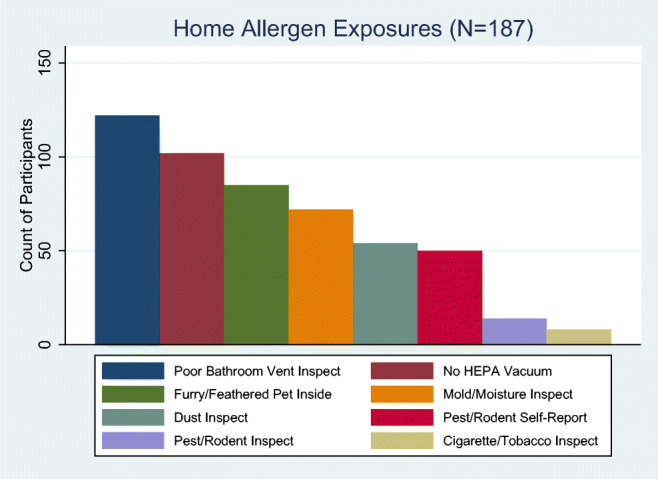
Count of participants with each of the self-report and inspection home allergen exposure assessments
Unweighted allergen exposure composite
Using the 10 variables listed in Table 1, the unweighted composite score had a possible range of 0–10 and an actual range of 0.4–8.8 in this sample (M = 3.80, SD = 1.67, median = 3.5, IQR = 2.2). Table 3 has the results of the regression analysis of the scale with asthma control and asthma quality of life, demonstrating no association with asthma control and a small association with asthma quality of life. A racial difference was observed in this measure for AA/B (M = 4.59, SD = 2.01) and Caucasian/Other (M = 3.60, SD = 1.52) participants for the unweighted allergen exposure composite score (t(46.62) = 2.80, p = 0.007).
Table 3.
Unadjusted regression analysis comparing three environment exposures scales to asthma control and quality of life outcomes (N = 187)
| Scale | Asthma control test | Asthma quality of life | ||||
|---|---|---|---|---|---|---|
| Model summary | Point estimate | 95% CI | Model summary | Point estimate | 95% CI | |
| Allergen exposure | R2 = 0.02 | β = − 0.33 | − 0.69, 0.04 | R2 = 0.02* | β = − 0.11* | − 0.22, − 0.01 |
| Weighted allergen exposure score | R2 = 0.02* | β = − 0.88* | − 1.68, − 0.17 | R2 = 0.03* | β = − 0.30* | − 0.54, − 0.07 |
| Precision allergen exposure score | R2 = 0.02* | β = − 0.28* | − 0.54, − 0.02 | R2 = 0.01 | β = − 0.06 | − 0.14, 0.01 |
CI, confidence interval
*p < 0.05
Weighted allergen exposure score
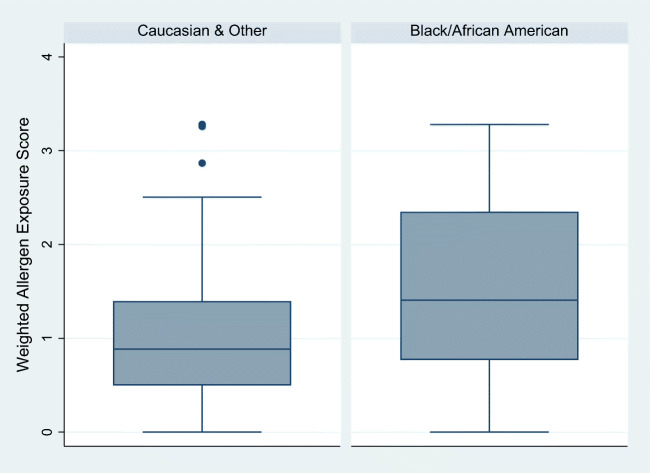
Factor analysis resulted in a single factor with an eigenvalue above 1 (2.36) to explain 67% of the variance. Items were all linked to home maintenance and access to home maintenance resources. Seven items composed the final scale with the following weights: (1) inspection evidence of roach or mice (0.9376); (2) dust (0.5412); (3) mold (0.5234); (4) evidence of tobacco smoke exposure (0.5196); (5) properly vented bathroom fan (0.5037); (6) self-report presence of roach, mice, or rats (0.4130); and (7) access to a working vacuum with HEPA filter (0.3622). Pets indoors loaded on a separate factor, and not included in the scale as a single-item factor, while particulate matter and relative humidity scores did not load on any factor. In the current sample, the score had a range of 0–3.3 (M = 1.09, SD = 0.76; median = 0.90; IQR = 0.90). Similar results were obtained with oblimin oblique rotation and Kaiser normalization. The tetrachoric matrix was not used due to limited variability in the values in the matrix. This empirically weighted scale, composed of 7 items, demonstrated the best performance in unadjusted models with outcomes asthma control (F = 4.65, p = 0.03, R2 = 0.02) and quality of life (F = 6.45, p = 0.01, R2 = 0.03) (Table 3). Furthermore, a significant racial difference for AA/B (M = 1.51, SD = 1.00) and Caucasian/Other participants (M = 0.98, SD = 0.65) was observed in the empirically weighted allergen exposure score composite (t(43.83) = − 3.09, p = 0.004) (Fig. 2).
Fig. 2.
Distribution of the weighted allergen exposure scale by race
Precision allergen exposure score
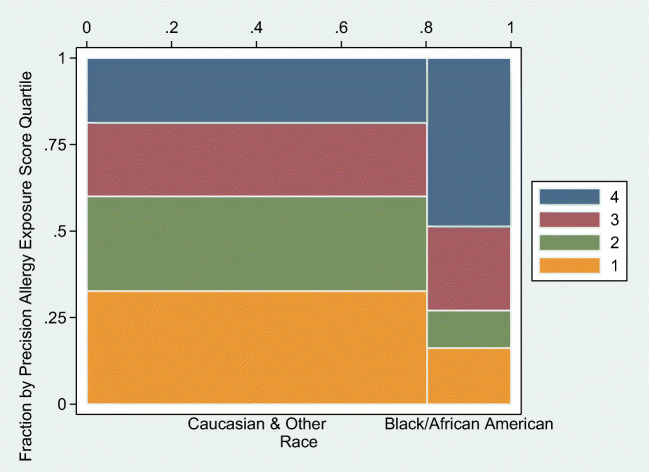
The previously described panel of 14 region-specific allergen biomarkers was used to calculate the precision allergen exposure score (Online Supplemental Tables). This precision score was associated with asthma control, but not asthma quality of life (Table 3). The precision score was associated with racial disparity for AA/B (M = 3.45, SD = 2.72) and Caucasian/Other (M = 1.66, SD = 2.07) (t(46.77) = − 3.77, p < 0.001). This disparity is also presented in a visualization by quartile of precision allergy exposure score (Fig. 3) with the highest quartile of the precision score including 48.65% of the AA/B participants (N = 18/37) compared with 18.67% of the participants of other races (28/150).
Fig. 3.
Visualization by quartile of precision allergy exposure score with racial disparity
Discussion
We developed three home environment allergen exposure scales for older adults with asthma, and observed racial disparities in this study. Given the lack of clinically meaningful and tailored assessment for the risk of allergy and asthma triggers in the home environment, our findings have both clinical relevance and empirical research significance. These scales enable efficient home environment assessments to clinically prioritize or efficiently aggregate multiple home exposure assessment items for focused and tailored interventions, and to address the most clinically salient exposure(s). While only a small proportion of variance was explained by the novel scores in asthma control or asthma quality of life, the novel scores were associated with substantial racial disparities. Because non-eosinophilic asthma is not likely influenced by home allergens, further consideration for variables that address a clinical history of allergic reactivity, in addition to the biomarker results, is indicated to enhance potential for a precision allergen score of home environment exposures. Our study focused on allergen exposure assessments, thus the presence of non-eosinophilic endotypes and phenotypes may have attenuated our results (Carr et al. 2018).
Our findings are consistent with the Global Initiative for Asthma (2019) guidelines, with evidence that home environmental control interventions reduce triggers, but little quality evidence of impact in improving clinical asthma control or quality of life outcomes. In the only other study identified that developed a home environmental index, Frisk et al. (2009) combined nine home environmental asthma risk indicators for the Housing Environmental Index (HE-index). Frisk et al. did not report any statistically significant relationships between the number of allergen risk indicators and participant (mean age = 39 years) SPT results or lung function. Frisk et al. noted that allergic sensitization is more prevalent in children with asthma compared with adults. The diminished allergic sensitivity of older adults may be relevant to the results in this study and partially account for the small proportion of variance explained. Participants may also avoid exposures that have triggered exacerbations in the past, which may provide an alternate explanation to the small magnitude of associations for the precision allergen exposure scale and asthma outcomes. Of note, the seven items that loaded on the weighted allergen exposure scale can be linked to home maintenance and access to resources, which are issues common in low-income housing. Pets indoors are a separate concern, which is reflected in our results as loading on a separate factor. Having pets indoors is often an issue of personal preference rather than resources or home maintenance. Furthermore, the HEC is often used as a measure in controlled intervention trials (Krieger et al. 2015) that support evidence on the clinical and public health importance of interventions to reduce environmental triggers on asthma prevention and control. Our work to refine HEC items into clinically meaningful subscales promises to advance the analytic design of ongoing intervention trials while reducing the relevant items for pragmatic adoption to practice.
Racial disparities were identified in all three of our home environment allergen exposure scales, consistent with the results of other studies (Akinbami et al. 2014; Bhan et al. 2015). Our study provides corroborating evidence that environmental exposures, rather than race alone, may be the causal factor in racial disparities (Fitzpatrick et al. 2019). The allergen exposure scores were consistently greater for AA/B indicating increased exposures to common asthma allergen triggers. AA/B participants were more likely to rent their home or live in public housing. Unique challenges exist when addressing potential sources of environmental exposure for renters. Due to the power differential in the renter–landlord relationship, renters note little to no control over decision making about rental unit maintenance for mold, pests, and exposure to second-hand smoke such as ventilation, bathroom fan replacement, and/or smoke-free policies for multi-housing units (Hahn et al. 2017).
There are several additional plausible alternative explanations for racial disparities in asthma, unmeasured in this study, to guide future inquiry. Allostatic load theory, or cumulative physical and psychosocial stressors increasing risk of disease, is relevant to contextualize findings and understand racial disparities associated with asthma (Bahreinian et al. 2013; Wright and Schreier 2013). Adverse childhood events, experiencing or witnessing racism, and higher rates of ambient pollutant exposure, socio-economic hardship, and limited access to healthcare increase risk for asthma development and severity in AA/B individuals throughout their lifespan (Beck et al. 2014; Exley et al. 2015). Racial differences in genomic, vitamin D metabolism, lung physiology, and immune responses may also contribute to disparities (Torgerson et al. 2011). Further research is needed to integrate these factors for asthma outcome research in older adults with home environmental exposures and other populations, such as Indigenous people. For example, integrating our scale development methods with the testing of house dust contents for detectable levels or clinical threshold allergen levels provides a promising avenue of inquiry (Cohn et al. 2006; Salo et al. 2018). Likewise, biomarkers of non-eosinophilic asthma endotypes and inflammatory phenotypes may strengthen our measurement scales. Our results re-emphasize the importance of ongoing research and interventions that address home asthma exposures for minorities living in low-income housing.
Kitchen ventilation is assessed on the HEC (Krieger et al. 2015). This study did not include kitchen ventilation in scale development. Our data collection team had many concerns about the measurement error and relevance of these questions to personal exposure. Many of the older adult participants in this study never cooked in their kitchen or used only the microwave. We were able to verify through observation whether bathroom fans ventilated to the outside, but the age of the homes and circular HVAC (heating, ventilation, and air conditioning) systems specific to kitchens in many homes meant we were unable to verify self-reported questions about where kitchen fans exhausted with inspector observations. We did include bathroom ventilation items as all bathrooms were enclosed and many of the kitchens were part of an open floor plan, particularly in newer homes. Thus, unmeasured but observed open floor plan layouts for kitchens introduced potential validity challenges to the kitchen ventilation aspect of the HEC. Last, given the age and prevailing culture in the generation of our participants, we were concerned there would be gender-normed behaviour relative to kitchen cooking that would be more influential to the relevance of this item to the individual’s exposure. Our participants were not equally distributed by gender. Overall, given our concerns about potential measurement error and relevance to individual exposure, we did not prioritize including the kitchen ventilation HEC items in our scale development.
Replication studies and future studies that incorporate the home environment allergen exposure scale should be considered for a number of compelling reasons. First, future studies with samples that include child and adolescent populations can expand the utility of the home environment allergen exposure scale scores. Second, the replication of these methods in populations residing in high-risk/low-income housing may generate a clinically meaningful, tailored assessment of asthma triggers. This high-risk housing includes military family populations that experience unique living arrangements in a wide range of geographic locations, with the military functioning as the largest landlord in the United States (Military Family Advisory Network 2019; US Government Accountability Office 2018, 2019). Methodologically, further consideration is needed for variables that address allergic reactivity and biomarker results to enhance the potential for a precision allergen score. A precision allergen score using objective data from select biomarkers can eliminate a degree of bias in subjective self-report measures and provide information on the internal dose of an environmental exposure in an individual. A precision allergen score can be a tool for researchers, public health practitioners, and clinicians to more fully integrate home environmental exposures into the understanding of health outcomes and trajectory of an asthma diagnosis. Finally, the procedure and results should be replicated in future study to ascertain tetrachoric matrix factor analyses for the binary variables used to meet model assumptions.
Limitations
There are several limitations to this study. Findings can only be generalized to older adult non-smokers and may be limited in their application outside of the study’s geographic region. Future studies should refine the personalized allergen testing panel to include clinical history of reactivity to each allergen. The use of self-report items may introduce recall bias. Best practices in measuring the temporal nature of objective humidity and particulate home environment exposures relevant to asthma are not well established, and there is a potential measurement error in using averages compared with peaks, seasonal effects, and short-term compared with long-term exposures. Objective environmental measures were taken in the room where the participant reported spending the most time, which may have missed major combustion or cooking exposures. Finally, selection bias is a factor, as the longitudinal cohort study participants were mostly white, female, and college educated.
Conclusion
The results of our study contribute novel home environmental assessment tools that aid in measuring and calculating clinically salient allergen exposures for asthma outcomes. Our study included novel findings of racial disparities in older adults with asthma, emphasizing environmental determinants of health in the home setting. We anticipate this line of inquiry will inform future research addressing clinical interventions to prevent and mitigate home allergen-triggered eosinophilic asthma.
Electronic supplementary material
(DOCX 813 kb)
Acknowledgements
The authors gratefully acknowledge Diane Endicott, RN (University of Louisville School of Nursing, Louisville, KY), Carol Norton, MUP (University of Louisville School of Nursing, Louisville, KY), Bryan Beatty, RRT, CPFT (Clinical Program Manager, Division of Pulmonary, Critical Care & Sleep Disorders Medicine, University of Louisville, Louisville, KY), Zena Ntiranyibagira, PhD (Assistant Professor of French at SUNY Erie Community College), and Olive Ndayishimiye, BSN, RN.
Funding information
This research was supported by the National Institute on Aging at the National Institutes of Health, Award NIH/NIA no. 3 R01 AG047297-04S1.
Compliance with ethical standards
Disclaimer
Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIA.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical conduct of research
The University of Louisville Institutional Review Board (no. 13.0419) reviewed and approved the study protocol.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akinbami, L. J., Moorman, J. E., Simon, A. E., & Schoendorf, K. C. (2014). Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. Journal of Allergy and Clinical Immunology. 10.1016/j.jaci.2014.05.037. [DOI] [PMC free article] [PubMed]
- Bahreinian, S., Ball, G. D., Vander Leek, T. K., Colman, I., McNeil, B. J., Becker, A. B., et al. (2013). Allostatic load biomarkers and asthma in adolescents. American Journal of Respiratory and Critical Care Medicine. 10.1164/rccm.201201-0025OC. [DOI] [PubMed]
- Baptist, A. P., & Busse, P. J. (2018). Asthma over the age of 65: all’s well that ends well. The Journal of Allergy and Clinical Immunology. In Practice. 10.1016/j.jaip.2018.02.007. [DOI] [PMC free article] [PubMed]
- Beck, A. F., Huang, B., Simmons, J. M., Moncrief, T., Sauers, H. S., Chen, C., et al. (2014). Role of financial and social hardships in asthma racial disparities. Pediatrics. 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed]
- Bhan, N., Kawachi, I., Glymour, M. M., & Subramanian, S. V. (2015). Time trends in racial and ethnic disparities in asthma prevalence in the United States from the Behavioral Risk Factor Surveillance System (BRFSS) study (1999-2011). American Journal of Public Health. 10.2105/AJPH.2014.302172. [DOI] [PMC free article] [PubMed]
- Carlsten, C., Brauer, M., Brinkman, F., Brook, J., Daley, D., McNagny, K., . . . Denburg, J. (2014). Genes, the environment and personalized medicine: We need to harness both environmental and genetic data to maximize personal and population health. EMBO Reports, 15(7), 736-739. 10.15252/embr.201438480. [DOI] [PMC free article] [PubMed]
- Carr, T. F., Zeki, A. A., & Kraft, M. (2018). Eosinophilic and noneosinophilic asthma. American Journal of Respiratory and Critical Care Medicine. 10.1164/rccm.201611-2232PP. [DOI] [PMC free article] [PubMed]
- Castner, J. (2019). The symptom science model: a shared mental model to advance the next generation of knowledge in the emergency nursing specialty. Journal of Emergency Nursing, 10.1016/j.jen.2019.05.008. [DOI] [PubMed]
- Castner, J., Amiri, A., Rodriguez, D. J., Huntingon-Moskos, L., Thompson, L., Zhao, S., et al. (2019). Advancing the symptom science model with environmental health. Public Health Nursing. 10.1111/phn.12641. [DOI] [PMC free article] [PubMed]
- Cavallazzi, R., Jorayeva, A., Beatty, B. L., Antimisiaris, D., Gopalraj, R., Myers, J., et al. (2018). Predicting asthma in older adults on the basis of clinical history. Respiratory Medicine. 10.1016/j.rmed.2018.07.010. [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention (2017). Tables of summary health statistics, National Health Interview Survey https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_A-2.pdf.
- Cohn, R. D., Arbes, S. J., Jr., Jaramillo, R., Reid, L. H., & Zeldin, D. C. (2006). National prevalence and exposure risk for cockroach allergen in U.S. households. Environmental Health Perspectives, 114(4), 522–526. 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed]
- Exley, D., Norman, A., & Hyland, M. (2015). Adverse childhood experience and asthma onset: a systematic review. European Respiratory Review. 10.1183/16000617.00004114. [DOI] [PMC free article] [PubMed]
- Fitzpatrick, A. M., Gillespie, S. E., Mauger, D. T., Phillips, B. R., Bleecker, E. R., Israel, E., . . . Teague, W. G. (2019). Racial disparities in asthma-related health care use in the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. The Journal of Allergy and Clinical Immunology, 143(6), 2052–2061. doi:10.1016/j.jaci.2018.11.022. [DOI] [PMC free article] [PubMed]
- Frisk ML, Stridh G, Ivarsson A-B, Kamwendo K. Can a housing environmental index establish associations between indoor risk indicators and clinical tests in persons with asthma? International Journal of Environmental Health Research. 2009;19(6):389–404. doi: 10.1080/09603120902781622. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma. (2019). Global strategy for asthma management and prevention. www.ginasthma.org.
- Gomez, M., Reddy, A. L., Dixon, S. L., Wilson, J., & Jacobs, D. E. (2017). A cost-benefit analysis of a state-funded healthy homes program for residents with asthma: findings from the New York State Healthy Neighborhoods Program. Journal of Public Health Management and Practice, 10.1097/PHH.0000000000000528. [DOI] [PubMed]
- Hahn EJ, Hooper M, Riker C, Butler KM, Rademacher K, Wiggins A, Rayens MK. Lung cancer worry and home screening for radon and secondhand smoke in renters. Journal of Environmental Health. 2017;79(6):8–13. [PMC free article] [PubMed] [Google Scholar]
- Huss, K., Naumann, P. L., Mason, P. J., Nanda, J. P., Huss, R. W., Smith, C. M., et al. (2001). Asthma severity, atopic status, allergen exposure and quality of life in elderly persons. Annals of Allergy, Asthma & Immunology. 10.1016/S1081-1206(10)62900-6. [DOI] [PubMed]
- Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. The European Respiratory Journal. 1999;14(1):32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- Kannan JA, Bernstein DI, Bernstein CK, Ryan PH, Bernstein JA, Villareal MS, et al. Significant predictors of poor quality of life in older asthmatics. Annals of Allergy, Asthma & Immunology. 2015;115(3):198–204. doi: 10.1016/j.anai.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger, J., Song, L., & Philby, M. (2015). Community health worker home visits for adults with uncontrolled asthma: the HomeBASE Trial randomized clinical trial. JAMA Internal Medicine. 10.1001/jamainternmed.2014.6353. [DOI] [PubMed]
- Military Family Advisory Network. (2019). Preliminary research report: living conditions of families in privatized military housing. Retrieved from www.militaryfamilynetwork.org: militaryfamilyadvisorynetwork.org/wp-content/uploads/Privatized-Military-Housing-Survey-Report-5_20.pdf.
- Nathan, R. A., Sorkness, C. A., Kosinski, M., Schatz, M., Li, J. T., Marcus, P., et al. (2004). Development of the asthma control test: a survey for assessing asthma control. The Journal of Allergy and Clinical Immunology. 10.1016/j.jaci.2003.09.008. [DOI] [PubMed]
- New York State Department of Health. (2013). New York state asthma surveillance summary report. October, 2013 (p. 250. Available at: https://www.health.ny.gov/statistics/ny_asthma/pdf/2013_asthma_surveillance_summary_report.pdf). Albany: Public Health Information Group Center for Community Health.
- Polivka, B. J., Folz, R., Myers, J., Barnett, R., Antimisiaris, D., Jorayeva, A., et al. (2018). Identifying phenotypes and factors impacting outcomes in older adults with asthma: a research protocol and recruitment results. Research in Nursing & Health. 10.1002/nur.21880. [DOI] [PMC free article] [PubMed]
- Reddy, A. L., Gomez, M., & Dixon, S. L. (2017). An evaluation of a state-funded healthy homes intervention on asthma outcomes in adults and children. Journal of Public Health Management and Practice. 10.1097/PHH.0000000000000530. [DOI] [PubMed]
- Salo, P. M., Wilkerson, J., Rose, K. M., Cohn, R. D., Calatroni, A., Mitchell, H. E., . . . Zeldin, D. C. (2018). Bedroom allergen exposures in US households. The Journal of Allergy and Clinical Immunology, 141(5), 1870-1879.e1814. 10.1016/j.jaci.2017.08.033. [DOI] [PMC free article] [PubMed]
- Shedd, A. D., Peters, J. I., Wood, P., Inscore, S., Forkner, E., Smith, B., et al. (2007). Impact of home environment characteristics on asthma quality of life and symptom scores. The Journal of Asthma. 10.1080/02770900701209699. [DOI] [PubMed]
- Skloot, G. S., Busse, P. J., Braman, S. S., Kovacs, E. J., Dixon, A. E., Vaz Fragoso, C. A., et al. (2016). An official American Thoracic Society workshop report: evaluation and management of asthma in the elderly. Annals of the American Thoracic Society, 10.1513/AnnalsATS.201608-658ST. [DOI] [PMC free article] [PubMed]
- Statistics Canada. (2014). Archived - Health indicators, annual estimates, 2003–2014. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310045101&pickMembers%5B0%5D=1.1&pickMembers%5B1%5D=2.6&pickMembers%5B2%5D=3.1
- Takaro TK, Scott JA, Allen RW, Anand SS, Becker AB, Befus AD, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. Journal of Exposure Science & Environmental Epidemiology. 2015;25(6):580–592. doi: 10.1038/jes.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson, D. G., Ampleford, E. J., Chiu, G. Y., Gauderman, W. J., Gignoux, C. R., Graves, P. E., et al. (2011). Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nature Genetics. 10.1038/ng.888. [DOI] [PMC free article] [PubMed]
- United States Government Accountability Office. (2018). Military Housing Privatization DOD should take steps to improve monitoring, reporting, and risk assessment,. (GAO-18-218). www.gao.gov: GAO Retrieved from https://www.gao.gov/assets/700/690621.pdf.
- United States Government Accountability Office. (2019). Military Housing Privatization preliminary observations on DOD’s oversight of the condition of privatized military housing (testimony). (GAO-20-280T). www.gao.gov: GAO Retrieved from https://www.gao.gov/assets/710/702950.pdf.
- Wang, J., Engvall, K., Smedje, G., & Norback, D. (2017). Exacerbation of asthma among adults in relation to the home environment in multi-family buildings in Sweden. The International Journal of Tuberculosis and Lung Disease. 10.5588/ijtld.16.0307. [DOI] [PubMed]
- Wright, R. J., & Schreier, H. M. (2013). Seeking an integrated approach to assessing stress mechanisms related to asthma: is the allostatic load framework useful? American Journal of Respiratory and Critical Care Medicine. 10.1164/rccm.201210-1816ED. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 813 kb)