Abstract
Introduction
Hip displacement is common in cerebral palsy (CP) and is related to the severity of neurological and functional impairment. It is a silent, but progressive disease, and can result in significant morbidity and decreased quality of life, if left untreated. The pathophysiology of hip displacement in CP is a combination of hip flexor-adductor muscle spasticity, abductor muscle weakness, and delayed weight-bearing, resulting in proximal femoral deformities and progressive acetabular dysplasia. Due to a lack of symptoms in the early stages of hip displacement, the diagnosis is easily missed. Awareness of this condition and regular surveillance by clinical examination and serial radiographs of the hips are the key to early diagnosis and treatment.
Hip surveillance programmes
Several population-based studies from around the world have demonstrated that universal hip surveillance in children with CP allows early detection of hip displacement and appropriate early intervention, with a resultant decrease in painful dislocations. Global hip surveillance models are based upon the patients’ age, functional level determined by the Gross Motor Function Classification system (GMFCS), gait classification, standardized clinical exam, and radiographic indices such as the migration percentage (MP), as critical indicators of progressive hip displacement.
Conclusion
Despite 25 years of evidence showing the efficacy of established hip surveillance programmes, there is poor awareness among healthcare professionals in India about the importance of regular hip surveillance in children with CP. There is a need for professional organizations to develop evidence-based guidelines for hip surveillance which are relevant to the Indian context.
Keywords: Cerebral palsy, Spasticity, Diplegia, Quadriplegia, Hemiplegia, Hip displacement, Hip surveillance, Gross Motor Function Classification System (GMFCS), Migration percentage, Painful hip
Introduction
Cerebral palsy (CP) is the most common cause of physical disability affecting children, with a prevalence of about 2 per 1000 live births [1]. In a child with CP, hip dysplasia is the second most common problem, after equinus, causing significant pain and disability [2, 3]. The incidence of hip displacement in CP is approximately 35% [2–4]. Factors deciding the risk of hip displacement are the age of the child, severity of neurological involvement and limitations in walking ability which are directly related to gross motor function [4, 5]. Early identification and orthopaedic intervention of “at risk” children, identified by hip surveillance programmes globally, have been shown to prevent hip dislocation and the need for complex future salvage surgery [3, 6–10].
A hip surveillance programme includes evaluation of all children with cerebral palsy by physical examination and serial radiographs, with the goal of timely referral to the orthopaedic surgeon [11]. Hip surveillance also includes children who are irregular in follow-up and children who are at risk of developing hip displacement outside of tertiary referral centres. Various countries around the world have adopted national hip surveillance programmes [3, 6–10]; however, in India no such established national or state-wide surveillance programmes exist, resulting in wide variation in practices.
This review article provides an overview of the epidemiology, natural history, pathophysiology and anatomy of hip displacement in children with cerebral palsy, clinical and radiological assessment, hip displacement related to various GMFCS levels, and an overview of various global hip surveillance models and their outcomes which will help in developing methods to prevent and proactively manage hip disorders in children with CP.
Epidemiology and Risk of Hip Displacement in Cerebral Palsy
The prevalence of hip displacement for the CP population as a whole is approximately 35%. The prevalence of hip subluxation is between 25 and 60% and hip dislocation is 10–15% in children with CP [2, 3, 12–14]. Previous studies have suggested that the most important risk factor associated with hip displacement is the severity of the disease [12, 15, 16]. Accordingly it has been shown that children with quadriplegia have the highest risk of developing hip displacement while those with less severe neurologic involvement have a lower risk. Rather than motor type and topography (e.g. quadriplegia), the severity of neurological involvement is better stratified by reliable and valid classifications of gross motor function. The Gross Motor Function Classification System (GMFCS) developed by Palisano et al. [17] is a well-accepted five-level ordinal system to classify motor function and degree of motor impairment in CP. A level I child (between 6 and 12 years of age) has near normal gross motor function and can ambulate independently; while a level V child cannot sit independently, stand or walk and is dependent for all aspects of care. The GMFCS has been proven to be a valid, reliable, stable, and clinically relevant method for the classification and prediction of motor function in children with cerebral palsy from 2 to 18 years of age.
In the first population-based study using the Australian CP Register, Soo et al. [4] confirmed the linear correlation between hip displacement and the GMFCS level. Throughout childhood, the risk for hip displacement was determined to be approximately 15% for GMFCS level II, 40% for level III, 70% for level IV and 90% for level V children (Fig. 1). The risk in GMFCS level I was negligible, except in hemiplegic children with hip involvement designated as Winter, Gage and Hicks Type IV (WGH IV) hemiplegia. The same study also corroborated the fact that the risk of hip displacement was related to topography, with a 1% risk in children with hemiplegia compared to an 82% risk in children with quadriplegia [4]. However, the incidence of hip displacement was unrelated to the motor type, as children with spasticity and dystonia had the same risk as children with hypotonia [4]. Subsequent population-based studies have corroborated these findings and serve as a basis for comprehensive hip surveillance programmes [8–10, 18, 19].
Fig. 1.

Incidence of hip displacement related to the Gross Motor Function Classification System (GMFCS).
Adapted from Soo et al. [4]
Natural History of Hip Dysplasia in Cerebral Palsy
It is important to understand the natural history of untreated hip displacement in children with CP, so as to make informed decisions regarding its management. There has been some controversy in literature regarding a ‘proactive approach’ of early diagnosis and early intervention versus a ‘reactive approach’ of offering surgery only after the hip becomes painful or causes functional limitation [20, 21]. However, recent evidence from population-based studies does show that the reactive approach leads to inferior outcomes and puts the child with CP at risk for development of pain, gait abnormalities, difficulty in seating and perineal hygiene, pressure ulcers, pelvic obliquity and scoliosis [22]. Using newly developed, validated, disease-specific outcome measures such as the Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD), it has been shown that severe hip displacement significantly reduces health-related quality of life in children with CP [23]. Conversely, early surgery showed significant improvements in CPCHILD scores, especially in the personal care/activities of daily living, and positioning, transfers and mobility domains [24].
Proactive treatment of hip subluxation in ambulatory children is well accepted. Well-reduced hips are essential to provide a stable platform for walking and to prevent gait deterioration, loss of hip motion and future osteoarthritis resulting from femoral head deformation and articular cartilage damage seen in long-standing dislocations. The rationale for hip management in non-ambulatory children is less defined. Adequate hip abduction is important for perineal hygiene and ease of care for bedridden children. Ease of transfers, proper seating balance, prevention of pressure sores and maintenance of pelvic and spinal alignment are other important considerations [16, 25, 26]. Hip subluxation and dislocation frequently lead to pelvic obliquity and scoliosis which affect seating balance (Fig. 2).
Fig. 2.

Radiograph showing bilateral hip dislocation with pelvic obliquity and scoliosis in a child with quadriplegic cerebral palsy
Prevention of pain is the overarching goal of early detection and management of hip displacement in CP. Samilson [26] reported that only 6 of 274 patients with CP had pain attributable to hip subluxation while Pritchett [21] reported that 62% of patients with dislocated hips had no pain and 33% had only mild pain. However, on a long-term follow-up of hip subluxation in children with CP, Bagg and Miller [16] reported that 89% of dislocated hips had moderate to severe pain and 45% of subluxated hips had pain. More recent publications support an increased incidence of hip pain related to the severity of hip displacement and worsening hip morphology [22, 27]. It must be noted that early displacement is rarely painful and advocates of the proactive approach do not recommend using pain as a deciding factor in the management of neuromuscular hip dysplasia. Pain is a late symptom and usually indicates that the hip is going beyond the scope of reconstructive procedures. Regular and judicious clinical examination combined with periodic radiographs are required to diagnose early hip displacement in children with CP.
Pathophysiology and Pathoanatomy of Hip Displacement in CP
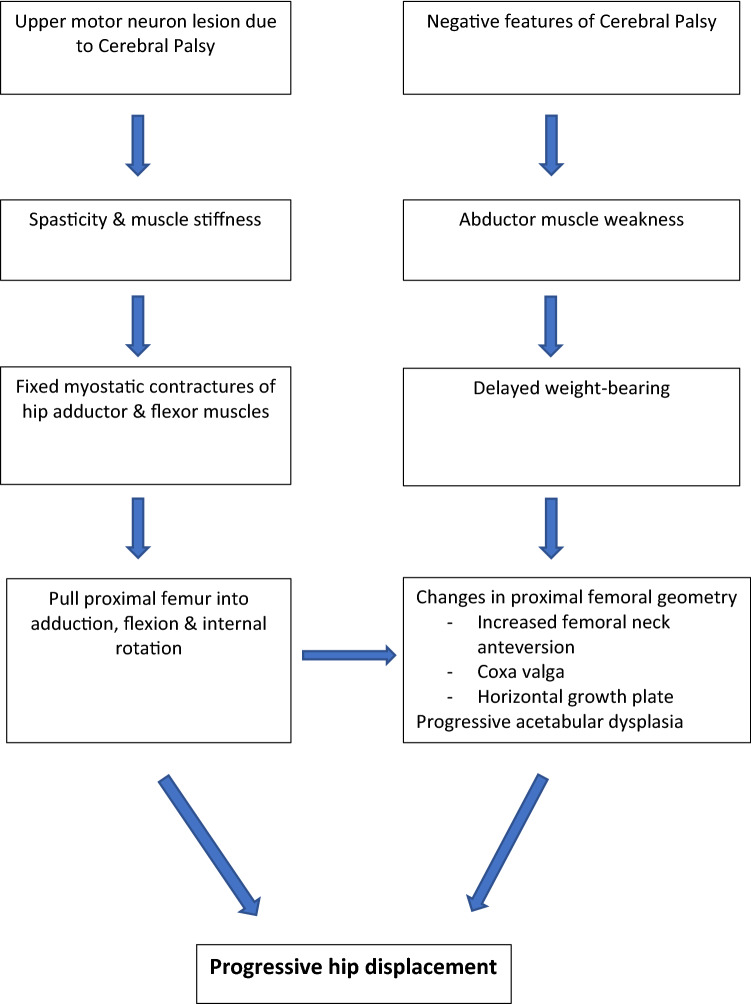
The ‘traditional view’ of the pathophysiology of hip displacement in CP is based upon the spastic muscle model. According to this model, spasticity resulting from cerebral palsy leads to an increase in muscle stiffness resulting in fixed myostatic contractures. The spastic and contracted hip adductor and flexor muscles pull the proximal femur into adduction, flexion and internal rotation, effectively driving the femoral head out of the hip joint. Fixed muscle contractures have been assumed to lead to the development of secondary bony deformities commonly associated with hip dysplasia, namely, increased femoral anteversion, coxa valga and gradual acetabular dysplasia [28]. While the spastic muscle model is logical and intuitive, recent evidence has shown that hip displacement is unrelated to the motor type, with hypotonic children having the same risk as children with spasticity or dystonia, and can occur even in the absence of muscle contractures [4].
A more holistic and modern view focuses on the role of negative features of CP i.e. abductor muscle weakness and delayed weight-bearing as additional factors driving hip displacement in CP. Proximal femoral deformities are commonly associated with hip displacement in CP and include increased femoral neck anteversion (FNA), coxa valga and horizontal or lateral inclination of the proximal femoral physis [29, 30]. In a large population-based study, Robin et al. [31] correlated changes in proximal femoral geometry to the severity of neurological problem stratified according to the GMFCS. Mean femoral anteversion was found to be abnormally increased to 36.5° across all GMFCS levels, being approximately 30° for level I, 35° for level II and 40° for levels III–V. Similarly, the mean neck-shaft angle (NSA) was found to be abnormally high at 147.5° in children with CP and also correlated strongly with the GMFCS, with values ranging from 136° to 163° across levels I–V. Furthermore, it was demonstrated that increases in FNA and NSA were significant contributors to the development of progressive hip subluxation [31]. It has been postulated that abnormal joint forces resulting from delayed or lack of weight-bearing combined with muscle imbalance resulting from abductor insufficiency are likely determinants of abnormal proximal femoral geometry and consequent hip displacement seen in CP [31–33]. Coxa valga and lateral tilt of the proximal femoral physis further contribute to progressive acetabular dysplasia resulting from progressive lateral displacement of the femoral head and a shift of forces to the lateral aspect of the acetabulum [34–36] (Fig. 3).
Fig. 3.
Pathophysiology of Hip Displacement in Cerebral Palsy
Clinical Examination
The clinical evaluation of a child with suspected hip dysplasia related to CP is performed as a part of the routine assessment of a child with CP. It may be performed by the Orthopaedic surgeon, Physiotherapist, Neurologist or any healthcare provider with expertise in this condition. The examination should be comprehensive and systematic and include relevant items in history and physical examination. This section will focus on aspects relevant to hip displacement in CP. For a general overview of clinical examination of children with CP, readers are encouraged to read our related publication [37].
History
The history includes information regarding the birth, developmental milestones, other associated medical problems, surgical history, ongoing medications, physiotherapy treatment, and use of orthoses and assistive devices, if any. Specific items in the history and clinical examination related to hip dysplasia include:
Determination of the developmental status and gross motor function i.e. the GMFCS level.
Determination of motor type (spastic, dystonic, mixed etc.).
Determination of topographical pattern of limb involvement (diplegia, quadriplegia or hemiplegia).
Issues related to pain, perineal hygiene, ability to transfer and functional limitations in sitting, standing and walking.
Information regarding co-morbidities such as epilepsy, gastrointestinal issues, respiratory status etc.
Information relating to any prior interventions e.g. botulinum injections.
Physical Examination
-
(A)
For ambulatory patients, gait should be observed and the need for assistive devices should be noted. Gait deviations such as limb length discrepancy, scissoring, abductor lurch, pelvic obliquity and spinal asymmetry are recorded. Gait analysis can be performed using different techniques such as observational, videographic, or instrumented 3D gait analysis.
-
(B)
Clinical examination should be performed in the standing and sitting positions to determine leg length discrepancy, pelvic obliquity in the sagittal and coronal planes, sitting balance and presence of scoliosis.
-
(C)Hip assessment [37] (Fig. 4a–d)
- Hip range of motion (ROM) in all planes is assessed in the supine position. Hip flexion deformity is determined by the Thomas test and prone Staheli test. Range of hip abduction is measured with the hips in both extension (for medial hamstrings) and flexion (for adductors). Spasticity may be differentiated from contracture using the modified Ashworth and Tardieu scores.
- Transverse plane measurements are best performed in the prone position. The arc of internal and external rotation is noted and the degree of femoral neck anteversion is measured using the trochanteric prominence test or Gage test.
-
(D)
Pelvic obliquity and scoliosis: presence and correctability should be noted. Pelvic obliquity may result from an infrapelvic cause (fixed hip contractures), suprapelvic cause (structural scoliosis), or a combination of both.
Fig. 4.
Figure showing various tests in the clinical examination. a Thomas’ test demonstrating fixed flexion deformity of the right hip. b Popliteal angle test of right knee. c Bilateral hip abduction in extension demonstrating restricted left hip abduction. d Bilateral hip internal rotation demonstrating increased femoral anteversion in left hip
The final ‘diagnostic matrix’ should capture all relevant points on history and clinical examination that impact the decision-making in management of hip disease in children with CP.
Radiographic Assessment
Early hip displacement is typically silent clinically and becomes symptomatic only in the advanced stage of the disease. Radiography thus becomes the mainstay for the early diagnosis of hip displacement in CP and forms the basis for all surveillance protocols [2, 3, 6, 8, 10, 11, 38, 39]. An anteroposterior (AP) radiograph of the pelvis with both hips, taken in a standardized manner, is sufficient to diagnose the presence and progression of hip displacement in CP. A frog-leg lateral view is generally not required. Accurate positioning of the patient with the pelvis horizontal, both lower limbs parallel to each other, patellae facing upward, and correction of excessive lumbar lordosis in the presence of hip flexion contractures, is important [6, 40]. The following measurements are made on the AP radiograph (Fig. 5):
The Reimers Migration Percentage (RMP) is the most commonly used, reliable, and valid measure to quantify the extent of hip displacement [41–43]. To measure RMP, the percentage of the proximal femoral epiphysis lateral to the Perkin’s line is determined as a ratio to its diameter. While measurement of RMP on a single AP X-ray has been found to be reliable [42], it is recommended that treatment decisions should be based on serial radiographs demonstrating progressive changes in RMP over time [44]. The RMP threshold to identify “hips-at-risk” is > 30 or 33%. The risk of progression of RMP is found to be four times greater in children with quadriplegia than in diplegic CP. Hip RMP > 50% will not reduce spontaneously and 1/3rd will progress to dislocation.
The Acetabular Index (AI) is often used as a means to measure acetabular dysplasia. It is the angle between the Hilgenreiner’s line and a line extending from the lateral extent of the triradiate cartilage to the lateral edge of the acetabulum.
The Neck-Shaft Angle (NSA) is an important measure of coxa valga and distorted proximal femoral morphology seen in CP. Since its value is affected by the concomitant presence of excessive femoral anteversion, it is most reliable when measured in 30°–40° of internal rotation.
Head-Shaft angle (HSA) and physeal tilt: HSA is the angle between the proximal femoral physeal line and the femoral shaft axis. However, it is not strongly correlated to hip displacement and its role in hip surveillance is controversial [45].
Fig. 5.
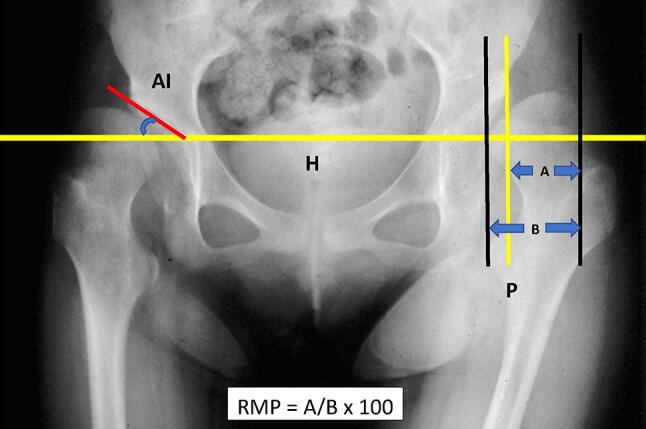
AP hip radiograph showing measurement of Reimer’s Migration Percentage (RMP). RMP in this case is 70%. H Hilgenreiner’s line, P Perkin’s line, AI Acetabular Index
Role of CT scan and MRI: CT scan and MRI have a limited role to play in the screening and diagnosis of hip displacement in CP. The main role of CT lies in determining the location of acetabular dysplasia (anterior, posterior, or global) and the severity of femoral head deformity, as part of the pre-operative assessment prior to reconstructive surgery. It is also helpful to decide the location of the acetabuloplasty used in surgical management and/or whether the hip morphology is conducive to reconstructive, rather than salvage procedures, in hips with complex acetabular and femoral deformities [29]. MRI is occasionally useful in evaluating the status of articular cartilage degeneration (using delayed gadolinium-enhanced magnetic resonance imaging of cartilage [dGEMRIC]) to decide whether a hip is amenable to reconstruction rather than salvage [46].
Why is Hip Surveillance Important?
Hip surveillance is defined as ‘the process of monitoring and identifying the early critical indicators of hip displacement’. Considering the fact that the early identification of hip displacement in CP permits early treatment with improved functional outcomes (Fig. 6), several countries and regions around the world have initiated provincial or country-wide hip surveillance programmes. Cross-sectional and population-based studies have confirmed that a well-run hip surveillance programme can reduce or even eliminate the incidence of late-presenting dislocation with all its attendant problems [3, 6, 14, 38]. An ideal hip surveillance programme should be population-based covering the entire population at risk, have a well-defined schedule of periodic hip examination and serial radiographs based on current evidence and consensus expert opinion, and ensure that progressive hip displacement is detected early enough to ensure timely referral and treatment by the Orthopaedic surgeon. In order to be successful, community access to surveillance must be universal, referral pathways must exist, access to Orthopaedic treatment must be easily available, and there must be significant buy-in from all stakeholders including caregivers, healthcare providers and administrative policy makers [6, 38].
Fig. 6.
Flowchart showing surveillance-based treatment algorithm as per evidence from literature
The first national hip surveillance model called the Cerebral Palsy Follow-up Programme (CPUP) was established in Sweden in 1994 and is now utilized all across Scandinavia and parts of Europe. The results from the first 10 years were published in 2005 and showed a statistically significant decrease in the incidence of hip dislocation from 8% in a historical control group before surveillance to 0.5% in the study group after initiating the surveillance programme [3]. Another cohort was added after the first 10 years and at a 20-year review the dislocation rate was 0%, effectively eradicating hip dislocation in CP [38]. Preventive and reconstructive surgeries were performed in 12–15% of both study groups, but more importantly not a single child needed a salvage surgery. 38% of children who underwent preventive or reconstructive surgery needed a repeat operation, especially those undergoing soft-tissue releases. This was not considered a failure and underscores the importance of continuing surveillance even after hip surgery [38].
Following the success of the Swedish model, a similar surveillance programme was initiated in Australia since 1997. A pilot study from the group in 2002 showed that more number of cases were being picked up and at a younger age than in the pre-surveillance period [6]. 46% of cases needed surgery and the rate of preventive surgery increased substantially due to early identification of ‘silent’ hip subluxation. The mean age at which preventive surgery was undertaken was 4.2 years compared to 8.3 years in the pre-surveillance period. The need for salvage surgery gradually reduced and was virtually eliminated at the end of the study period [6]. Data from Tasmania [8] and Queensland [9] further support the Australian model. Five year outcomes of a population-based, state-wide hip surveillance programme in Queensland showed that significant hip displacement (RMP > 30%) was detected in 28% of the population and that the programme was successful in correctly identifying ‘at-risk’ hips, fast tracking children to Orthopaedic review, preventing silent hip dislocation, and discharging children at minimal risk of dislocation [9]. A 5-year review of pooled data (3366 children) after implementation of the Australian hip surveillance guidelines in 2008, highlighted the efficacy of the surveillance protocol, wherein progression of RMP > 30% was always correctly identified in GMFCS level III–V children, enabling timely referral and orthopaedic management [10].
International Hip Surveillance Models
As part of their country-wide, state-wide or provincial hip surveillance programmes, Sweden [3, 38], Australia [10, 39] and British Columbia [47] have published guidelines based upon best-available current research evidence and expert consensus. The objective of these guidelines and consensus statements is to outline recommendations for hip surveillance to ensure that children with CP receive appropriate screening and are referred to an orthopaedic surgeon at the appropriate time to minimize or prevent complications associated with hip dislocations. Swedish researchers have also developed the ‘CPUP Hip Risk Score’ based upon age, GMFCS level, migration percentage (MP) and head-shaft angle (HSA), to determine the risk of developing hip displacement within 5 years of the first pelvic radiograph [48]. In 2017, the American Academy of Cerebral Palsy and Developmental Medicine (AACPDM) developed a Hip Surveillance Care Pathway as an algorithm to inform clinical practice based on best evidence and expert consensus (www.aacpdm.org) [49]. The details of each surveillance protocol vary somewhat in terms of age of entry and discharge, frequency of clinical and radiographic assessment, and when to refer for orthopaedic treatment. However, the common thread for all protocols is to utilize age, GMFCS, gait classification (WGH IV) and Reimer’s migration percentage (RMP) as critical early indicators of progressive hip displacement.
Table 1 provides an overview of the various surveillance models and how they compare with each other. For more detailed information, the reader is encouraged to access the respective published guidelines.
Table 1.
Table showing similarities and differences between various international hip surveillance models
| Category | Scandinavian | Australian | British Columbian | AAPCDM |
|---|---|---|---|---|
| 1. Year established | 1994 |
2008 Revised 2014 |
2012 | 2017 |
| 2. Evaluation done by whom and what it includes |
Done by physical therapists Evaluation includes Gross and fine motor function Upper limb and lower limb range of motion Spine examination Radiographs |
Done by physical therapists Evaluation includes Gross and fine motor function Upper limb and lower limb range of motion Radiographs Spine examination Limb length Gait |
Done by physical therapists Evaluation includes Gross and fine motor function Upper limb and lower limb range of motion Radiographs Spine examination Limb length Gait |
Done by physical therapists Evaluation includes Gross and fine motor function Upper limb and lower limb range of motion Radiographs Spine examination Limb length Gait |
| 3. Evaluation of various GMFCS levels | ||||
| GMFCS I | ||||
| Clinical examination (CE) | Every 6 months till 6 years of age and then 12 monthly thereafter till skeletal maturity |
Starts at 12–24 months of age Review at 3 and 5 years |
12 monthly till 5 years of age |
Starts at 2 years of age Review at 4 and 6 years of age |
| Radiographs (AP pelvis with both hips) | No X-ray (unless deterioration of hip/spine examination) | No X-ray |
X-ray at 5 years of age If normal, discharge from surveillance |
No X-ray |
| GMFCS II | ||||
| Clinical examination (CE)` |
Every 6 months till 6 years age and then 12 monthly thereafter till skeletal maturity Discharge at skeletal maturity |
12 months after commencement If MP unstable/abnormal, repeat 12 monthly until stabilized. If MP stable, review at 4–5 years of age. At 4–5 years of age, if MP unstable/abnormal, repeat 12 monthly until stabilized. If MP stable, review at 8–10 years of age At 8–10 years, if MP unstable then repeat 12 monthly until stabilized or patient reaches skeletal maturity |
12 monthly till 5 years of age | Every 24 months from 2 to 10 years of age |
| Radiographs (AP pelvis with both hips) |
At age 2 and 6 years After 6 years—if no deterioration on CE and MP < 33%, no further X-rays required |
12 months after commencement If MP unstable/abnormal, repeat 12 monthly until stabilized. If MP stable, review at 4–5 years of age. At 4–5 years of age, if MP unstable/abnormal, repeat 12 monthly until stabilized. If MP stable, review at 8–10 years of age At 8–10 years, if MP unstable then repeat 12 monthly until stabilized or patient reaches skeletal maturity If MP stable, discharge from surveillance |
X-ray at 5 years of age If normal, discharge from surveillance |
X-ray at 2, 6, 10 years of age If MP < 30% at 10 years of age, discharge from surveillance |
| GMFCS III | ||||
| Clinical examination |
Every 6 months till 6 years age and then 12 monthly thereafter till skeletal maturity After skeletal maturity If MP stable/normal and no scoliosis, continue only clinical surveillance |
6 months after commencement If unstable/abnormal repeat every 6 monthly until stabilized If MP stable, repeat every 12 monthly Review at 7 years of age If unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, discontinue surveillance till pre-pubertal age Review at pre-puberty |
After initial clinical examination, review at 24 months of age and 12 monthly thereafter till 6 years of age 12 monthly from 6 years of age till skeletal maturity |
Every 12 monthly from 2 to 8 years of age Every 24 months from 8 to 16 years of age or till skeletal maturity |
| Radiographs (AP pelvis with both hips) |
Every 12 months till 8 years of age After 8 years till skeletal maturity—X-ray frequency individualized based on previous clinical and X-ray findings If MP stable/normal, X-rays every 24 months till skeletal maturity After skeletal maturity, no further X-rays if MP < 33% and no scoliosis. If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
6 months after commencement If unstable/abnormal repeat every 6 monthly until stabilized If MP stable, repeat every 12 monthly Review at 7 years of age If unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, discontinue surveillance till pre-pubertal age Review at pre-puberty X-ray 12 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, gait deterioration, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
Initial X-ray at 24 months of age and 12 monthly thereafter till 6 years of age. Every 24 months from 6 years of age till skeletal maturity |
Every 12 monthly from 2 to 8 years of age Every 24 months from 8 to 16 years of age or till skeletal maturity If MP unstable/abnormal, repeat every 12 monthly until stabilized At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
| GMFCS IV | ||||
| Clinical examination |
Every 6 months till 6 years of age and then 12 monthly thereafter till skeletal maturity After skeletal maturity If MP stable/normal and no scoliosis, continue only clinical surveillance |
6 months after commencement If unstable/abnormal repeat every 6 monthly until stabilized If MP stable, repeat every 12 monthly Review at 7 years of age If MP unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, discontinue surveillance till pre-pubertal age Review at pre-puberty |
After initial clinical examination, review at 24 months of age and 6 monthly thereafter till 6 years of age 12 monthly from 6 years of age till skeletal maturity |
6 monthly from 2 to 4 years of age 12 monthly from 4 years of age till 16 years of age or skeletal maturity |
| Radiographs (AP pelvis with both hips) |
Every 12 months till 8 years of age After 8 years till skeletal maturity—X-ray frequency individualized based on previous clinical and X-ray findings If MP stable/normal, X-rays every 24 months till skeletal maturity After skeletal maturity, no further X-rays if MP < 33% and no scoliosis If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
6 months after commencement If unstable/abnormal repeat every 6 monthly until stabilized If MP stable, repeat every 12 monthly Review at 7 years of age If MP unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, discontinue surveillance till pre-pubertal age Review at pre-puberty X-ray 12 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, gait deterioration, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
Initial X-ray at 24 months of age and 6 monthly thereafter till 6 years of age 12 monthly from 6 years of age till skeletal maturity |
6 monthly from 2 to 4 years of age If MP unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, 12 monthly from 4 years of age till 16 years of age or skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
| GMFCS V | ||||
| Clinical examination |
Every 6 months till 6 years of age and then 12 monthly thereafter till skeletal maturity After skeletal maturity If MP stable/normal and no scoliosis, continue only clinical surveillance |
6 months after commencement. 6 monthly till 7 years of age Review at 7 years of age If MP stable/normal, 12 monthly till skeletal maturity If MP abnormal, presence of scoliosis or pelvic obliquity, 6 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
After initial clinical examination, review at 24 months of age and 6 monthly thereafter till 6 years of age 12 monthly from 6 years of age till skeletal maturity |
6 monthly from 2 to 4 years of age 12 monthly from 4 years of age till 16 years of age or skeletal maturity |
| Radiographs (AP pelvis with both hips) |
Every 12 months till 8 years of age After 8 years till skeletal maturity—X-ray frequency individualized based on previous clinical and X-ray findings If MP stable/normal, X-rays every 24 months till skeletal maturity After skeletal maturity, no further X-rays if MP < 33% and no scoliosis If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
6 months after commencement 6 monthly till 7 years of age Review at 7 years of age If MP stable/normal, 12 monthly till skeletal maturity If MP abnormal, presence of scoliosis or pelvic obliquity, 6 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
Initial X-ray at 24 months of age and 6 monthly thereafter till 6 years of age 12 monthly from 6 years of age till skeletal maturity |
6 monthly from 2 to 4 years of age If MP unstable/abnormal, repeat every 6 monthly until stabilized If MP stable/normal, 12 monthly from 4 years of age till 16 years of age or skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
| 4. WGH type IV | ||||
| Clinical examination | Does not mention |
Review at 5 years of age If MP unstable/abnormal, repeat 12 monthly until stabilized If MP stable, review at 10 years of age After 10 years of age, 12 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
12 monthly from 2 years of age till skeletal maturity but review at 5 years of age | Every 24 months from 2 years of age till skeletal maturity |
| Radiographs (AP pelvis with both hips) |
Review at 5 years of age If MP unstable/abnormal, repeat 12 monthly until stabilized If MP stable, review at 10 years of age After 10 years of age, 12 monthly till skeletal maturity At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance 12 monthly |
Review at 5 years of age with X-ray 12 monthly from 5 years of age till skeletal maturity |
X-ray at 2, 6, 10 years of age Every 24 months from 10 to 16 years of age or till skeletal maturity. At skeletal maturity If MP stable/normal, discontinue surveillance If MP abnormal, progressive scoliosis or pelvic obliquity, continue surveillance |
|
| 5. Orthopaedic referral | MP > 33% | MP > 30% and/or unstable MP and/or hip pain |
MP > 30% Hip abduction < 30° Presence of hip pain Difficulty in ADLs related to the hip Decreased ability to walk, sit, or stand related to the hip |
MP > 30% Hip abduction < 30° Presence of hip pain on history or examination |
CP Hip Surveillance in India and Review of Indian Literature
The true prevalence and etiology of CP in India are unknown, since most studies are hospital-based and of a small cohort of children rather than community-based [50]. In a retrospective study of 544 cases over an 8-year period, Laisram et al. [51] showed that the etiology of CP in ~ 70% of cases was due to perinatal and post-natal factors, compared to developed countries which reported a relatively higher incidence of prenatal causes. The clinical spectrum of CP is also different between developing and developed countries, as shown in an analysis of 1212 children from North India over a period of 2 decades [52]. Spastic quadriplegia was the commonest clinical phenotype, seen in over 50% of cases, as compared to developed countries where spastic diplegia is more common. Perinatal causes (birth asphyxia, sepsis) and post-natal causes (meningitis, hyperbilirubinemia etc.) contribute to a large proportion of the disease—a scenario very different from developed countries where CP is more related to prematurity and survival [52].
There are very few reports from India regarding the incidence of hip displacement in children with CP. There are no population-based or longitudinal studies available and all reports are cross-sectional studies on small cohorts. Hence it is difficult to estimate the true incidence of hip displacement in India. Divecha [53] reported on the use of combined hip abduction angle (CHAA) and compared it with RMP in a group of 106 children with CP (GMFCS levels IV and V). They found a positive correlation between CHAA and RMP, with a sensitivity of 74%, specificity of 67%, false positive rate of 32% and false negative rate of 25%. They recommended using the CHAA < 40° as an easy clinical screening test which can be used by paraclinical health practitioners for early detection and referral to the orthopaedic surgeon [53]. In a cross-sectional study of 118 children, between 2 and 12 years of age and GMFCS levels III–V, Vykuntaraju [54] reported only a 12.71% incidence of hip displacement as measured by the RMP. However, serial X-rays were not taken and the X-ray protocol was not standardised. In a similar cross-sectional study analyzing 91 hips in 52 patients randomly selected from the PMR Clinic, Sahoo et al. [55] found significant hip displacement (RMP > 33%) in 24% of hips, but 70% of the selected cohort were GMFCS levels I and II. In a study by Kunju et al. [56] 101 children with CP between 4 and 9 years of age reporting to the outpatient paediatric neurology clinic, underwent one-time radiological screening. Out of 36 hemiplegic CP only two (5%) had RMP > 33%, while 100% of children with spastic quadriplegia had significant hip displacement RMP > 33%. Incidence of hip displacement (RMP > 33%) was 2.5%, 7.7%, 50%, 61% and 66% in GMFCS levels I, II, III, IV and V respectively.
At present, there are no formal CP hip surveillance programmes existing in India nor are there any existing guidelines relevant to the Indian context. Some Orthopaedic surgeons and centres use their own schedule for clinical and radiological screening for hip displacement, which may or may not be based on established international hip surveillance models. There thus remains a large variation in the screening, diagnosis, and treatment practices of hip displacement in children with cerebral palsy. There is a strong need to promote interdisciplinary discussion between various organizations, such as, the Paediatric Orthopaedic Society of India (POSI), Indian Academy of Cerebral Palsy (IACP), Indian Association of Physiotherapists (IAP), Association of Child Neurology (AOCN) etc., that will lead to the development of standardized guidelines for hip surveillance relevant to the Indian context. These guidelines will help reduce practice variation and improve long-term patient outcomes.
Conclusions
Hip displacement in cerebral palsy is an insidious and progressive problem, with poor outcomes if left untreated. Universal hip surveillance in children with cerebral palsy, using a standardized protocol, has the potential to diagnose hip displacement early so that appropriate treatment can be instituted before significant damage to the hip occurs. Existing hip surveillance models can serve as a framework to formulate guidelines and develop a referral care pathway that is appropriate for our healthcare system.
Funding
There was no sources of financial or material support for this report.
Compliance with Ethical Standards
Conflict of interest
The authors state that they have no conflict of interest, financial or otherwise, concerning the material or methods used in this study or the findings specified in this paper.
Ethical standard statement
This study was approved by ethical committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the Institutional Ethics Committee.
Informed consent
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenbaum P, et al. A report: the definition and classification of cerebral palsy. Developmental Medicine and Child Neurology Supplement. 2006;109:8–14. [PubMed] [Google Scholar]
- 2.Hägglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskeletal Disorders. 2007;8:101. doi: 10.1186/1471-2474-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hägglund G, Andersson S, Düppe H, Lauge-Pedersen H, Nordmark E, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy. The first 10 years of a population-based prevention programme. Journal of Bone Joint Surgery (British Volume) 2005;87:95–101. [PubMed] [Google Scholar]
- 4.Soo B, Howard JJ, Boyd RN, et al. Hip displacement in cerebral palsy. Journal of Bone and Joint Surgery (American Volume) 2006;88:121–129. doi: 10.2106/JBJS.E.00071. [DOI] [PubMed] [Google Scholar]
- 5.Terjesen T. Development of the hip joints in unoperated children with cerebral palsy: A radiographic study of 76 patients. Acta Orthopaedica. 2006;77:125–131. doi: 10.1080/17453670610045803. [DOI] [PubMed] [Google Scholar]
- 6.Dobson F, Boyd RN, Parrott J, et al. Hip surveillance in children with cerebral palsy. Impact on the surgical management of spastic hip disease. Journal of Bone Joint Surgery (British Volume) 2002;84:720–726. doi: 10.1302/0301-620x.84b5.12398. [DOI] [PubMed] [Google Scholar]
- 7.Gordon GS, Simkiss DE. A systematic review of the evidence for hip surveillance in children with cerebral palsy. Journal of Bone and Joint Surgery (British Volume) 2006;88:1492–1496. doi: 10.1302/0301-620X.88B11.18114. [DOI] [PubMed] [Google Scholar]
- 8.Connelly A, Flett P, Graham HK, et al. Hip surveillance in Tasmanian children with cerebral palsy. Journal of Paediatrics and Child Health. 2009;45:437–443. doi: 10.1111/j.1440-1754.2009.01534.x. [DOI] [PubMed] [Google Scholar]
- 9.Kentish M, Wynter M, Snape N, et al. Five-year outcome of statewide hip surveillance of children and adolescents with cerebral palsy. Journal of Pediatric Rehabilitation Medicine. 2011;4:205–217. doi: 10.3233/PRM-2011-0176. [DOI] [PubMed] [Google Scholar]
- 10.Wynter M, Gibson N, Willoughby KL, et al. Australian hip surveillance guidelines for children with cerebral palsy: 5-year review. Developmental Medicine and Child Neurology. 2015;57:808–820. doi: 10.1111/dmcn.12754. [DOI] [PubMed] [Google Scholar]
- 11.Scrutton D, Baird G. Surveillance measures of the hips of children with bilateral cerebral palsy. Archives of Disease in Childhood. 1997;76:381–384. doi: 10.1136/adc.76.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke PH, Cole WG, Carey RP. Dislocation of the hip in cerebral palsy. Natural history and predictability. Journal of Bone Joint Surgery (British Volume) 1989;71:441–446. doi: 10.1302/0301-620X.71B3.2722938. [DOI] [PubMed] [Google Scholar]
- 13.Scrutton D, Baird G, Smeeton N. Hip dysplasia in bilateral cerebral palsy: Incidence and natural history in children aged 18 months to 5 years. Developmental Medicine and Child Neurology. 2001;43:586–600. doi: 10.1017/s0012162201001086. [DOI] [PubMed] [Google Scholar]
- 14.Elkamil AI, Andersen GL, Hägglund G, Lamvik T, Skranes J, Vik T. Prevalence of hip dislocation among children with cerebral palsy in regions with and without a surveillance programme: A cross sectional study in Sweden and Norway. BMC Musculoskeletal Disorder. 2011;12:284–295. doi: 10.1186/1471-2474-12-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard CB, McKibbin B, Williams LA, et al. Factors affecting the incidence of hip dislocation in cerebral palsy. Journal of Bone and Joint Surgery (British Volume) 1985;67:530–532. doi: 10.1302/0301-620X.67B4.4030844. [DOI] [PubMed] [Google Scholar]
- 16.Bagg MR, Farber J, Miller F. Long-term follow-up of hip subluxation in cerebral palsy patients. Journal of Pediatric Orthopedics. 1993;13:32–36. doi: 10.1097/01241398-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 18.Larnert P, Risto O, Hagglund G, Wagner P. Hip displacement in relation to age and gross motor function in children with cerebral palsy. Journal of Children’s Orthopaedics. 2014;8:129–134. doi: 10.1007/s11832-014-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruszczynski B, Sees J, Miller F. Risk factors for hip displacement in children with cerebral palsy: Systematic review. Journal of Pediatric Orthopedics. 2016;36:829–833. doi: 10.1097/BPO.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien JJ, Sirkin RB. The natural history of the dislocated hip in cerebral palsy. Developmental Medicine and Child Neurology. 1978;20:241. doi: 10.1111/j.1469-8749.1979.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 21.Pritchett JW. The untreated unstable hip in severe cerebral palsy. Clinical Orthopaedics and Related Research. 1983;173:169–172. [PubMed] [Google Scholar]
- 22.Wawrzuta J, Willoughby KL, Molesworth C, Ang SG, Shore BJ, Thomason P, Graham HK. Hip health at skeletal maturity: A population-based study of young adults with cerebral palsy. Developmental Medicine and Child Neurology. 2016;58:1273–1280. doi: 10.1111/dmcn.13171. [DOI] [PubMed] [Google Scholar]
- 23.Ramstad K, Jahnsen RB, Terjesen T. Severe hip displacement reduces health-related quality of life in children with cerebral palsy: A population-based study of 67 children. Acta Orthopaedica. 2017;88(2):205–210. doi: 10.1080/17453674.2016.1262685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiFazio R, Vessey JA, Miller P, Van Nostrand K, Snyder B. Postoperative complications after hip surgery in patients with cerebral palsy: A retrospective matched cohort study. Journal of Pediatric Orthopedics. 2016;36:56–62. doi: 10.1097/BPO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 25.Letts M, Shapiro L, Mulder K, Klassen O. The windblown of children with hip displacement in cerebral palsy. Journal of Pediatric Orthopedics. 1984;4:55–62. doi: 10.1097/01241398-198401000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Samilson RL, Tsou P, Aamoth G, Green W. Dislocation and subluxation of the hip in cerebral palsy. Journal of Bone and Joint Surgery (American Volume) 1972;54:863–873. [PubMed] [Google Scholar]
- 27.Ramstad K, Terjesen T. Hip pain is more frequent in severe hip displacement: A population-based study of 77 children with cerebral palsy. Journal of Pediatric Orthopedics Part B. 2016;25:217–221. doi: 10.1097/BPB.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 28.Miller F, Dabney KW, Rang M. Complications in cerebral palsy treatment. In: Epps CH Jr, editor. Complications in pediatric orthopaedic surgery. Philadelphia: JB Lippincott Company; 1995. pp. 477–544. [Google Scholar]
- 29.Gose S, Sakai T, Shibata T, Murase T, Yoshikawa H, Sugamoto K. Morphometric analysis of the femur in cerebral palsy: 3-dimensional CT study. Journal of Pediatric Orthopedics. 2010;30:568–574. doi: 10.1097/BPO.0b013e3181e4f38d. [DOI] [PubMed] [Google Scholar]
- 30.Lapalza FJ, Root L. Femoral anteversion and neck-shaft angles in hip instability in cerebral palsy. Journal of Pediatric Orthopedics. 1994;14:719–723. doi: 10.1097/01241398-199414060-00006. [DOI] [PubMed] [Google Scholar]
- 31.Robin J, Graham HK, Selber P, Dobson F, Smith K, Baker R. Proximal femoral geometry in cerebral palsy: a population-based cross-sectional study. Journal of Bone Joint Surgery (British Volume) 2008;90-B:1372–1379. doi: 10.1302/0301-620X.90B10.20733. [DOI] [PubMed] [Google Scholar]
- 32.Phelps WM. Prevention of acquired dislocation of the hip in cerebral palsy. Journal of Bone and Joint Surgery (American Volume) 1959;41:440–448. [PubMed] [Google Scholar]
- 33.Tachdjian MO, Minear WL. Hip dislocation in cerebralpalsy. Journal of Bone and Joint Surgery (American Volume) 1956;38(6):1358–1364. [PubMed] [Google Scholar]
- 34.Lee KM, Kang JY, Chung CY, Kwon DG, Lee SH, Choi IH, Cho T, Yoo WJ, Park MS. Clinical relevance of valgus deformity of proximal femur in cerebral palsy. Journal of Pediatric Orthopedics. 2010;30:720–725. doi: 10.1097/BPO.0b013e3181edba2a. [DOI] [PubMed] [Google Scholar]
- 35.Miller F, Slomczykowski M, Cope R, Lipton GE. Computer modeling of the pathomechanics of spastic hip dislocation in children. Journal of Pediatric Orthopedics. 1999;19:486–492. doi: 10.1097/00004694-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Chung MK, Zulkarnain A, Lee JB, Cho BC, Chung CY, Lee KY, Sung KH, Park MS. Functional status and amount of hip displacement independently affect acetabular dysplasia in cerebral palsy. Developmental Medicine and Child Neurology. 2017;59:743–749. doi: 10.1111/dmcn.13437. [DOI] [PubMed] [Google Scholar]
- 37.Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian Journal of Orthopaedics. 2019;53:35–44. doi: 10.4103/ortho.IJOrtho_409_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagglund G, Alriksson-Schmidt A, LaugePedersen H, et al. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint Journal. 2014;96-B:1546–1552. doi: 10.1302/0301-620X.96B11.34385. [DOI] [PubMed] [Google Scholar]
- 39.Wynter M, Gibson N, Kentish M, et al. The development of Australian standards of care for hip surveillance in children with cerebral palsy: How did we reach consensus? Journal of Pediatric Rehabilitation Medicine. 2011;4:171–182. doi: 10.3233/PRM-2011-0173. [DOI] [PubMed] [Google Scholar]
- 40.Kinch K, Campbell DM, Maclean JGB, et al. How critical is patient positioning in radiographic assessment of the hip in cerebral palsy when measuring migration percentage? Journal of Pediatric Orthopedics. 2015;35:756–760. doi: 10.1097/BPO.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 41.Reimers J. The stability of the hip in children. A radiological study of the results of muscle surgery in cerebral palsy. Acta Orthopaedica Scandinavica. 1980;184(Suppl):1–100. doi: 10.3109/ort.1980.51.suppl-184.01. [DOI] [PubMed] [Google Scholar]
- 42.Parrott J, Boyd RN, Dobson F, et al. Hip displacement in spastic cerebral palsy: Repeatability of radiologic measurement. Journal of Pediatric Orthopedics. 2002;22:660–667. [PubMed] [Google Scholar]
- 43.Craven A, Rym A, Boyd RN. Reliability of radiologic measures of hip displacement in a cohort of preschool-aged children with cerebral palsy. Journal of Pediatric Orthopedics. 2014;34:597–601. doi: 10.1097/BPO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 44.Faraj S, Atherton WG, Stott NS. Inter- and intra-measurer error in the measurement of Reimers’ hip migration percentage. Journal of Bone Joint Surgery. 2004;86-B:434–437. doi: 10.1302/0301-620x.86b3.14094. [DOI] [PubMed] [Google Scholar]
- 45.Chougule S, Dabis J, Petrie A, Daly K, Gelfer Y. Is head-shaft angle a valuable continuous risk factor for hip migration in cerebral palsy? Journal of Children’s Orthopaedics. 2016;10:651–656. doi: 10.1007/s11832-016-0774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YJ, Jaramillo D, Millis MB, et al. Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. Journal of Bone Joint Surgery (American Volume) 2003;85A:1987–1992. doi: 10.2106/00004623-200310000-00019. [DOI] [PubMed] [Google Scholar]
- 47.BC_Hip_Surveillance_Planning_Committee: British Columbia Consensus Statement on Hip Surveillance for children with cerebral palsy: Information for health care professionals caring for children with cerebral palsy. Vancouver, BC, Child Heath BC, 2012.
- 48.Hermanson M, Hagglund G, Riad J, Rodby-Bousquet E, Wagner P. Prediction of hip displacement in children with cerebral palsy: development of the CPUP hip score. Bone Joint Surgery. 2015;97-B(10):1441–1444. doi: 10.1302/0301-620X.97B10.35978. [DOI] [PubMed] [Google Scholar]
- 49.The American Academy of Cerebral Palsy and Developmental Medicine: Hip surveillance care pathways. https://www.aacpdm.org/publications/care-pathways/hip-surveillance. Accessed 13 Mar 2020.
- 50.Jindal P, Macdermid JC, Rosenbaum P, Direzze B, Narayan A, Nayak SL. Treatment and re/habilitation of children with cerebral palsy in India: A scoping review. Developmental Medicine and Child Neurology. 2019;61(9):1050–1060. doi: 10.1111/dmcn.14211. [DOI] [PubMed] [Google Scholar]
- 51.Laisram N, Srivastava VK, Srivastava RK. Cerebral palsy: An etiological study. Indian Journal of Pediatrics. 1992;59:723–728. doi: 10.1007/BF02859408. [DOI] [PubMed] [Google Scholar]
- 52.Singhi P, Saini AG. Changes in the clinical spectrum of cerebral palsy over two decades in North India: An analysis of 1212 cases. Journal of Tropical Pediatrics. 2013;59:434–440. doi: 10.1093/tropej/fmt035. [DOI] [PubMed] [Google Scholar]
- 53.Divecha A, Bhaskar A. Utility of combined hip abduction angle for hip surveillance in children with cerebral palsy. Indian Journal of Orthopaedics. 2011;45(6):548–552. doi: 10.4103/0019-5413.87129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vykuntaraju KN, Manohar V, Lakshman RR, Ramaswamy P. Developmental dysplasia of spastic hip in children with cerebral palsy in Southern India. Indian Pediatrics. 2016;53:259–260. [PubMed] [Google Scholar]
- 55.Sahoo PK, Sahoo MM, Chitapure T, Das SP. Radiological Evaluation of hip in cerebral palsy: A randomized cross-sectional study. Indian Journal of Physical Medicine and Rehabilitation. 2017;28(3):95–99. [Google Scholar]
- 56.Kunju PAM, Shibu G, Amruthalal Severity of hip displacement in relation to subtypes and motor function in cerebral palsy: Role of hip surveillance. Orthopedics and Rheumatology Open Access Journal. 2018;12(5):555848. [Google Scholar]