Graphical abstract
Abbreviations: RNA, ribonucleic acid; circRNAs, circular RNAs; RBP, RNA-binding protein; ncRNAs, noncoding RNAs; snRNA, small nuclear RNA; rRNA, ribosomal RNA; miRNAs, microRNAs; siRNAs, small interfering RNAs; lncRNAs, long ncRNA; RNase, ribonuclease; UTR, untranslated regions; ecircRNAs, exonic circular RNAs; ciRNAs, circular intronic RNAs; EIciRNAs, exon–intron RNAs; tricRNAs, tRNA intronic circRNAs; ceRNAs, endogenous RNAs; MER, miRNA response elements; ciRS-7, circular RNA sponge for miR-7; HCC, hepatocellular carcinoma; CRC, colorectal cancer; PCR, polymerase chain reaction; qPCR, quantitative PCR; RT-PCR, reverse transcription-PCR; GC, gastric cancer; TNM, tumor node metastases; AML, acute myloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; MM, multiple myeloma; LUAD, lung adenocarcinoma; EMT, epithelial-mesenchymal transition; NSCLC, non-small cell lung cancer; ccRCC, clear cell renal cell carcinoma; ISH, in situ hybridization; BSJ, back-splice junction; PDAC, pancreatic ductal adenocarcinoma
Keywords: Circular RNAs, Cancer, Functions, Biomarker
Abstract
Circular RNAs (circRNAs) are a very interesting class of conserved single-stranded RNA molecules derived from exonic or intronic sequences by precursor mRNA back-splicing. Unlike canonical linear RNAs, circRNAs form covalently closed, continuous stable loops without a 5′end cap and 3′end poly(A) tail, and therefore are resistant to exonuclease digestion. The majority of circRNAs are highly abundant, and conserved across different species with a tissue or developmental-stage-specific expression. circRNAs have been shown to play important roles as microRNA sponges, regulators of gene splicing and transcription, RNA-binding protein sponges and protein/peptide translators. Emerging evidence reveals that circRNAs function in various human diseases, particularly cancers, and may function as better predictive biomarkers and therapeutic targets for cancer treatment. In consideration of their potential clinical relevance, circRNAs have become a new research hotspot in the field of tumor pathology. In the present study, the current understanding of the biogenesis, characteristics, databases, research methods, biological functions subcellular distribution, epigenetic regulation, extracellular transport and degradation of circRNAs was discussed. In particular, the multiple databases and methods involved in circRNA research were first summarized, and the recent advances in determining the potential roles of circRNAs in tumor growth, migration and invasion, which render circRNAs better predictive biomarkers, were described. Furthermore, future perspectives for the clinical application of circRNAs in the management of patients with cancer were proposed, which could provide new insights into circRNAs in the future.
1. Introduction
Circular RNAs (circRNAs) were initially found in a plant-based virus in 1976 and described as “covalently closed circRNAs molecules”[1], [2]. However, circRNAs were originally considered to be the byproducts of aberrant RNA splicing and did not attract much attention by researchers during the next decades[3], [4]. Following developments in bioinformatics, a large number of circRNAs have been identified, and some of their features have become increasingly clear.
Due to the pressing need to understand the complex gene expression dynamics in various types of cancer, the cellular roles of RNA molecules with gene-regulatory potential have been widely revealed. The vast majority (>90%) of the mammalian genome could be transcribed into noncoding RNAs (ncRNAs), instead of coding RNAs[5], [6], [7], [8], [9]. ncRNAs are classified into five primary categories: Housekeeper ncRNAs (small nuclear RNA; snRNA), small nucleolar RNA, ribosomal RNA (rRNA), transfer RNA and regulatory ncRNAs. Regulatory ncRNAs could be categorized into: Small ncRNAs (<200 bp), including microRNA (miRNAs/miRs), small interfering (si)RNAs and PIWI-interacting RNAs, snRNAs and long ncRNAs (lncRNAs; >200 bp) [10], [11]. circRNAs, a peculiar group of lncRNAs extensively existing in mammalian cells, have recently been regarded as an intriguing class of endogenous RNAs that form a closed continuous loop[12], [13], [14].
First, a number of studies have reported that circRNAs are strongly specific to tissues [15], [16]. Secondly, due to their resistance to ribonuclease (RNase) activity, circRNAs are much more stable, as compared to their linear counterparts[15], [17]. Thirdly, genome-wide analysis has discovered that circRNAs exhibit a higher sequence-conservation and more abundance than their linear counterparts [17], [18]. In addition, circRNAs are regarded as competing endogenous RNAs that regulate alternative splicing or transcription, bind or sequester proteins, and are translated into functional peptides[19], [20]. These features demonstrated that circRNAs may be capable of playing a role in pathological and biological cellular processes. Increasing evidence indicates that circRNAs are closely associated with the pathology of various diseases, including Alzheimer’s disease[21] neurological dysfunction[22] osteoarthritis[23] diabetes[24] cardiac disease[25] and cancer[26].
In particular, circRNAs have been found to play crucial roles in cancer initiation, development and drug resistance[27], [28]. Furthermore, circRNAs can have an impact on the tumor microenvironment through intercellular communication due to its abundance in exosomes and human fluids. Therefore, circRNAs can be visualized as promising biomarkers for cancer. In the present review, the current research on the clinical significance and functional mechanism of circRNAs in the biogenesis, biological functions, advances, challenges and clinical implications of various cancers was summarized.
2. Biogenesis of circRNAs
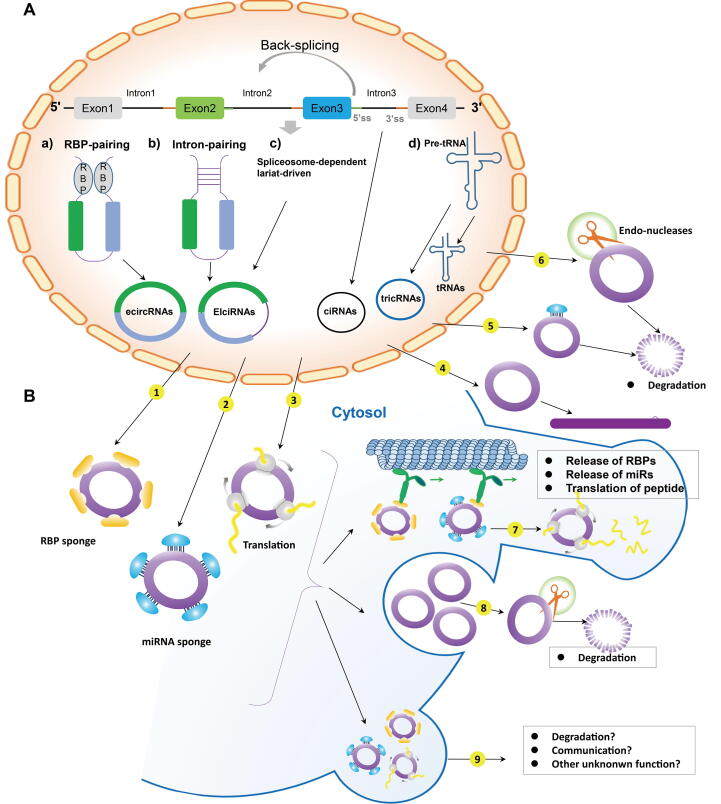
circRNAs are typically derived from the back-splicing of precursor mRNAs to form closed RNA transcripts. However, the mechanisms of circRNAs biogenesis and regulatory factors involved in circularization remains unclear [15], [17]. circRNAs can also originate from exons, introns, 5′untranslated regions (UTR), 3′UTR or antisense sequences[15], [17], [29]. To date, circRNAs have been divided into four categories: Exonic circRNAs (ecircRNA), circular intronic RNAs (ciRNAs), exon–intron RNAs (EIciRNAs) and tRNA intronic circRNAs (tricRNAs)[30]. Among the various types of circRNAs, the most studied are ecircRNAs, which account for > 80% of all circRNAs. Four related biogenesis mechanisms are discussed below and the brief process of biogenesis is illustrated in Fig. 1.
Fig. 1.
Biogenesis, functions and degradation of circRNA. (A) Biogenesis of circRNAs. (a) circRNA formation through RBP-mediated pre-miRNA folding. (b) Pairing between the 2 introns flanking the circularized exons. (c) The back-splicing site promotes the joining of the downstream 5’donor sites with the upstream 3’acceptor sites. (d) tricRNA exon termini link to each other to form a mature tRNA, and intron termini are ligated together to form tricRNA. (B) Functions and degradation of circRNAs. circRNAs could (1) bind RBPs as transcription regulators, (2) function as miRNA sponges, (3) be translated into proteins/peptides, (4) generate pseudogenes, (5) sponge miRNA for direct degradation and (6) be degraded by endonucleases. (7) The circRNA-complex may diffuse in the cytoplasm or be actively transported into particular regions of the cell (e.g., the synapse) where it can release its bound cargo or start to be translated. (8) The enclosure of circRNAs or circRNA factor complexes in vesicles could be released into the extracellular space, which would remove circRNAs from the cytoplasm. (9) The circRNA or circRNA complexes could reach other cells or tissues and therefore act as messenger molecules or fulfill other unknown functions.
2.1. RNA-binding protein (RBP)-induced circularization
circRNA biogenesis could be elicited by the mediation of RBPs of circularization. RBPs, such as Quaking, Muscleblind and Fused-in sarcoma, which are regarded as trans-acting factors, could enhance circularization by bridging related intronic sequences[31]. The dimerization of RBPs combined with the upstream and downstream of the circularized exons, can induce a closer link between 3′ and 5′ends of the circularized exons and facilitate splicing (Fig. 1Aa) [32].
2.2. Intron-pairing circularization
Pairing with a complementary inverted sequence could enhance back-splicing [33]. The unique intronic sequence allows the splice donor near the splice acceptor, finally promoting the nucleophilic attack and cleavage[34]. The competition of reverse complementary sequences at different locations results in one gene producing different circRNA isoforms (Fig. 1Ab)[35], [36].
2.3. Spliceosome-dependent lariat-driven circularization
Exon circularization is spliceosome-dependent, as confirmed by the variation in 5′ splice sites[36]. At the back-splicing site, the spliceosomes are gathered to facilitate the connection between the 5′donor and 3′acceptor sites[37]. Internal splicing consequently occurs in the lariat, which leads to the release of ecircRNAs or EIciRNAs[38]. In addition, back-splicing covering single exons or several exons with intervening introns could occur post-transcriptionally and co-transcriptionally (Fig. 1Ac)[39].
2.4. tricRNA splicing pathway
The formation of tricRNA requires tRNA splicing enzymes to divide pre-tRNA into two parts: tricRNAs are derived from a 3′-5′phosphodiester bond[40]. A structural motif resembling the archaeal bulge-helix-bulge is present in pre-tRNA. The leader and trailer are removed by RNase P and RNase Z, respectively. Cleavage of the pre-tRNA yields two exon halves and an intron, each bearing 5′OH and 2′,3′cyclic phosphate at the cut sites (Fig. 1Ad)[41].
3. Biological functions of circRNAs
circRNAs are known to have multiple functions, which include serving as miRNA sponges, interacting with RBPs, modulating alternative splicing and transcription, translation, generating pseudogenes, transportation and communication. In addition, circRNAs can regulate gene expression due to their role in aiding the process of translation.
3.1. circRNAs interacting with proteins
circRNAs function as protein antagonists or baits to inhibit the activity of proteins (Fig 1B.1). For instance, circ-Foxo3 could interact with cell cycle-related proteins, including p21 and p27, thereby blocking the roles of the proteins in cancer cell cycle progression[42]. Another circRNA, circPABPN1, has been shown to bind to HuR, a well-known RBP [43]. Similarly, the binding of circPABPN1 to the well-known RNA binding protein HuR reduced the translation of PABPN1 by preventing HuR from interacting with PABPN1 mRNA[44]. circANRIL, which was shown to bind to peccadillo homolo 1 (PES1), repressed PES1-mediated rRNA maturation[45].
3.2. circRNAs act as miRNA sponges or competing endogenous RNAs
To date, the majority of circRNAs have been reported to serve as miRNA sponges (Fig 1B.2) [29], [46]. miRNAs play a pivotal role in tumor progression [47], [48]. It has been reported that competitive endogenous RNAs (ceRNAs) can serve as sponges for miRNAs [49]. circRNAs are predominantly cytoplasmic and have multiple miRNA response elements (MER)[20] suggesting that circRNAs may competitively bind to miRNAs. Thus, circRNAs could regulate miRNA function through suppressing the effect of ceRNA.
The most well-known miRNA in the circRNA field is miR-7 [50], [51]. miR-7 has been identified as either a tumor inducer or a tumor suppressor during tumorigenesis. circular RNA sponge for miR-7 (ciRS-7; also known as CDR1as), the most well-known circRNA, contains > 70 miR-7 binding sites, and is expressed in different tissues and organs[52]. By recruiting miR-7, ciRS-7 is capable of inhibiting the miR-7 function and upregulating the expression of related genes, such as IRS2 and EGFR[53]. In esophageal squamous cell carcinoma, ciRS-7 was shown to act as ceRNA to absorb miR-7 and regulate the NF-κB/p65 pathway[54]. In the mammalian brain, ciRS-7, a lncRNA cyrano, and miR-7 and miR-671, two miRNAs, can collaborate to form a sophisticated regulatory network [55]. In addition to ciRS-7, several circRNAs are considered to act as miRNA sponges. CircHIPK3 has been shown to act as ceRNA to absorb miRNAs, including the miR-379, miR-4288, miR-558 and miR-7[56], [57], [58], [59]. circHIPK2 functions as an miR124-2HG sponge to modulate astrocyte activation through the interplay between autophagy and endoplasmic reticulum stress [60]. In addition, multiple lines of evidence have proven that circITCH is capable of sponging miR-214 in glioma and bladder cancer[61].
3.3. circRNA translation into proteins/peptides
A 5′cap and 3′poly(A)tail are required for linear mRNA translation[62]. Unlike mRNAs, circRNAs lack unique molecular structure[63]. However, circRNAs can be translated through N6-methyladenosine modification or internal ribosome entry site (IRES) to promote direct binding of initial factors to the circRNAs, as demonstrated with engineered circRNAs [62], [64], [65], [66]. Although the majority of circRNAs do not have the capacity to bind to ribosomes for translation, data have shown that a small proportion of endogenous circRNAs can be translated into proteins or peptides (Fig. 1B.3)[19], [67], [68], [69], [70]. It was reported by Pamudurti et al[19] that endogenous circMbl3 was translated into a small peptide in the fly head analyzed by mass spectrometry. In addition, it was indicated by Legnini et al[70] that circZNF609, an circRNA, regulated myogenesis and was translated into a protein.
The functional correlation between the majority of circRNA-originated proteins and the linear proteins remains unclear. Since circRNA-originated proteins are usually truncated versions of the linear proteins, the circRNA-originated proteins share the same start codons as their linear counterparts. However, they have a stop codon that is formed by the circular junction. This raises the question of whether circRNA-originated proteins share similar functions with, or act as competitors to, their linear counterpart-encoded proteins. Considering the rapid developments in the protein-coding circRNA field, the foremost aims of this research field are to expand our current understanding of the protein-coding ability of circRNAs and the function of the resulting proteins/peptides.
3.4. Pseudogenes derived from circRNAs
Pseudogenes are mainly derived from the reverse transcription of linear mRNAs, which are located in 10% of known gene loci inside the host genomes[71], [72]. Myriad circRNA-originated pseudogenes have been characterized by checking the back-splicing junction sequences of the genomes (Fig. 1B.4)[73]. For example, by retrieving the corresponding circle locus in the mouse genome, 9 low-confidence circRFWD2-derived pseudogenes and 33 high-confidence circRFWD2-originated pseudogenes were identified. However, most circRFWD2-derived pseudogenes did not contain a poly(A)tail, indicating that the way in which circREWD2 is reverse-transcribed into cDNA remains unclear. Therefore, the molecular mechanism of generating pseudogenes is supposed to be explored in circRNA research.
3.5. circRNAs regulate alternative splicing or transcription
Most circRNAs in the cytoplasm are derived from exons. On the contrary, EIciRNAs are predominantly located in the nucleus and act as transcriptional regulators[17]. It was demonstrated by Li et al[74] that EIciRNAs interact with U1 snRNPs, and that the EIciRNA-U1 snRNPs complexes may regulate RNA polymerase II activity and promote the transcription of their parental genes[75]. In addition, circRNAs were shown to interact with the Pol II transcription compound to activate the transcription of their parent genes[75]. circSEP3, an ecircRNA, was confirmed to modulate the splicing of its parent gene. circSEP3 can bind intensively to the cognate DNA locus, while the linear counterpart interacts more weakly with DNA. The results identified the ability of circRNA to skew splicing preference and favor the cognate alternative splicing mRNA variant[76]. These studies together suggested that certain circRNAs could regulate gene expression at both splicing and transcription levels.
3.6. Other potential emerging functions of circRNAs
A compelling characteristic of circRNA is that it is extremely stable and accumulates over time. Threfore, circRNAs can act as “flight recorders” of cellular transcription history. In a physiological sense, long-lived circRNAs may act as a repository for translation. Considering that some of the circRNAs mentioned above encode proteins from IRES elements, this repository could be translated to respond to physiological changes or stress response. The local translation of circRNAs in synapses may be important, as other RNAs are also translated in synapses [77]. Since circRNAs can bind to RBPs[42] similar to miRNAs, circRNAs may work through binding, interacting, delivering and releasing their cargo to specific intracellular compartments. In addition, circRNAs may compete in specific subcellular locations for the limiting amounts of RBPs. However, further molecular biology experiments are urgently needed to validate these hypotheses.
Considering that some circRNAs are found in vesicles [78], [79] and these vesicles could be transported to the target tissues, they may also act as a delivery capsule. Along with circRNAs, miRNAs and RBPs can be transferred to an organ or a tissue. At the targeted organ or tissue, the miRNAs and RBPs would be released from circRNA through circRNA degradation or other mechanisms. It was revealed by Liu et al[80] that in vitro synthesis of circRNAs can serve as a quick, convenient and effective strategy of inhibiting miRNA function.
4. Degradation of circRNAs
It was reported by Enuka et al[81] that the majority of circRNAs have a longer half-life (18.8–23.7 h) than their full-length linear counterparts, according to an in vitro study of 60 circRNAs in cell culture following 4-thiouridine metabolic labeling (4.0–7.4 h). In addition, circRNAs may have an even longer half-life in vivo[82], [83]. The accumulation of circRNAs in the brain is possibly due to the good stability of these circRNAs [82], [83].
The mechanisms and rates of circRNA degradation in vivo remains unclear. In fact, the degradation of circRNA can be initiated by an endonuclease. The first study on circRNA degradation was performed using RNase H and Rrp44 to detect endonuclease activity in vitro [84], [85].The authors demonstrated that the cleavage of artificial circRNA was very low. The best characterized circRNA degradation pattern is the small RNA-mediated degradation of circRNAs. For example, it was revealed by Hansen et al[52] that the degradation of CDR1as is mediatedby miR-671 through Argonaute 2 (Ago2)-mediated degradation. CDR1as, miR-671 and its binding site are highly conserved, and the deletion of one of these sites leads to a significant increase in CDR1as levels[55].
A recent study indicated that the RNA modification ofN6-adenosine methylation (m6A) promotes the recruitment of endonucleases to degrade circRNAs[86]. It was found by Liu et al[87] that the circRNAs are globally degraded by RNase L upon poly(I:C) stimulation or viral infection. The authors discovered that spontaneous RNase L activation, circRNA reduction and an increased phosphorylation of PKR in peripheral blood mononuclear cells (PBMCs) from patients with systemic lupus erythematosus[87].
In addition to degradation, circRNAs could be eliminated from cells by exocytosis. CircRNAs could be found in exosomes, but it remains unclear whether the secretion of circRNAs could lower their intracellular levels (Fig 1B.8)[88]. Moreover, circRNA secretion may become a communication mechanism (Fig 1B.9)[89], [90]. Therefore, more attention should be pay on the degradation and extracellular transport of circRNAs. CircRNAs are abundant in the cytoplasm and are contained in exosomes during their formation. CircRNAs could be transferred from the cytoplasm into exosomes.
5. circRNA research database
With the rapid developments in bioinformatics, several useful databases have been developed to date to improve circRNA research. Online databases that are useful in circRNA prediction, identification, characterization, localization and investigation of the interaction of circRNAs with MER and RBP have been included in the present study. The online databases of circRNA research are shown in Table 1.
Table 1.
Database for circRNA research.
| Database | Website | Function | Ref |
|---|---|---|---|
| circRNADb | http://202.195.183.4:8000/circrnadb/circRNADb.php | Offering the detailed information of circRNAs, especially the exon splicing, IRES and ORF | [95] |
| CircPro | http://bis.zju.edu.cn/CircPro | Analysis of protein-coding potential of circRNAs | [96] |
| Circbase | http://www.circbase.org/ | Providing circRNAs information from multiple species | [97] |
| Starbase v2.0 | http://starbase.sysu.edu.cn/ | Providing the RNA-RNA and protein-RNA interaction networks | [98] |
| CIRCpedia v2 | http://www.picb.ac.cn/rnomics/circpedia/ | Containing circRNA annotation across 6 different species | [99] |
| DeepBase v2.0 | http://deepbase.sysu.edu.cn/ | Containing 14,867 human circRNAs | [100] |
| Circnet | http://circnet.mbc.nctu.edu.tw/ | Describing the regulation between circRNAs, miRNAs and genes | [101] |
| CircInteractome | http://circinteractome.nia.nih.gov/ | Providing bioinformatic analysis of binding sites on circRNAs | [102] |
| CSCD | http://gb.whu.edu.cn/CSCD/ | Predicting cellular distribution of circRNAs, MRE, RBP and variable splicing of related genes | [103] |
| Circ2Traits | http://gyanxet-beta.com/circdb/ | Predicting the interaction among miRNAs, lncRNAs and circRNAs | [104] |
| CirclncRNAnet | http://app.cgu.edu.tw/circlnc/ | Offering a “one-stop” resource for analysis of ncRNA biology | [105] |
| CircRNADisease | http://cgga.org.cn:9091/circRNADisease | Providing experimentally supported circRNA and disease associations | [106] |
| ExoRBase | http://www.exorbase.org/ | Including annotation, expression level and possible original tissues about 58,330 circRNAs in human blood exosomes | [107] |
6. circRNAs in cancer
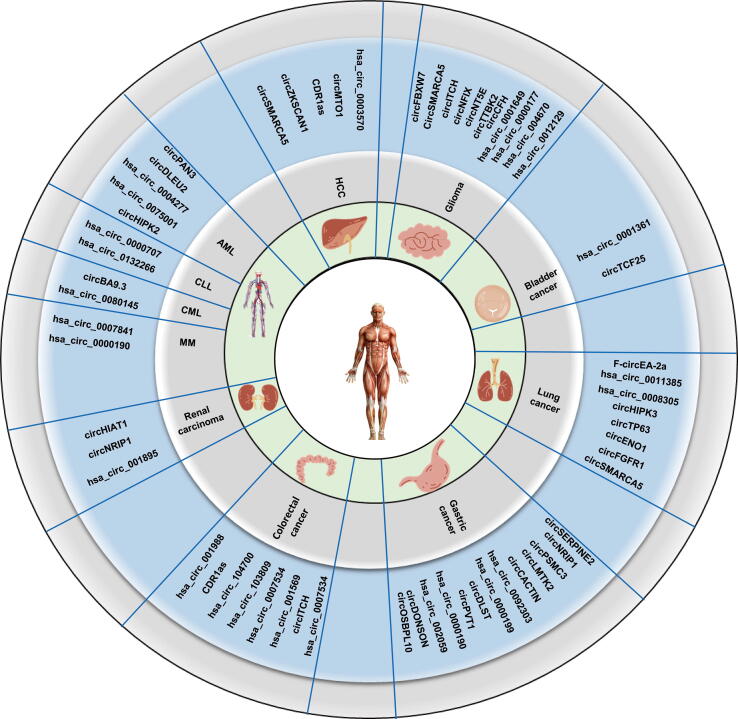
To date, various cancer-related circRNAs have been discovered and characterized (Fig. 2). Accumulating evidence indicates that these circRNAs function in a large number of cancers and play indispensable roles in their occurrence and progression[61], [104], [105].
Fig. 2.
Overview of functional circRNAs in various types of cancer. The map shows the circRNAs that have been confirmed to function in various types of cancer.
6.1. Glioma
Emerging studies have confirmed that circRNAs play a pivotal role in glioma. circRNA is a (Table 2) double-edged sword in glioma. It was found by Yang et al[63] that circFBXW7, which contains a spanning junction open reading frame (ORF), could be translated into a 21-KDa protein, namely FBXW7-185aa. FBXW7-185aa could cooperate with the protein encoded by the linear FBXW7 to facilitate the degradation of c-Myc and suppress glioma cell growth. In addition, it was discovered by Zhang et al[69] that SHPRH-146aa, encoded by circSHPRH, protected its linear counterpart against degradation by the ubiquitin proteasome that functioned as a tumor inhibitor in human glioblastoma (GBM). In addition, the overexpression of circSMARCA5[106] increased the expression of serine and arginine rich splicing factor 3 to suppress tumorigenesis in GBM. As reported in a previous study, hsa_circ_0001649 and circITCH also acted as tumor suppressors in glioma[107]. Some circRNAs have also been found to play oncogenic roles in glioma. Another study showed that circNFIX functioned as ceRNA to absorb miR-34a-5p, and influenced the expression of targeted gene NOTCH1[108]. circNT5E directly sponged miR-422a, thus affecting the pathological development of GBM[109]. CircTTBK2 was found by Zheng et al[110] to be overexpressed in glioma tissues and cell lines, which facilitated glioma cell growth. In addition, hsa_circ_0000177[111] hsa_circ_0012129[112] circCFH [113] and hsa_circ_0046701[114] could also accelerate glioma tumorigenesis. These findings indicated that circRNA might have an marked effect on the progression of GBM, increasing its potential as a convenient biomarker for GBM screening.
Table 2.
Summary of some tumor-related circRNAs.
| Cancer | CircRNA ID | expression | Function | Mechanism | Refs. |
|---|---|---|---|---|---|
| Glioma | circFBXW7 | Down-regulated | tumor suppressor | encoded peptides | [63] |
| circSHPRH | Down-regulated | tumor suppressor | encoded peptides | [69] | |
| circSMARCA5 | Down-regulated | tumor suppressor | circSMARCA5/SRSF1/SRSF3 | [106] | |
| circITCH | Down-regulated | tumor suppressor | circITCH/miR-214 | [107] | |
| circNFIX | Up-regulated | oncogene | circNFIX/miR-34a-5p | [108] | |
| circNT5E | Up-regulated | oncogene | circNT5E/miR-422a | [109] | |
| circTTBK2 | Up-regulated | oncogene | miR-217/HNF1β/Derlin-1 | [110] | |
| hsa_circ_0000177 | Up-regulated | oncogene | hsa_circ_0000177/miR-638-FZD7/Wnt | [111] | |
| hsa_circ_0012129 | Up-regulated | oncogene | hsa_circ_0012129/miR-661 | [112] | |
| circCFH | Up-regulated | oncogene | circ-CFH/miR-149/AKT1 | [113] | |
| hsa_circ_0046701 | Up-regulated | oncogene | hsa_circ_0046701/miR-142-3p/ITGB8 | [114] | |
| HCC | circMTO1 | Down-regulated | oncogene | circMTO1/miR-9 | [117] |
| circSMARCA5 | Down-regulated | tumor suppressor | circSMARCA5/miR-17-3p | [118] | |
| circZKSCAN1 | Down-regulated | tumor suppressor | PI3K pathway | [119] | |
| hsa_circ_0003570 | Down-regulated | oncogene | Unknown | [120] | |
| Cdr1as | Up-regulated | oncogene | Cdr1as /miR-7 | [121] | |
| Colorectal | hsa_circ_0000069 | Up-regulated | oncogene | Unknown | [124] |
| cancer | hsa_circ_0007534 | Up-regulated | oncogene | Unknown | [125] |
| hsa_circ_103809 | Down-regulated | oncogene | Unknown | [126] | |
| hsa_circ_104700 | Down-regulated | oncogene | Unknown | [126] | |
| CircCCDC66 | Up-regulated | oncogene | CircCCDC66/miR-93 | [127] | |
| Circular BANP | Up-regulated | oncogene | Circular BANP/p-Akt | [128] | |
| hsa_circ_001569 | Up-regulated | oncogene | hsa_circ_001569/FMNL2 | [129] | |
| Cdr1as | Up-regulated | oncogene | ciRS-7/miR-7 /EGFR | [130] | |
| Gastric | hsa_circ_0000190 | Down-regulated | oncogene | Unknown | [133] |
| hsa_circ_002059 | Down-regulated | oncogene | Unknown | [134] | |
| cancer | hsa_circ_0000199 | Up-regulated | oncogene | hsa_circ_0000199/miR-198 | [135] |
| circDLST | Up-regulated | oncogene | circDLST/miR-502-5p | [136] | |
| circPSMC3 | Up-regulated | oncogene | circPSMC3/miR-296-5p | [137] | |
| hsa_circ_0092303 | Up-regulated | oncogene | hsa_circ_0092303/miR-331-3p | [138] | |
| circNRIP1 | Up-regulated | oncogene | circNRIP1/miR-149-5p | [139] | |
| circLMTK2 | Up-regulated | oncogene | circLMTK2/miR-150-5p | [140] | |
| circSERPINE2 | Up-regulated | oncogene | circSERPINE2/miR-375 | [26] | |
| circDONSON | Up-regulated | oncogene | circDONSON/NURF complex | [141] | |
| hsa_circ_0008549 | Up-regulated | oncogene | hsa_circ_0008549/miR-136-5p | [142] | |
| Lung | hsa_circ_0008305 | Down-regulated | oncogene | hsa_circ_0008305/miR-429 | [144] |
| F-circEA-2a | Up-regulated | oncogene | Unknown | [145] | |
| cancer | hsa_circ_0011385 | Up-regulated | oncogene | hsa_circ_0011385/miR-361-3p | [146] |
| CircTP63 | Up-regulated | oncogene | CircTP63/miR-873-3p | [147] | |
| circENO1 | Up-regulated | oncogene | circENO1/miR-22-3p | [148] | |
| circFGFR1 | Up-regulated | oncogene | circFGFR1/miR-381-3p | [149] | |
| circSMARCA5 | Down-regulated | tumor suppressor | circSMARCA5/miR-19b-3p/HOXA9 | [150] | |
| AML | circDLEU2 | Up-regulate | oncogene | circDLEU2/miR-496/PPKACB | [151] |
| circHIPK2 | Up-regulated | oncogene | circHIPK2/miR-124-3p | [152] | |
| circPAN3 | Up-regulated | oncogene | circPAN3/miR-153-5p | [153] | |
| CLL | circCBFB | Up-regulated | oncogene | circCBFB/miR-607/FZD3/Wnt | [154] |
| hsa_circ_0132266 | Down-regulated | tumor suppressor | hsa_circ_0132266/miR-337-3p/PML | [155] | |
| circ-RPL15 | Up-regulated | oncogene | miR-146b-3p/RAF1 axis. | [156] | |
| CML | circBA9.3 | Up-regulated | oncogene | circBA9.3/c-ABL1 | [157] |
| hsa_circ_0080145 | Up-regulated | oncogene | hsa_circ_0080145/miR-29b | [158] | |
| circHIPK3 | Up-regulated | oncogene | circHIPK3/miR-124 axis | [159] | |
| MM | hsa_circ_0007841 | Up-regulated | oncogene | hsa_circ_0007841/miRNAs | [160] |
| hsa_circ_0000190 | Down-regulated | tumor suppressor | hsa_circ_0000190/miR-767-5p | [161] | |
| circITCH | Down-regulated | tumor suppressor | circITCH/miR-615-3p | [162] | |
| BC | circTCF25 | Up-regulated | oncogene | circTCF25/miR-103a-3p | [164] |
| hsa_circ_0001361 | Up-regulated | oncogene | hsa_circ_0001361/miR-491-5p | [165] | |
| circSLC8A1 | Down-regulated | tumor suppressor | miR-130b/miR-494 | [166] | |
| kidney | circHIAT1 | Down-regulated | tumor suppressor | circHIAT1/miR-195-5p/29a-3p | [167] |
| Cancer | hsa_circ_001895 | Up-regulated | oncogene | hsa_circ_001895/miRNA-296-5p | [168] |
| circNRIP1 | Up-regulated | oncogene | circNRIP1/miR-505 | [169] |
(HCC: Hepatocellular carcinoma; AML:acute myloid leukemia; CLL: chronic lymphocytic leukemia; MM: multiple myeloma; BC: bladder cancer)
6.2. Hepatocellular carcinoma (HCC)
HCC, which accounts for 90% of primary malignancies of the liver, is a major cause of cancer-related mortality worldwide [115], [116]. circRNAs have been reported in several studies to be able to function as a tumor inhibitor in HCC. circMTO1 repressed HCC tumorigenesis by directly sponging miR-9 to elevate p21, suggesting that circMTO1 was associated with the prognosis of HCC [117]. In addition, circSMARCA5 increased TIMP3 expression by sponging miR-181b-5p to inhibit HCCprogression [118]. Furthermore, circZKSCAN1 could collaborate tightly with its linear mRNA to inhibit the growth, migration and invasion of HCC[119]. The downregulation of hsa_circ_0003570[120] has been shown to be closely linked to tumor size and neoplastic angiopoiesis in HCC. Of note, Cdr1as was found to be significantly overexpressed in HCC, as compared with the adjacent normal tissues, and Cdr1as to be a sponge for miR-7, which was involved in the promotion of HCC cell growth and migration[121], [122]. These findings indicated that circRNA was firmly interrelated with the progression and tumorigenesis of HCC.
6.3. Colorectal cancer (CRC)
CRC is the fourth leading cause of global mortality [123]. RNA sequencing was conducted toscrutinize the expression of circRNAs in tumor and normal tissues. In a study by Anna et al[82] 11 upregulated and 28 downregulated circRNAs were identified in CRC tissues. Furthermore, the ratio of selected circRNAs to their host gene in the CRC tissues (hsa_circ_0817/CUL5, hsa_circ_3204/USP3, hsa_circ_6229/METTL3 and hsa_circ_7374/TNS4) was smaller than that in the adjacent normal tissues. Similarly, microarray analysis showed that the expression of 412 circRNAs was upregulated, while 480 circRNAs were downregulated in CRC tissues, as compared with normal tissues[124]. In detail, quantitative polymerase chain reaction (qPCR) results of CRC patients indicated that the hsa_circ_0000069 expression was elevated and promoted cell proliferation, migration and invasion in CRC[124]. In addition, the expression of hsa_circ_0007534 was linked to tumor stage and lymphatic metastasis in CRC tissues [125]. In addition, it was found by Zhang et al[126] that the expression of hsa_circ_104700 and hsa_circ_103809 was clearly downregulated in CRC tissues and closely linked to cancer pathogenesis. It was shown by Hsiao et al[127] that the elevation of circCCDC66 in CRC was closely associated with tumor pathogenesis. CircCCDC66 can sponge miRNA-33b and miR-93 to protect MYC mRNA from degradation. On the other hand, circBANP was found to be elevated in CRC, the knockdown of which could significantly inhibit the growth of CRC cells[128]. In addition, hsa_circ_001569 could upregulate the expression of its functional targets FMNL2 and BAG4, subsequently exerting a strong effect on tumorigenesis[129]. Furthermore, CDR1as was aberrantly increased in CRC tissues. The expression of CDR1as was closely associated with tumor volume, tumor metastasis and survival rate [130]. The downregulation of CDR1as suppressed CRC cell growth and migration by blocking miR-7 targets [131]. Collectively, these findings revealed that circRNA is involved in the progression and pathogenesis of CRC.
6.4. Gastric cancer (GC)
GC is the third most common cause of cancer-related mortality worldwide[132]. hsa_circ_0000190 Was found by Chen et al[133] to be downregulated in GC tissues. The low levels of hsa_circ_0000190 were correlated with tumor volume, metastasis and the tumor-node-metastasis stage. It was reported by Li et al[134] that the expression level of hsa_circ_002059 was closely associated with GC distant metastasis and tumorigenesis. Furthermore, Huang et al[135] reported that circAKT3 (hsa_circ_0000199) sponged miR-198 to promote PIK3R1 expression and DNA damage repair, consequently suppressing the apoptosis of GC cells. A recent study revealed that circDLST sponged miR-502-5p to facilitate cell proliferation, cell cycle and DNA synthesis both in vitro and in vivo[136]. Rong et al[137] identified circPSMC3 and reported that its expression was decreased in GC tissues, GC plasmas and GC cell lines. circPSMC3 sponged miRNA-296-5p with phosphatase and tensin homolog (PTEN) to promote GC proliferation.
Conversely, it was discovered by Zhang et al[138] that circCACTIN (hsa_circ_0092303) was a tumor inducer that promotes GC growth by modulating TGFBR1 mRNA expression and sponging miRNA-331-3p. It was found by Zhang et al[139] that circNRIP1 sponged miR-149-5p to enhance GC cell progression, migration and invasion. It was further proven that circNRIP1 could be transmitted between GC cells by exosomal communication. CircLMTK2 was found by Wang et al[140] to be elevated in GC tissues and correlated with poor prognosis, as well as poor tumor node metastasis (TNM) stage. circLMTK2 sponged miR-150-5p and eventually regulated the expression of c-Myc to promote GC tumorigenesis. YWHAZ and circSERPINE2 were found by Liu et al[26] to be upregulated, while miR-375 was clearly downregulated in GC tissues and cells. Mechanistically, circSERPINE2 sponged miR-375 and modulated YWHAZ expression to promote GC cell growth and cell cycle progression. It was discovered by Ding et al[141] that the expression of circDONSON was positively correlated with advanced TNM stage and poor prognosis. Mechanistically, circDONSON promoted GC proliferation by recruiting the NURF complex to initiate SOX4 expression. circOSBPL10 (hsa_circ_0008549) was found by Wang et al[142] to be markedly upregulated in GC tissues, and its decreased expression impaired GC tumorigenesis. Similarly, circOSBPL10 promoted GC cell growth through the circOSBPL10-miR-136-5p-WNT2 axis in GC cells. Therefore, circOSBPL10 may act as a novel predictor of prognosis and proliferation in GC. Collectively, these results revealed that circRNAs can serve as novel diagnostic and prognostic biomarkers of GC.
6.5. Lung cancer
Lung cancer has the highest cancer-related mortality rate worldwide[143]. Wang et al[144] confirmed that circPTK2 (hsa_circ_0008305) sponged miR-429, consequently favoring non-small cell lung cancer (NSCLC) cell invasion. F-circEA-2a was found by Tan et al[145] to be predominantly located in the cytoplasm and to facilitate cell migration and invasion. Chen et al[146] discovered that the oncogenic circRNA circHIPK3 (hsa_circ_0011385) could sequestrate miR-361-3p and interact with splicing factors. It was found by Cheng et al[147] that circTP63 sponged miR-873-3p and prevented the decrease of FOXM1, subsequently promoting cell cycle progression. Zhou et al[148] revealed that circENO1 and its linear counterpart were elevated in lung adenocarcinoma (LUAD) cells. The downregulation of circENO1 induced apoptosis, and suppressed cell growth, migration and EMT. CircENO1 sponged miR-22-3p and upregulated ENO1. Collectively, circENO1 may serve as a target of LUAD. circFGFR1 was found by Zhang et al[149] to be increased in NSCLC patients, an increase that was linked to poor prognosis. Mechanistically, circEGFR1 sponged miR-381-3p to elevate the expression of the downstream gene CXCR4, and finally accelerated NSCLC tumorigenesis. Nevertheless, Wang et al[150] showed that circSMARCA5 sponged miR-19b-3p, subsequently exerting its tumor inhibitory effects. Collectively, these results predicted that circRNAs can serve as a therapeutic target in lung cancer.
6.6. Hematological malignancies
A multitude of circRNAs have been used as diagnostic and prognostic biomarkers in hematological malignancies, such as acute myloid leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myeloid leukemia (CML) and multiple myeloma (MM).
AML. CircDLEU2 was reported by Wu et al[151] to act as a sponge to suppress the biological function of miR-496 and subsequently promoted AML tumorigenesis by targeting the miR-496/PPKACB channel. Li et al[152] discovered that circHIPK2 was elevated in other AML types, as compared to acute promyelocytic leukemia (APL). CircHIPK2 sponged miR-124-3p, which was closely associated with cell differentiation, thereby playing a crucial role in activating transcription. Therefore, circHIPK2 might act as an APL-associated biomarker. It was revealed by Shang et al[153] that oncogenic circRNA circPAN3 could be an important regulator of drug resistance, which is mainly due to the circPAN3-miR-153-5p axis in AML cells.
CLL. Xia et al[154] showed that circCBFB (hsa_circ_0000707) played a crucial role in CLL tumorigenesis. Mechanistically, circCBFB sponged miR-607 to elevate the expression level of FZD3 and subsequently activated the Wnt/β-catenin pathway. In a study by Wu et al[155] circMTO1 (hsa_circ_0132266) was shown to be markedly decreased in the peripheral PBMCs of CLL patients, as compared to the control group. Mechanistically, hsa_circ_0132266 facilitated CLL progression and pathogenesis mainly through the hsa_circ_0132266/miR-337-3p/PML signaling cascade. circ-RPL15 was identified as a novel oncogenic biomarker for CLL, with its mechanism working via the miR-146b-3p-mediated repression of the RAS/RAF1/MEK/ERK pathway[156].
CML. It was revealed by Pan et al[157] that circBA9.3 might efficiently facilitate the growth of cancer cells by suppressing apoptosis. circBA9.3 was mainly located in the cytoplasm and elevated the expression of c-ABL1 and BCR-ABL1. Therefore, circBA9.3 was linked to an increased tyrosine kinase activity, which promoted tyrosine kinase inhibitor treatment resistance. Liu et al[158] indicated that hsa_circ_0080145 functioned as a sponge to absorb miR-29b and subsequently favored the proliferation of CML. This study indicated that hsa_circ_0080145 could be a promising biomarker for CML treatment. As previously reported, the oncogenic role of circHIPK3 was confirmed in the development and treatment of CML. These oncogenic effects were achieved by sponging miR-124[159].
MM. It was confirmed by Gao et al[160] that the hsa_circ_0007841 expression was markedly elevated in MM cell lines, which was closely associated with disease prognosis. Bioinformatics analysis demonstrated that several miRNAs interacted with hsa_circ_0007841, suggesting that hsa_circ_0007841 may serve as a novel biomarker for MM. Feng et al[161] indicated that hsa_circ_0000190 suppressed cell growth and promoted apoptosis in MM by sponging miR-767-5P and regulating mitogen-activated protein kinase 4. It was discovered by Liu et al[162] that circITCH was downregulated in MM cells and circITCH acted as a sponge for miR-615-3p.
6.7. Other types of cancer
The ectopic expression of circRNAs has been validated in multiple types of cancer [163]. In bladder cancer, Zhong et al[164] speculated that the oncogenic circRNA circTCF25 could sponge miR-103a-3p/miR-107 based on the multiple bioinformatics approaches, which consequently increased 13 target genes associated with cell proliferation, migration and invasion. It was discovered by Liu et al[165] that an oncogenic circRNA, hsa_circ_0001361, was elevated in bladder cancer tissues. Hsa_circ_0001361 directly sponged miR-491-5p to elevate MMP9 and subsequently promoted the occurrence and progression of bladder cancer. In addition, circRNA circSLC8A1 has been reported to act as a sponge of miR-130b/miR-494 in preventing bladder cancer progression by regulating PTEN[166]. In kidney cancer, a decreased expression of circHIAT1 in clear cell renal cell carcinoma (ccRCC) tissues was identified by Wang et al[167]. CircHIAT1 can directly interact with miR-195-5p/29a-3p/29c-3p to elevate CDC42 expression. Androgen receptor (AR) inhibited circHIAT1 expression and subsequently led to the suppression of CDC42. The AR-circHIAT1-mediated miR-195-5p/29a-3p/29c-3p/CDC42 signaling pathway might provide an effective strategy for a more effective inhibition of ccRCC metastasis. It was found by Chen et al[168] found that hsa_circ_001895 sequestrated miR-296-5p, subsequently inducing ccRCC development. Dong et al[169] discovered that circNRIP1 was overexpressed in renal carcinoma tumors and circNRIP1 played oncogenic roles in the renal carcinoma cell lines by targeting miR-505 through the activation of the AMPK and PI3K/AKT/mTOR cascades. Collectively, the studies mentioned above demonstrated that circRNAs are potentially involved in tumorigenesis. However, the clinical implications of the use of circRNAs as novel therapeutic avenues require further research.
7. circRNAs are promising biomarkers in cancer
The clinical use of biomarkers is critical during all stages of cancer, and has become one of the major approaches for cancer diagnosis and prognosis. Different from linear mRNAs, the unique covalently closed-loop structures make circRNAs avoid RNaseR degradation and thus possess high stability[170]. In addition, circRNAs are widely distributed in the plasma, urine, tissue samples, cell-free saliva and other human components in a cell-specific manner[171], [172]. The expression patterns and characteristics of circRNAs (high and selective abundance, high stability, high conservation and specific expression)could partly explain the ability of circRNAs as potential biomarkers or therapeutic targets. It was found by Memczak et al[173] that circRNAs were elevated, as compared with those of their linear counterparts in the blood. A diverse set of circRNAs exhibited a high level in the blood, which can be easily detected. However, the expression of their linear counterparts was very low. Therefore, blood circRNAs may offer disease-related knowledge that canonical RNA analysis cannot provide.
A large number of studies performed on HCC patients have shown that hsa_circ_0000798[174] hsa_circ_0027089[175] and hsa_circ_0058124[176] are upregulated in HCC tissues, whereas some circRNAs are downregulated, including hsa_circSMARCA5[177] hsa_circ_0068669[178] hsa_circ_0028502[179] and hsa_circ_0076251[179]. These circRNAs are supposed to serve as potential biomarkers for HCC. Several circRNAs also serve as biomarkers in patients with CRC. Increased plasma levels of hsa_circ_0082182 and hsa_ circ_0000370[180] were significantly connected with lymph node metastasis, while the upregulation of hsa_circ_0004585[181] was correlated with patient tumor size. The downregulation of hsa_circ_0000567[182] was correlated with TNM stage. In GC, hsa_circ_0003159[183] hsa_circ_0000096[184] hsa_circ_002059[185] hsa_circ_0000190[133] and hsa_circ_0000181[186] were all downregulated and linked to distal metastasis or invasion, which may predict tumor metastasis. In addition, the expression level of hsa_circ_0000467 was higher in the GC tissues, plasma and GC cells, as compared with the healthy control, and was correlated with TNM stage in GC. The area under the curve (AUC) of hsa_circ_0000467 was 0.799, which was much higher than some already existing biomarkers. The sensitivity and specificity of hsa_circ_0000467 were 70.5 and 64.8%, respectively[187]. Furthermore, a circRNA downregulated in GC tissues, circPSMC3, was negatively correlated with TNM stage and lymphatic metastasis, and exhibited a high AUC (0.933). The sensitivity and specificity of circPSMC3 were 85.85 and 95.24%, respectively[137]. The expression level of hsa_circ_0001895 was correlated with GC cell differentiation, Borrmann type and carcinoembryonic antigen level. The AUC, sensitivity and specificity of hsa_circ_0001895 were 0.792, 67.8 and 85.7%, respectively[188]. The hsa_circ_0008673[189] increased in breast cancer patients, circASXL1[190] and hsa_circ_0137439[191] were increased in bladder cancer, and circBNC2 were decreased in ovarian cancer, which confirmed the diagnostic value of the circRNAs in neoplastic diseases. Other circRNAs serving as biomarkers in cancer are listed in Table 3. Besides, the AUC, sensitivity and specificity of plasma hsa_circ_0000520[192] (0.897, 82.4, 84.4%) were clearly higher than those in the tissue (0.613, 53.6 and 85.7%) respectively, indicating a relatively superior diagnostic value[192]. On the contrary, tissue hsa_circ_0000190[133] and hsa_circ_0000181[186] both exhibited a superior diagnostic value than their plasma counterparts in GC. In detail, tissue hsa_circ_0000190 was significantly linked to tumor diameter and TNM stage, whereas plasma hsa_circ_0000190 exhibited no linkage[133]. Hence, there is a need to compare circRNAs detected in different human components to identify a precise biomarker for distinguishing cancer patients from healthy controls.
Table 3.
The potential role of circRNAs as biomarkers in various cancers.
| Cancer | Name of CircRNAs | Changes of expression | Diagnostic significance | ROC curve | Numbers of patients | Refs |
|---|---|---|---|---|---|---|
| HCC | CircSMARCA5 | Down | Correlated with tumor differentiation, TNM stage, cancer invasion and cancer diameter | Plasma circRNA; AUC value: 0.938, 0.853, 0.711 |
133 | [177] |
| Hsa_circ_0000798 | Up | Correlated with tumor size and cirrhosis | Plasma circRNA; AUC value: 0.703 |
102 | [174] | |
| Hsa_circ_0068669 | Down | Associated with microvascular invasion and TMN stages | Tissue circRNA; AUC value: 0.64; Sensitivity: 0.59; Specificity: 0.71 |
100 | [178] | |
| Hsa_circ_0027089 | Up | – | Plasma circRNA; AUC value: 0.794; Sensitivity: 0.578; Specificity: 0.848 |
239 | [175] | |
| Hsa_circ_0058124 | Up | Associated with tumor siz, tumor, node, TNM stage, and vascular invasion | Tissue circRNA; AUC value: 0.878 |
128 | [176] | |
| Hsa_circ_0028502 | Down | Related to TNM stage | Tissue circRNA; AUC value: 0.675; Sensitivity: 0.721; Specificity: 0.580 |
200 | [179] | |
| Hsa_circ_0076251 | Down | Related to Barcelona Clinic Liver Cancer (BCLC) stage and the presence of serum HbsAg | Tissue circRNA; AUC value: 0.738; Sensitivity: 0.713; Specificity: 0.640 |
200 | ||
| Colorectal cancer |
Hsa_circ_0000567 | Down | Correlated with tumor size, lymph metastasis, distal metastasis, and TNM stage | Tissue circRNA; AUC value: 0.8653; Sensitivity: 0.8333; Specificity: 0.7647 |
204 | [182] |
| Hsa_circ_0082182 | Up | Connected with lymph node metastasis |
Plasma circRNA; AUC value: 0.7371 |
156 | [180] | |
| Hsa_circ_0000370 | Up | Connected with lymph node metastasis |
Plasma circRNA; AUC value: 0.8152 |
156 | ||
| Hsa_circ_0035445 | Up | Connected with the TNM stage | Plasma circRNA; AUC value: 0.7028 |
156 | ||
| Hsa_circ_0004585 | Up | Correlated with patient’s tumor size | Plasma circRNA; AUC value: 0.731; Sensitivity: 0.851; Specificity: 0.511 |
284 | [181] | |
| Hsa_circ-0004771 | Up | Correlated with TNM stage and distant metastasis | Exosome circRNA; AUC value: 0.90 |
135 | [195] | |
| Gastric cancer |
Hsa_circ_0003159 | Down | Associated with gender, distal metastasis and TNM stage | Tissue circRNA; AUC value: 0.75; Sensitivity: 0.852; Specificity: 0.565 |
108 | [183] |
| Hsa_circ_0000096 | Down | Associated with gender, invasion and TNM stage | Tissue circRNA; AUC value: 0.82 |
101 | [184] | |
| Hsa_circ_002059 | Down | Correlated with distal metastasis, TNM stage, gender and age | Plasma circRNA; AUC value: 0.73; Sensitivity: 0.81; Specificity: 0.62 |
101 | [185] | |
| Hsa_circ_0000190 | Down | Tissue circRNA: Related to tumor diameter, lymphatic metastasis, distal metastasis and TNM stage | Tissue circRNA; AUC value: 0.75; Sensitivity: 0.721; Specificity: 0.683; Plasma circRNA; AUC value: 0.6; Sensitivity: 0.414; Specificity: 0.875 |
208 | [133] | |
| Hsa_circ_0000181 | Down | Correlated with tumor diameter, lymphatic metastasis, distal metastasis | Tissue circRNA; AUC value: 0.756; Sensitivity: 0.852; Specificity:0.539 Plasma circRNA; AUC value: 0.582; Sensitivity: 0.206; Specificity: 0.99 |
115 | [186] | |
| Hsa_circ_0001895 | Down | Correlated with GC cell differentiation, Borrmann type, and CEA level | Tissue circRNA; AUC value: 0.792; Sensitivity: 0.678; Specificity:0.857 |
257 | [188] | |
| Hsa_circ_0000467 | Up | Correleated with TNM stage | Tissue circRNA; AUC value: 0.799; Sensitivity: 0.705; Specificity:0.648 |
102 | [187] | |
| CircPSMC3 | Down | Associated with TNM stage and lymphatic metastasis | Tissue circRNA; AUC value: 0.933; Sensitivity: 0.536; Specificity:0.857 |
106 | [137] | |
| Hsa_circ_0000520 | Down | Tissue:associated with TNM stage Plasma: linked with CEA expression. |
Tissue circRNA; AUC value: 0.613; Sensitivity: 0.852; Specificity:0.539 Plasma circRNA; AUC value: 0.897; Sensitivity: 0.824; Specificity: 0.844 |
112 | [192] | |
| Oral squamous cell carcinoma | Hsa_circ_0003829 | Down | Correlated with lymph node metastasis status and TNM stage | Tissue circRNA; AUC value: 0.81; Sensitivity: 0.7; Specificity:0.8 |
120 | [197] |
| Hsa_circ_0001874 | Up | Correlated with TNM stage and tumor grade | Salivary circRNA; AUC value: 0.863; Sensitivity: 0.744; Specificity:0.902 |
93 | [196] | |
| Hsa_circ_0001971 | Up | Correlated with TNM stage | Salivary circRNA; AUC value: 0.845; Sensitivity: 0.756; Specificity:0.878 |
93 | [196] | |
| Lung cancer |
Hsa_circ_0001715 | Up | Correlated with TNM stage and distant metastasis | Plasma circRNA; AUC value: 0.871; Sensitivity: 0.8772; Specificity: 0.7167 |
117 |
[198] |
| Hsa_circ_0005962 | Up | Related to EGFR mutations and gender | Tissue circRNA; AUC value: 0.73; Sensitivity: 0.719; Specificity:0.722 |
153 |
[199] |
|
| Hsa_circ_0086414 | Down | Related to gender | Tissue circRNA; AUC value: 0.81; Sensitivity: 0.778; Specificity:0.722 |
153 | ||
| Hsa_circ_002178 | Up | – | Exosome circRNA; AUC value: 0.9956 |
210 | [194] | |
| Hsa_circ_0037515 | Down | – | Tissue circRNA; AUC value: 0.81; Sensitivity: 0.57; Specificity:0.90 |
122 | [200] | |
| Hsa_circ_0037516 | Down | – | Tissue circRNA; AUC value: 0.82; Sensitivity: 0.65; Specificity:0.84 |
122 | ||
| Breast cancer | Hsa_circ_0008673 | Up | Correlated with tumor size,distant metastasis, ER positive and PR positive | Plasma circRNA; AUC value: 0.833; Sensitivity: 0.550; Specificity: 0.971 |
378 | [189] |
| Ovarian Cancer | CircBNC2 | Down | Associated with histological grade , serous subtype, LNM, and distant metastasis | Tissue circRNA; AUC value: 0.879; Sensitivity: 0.964; Specificity:0.807 |
249 | [201] |
| Bladder cancer | Hsa_circ_0001136 | Up | Correlated with tumor grade, tumor stage, lymph node invasion and distant metastasis | Tissue circRNA; AUC value: 0.770; Sensitivity: 0.686; Specificity:0.769 |
122 | [190] |
| Hsa_circ_0137439 | Up | Correlated with tumor stage, tumor grade, lymph node status | Tissue circRNA; AUC value: 0.890; Sensitivity: 0.886; Specificity:0.734 |
116 | [191] | |
| Papillary thyroid carcinoma | Hsa_circ_0137287 | Down | Correlated with extrathyroidal extension, lymph node metastasis , advanced T stage and tumor size | Tissue circRNA;AUC value: 0.8973; Sensitivity: 0.792; Specificity:0.900 |
120 | [202] |
| Pancreatic cancer | Circ-IARS | Up | Correlated with liver metastasis, vascular invasion and TNM stage | Exosome circRNA | 92 | [193] |
Li et al[193] was the first to identify that exosomes contained large amounts of circRNAs, due to the fact that > 1,000 circRNAs were found in human serum exosomes. Of note, circRNAs have been found to be stably overexpressed in exosomes, by at least 2-fold, as compared to producing cells such as circIARS, circRASSF2 and circPTGR1[172]. Therefore, circRNAs could be placed in the novel category of exosomal cancer biomarkers [172]. In pancreatic cancer tissues and plasma exosomes, the expression of exosomal circRNA IARS was higher than that of the control group. The results of the study indicated that the presence of exosomal circRNA may be a useful diagnostic marker for pancreatic ductal adenocarcinoma (PDAC)[193]. The amplification of hsa_circ_002178[194] in the exosomes was found to function as a novel diagnostic biomarker for lung cancer, with a reported AUC value of 0.9956. In addition, the AUC value of exosomal hsa_circ_0004771[195] is 0.9 in CRC, serving as an invasive diagnostic biomarker for CRC treatment.
A total of 422 salivary circRNAs were discovered by Bahn et al[172] which were confirmed to play a key role in signal transduction and inflammatory response in human cell-free saliva. As the occurrence and development of tumors are largely influenced by inflammation, it is believed that circRNAs originating from saliva could play an essential role in tumorigenesis; 2 such circRNAs are hsa_circ_0001874 and hsa_circ_0001971[196] in oral squamous cell carcinoma. In addition, circRNAs could also be found in gastric juice. Shao et al[172] discovered that hsa_circ_0014717 have favorable stability in gastric juice. This team proved that the expression levels of hsa_circ_0014717 in gastric juice did not change under freeze–thaw for 8 h. Collectively, circRNAs may serve as effective biomarkers for cancer diagnosis.
8. Available strategies in circRNA research
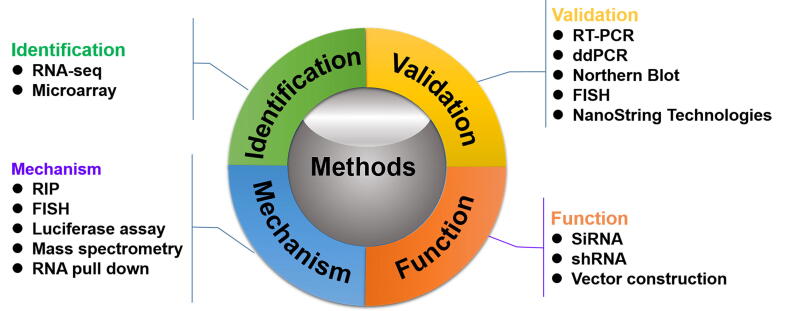
There are several challenges in cancer-related circRNAs research that are often neglected. Most have to do with the fact that most circRNA sequences are the same as the host gene sequence. Therefore, circRNA identification, characterization, quantification, overexpression and knockdown methods all depend on the specific junction site (Fig. 3)[197] .
Fig. 3.
Strategies of circRNA research (identification, validation, function and mechanism).
8.1. circRNA identification
8.1.1. RNA-seq
By using algorithms designed to examine “out-of-order” splicing, a variety of circRNAs, such as exonic, intronic and intergenic circRNAs, have been broadly discovered according to the total RNA-seq data[198]. The methodologies used included find_circ, circRNA_finder, CIRCexplorer and CIRI[198]. However, the accurate quantification of circRNAs from the total RNA-seq datasets frequently requires a high sequencing depth, and at least 100-bp sequencing was recommended to ensure the accurate prediction of circRNAs[199]. Currently, there are regular advancements in novel bioinformatics algorithms, as they are attractive tools for identifying circRNAs. For instance, the analysis of circRNAs can be performed by 6 algorithms: ACSF, CIRCexplorer2, CIRI2, DCC, KNIFE and Uroborus[199]. In addition, the mapper like STAR is capable of annotating more complex RNA sequence arrangements with the features of a high accuracy and speed, such as chimeric and circRNA[200]. Alternatively, the BWA-MEM algorithm has been found to detect circRNAs with fast and low RAM requirements[201]. Segemehl, which exploited an improved suffix array for seeding, was found to outperform its competitors on splice site validation[202].
8.1.2. Microarrays
The application of microarrays is an attainable supplement to RNA-seq for validating circRNAs, since they require less bioinformatics expertise[203]. The first commercial microarrays were manufactured from Arraystar Inc. (https://www.arraystar.com/arraystar-human-circular-rna-microarray), which contains > 10,000 circRNAs that have been selected from publications. Microarray analysis can eliminate the uncertainty of RNA-Seq analysis, due to lack of generalization. As previously reported, when manufacturers ensure reproducibility and efficiency, the process is highly targeted, and relevant standard analysis methods can be used regardless of the type of hybridized[202]. In a recent study, 87,935 circRNA sequences covering most circRNAs characterized to date in circBase have been integrated to design microarray probes, which is clearly more accurate than RNA-seq. Furthermore, the majority of circRNAs measured by this microarray could be further confirmed through reverse transcription (RT)-qPCR or RNA-seq[204].
8.2. circRNA validation and characterization
8.2.1. RT-qPCR
RT-qPCR of circRNAs has been broadly employed for the detection, validation and sometimes even quantification of circRNAs[205]. However, detecting the putative circRNA junction by harnessing qPCR does not guarantee the existence of a circRNA, as certain linear RNAs share the same sequences through junction sites. Currently, divergent primers are particularly designed to extend the circRNA back-splice junction (BSJ) sequence, which was found to exhibit high specificity on the amplification of the circRNAs and not target the linear RNA, allowing direct and precise detection and quantification of circRNAs[205]. In detail, total RNA was digested by RNaseR, reverse-transcribed into cDNA, and subsequently amplified by divergent and convergent primers. In a previous study, RNase R could degrade most linear RNAs, but had no effect on circRNAs[206]. Both divergent and convergent primers could produce a band in the RNaseR(-) group. In the RNaseR(+) group, divergent primers produce a band, while the convergent primers did not. Furthermore, the amplification product should be detected by sanger sequencing to ensure its true presence.
8.2.2. Droplet digital PCR (ddPCR)
ddPCR is a novel technology for the accurate quantification of RNA, which exhibits a higher sensitivity. A previous study tested the application of ddPCR in circRNA quantification and determined the stability of circRNA, as well as compared RT-qPCR with ddPCR[207]. It was observed that a prolonged RT incubation time would result in the circRNA accumulating a variety of PCR products, which would lead to a relatively low accuracy of RT-qPCR in the quantification of circRNA. DdPCR can overcome this shortcoming and be used instead of qPCR for the quantification of circRNA[207]. In addition, it has been shown that plasma levels of secretory circRNAs are detectable by RT-ddPCR in advanced lung cancers[208].
8.2.3. Northern blot
Evidence has demonstrated that northern blot hybridization is the gold standard for circRNA analysis, convincingly indicating the circular configuration of putative circRNAs[209]. Strictly, circRNA validation generally requires the northern blots to be combined with other tools, such as RNase R and RNase H treatments[210]. In the RNaseR(-) group, both circRNA and linear mRNA could be detected, while in the RNaseR(+) group only the band of circRNA could be found, due to linear mRNA digestion[189]. However, northern blotting is not without drawbacks, including the requirement of a very large number of RNA, and the amount required and frequency of radioactively-labelled probes[199]. Nowadays, various northern gels and detection systems are under investigation, so they can be improved.
8.2.4. Fluorescence in situ hybridization (FISH)
Visualizing circRNAs in cells is extremely critical for studying their biology. An oligonucleotide probe coupled with alkaline phosphatase, fluorescent dyes or an antigen can be used to visualize an circRNA in fixed and permeabilized cells, as shown by the use of digoxigenin in ISH[199]. In a previous study a simple smRNA FISH protocol was used to measure the circRNAs produced from identical genetic loci and coexisted with overlapping, non-circular mRNA isoforms[211]. Most importantly, the BSJ site needs to be extended by the designed hybridization probe.
8.2.5. NanoString technology
NanoString, a relatively new digital counting technology, is precise in quantifying linear mRNAs without any enzymatic reactions. Recent studies have found that after designing color-coded probes spanning the specific back-spliced junctions of circRNAs, NanoString technology was used for the detection of circRNAs in both high- and low-quality RNA samples from cell lines and samples from patients with B-cell malignancies, a method that is sensitive, specific and quantitatively accurate[212].
8.3. Overexpression of circRNAs
The biological functions of circRNAs can be investigated by overexpressing the selected circRNAs. circRNAs could be produced in vitro using self-splicing introns or splint ligation methods[31], [213]. To construct stable cell lines overexpressing the selected circRNA, cells could be transfected using a linearized circRNA-producing plasmid[35]. However, this approach often leads to the random insertion of a circRNA expression locus. The change of intronic sequences could make circRNA circulation more accurate under certain circumstances[214]. Therefore, the amount of circRNA generation is supposed to be detected and confirmed by Northern blotting.
8.4. circRNA-knockdown
The key means of investigating the biological function of circRNAs is a loss-of-function study by RNA interference. Gene-knockdown through the use of siRNAs specifically targeting the BSJ is widely used as a method of reducing the expression of circRNAs. Of note, the design space is extremely restricted when targeting the BSJ, and the passenger disabled siRNA could be beneficial [215]. The construction of siRNAs relies on a high transfection efficiency, since these RNAs merely knock down the targets transiently. The more stable knockdown method is the use of AGO shRNAs or vectors expressing shRNAs [216], [217]. The RNA-targeting Cas13 system is a useful tool for degrading circRNAs[218], [219]. The Cas13 enzymes belong to the class 2 type VI CRISPR/Cas effectors. Efficient Cas13 knockdown requires 28–30-nt long spacers and is intolerant to mismatches in spacers. Therefore, CRISPR RNAs carrying spacers that specifically target and span the BSJ site, in principle, should be able to discriminate circular and linear RNAs.
8.5. Mechanistic study
For mechanistic studies, bioinformatics prediction, luciferase reporter assay, RNA immunoprecipitation and RNA pull-down followed by mass spectrometry are conducted to explore circRNA-miRNA and circRNA-protein interactions. For instance, circRNA-miRNA interactions can be predicted by employing Arraystar’s homemade miRNA target prediction software based on TargetScan and miRanda to establish a circRNA-miRNA-mRNA coexpression network of hsa_circ_0044556[220]. In the study performed on the role of circSLC8A1 in bladder cancer, RNA pull-down and luciferase reporter assays were performed to explore the interactions between the specific circRNA, miRNA and mRNA[166]. In addition, it has been shown that researchers from the South China University of Technology determined circRNA-miRNA interactions via AGO cross-linking and immunoprecipitation, along with CLIP-Seq and RNA-Seq data. However, these techniques do not distinguish between circRNA and linear RNA. Therefore, the circRNAs should be further quantified using a circRNA-specific method, such as RT-qPCR, with a divergent primer[199]. It was reported in recent studies that the RNase protection assay can be employed to map protein-RNA interactions, which will block the cleavage of circRNAs via RNase H [209]. A site of interaction between the protein and RNA is then illustrated under the condition of a protein binding to the RNA at the target sequence[221]. An RNA pull-down assay is another attractive method of investigating putative protein-binding partners by employing probes for known circRNAs, followed by confirmation by western blotting and mass spectrometry. As compared with the RNA pull-down assay, the RBP immunoprecipitation assay discovers the RNAs by targeting the protein[222]. In order to study the circRNA protein-coding ability, circRNA N6-methyladenosin, IRES and ORF should be predicted by bioinformatic analysis[70], [104], [171].
9. Challenges and future perspectives
The functions and properties of circRNAs have been elucidated little by little through the advances in high-throughput screening[20]. A plethora of circRNAs have been found to participate in tumorigenesis through multiple molecular mechanisms, such as their interaction with RBPs, which serve as miRNA sponges that translate into small peptides and regulate the expression of parental genes[69], [104]. Despite the fact that great progress has been made in the study of circRNAs, there are several aspects of circRNAs need to be investigated before it can be incorporated into clinical practice.
A noteworthy characteristic of circRNAs is subcellular distribution. circRNAs are mainly distributed in the cytoplasm [15], [49]. Of note, certain circRNAs exhibit a modulated switch in their nucleocytoplasmic localization during their development [223]. The distribution of circRNAs at synaptosomes, dendrites and axons is appealing [224], [225]. It remains unclear whether the accumulation of circRNA is due to a directed transport or diffusion of the molecules in the spots (e.g. by binding to membrane proteins). Further studies are required to investigate what drives the subcellular distribution of circRNAs. Since circRNAs and mRNAs share 5′UTR regions in most cases[19] it is conceivable that these sequences show that the distribution of both the circular and linear forms originate from a given locus. In addition, mRNA and circRNA may compete for the interaction with effector or transport proteins, which is a way for circRNAs to modulate gene expression. Further studies are required to verify these assumptions and screen circRNA biogenesis and transportation using live-cell imaging. In addition, this area is still lacking an accurate description of the amount and classification of circRNAs.
Emerging evidence confirms that epigenetics is associated with tumorigenesis. To date, several circRNAs have been proven to regulate epigenetic changes, such as histone modifications and DNA methylation[226]. However, little is known about how circRNAs are transported inside the cells and their degradation mechanisms of circRNAs. First, although circRNA may serve as a miRNA sponge, miRNA-mediated circRNA degradation has rarely been explored. The expression of CDR1as is regulated by miR-671 via AGO2-mediated degradation[29]. Meanwhile, CDR1as levels are modulated by miR-7 possibly through slicing [55]. Secondly, variations in the m6A modifications of circRNAs may have an effect on RNA stability, cell-specific expression and the length of single exons[227]. Furthermore, m6A modification identified the binding of YTHDF2 to the molecular target and interacted with HRSP12 to regulate the cleavage of circRNA, indicating its positive effect on the degradation of circRNAs[228]. In addition, in a study by Chen et al[229] the m6A modification of circNSUN2 was found to facilitate its export from the nucleus to the cytoplasm.
It’s worth noting that circRNAs are enriched in extracellular vesicles, so that cells can eliminate circRNAs through extracellular vesicles[230]-[231]. In addition, extracellular vesicles or microvesicles may have an impact on the tumor microenvironment through intercellular communication [232], [233]. For example, in a study by Li et al[234] it was found that tumor-excreted circPDE8A diffused into blood circulation by exosome transportation, and plasma exosomal circPDE8A was strongly associated with tumor invasion in pancreatic ductal adenocarcinoma (PDAC). This suggested that exosome communication indeed occurs in PDAC cells. In addition, UAP56 or URH49 were confirmed to play a key role in the nuclear exportat of circRNAs in HeLa cells [235], [236]. In addition, exosomal circRNAs also existed in platelet-derived extracellular vesicles[237] pancreatic cancer cells[193], [238] and hepatic cells[238]. Exosomes can be received by many types of cells, including macrophages, and they could function as messengers for cell-to-cell communication. Collectively, the extracellular transport and degradation of circRNAs should be studied in detail in future studies, which will contribute to a novel insight of circRNAs biology.
Despite the aforementioned exciting progress in circRNA research, there are still challenges in the clinical application of exosomal circRNAs. Firstly, it is difficult to detect circRNA exosomes due to its low abundance. Secondly, the conformation and sequence of circRNA overlap with linear mRNA counterparts, making the accurate assessment of circRNA expression challenging. In addition, the mechanism through which circRNAs are enriched during exosomal formation remains unclear.
Most reports of circRNAs as essential regulators of cancer have provided limited information about their function. CeRNA could not represent the primary function of circRNAs, since most circRNAs are short in length and low in abundance[239]. The function of circRNAs in cancer pathogenesis is still not fully understood, particularly with regards to drug resistance. The functional study of these newly selected circRNAs may not only broaden our knowledge of the non-conding RNA and eukaryotic transcriptome, but also offer new insights into the diagnosis and treatment of cancer. circRNAs could be appropriately modified to change the key binding sites associated with cancer, and specific targeting molecular drugs can be developed to alter downstream gene expression for the purpose of treating cancer.
Meanwhile, improved methods of artificially overexpressing or silencing circRNAs make it possible to regulate the expression of circRNAs, which is crucial to further investigating the functions of circRNAs. In addition, nanoparticles are closed spherical lipid vesicles and have been widely used in the clinic as drug carriers. For instance, due to passive targeting of drug carriers, stable nucleic acid lipid particles accumulate in the tumor tissue, so nanocarriers of a suitable size can easily pass through the tumor[240]. Of note, it was found by Du et al[18] that circFoxo3 can be delivered through plasmid conjugated with gold nanoparticles to induce tumor apoptosis. Future research, in combination with materials science, should focus on delivering circRNA to target cells in an efficient manner. Furthermore, a way in which to control therapeutic circRNAs once they have been delivered, as well as a mechanism of blocking the functions of an circRNA once it has completed exerting its therapeutic effects need to be identified. Stimulus-responsive nanoparticles may be a potential approach for delivering circRNAs. Presumably, circRNAs could be delivered as a promising drug for the clinical treatment of cancer in the next few years.
Despite the natural sponges acting as efficient miR sponges in tumor cells, synthetic circRNA sponges are also worth investigating. The synthetic circRNA sponges can obtain therapeutic loss-of-function targeted against miRNAs more conveniently and steadily, thereby controlling tumor progression. It has been shown that synthetic circRNA can function as an miR-21 sponge to hamper gastric carcinoma cell proliferation, which indicates the potential broad application of synthetic circRNA in the treatment of human cancer [80]. In addition, a new type of artificial circular multi-miR sponge exhibiting miR-21 and miR-93 loss-of-function was synthesized to inhibit cellular proliferation and migration in esophageal carcinoma cells[241]. However, the synthetic technology is not complete and still has drawbacks, such as a limited number of miR binding sites, an altered yield of ligation for RNA circularization and an appearance of potential toxicity, which require further investigations.
circRNA is a new important player in the ncRNA network, which has been identified as a key regulator of various types of cancer. Furthermore, circRNAs have been confirmed to participate in anticancer drug resistance. The latest studies on circRNAs in anticancer drug resistance were summarized by Xu et al[242] ranging from traditional chemotherapeutic drugs to targeted and immunotherapeutic drugs, which will expand their clinical potential and serve as a research hotspot. Hence, the appropriate and precise use of circRNAs is essential in the field of cancer studies, as well as the new foothold for precision medicine in the near future.
10. Conclusions
The study of circRNAs is a novel research field that has emerged with the rapid development of technology. Research on circRNAs has led to several surprising findings, indicating that circRNAs govern a wide spectrum of physiological and pathological processes, particularly in tumorigenesis. What we know so far suggests that circRNA-based diagnostic and therapeutic strategies may play promising role in cancer management.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China 81970196 (to CG) and 82073885 (to YY), Natural Science Foundation of Jiangsu Province BK20200097 (to CG), Innovation Team of Six Talent Peaks Project in Jiangsu Province TD-SWYY-015 (to CG), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Integration of Chinese and Western Medicine).
Contributor Information
Ye Yang, Email: yangye876@sina.com.
Chunyan Gu, Email: guchunyan@njucm.edu.cn.
References
- 1.Sanger H.L., Klotz G., Riesner D., Gross H.J., Kleinschmidt A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu M.T., Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280(5720):339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 3.Nahand J., Jamshidi S., Hamblin M., Mahjoubin-Tehran M., Vosough M., Jamali M. Circular RNAs: New Epigenetic Signatures in Viral Infections. Front Microbiol. 2020;11:1853. doi: 10.3389/fmicb.2020.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naeli P., Pourhanifeh M., Karimzadeh M., Shabaninejad Z., Movahedpour A., Tarrahimofrad H. Circular RNAs and gastrointestinal cancers: Epigenetic regulators with a prognostic and therapeutic role. Crit Rev Oncol/Hematol. 2020;145 doi: 10.1016/j.critrevonc.2019.102854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes C.P.C., Schroen B., Kuster G.M., Robinson E.L., Ford K., Squire I.B. Regulatory RNAs in Heart Failure. Circulation. 2020;141(4):313–328. doi: 10.1161/CIRCULATIONAHA.119.042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams B., Parsons C., Slack F. The tumor-suppressive and potential therapeutic functions of miR-34a in epithelial carcinomas. Expert opinion on therapeutic targets. 2016;20(6):737–753. doi: 10.1517/14728222.2016.1114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vafadar A., Shabaninejad Z., Movahedpour A., Mohammadi S., Fathullahzadeh S., Mirzaei H.R. Long Non-Coding RNAs As Epigenetic Regulators in Cancer. Curr Pharm Des. 2019;25(33):3563–3577. doi: 10.2174/1381612825666190830161528. [DOI] [PubMed] [Google Scholar]
- 8.Jafari Najaf Abadi M., Shafabakhsh R., Asemi Z., Mirzaei H., Sahebnasagh R., Mirzaei H. CFIm25 and alternative polyadenylation: Conflicting roles in cancer. Cancer Lett. 2019;459:112–121. doi: 10.1016/j.canlet.2019.114430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefi F., Shabaninejad Z., Vakili S., Derakhshan M., Movahedpour A., Dabiri H. TGF-β and WNT signaling pathways in cardiac fibrosis: non-coding RNAs come into focus. Cell Commun Signal: CCS. 2020;18(1):87. doi: 10.1186/s12964-020-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei L.H., Guo J.U. Coding functions of “noncoding” RNAs. Science. 2020;367(6482):1074–1075. doi: 10.1126/science.aba6117. [DOI] [PubMed] [Google Scholar]
- 11.Hammell C., Lubin I., Boag P., Blackwell T., Ambros V. nhl-2 Modulates microRNA activity in Caenorhabditis elegans. Cell. 2009;136(5):926–938. doi: 10.1016/j.cell.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slack F.J., Chinnaiyan A.M. The Role of Non-coding RNAs in Oncology. Cell. 2019;179(5):1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbaszadeh-Goudarzi K., Radbakhsh S., Pourhanifeh M., Khanbabaei H., Davoodvandi A., Fathizadeh H. Circular RNA and Diabetes: Epigenetic Regulator with Diagnostic Role. Curr Mol Med. 2020;20(7):516–526. doi: 10.2174/1566524020666200129142106. [DOI] [PubMed] [Google Scholar]
- 14.Shabaninejad Z., Vafadar A., Movahedpour A., Ghasemi Y., Namdar A., Fathizadeh H. Circular RNAs in cancer: new insights into functions and implications in ovarian cancer. J Ovar Res. 2019;12(1):84. doi: 10.1186/s13048-019-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 16.Hallajzadeh J., Amirani E., Mirzaei H., Shafabakhsh R., Mirhashemi S., Sharifi M. Circular RNAs: new genetic tools in melanoma. Biomarkers Med. 2020;14(7):563–571. doi: 10.2217/bmm-2019-0567. [DOI] [PubMed] [Google Scholar]
- 17.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du W.W., Fang L., Yang W., Wu N., Awan F.M., Yang Z. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24(2):357–370. doi: 10.1038/cdd.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L. Translation of CircRNAs. Mol Cell. 2017;66(1):9–21 e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L.L. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17(4):205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 21.Dube U., Del-Aguila J.L., Li Z., Budde J.P., Jiang S., Hsu S. An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat Neurosci. 2019;22(11):1903–1912. doi: 10.1038/s41593-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millan M.J. Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer's disease: An integrative review. Prog Neurobiol. 2017;156:1–68. doi: 10.1016/j.pneurobio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Shen S., Wu Y., Chen J., Xie Z., Huang K., Wang G. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann Rheum Dis. 2019;78(6):826–836. doi: 10.1136/annrheumdis-2018-214786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C., Ge H.M., Liu B.H., Dong R., Shan K., Chen X. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci USA. 2019;116(15):7455–7464. doi: 10.1073/pnas.1814874116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S.K., Garg A., Bar C., Chatterjee S., Foinquinos A., Milting H. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ Res. 2018;122(2):246–254. doi: 10.1161/CIRCRESAHA.117.311335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Song S., Lin S., Zhang M., Du Y., Zhang D. Circ-SERPINE2 promotes the development of gastric carcinoma by sponging miR-375 and modulating YWHAZ. Cell Prolif. 2019;52(4) doi: 10.1111/cpr.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarnerio J., Bezzi M., Jeong J.C., Paffenholz S.V., Berry K., Naldini M.M. Oncogenic Role of Fusion-circRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell. 2016;166(4):1055–1056. doi: 10.1016/j.cell.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Borran S., Ahmadi G., Rezaei S., Anari M., Modabberi M., Azarash Z. Circular RNAs: New players in thyroid cancer. Pathol Res Pract. 2020;216(10) doi: 10.1016/j.prp.2020.153217. [DOI] [PubMed] [Google Scholar]
- 29.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 30.Zheng X., Chen L., Zhou Y., Wang Q., Zheng Z., Xu B. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer. 2019;18(1):47. doi: 10.1186/s12943-019-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X.O., Wang H.B., Zhang Y., Lu X., Chen L.L., Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159(1):134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov A., Memczak S., Wyler E., Torti F., Porath H.T., Orejuela M.R. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10(2):170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Liang D., Wilusz J.E. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28(20):2233–2247. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashwal-Fluss R., Meyer M., Pamudurti N., Ivanov A., Bartok O., Hanan M. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Kelly S., Greenman C., Cook P.R., Papantonis A. Exon Skipping Is Correlated with Exon Circularization. J Mol Biol. 2015;427(15):2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Starke S., Jost I., Rossbach O., Schneider T., Schreiner S., Hung L.H. Exon circularization requires canonical splice signals. Cell Rep. 2015;10(1):103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Liang D., Tatomer D.C., Luo Z., Wu H., Yang L., Chen L.L. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol Cell. 2017;68(5):940–954 e943. doi: 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noto J.J., Schmidt C.A., Matera A.G. Engineering and expressing circular RNAs via tRNA splicing. RNA Biol. 2017;14(8):978–984. doi: 10.1080/15476286.2017.1317911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt C.A.G.J., Bao A., Hopper A.K., Matera A.G. Molecular determinants of metazoan tricRNA biogenesis. Nucleic Acids Res. 2019;47(12):6452–6465. doi: 10.1093/nar/gkz311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Xiao L., Chung H., Ma X., Liu X., Song J. circPABPN1Interaction between HuR and Modulates Autophagy in the Intestinal Epithelium by Altering ATG16L1 Translation. Mol Cell Biol. 2020;40(6) doi: 10.1128/MCB.00492-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdelmohsen K., Panda A.C., Munk R., Grammatikakis I., Dudekula D.B., De S. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14(3):361–369. doi: 10.1080/15476286.2017.1279788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piwecka M., Glazar P., Hernandez-Miranda L.R., Memczak S., Wolf S.A., Rybak-Wolf A. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357 doi: 10.1126/science.aam8526. (6357):eaam8526. [DOI] [PubMed] [Google Scholar]
- 47.Kohlhapp F.J., Mitra A.K., Lengyel E., Peter M.E. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogen. 2015;34(48):5857–5868. doi: 10.1038/onc.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupaimoole R., Calin G.A., Lopez-Berestein G., Sood A.K. miRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov. 2016;6(3):235–246. doi: 10.1158/2159-8290.CD-15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9(9) doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao J., Tao Y., Zhou Y., Qin N., Chen C., Tian D. MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int. 2015;15(1):103. doi: 10.1186/s12935-015-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Rana T.M. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 52.Hansen T.B., Wiklund E.D., Bramsen J.B., Villadsen S.B., Statham A.L., Clark S.J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30(21):4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Rossum D., Verheijen B.M., Pasterkamp R.J. Circular RNAs: Novel Regulators of Neuronal Development. Front Mol Neurosci. 2016;9:74. doi: 10.3389/fnmol.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R.C., Ke S., Meng F.K., Lu J., Zou X.J., He Z.G. CiRS-7 promotes growth and metastasis of esophageal squamous cell carcinoma via regulation of miR-7/HOXB13. Cell Death Dis. 2018;9(8):838. doi: 10.1038/s41419-018-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleaveland B., Shi C.Y., Stefano J., Bartel D.P. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018;174(2):350–362 e317. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G., Shi Y., Liu M., Sun J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018;9(2):175. doi: 10.1038/s41419-017-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ke Z., Xie F., Zheng C., Chen D. CircHIPK3 promotes proliferation and invasion in nasopharyngeal carcinoma by abrogating miR-4288-induced ELF3 inhibition. J Cell Physiol. 2019;234(2):1699–1706. doi: 10.1002/jcp.27041. [DOI] [PubMed] [Google Scholar]
- 58.Shan K., Liu C., Liu B.H., Chen X., Dong R., Liu X. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation. 2017;136(17):1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 59.Li Y., Zheng F., Xiao X., Xie F., Tao D., Huang C. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017;18(9):1646–1659. doi: 10.15252/embr.201643581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang R., Zhang Y., Han B., Bai Y., Zhou R., Gan G. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy. 2017;13(10):1722–1741. doi: 10.1080/15548627.2017.1356975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C., Yuan W., Yang X., Li P., Wang J., Han J. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17(1):19. doi: 10.1186/s12943-018-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J Natl Cancer Inst. 2018;110(3) doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wesselhoeft R.A., Kowalski P.S., Anderson D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9(1):2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe N., Matsumoto K., Nishihara M., Nakano Y., Shibata A., Maruyama H. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci Rep. 2015;5:16435. doi: 10.1038/srep16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21(2):172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang W.C., Wong C.W., Liang P.P., Shi M., Cao Y., Rao S.T. Translation of the circular RNA circbeta-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20(1):84. doi: 10.1186/s13059-019-1685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia X., Li X., Li F., Wu X., Zhang M., Zhou H. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18(1):131. doi: 10.1186/s12943-019-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M., Huang N., Yang X., Luo J., Yan S., Xiao F. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805–1814. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- 70.Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol Cell. 2017;66(1):22–37 e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z., Harrison P.M., Liu Y., Gerstein M. Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res. 2003;13(12):2541–2558. doi: 10.1101/gr.1429003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z., Carriero N., Gerstein M. Comparative analysis of processed pseudogenes in the mouse and human genomes. Trends Genet. 2004;20(2):62–67. doi: 10.1016/j.tig.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Dong R., Zhang X.O., Zhang Y., Ma X.K., Chen L.L., Yang L. CircRNA-derived pseudogenes. Cell Res. 2016;26(6):747–750. doi: 10.1038/cr.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Conn V.M., Hugouvieux V., Nayak A., Conos S.A., Capovilla G., Cildir G. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat Plants. 2017;3(5):17053. doi: 10.1038/nplants.2017.53. [DOI] [PubMed] [Google Scholar]
- 77.Bramham C.R., Wells D.G. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8(10):776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 78.Preusser C., Hung L.H., Schneider T., Schreiner S., Hardt M., Moebus A. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7(1):1424473. doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lasda E., Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X., Abraham J.M., Cheng Y., Wang Z., Wang Z., Zhang G. Synthetic Circular RNA Functions as a miR-21 Sponge to Suppress Gastric Carcinoma Cell Proliferation. Mol Ther Nucleic Acids. 2018;13:312–321. doi: 10.1016/j.omtn.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enuka Y., Lauriola M., Feldman M., Sas-Chen A., Ulitsky I., Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44(3):1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bachmayr-Heyda A., Reiner A., Auer K., Sukhbaatar N., Aust S., Bachleitner-Hofmann T. Correlation of circular RNA abundance with proliferation–exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057. doi: 10.1038/srep08057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanan M., Soreq H., Kadener S. CircRNAs in the brain. RNA Biol. 2017;14(8):1028–1034. doi: 10.1080/15476286.2016.1255398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackie G. Ribonuclease E is a 5'-end-dependent endonuclease. Nature. 1998;395(6703):720–723. doi: 10.1038/27246. [DOI] [PubMed] [Google Scholar]
- 85.Schaeffer D., Tsanova B., Barbas A., Reis F., Dastidar E., Sanchez-Rotunno M. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat Struct Mol Biol. 2009;16(1):56–62. doi: 10.1038/nsmb.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park O., Ha H., Lee Y., Boo S., Kwon D., Song H. Endoribonucleolytic Cleavage of mA-Containing RNAs by RNase P/MRP Complex. Mol Cell. 2019;74(3):494–507.e498. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 87.Liu C., Li X., Nan F., Jiang S., Gao X., Guo S. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell. 2019;177(4):865–880.e821. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 88.Shi X., Wang B., Feng X., Xu Y., Lu K., Sun M. circRNAs and Exosomes: A Mysterious Frontier for Human Cancer. Molecular therapy Nucleic acids. 2020;19:384–392. doi: 10.1016/j.omtn.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q., Wang W., Zhou Q., Chen C., Yuan W., Liu J. Roles of circRNAs in the tumour microenvironment. Mol Cancer. 2020;19(1):14. doi: 10.1186/s12943-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao M.S., Ai Y., Wilusz J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020;30(3):226–240. doi: 10.1016/j.tcb.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong R., Ma X.K., Li G.W., Yang L. CIRCpedia v2: An Updated Database for Comprehensive Circular RNA Annotation and Expression Comparison. Genomics Proteomics Bioinformatics. 2018;16(4):226–233. doi: 10.1016/j.gpb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.J.H. Yang, P. Shao, H. Zhou, Y.Q. Chen, L.H. Qu, deepBase: a database for deeply annotating and mining deep sequencing data Nucleic Acids Res. 38(Database 2010 issue):D123–130. [DOI] [PMC free article] [PubMed]
- 97.Liu Y.C., Li J.R., Sun C.H., Andrews E., Chao R.F., Lin F.M. CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res. 2016;44(D1):D209–D215. doi: 10.1093/nar/gkv940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dudekula D.B., Panda A.C., Grammatikakis I., De S., Abdelmohsen K., Gorospe M. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13(1):34–42. doi: 10.1080/15476286.2015.1128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia S., Feng J., Chen K., Ma Y., Gong J., Cai F. CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res. 2018;46(D1):D925–D929. doi: 10.1093/nar/gkx863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosal S., Das S., Sen R., Basak P., Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu S.M., Liu H., Huang P.J., Chang I.Y., Lee C.C., Yang C.Y. circlncRNAnet: an integrated web-based resource for mapping functional networks of long or circular forms of noncoding RNAs. GigaScience. 2018;7(1):1–10. doi: 10.1093/gigascience/gix118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Z., Wang K., Wu F., Wang W., Zhang K., Hu H. circRNA disease: a manually curated database of experimentally supported circRNA-disease associations. Cell Death Dis. 2018;9(5):475. doi: 10.1038/s41419-018-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li S., Li Y., Chen B., Zhao J., Yu S., Tang Y. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46(D1):D106–D112. doi: 10.1093/nar/gkx891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang M., Zhao K., Xu X., Yang Y., Yan S., Wei P. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9(1):4475. doi: 10.1038/s41467-018-06862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sadeghi S., Davoodvandi A., Pourhanifeh M., Sharifi N., ArefNezhad R., Sahebnasagh R. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur J Med Chem. 2019;178:131–140. doi: 10.1016/j.ejmech.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 106.Barbagallo D., Caponnetto A., Cirnigliaro M., Brex D., Barbagallo C., D'Angeli F. CircSMARCA5 Inhibits Migration of Glioblastoma Multiforme Cells by Regulating a Molecular Axis Involving Splicing Factors SRSF1/SRSF3/PTB. Int J Mol Sci. 2018;19(2):480. doi: 10.3390/ijms19020480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li F., Ma K., Sun M.H., Shi S. Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. American Journal of Translational Research. 2018;10(5):1373–1386. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Xu H., Zhang Y., Qi L., Ding L., Jiang H., Yu H. NFIX Circular RNA Promotes Glioma Progression by Regulating miR-34a-5p via Notch Signaling Pathway. Front Mol Neurosci. 2018;11:225. doi: 10.3389/fnmol.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang R., Zhang S., Chen X., Li N., Li J., Jia R. CircNT5E Acts as a Sponge of miR-422a to Promote Glioblastoma Tumorigenesis. Cancer Res. 2018;78(17):4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 110.Zheng J., Liu X., Xue Y., Gong W., Ma J., Xi Z. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1beta/Derlin-1 pathway. J Hematol Oncol. 2017;10(1):52. doi: 10.1186/s13045-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Z., Duan X. hsa_circ_0000177-miR-638-FZD7-Wnt Signaling Cascade Contributes to the Malignant Behaviors in Glioma. DNA Cell Biol. 2018;37(9):791–797. doi: 10.1089/dna.2018.4294. [DOI] [PubMed] [Google Scholar]
- 112.Xie G. Circular RNA hsa-circ-0012129 Promotes Cell Proliferation and Invasion in 30 Cases of Human Glioma and Human Glioma Cell Lines U373, A172, and SHG44, by Targeting MicroRNA-661 (miR-661) Med Sci Monit. 2018;24:2497–2507. doi: 10.12659/MSM.909229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bian A., Wang Y., Liu J., Wang X., Liu D., Jiang J. Circular RNA Complement Factor H (CFH) Promotes Glioma Progression by Sponging miR-149 and Regulating AKT1. Med Sci Monit. 2018;24:5704–5712. doi: 10.12659/MSM.910180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li G., Yang H., Han K., Zhu D., Lun P., Zhao Y. A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis by increasing the expression of miR-142-3p target ITGB8 in glioma. Biochem Biophys Res Commun. 2018;498(1):254–261. doi: 10.1016/j.bbrc.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 115.Khemlina G., Ikeda S., Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1):149. doi: 10.1186/s12943-017-0712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jayachandran A., Dhungel B., Steel J.C. Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol. 2016;9(1):74. doi: 10.1186/s13045-016-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han D., Li J., Wang H., Su X., Hou J., Gu Y. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66(4):1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 118.Yu J., Xu Q.G., Wang Z.G., Yang Y., Zhang L., Ma J.Z. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68(6):1214–1227. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 119.Yao Z., Luo J., Hu K., Lin J., Huang H., Wang Q. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11(4):422–437. doi: 10.1002/1878-0261.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fu L., Wu S., Yao T., Chen Q., Xie Y., Ying S. Decreased expression of hsa_circ_0003570 in hepatocellular carcinoma and its clinical significance. J Clin Lab Anal. 2018;32(2) doi: 10.1002/jcla.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu L., Zhang M., Zheng X., Yi P., Lan C., Xu M. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(1):17–27. doi: 10.1007/s00432-016-2256-7. [DOI] [PubMed] [Google Scholar]
- 122.Yu L., Gong X., Sun L., Zhou Q., Lu B., Zhu L. The Circular RNA Cdr1as Act as an Oncogene in Hepatocellular Carcinoma through Targeting miR-7 Expression. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0158347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Sun M., Song H., Wang S., Zhang C., Zheng L., Chen F. Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. J Hematol Oncol. 2017;10(1):79. doi: 10.1186/s13045-017-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo J.N., Li J., Zhu C.L., Feng W.T., Shao J.X., Wan L. Comprehensive profile of differentially expressed circular RNAs reveals that hsa_circ_0000069 is upregulated and promotes cell proliferation, migration, and invasion in colorectal cancer. Onco Targets Ther. 2016;9:7451–7458. doi: 10.2147/OTT.S123220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang R., Xu J., Zhao J., Wang X. Silencing of hsa_circ_0007534 suppresses proliferation and induces apoptosis in colorectal cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(1):118–126. doi: 10.26355/eurrev_201801_14108. [DOI] [PubMed] [Google Scholar]
- 126.P. Zhang, Z. Zuo, W. Shang, A. Wu, R. Bi J. Wu et al. Identification of differentially expressed circular RNAs in human colorectal cancer Tumour Biol. 39 (3) 2017 1010428317694546. [DOI] [PubMed]
- 127.Hsiao K.Y., Lin Y.C., Gupta S.K., Chang N., Yen L., Sun H.S. Noncoding Effects of Circular RNA CCDC66 Promote Colon Cancer Growth and Metastasis. Cancer Res. 2017;77(9):2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu M., Xu Y., Chen Y., Yan F. Circular BANP, an upregulated circular RNA that modulates cell proliferation in colorectal cancer. Biomed Pharmacother. 2017;88:138–144. doi: 10.1016/j.biopha.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 129.H. Xie, X. Ren, S. Xin, X. Lan, G. Lu, Y. Lin, S. Yang, Z. Zeng, W. Liao, Y.-Q. Ding Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 7(18). [DOI] [PMC free article] [PubMed]
- 130.Weng W., Wei Q., Toden S., Yoshida K., Nagasaka T., Fujiwara T. Circular RNA ciRS-7-A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clin Cancer Res. 2017;23(14):3918–3928. doi: 10.1158/1078-0432.CCR-16-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang W., Ji M., He G., Yang L., Niu Z., Jian M. Silencing CDR1as inhibits colorectal cancer progression through regulating microRNA-7. Onco Targets Ther. 2017;10:2045–2056. doi: 10.2147/OTT.S131597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 133.Chen S., Li T., Zhao Q., Xiao B., Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 134.Peifei L.i., Shengcan C., Huilin C., Xiaoyan M.o., Tianwen L.i. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clinical Chimica Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 135.Huang X., Li Z., Zhang Q., Wang W., Li B., Wang L. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18(1):71. doi: 10.1186/s12943-019-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J., Hou L., Liang R., Chen X., Zhang R., Chen W. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 2019;18(1):80. doi: 10.1186/s12943-019-1015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rong D., Lu C., Zhang B., Fu K., Zhao S., Tang W. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18(1):25. doi: 10.1186/s12943-019-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Zhang L., Song X., Chen X., Wang Q., Zheng X., Wu C. Circular RNA CircCACTIN Promotes Gastric Cancer Progression by Sponging MiR-331-3p and Regulating TGFBR1 Expression. Int J Biol Sci. 2019;15(5):1091–1103. doi: 10.7150/ijbs.31533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang S., Tang D., Wang W., Yang Y., Wu X., Wang L. circLMTK2 acts as a sponge of miR-150-5p and promotes proliferation and metastasis in gastric cancer. Mol Cancer. 2019;18(1):162. doi: 10.1186/s12943-019-1081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ding L., Zhao Y., Dang S., Wang Y., Li X., Yu X. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation of transcription factor SOX4. Mol Cancer. 2019;18(1):45. doi: 10.1186/s12943-019-1006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S., Zhang X., Li Z., Wang W., Li B., Huang X. Circular RNA profile identifies circOSBPL10 as an oncogenic factor and prognostic marker in gastric cancer. Oncogene. 2019;38(44):6985–7001. doi: 10.1038/s41388-019-0933-0. [DOI] [PubMed] [Google Scholar]
- 143.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 144.Wang L., Tong X., Zhou Z., Wang S., Lei Z., Zhang T. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-beta-induced epithelial-mesenchymal transition and metastasis by controlling TIF1gamma in non-small cell lung cancer. Mol Cancer. 2018;17(1):140. doi: 10.1186/s12943-018-0889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan S., Sun D., Pu W., Gou Q., Guo C., Gong Y. Circular RNA F-circEA-2a derived from EML4-ALK fusion gene promotes cell migration and invasion in non-small cell lung cancer. Mol Cancer. 2018;17(1):138. doi: 10.1186/s12943-018-0887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen L., Nan A., Zhang N., Jia Y., Li X., Ling Y. Circular RNA 100146 functions as an oncogene through direct binding to miR-361-3p and miR-615-5p in non-small cell lung cancer. Mol Cancer. 2019;18(1):13. doi: 10.1186/s12943-019-0943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng Z., Yu C., Cui S., Wang H., Jin H., Wang C. circTP63 functions as a ceRNA to promote lung squamous cell carcinoma progression by upregulating FOXM1. Nat Commun. 2019;10(1):3200. doi: 10.1038/s41467-019-11162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou J., Zhang S., Chen Z., He Z., Xu Y., Li Z. CircRNA-ENO1 promoted glycolysis and tumor progression in lung adenocarcinoma through upregulating its host gene ENO1. Cell Death Dis. 2019;10(12):885. doi: 10.1038/s41419-019-2127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang P.F., Pei X., Li K.S., Jin L.N., Wang F., Wu J. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol Cancer. 2019;18(1):179. doi: 10.1186/s12943-019-1111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y., Li H., Lu H., Qin Y. Circular RNA SMARCA5 inhibits the proliferation, migration, and invasion of non-small cell lung cancer by miR-19b-3p/HOXA9 axis. Onco Targets Ther. 2019;12:7055–7065. doi: 10.2147/OTT.S216320. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Wu D.M., Wen X., Han X.R., Wang S., Wang Y.J., Shen M. Role of Circular RNA DLEU2 in Human Acute Myeloid Leukemia. Mol Cell Biol. 2018;38(20) doi: 10.1128/MCB.00259-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li S., Ma Y., Tan Y., Ma X., Zhao M., Chen B. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018;9(6):651. doi: 10.1038/s41419-018-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shang J., Chen W., Wang Z., Wei T., Chen Z., Wu W. CircPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol. 2019;70(42–54) doi: 10.1016/j.exphem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 154.Xia L., Wu L., Bao J., Li Q., Chen X., Xia H. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/beta-catenin pathway. Biochem Biophys Res Commun. 2018;503(1):385–390. doi: 10.1016/j.bbrc.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 155.Wu W., Wu Z., Xia Y., Qin S., Li Y., Wu J. Downregulation of circ_0132266 in chronic lymphocytic leukemia promoted cell viability through miR-337-3p/PML axis. Aging (Albany NY). 2019;11(11):3561–3573. doi: 10.18632/aging.101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu Z., Sun H., Liu W., Zhu H., Fu J., Yang C. Circ-RPL15: a plasma circular RNA as novel oncogenic driver to promote progression of chronic lymphocytic leukemia. Leukemia. 2020;34(3):919–923. doi: 10.1038/s41375-019-0594-6. [DOI] [PubMed] [Google Scholar]
- 157.Pan Y., Lou J., Wang H., An N., Chen H., Zhang Q. CircBA9.3 supports the survival of leukaemic cells by up-regulating c-ABL1 or BCR-ABL1 protein levels. Blood Cells Mol Dis. 2018;73:38–44. doi: 10.1016/j.bcmd.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 158.Liu J., Kong F., Lou S., Yang D., Gu L. Global identification of circular RNAs in chronic myeloid leukemia reveals hsa_circ_0080145 regulates cell proliferation by sponging miR-29b. Biochem Biophys Res Commun. 2018;504(4):660–665. doi: 10.1016/j.bbrc.2018.08.154. [DOI] [PubMed] [Google Scholar]
- 159.Feng X., Nie S., Huang J., Li T., Zhou J., Wang W. Circular RNA circHIPK3 serves as a prognostic marker to promote chronic myeloid leukemia progression. Neoplasma. 2020;67(1):171–177. doi: 10.4149/neo_2018_181129N908. [DOI] [PubMed] [Google Scholar]
- 160.Gao M., Li C., Xiao H., Dong H., Jiang S., Fu Y. hsa_circ_0007841: A Novel Potential Biomarker and Drug Resistance for Multiple Myeloma. Front Oncol. 2019;9:1261. doi: 10.3389/fonc.2019.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng Y., Zhang L., Wu J., Khadka B., Fang Z., Gu J. CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway. J Exp Clin Cancer Res. 2019;38(1):54. doi: 10.1186/s13046-019-1071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu J., Du F., Chen C., Li D., Chen Y., Xiao X. CircRNA ITCH increases bortezomib sensitivity through regulating the miR-615-3p/PRKCD axis in multiple myeloma. Life Sci. 2020;262 doi: 10.1016/j.lfs.2020.118506. [DOI] [PubMed] [Google Scholar]
- 163.Galasso M., Costantino G., Pasquali L., Minotti L., Baldassari F., Corra F. Profiling of the Predicted Circular RNAs in Ductal In Situ and Invasive Breast Cancer: A Pilot Study. Int J Genomics. 2016;2016:4503840. doi: 10.1155/2016/4503840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhong Z., Lv M., Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu F., Zhang H., Xie F., Tao D., Xiao X., Huang C. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene. 2019 doi: 10.1038/s41388-019-1092-z. [DOI] [PubMed] [Google Scholar]
- 166.Lu Q., Liu T., Feng H., Yang R., Zhao X., Chen W. Circular RNA circSLC8A1 acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer progression via regulating PTEN. Molecular cancer. 2019;18(1):111. doi: 10.1186/s12943-019-1040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang K., Sun Y., Tao W., Fei X., Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 168.Chen Z., Xiao K., Chen S., Huang Z., Ye Y., Chen T. Circular RNA hsa_circ_001895 serves as a sponge of microRNA-296-5p to promote clear cell renal cell carcinoma progression by regulating SOX12. Cancer Sci. 2020;111(2):713–726. doi: 10.1111/cas.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Y.L. Zhen Dong, Qinghai Wang, Hongyang Wang, Jianlei Ji, Tao Huang, Aashish Khanal, Haitao Niu, Yanwei Cao, The Circular RNA-NRIP1 Plays Oncogenic Roles by Targeting microRNA-505 in the Renal Carcinoma Cell Lines. Journal of Cellular biochemistry. 2020; 121(3):2236-2246. [DOI] [PubMed]
- 170.Suzuki H., Zuo Y., Wang J., Zhang M.Q., Malhotra A., Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34(8) doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y. The Landscape of Circular RNA in Cancer. Cell. 2019;176(4):869–881 e813. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bahn J., Zhang Q., Li F., Chan T., Lin X., Kim Y. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61(1):221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Memczak S., Papavasileiou P., Peters O., Rajewsky N. Identification and Characterization of Circular RNAs As a New Class of Putative Biomarkers in Human Blood. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lei B., Zhou J., Xuan X., Tian Z., Zhang M., Gao W. Circular RNA expression profiles of peripheral blood mononuclear cells in hepatocellular carcinoma patients by sequence analysis. Cancer medicine. 2019;8(4):1423–1433. doi: 10.1002/cam4.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu K., Zhan H., Peng Y., Yang L., Gao Q., Jia H. Plasma hsa_circ_0027089 is a diagnostic biomarker for hepatitis B virus-related hepatocellular carcinoma. Carcinogenesis. 2020;41(3):296–302. doi: 10.1093/carcin/bgz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang C., Dong Z., Hong H., Dai B., Song F., Geng L. circFN1 Mediates Sorafenib Resistance of Hepatocellular Carcinoma Cells by Sponging miR-1205 and Regulating E2F1 Expression. Molecular therapy Nucleic acids. 2020;22:421–433. doi: 10.1016/j.omtn.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li Z., Zhou Y., Yang G., He S., Qiu X., Zhang L. Using circular RNA SMARCA5 as a potential novel biomarker for hepatocellular carcinoma. Clinica chimica acta; Int J Clin Chem. 2019;492:37–44. doi: 10.1016/j.cca.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 178.Yao T., Chen Q., Shao Z., Song Z., Fu L., Xiao B. Circular RNA 0068669 as a new biomarker for hepatocellular carcinoma metastasis. J Clin Lab Anal. 2018;32(8) doi: 10.1002/jcla.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang Z., Shen L., Wang S., Wu S., Hu Y., Guo J. Hsa_circ_0028502 and hsa_circ_0076251 are potential novel biomarkers for hepatocellular carcinoma. Cancer Med. 2019;8(17):7278–7287. doi: 10.1002/cam4.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ye D., Wang S., Huang Y., Chi P. A 3-circular RNA signature as a noninvasive biomarker for diagnosis of colorectal cancer. Cancer Cell Int. 2019;19:276. doi: 10.1186/s12935-019-0995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tian J., Xi X., Wang J., Yu J., Huang Q., Ma R. CircRNA hsa_circ_0004585 as a potential biomarker for colorectal cancer. Cancer Manag Res. 2019;11:5413–5423. doi: 10.2147/CMAR.S199436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang J., Li X., Lu L., He L., Hu H., Xu Z. Circular RNA hsa_circ_0000567 can be used as a promising diagnostic biomarker for human colorectal cancer. J Clin Lab Anal. 2018;32(5) doi: 10.1002/jcla.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tian M., Chen R., Li T., Xiao B. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. J Clin Lab Anal. 2018;32(3) doi: 10.1002/jcla.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li P., Chen H., Chen S., Mo X., Li T., Xiao B. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116(5):626–633. doi: 10.1038/bjc.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li P., Chen S., Chen H., Mo X., Li T., Shao Y. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clinica chimica acta; international journal of clinical chemistry. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 186.Zhao Q., Chen S., Li T., Xiao B., Zhang X. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32(4) doi: 10.1002/jcla.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lu J., Zhang P., Xie J., Wang J., Lin J., Chen Q. Hsa_circ_0000467 promotes cancer progression and serves as a diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal. 2019;33(3) doi: 10.1002/jcla.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Y. Shao, L. Chen, R. Lu, X. Zhang, B. Xiao, G. Ye et al. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances Tumour biol J Int Soc Oncodev Biol Med 39, (4) 2017 Doi: 1010428317699125. [DOI] [PubMed]
- 189.Hu Y., Song Q., Zhao J., Ruan J., He F., Yang X. Identification of plasma hsa_circ_0008673 expression as a potential biomarker and tumor regulator of breast cancer. J Clin Lab Anal. 2020;34(9) doi: 10.1002/jcla.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang G., Xie W., Qin C., Zhen Y., Wang Y., Chen F. Expression of circular RNA circASXL1 correlates with TNM classification and predicts overall survival in bladder cancer. Int J Clin Exp Path. 2017;10(8):8495–8502. [PMC free article] [PubMed] [Google Scholar]
- 191.Song Z., Zhang Q., Zhu J., Yin G., Lin L., Liang C. Identification of urinary hsa_circ _0137439 as potential biomarker and tumor regulator of bladder cancer. Neoplasma. 2020;67(1):137–146. doi: 10.4149/neo_2018_181214N970. [DOI] [PubMed] [Google Scholar]
- 192.Sun H., Tang W., Rong D., Jin H., Fu K., Zhang W. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark : section A of Disease markers. 2018;21(2):299–306. doi: 10.3233/CBM-170379. [DOI] [PubMed] [Google Scholar]
- 193.Li J., Li Z., Jiang P., Peng M., Zhang X., Chen K. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37(1):177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J., Zhao X., Wang Y., Ren F., Sun D., Yan Y. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11(1):32. doi: 10.1038/s41419-020-2230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pan B., Qin J., Liu X., He B., Wang X., Pan Y. Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer. Front Genet. 2019;10:1096. doi: 10.3389/fgene.2019.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Si-Yu Z., Jun W., Shao-Bo O., Zi-Kun H., Lan L. Salivary Circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell Physiol Biochem. 2018;47(6):2511–2521. doi: 10.1159/000491624. [DOI] [PubMed] [Google Scholar]
- 197.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cooper D., Cortés-López M., Miura P. Genome-Wide circRNA Profiling from RNA-seq Data. Methods in molecular biology (Clifton, NJ). 2018;1724:27–41. doi: 10.1007/978-1-4939-7562-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kristensen L., Andersen M., Stagsted L., Ebbesen K., Hansen T., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 200.A. Dobin, T. Gingeras, Mapping RNA-seq Reads with STAR Current protocols in bioinformatics. 51 (11) 2015 pp. 14.11-11.14.19 [DOI] [PMC free article] [PubMed]
- 201.Hansen T., Venø M., Damgaard C., Kjems J. Comparison of circular RNA prediction tools. Nucleic Acids Res. 2016;44(6) doi: 10.1093/nar/gkv1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carrara M., Fuschi P., Ivan C., Martelli F. Circular RNAs: Methodological challenges and perspectives in cardiovascular diseases. J Cell Mol Med. 2018;22(11):5176–5187. doi: 10.1111/jcmm.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen X., Chen R., Wei W., Li Y., Feng Z., Tan L. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(24):6319–6330. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]
- 204.Li S., Teng S., Xu J., Su G., Zhang Y., Zhao J. Microarray is an efficient tool for circRNA profiling. Briefings Bioinf. 2019;20(4):1420–1433. doi: 10.1093/bib/bby006. [DOI] [PubMed] [Google Scholar]
- 205.Panda A., Gorospe M. Detection and Analysis of Circular RNAs by RT-PCR. Bio-protocol. 2018;8(6) doi: 10.21769/BioProtoc.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Asha P.K., Blouin R.T., Zaniewski R., Deutscher M.P. Ribonuclease BN: identification and partial characterization of a new tRNA processing enzyme. Proc Natl Acad Sci U S A. 1983;80(11):3301–3304. doi: 10.1073/pnas.80.11.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen D.F., Zhang L.J., Tan K., Jing Q. Application of droplet digital PCR in quantitative detection of the cell-free circulating circRNAs. Biotechnol Biotechnol Equip. 2017;32(1):116–123. [Google Scholar]
- 208.Luo Y., Yang Y., Chien C., Yarmishyn A., Ishola A., Chien Y. Plasma Level of Circular RNA hsa_circ_0000190 Correlates with Tumor Progression and Poor Treatment Response in Advanced Lung Cancers. Cancers. 2020;12(7):1740. doi: 10.3390/cancers12071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schneider T., Schreiner S., Preußer C., Bindereif A., Rossbach O. Northern Blot Analysis of Circular RNAs. Methods in molecular biology (Clifton, NJ). 2018;1724:119–133. doi: 10.1007/978-1-4939-7562-4_10. [DOI] [PubMed] [Google Scholar]
- 210.Szabo L., Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17(11):679–692. doi: 10.1038/nrg.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kocks C., Boltengagen A., Piwecka M., Rybak-Wolf A., Rajewsky N. Single-Molecule Fluorescence In Situ Hybridization (FISH) of Circular RNA CDR1as. Methods in molecular biology (Clifton, NJ). 2018;1724:77–96. doi: 10.1007/978-1-4939-7562-4_7. [DOI] [PubMed] [Google Scholar]
- 212.Dahl M., Daugaard I., Andersen M., Hansen T., Grønbæk K., Kjems J. Enzyme-free digital counting of endogenous circular RNA molecules in B-cell malignancies. Lab Investig J Techn Methods Pathol. 2018;98(12):1657–1669. doi: 10.1038/s41374-018-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schmidt C.A., Noto J.J., Filonov G.S., Matera A.G. A Method for Expressing and Imaging Abundant, Stable, Circular RNAs In Vivo Using tRNA Splicing. Methods Enzymol. 2016;572:215–236. doi: 10.1016/bs.mie.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 214.Xiang J., Yin Q., Chen T., Zhang Y., Zhang X., Wu Z. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24(5):513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bramsen J.B., Laursen M.B., Damgaard C.K., Lena S.W., Babu B.R., Wengel J. Improved silencing properties using small internally segmented interfering RNAs. Nucleic Acids Res. 2007;35(17):5886–5897. doi: 10.1093/nar/gkm548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Herrera-Carrillo E., Harwig A., Berkhout B. Influence of the loop size and nucleotide composition on AgoshRNA biogenesis and activity. RNA Biol. 2017;14(11):1559–1569. doi: 10.1080/15476286.2017.1328349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu Y.P., Schopman N.C., Berkhout B. Dicer-independent processing of short hairpin RNAs. Nucleic Acids Res. 2013;41(6):3723–3733. doi: 10.1093/nar/gkt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Abudayyeh O.O., Gootenberg J.S., Patrick E., Shuo H., Julia J., Belanto J.J. RNA targeting with CRISPR-Cas13. Nature. 2017;550(7675):280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.D.B.T. Cox, JSG, Omar, O. Abudayyeh, Brian Franklin, Max J. Kellner, Julia Joung, Feng Zhang. RNA editing with CRISPR-Cas13 Science. 358 (6366) 2020 1019-1027. [DOI] [PMC free article] [PubMed]
- 220.Jing L., Wu J., Tang X., Ma M., Long F., Tian B. Identification of circular RNA hsa_circ_0044556 and its effect on the progression of colorectal cancer. Cancer Cell Int. 2020;20:427. doi: 10.1186/s12935-020-01523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao H., Zhu M., Limbo O., Russell P. RNase H eliminates R-loops that disrupt DNA replication but is nonessential for efficient DSB repair. EMBO Rep. 2018;19(5) doi: 10.15252/embr.201745335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang A., Zheng H., Wu Z., Chen M., Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10(8):3503–3517. doi: 10.7150/thno.42174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veno M.T., Hansen T.B., Veno S.T., Clausen B.H., Grebing M., Finsen B. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol. 2015;16(1):245. doi: 10.1186/s13059-015-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol Cell. 2015;58(5):870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 225.You X., Vlatkovic I., Babic A., Will T., Epstein I., Tushev G. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18(4):603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang T., Zhang J., Xu Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int J Environ Res Public Health. 2020;17(13):4878. doi: 10.3390/ijerph17134878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Wu M. Epigenetics in Neurodevelopment: Emerging Role of Circular RNA. Front Cell Neurosci. 2019;13:327. doi: 10.3389/fncel.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Park O.H., Ha H., Lee Y., Boo S.H., Kwon D.H., Song H.K. Endoribonucleolytic Cleavage of m(6)A-Containing RNAs by RNase P/MRP Complex. Mol Cell. 2019;74(3):494–507 e498. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 229.Chen R.X., Chen X., Xia L.P., Zhang J.X., Pan Z.Z., Ma X.D. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10(1):4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kooijmans S., Vader P., van Dommelen S., van Solinge W., Schiffelers R. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu K., Xing F., Wu S.Y., Watabe K. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim Biophys Acta, Rev Cancer. 2017;1868(2):538–563. doi: 10.1016/j.bbcan.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pourhanifeh M., Mohammadi R., Noruzi S., Hosseini S., Fanoudi S., Mohamadi Y. The role of fibromodulin in cancer pathogenesis: implications for diagnosis and therapy. Cancer cell international. 2019;19:157. doi: 10.1186/s12935-019-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li Z., Yanfang W., Li J., Jiang P., Peng T., Chen K. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 235.Wan Y., Hopper A.K. Size matters: conserved proteins function in length-dependent nuclear export of circular RNAs. Genes Dev. 2018;32(9–10):600–601. doi: 10.1101/gad.316216.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Huang C., Liang D., Tatomer D.C., Wilusz J.E. A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 2018;32(9–10):639–644. doi: 10.1101/gad.314856.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Preußer C., Hung L., Schneider T., Schreiner S., Hardt M., Moebus A. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Ves. 2018;7(1):1424473. doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dai X., Chen C., Yang Q., Xue J., Chen X., Sun B. Exosomal circRNA_100284 from arsenite-transformed cells, via microRNA-217 regulation of EZH2, is involved in the malignant transformation of human hepatic cells by accelerating the cell cycle and promoting cell proliferation. Cell Death Dis. 2018;9(5):454. doi: 10.1038/s41419-018-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zheng Q., Bao C., Guo W., Li S., Chen J., Chen B. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jia Z., Gong Y., Pi Y., Liu X., Gao L., Kang L. pPB Peptide-Mediated siRNA-Loaded Stable Nucleic Acid Lipid Nanoparticles on Targeting Therapy of Hepatic Fibrosis. Mol Pharm. 2018;15(1):53–62. doi: 10.1021/acs.molpharmaceut.7b00709. [DOI] [PubMed] [Google Scholar]
- 241.Wang Z., Ma K., Cheng Y., Abraham J., Liu X., Ke X. Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Lab Investig J Techn Meth Pathol. 2019;99(10):1442–1453. doi: 10.1038/s41374-019-0273-2. [DOI] [PubMed] [Google Scholar]
- 242.Xu T., Wang M., Jiang L., Ma L., Wan L., Chen Q. CircRNAs in anticancer drug resistance: recent advances and future potential. Molecular cancer. 2020;19(1):127. doi: 10.1186/s12943-020-01240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]