Highlights
-
•
Diagnosis and management of giant juvenile fibroadenoma can be challenging.
-
•
Maintaining symmetry after complete excision is tougher in developing breasts.
-
•
Periareolar approach for subareolar fibroadenomas provides good cosmetic results.
-
•
Benign tumor to breast size ratio up to 70% provides excellent cosmetic outcome.
Abbreviations: GF, giant fibroadenoma; JF, juvenile fibroadenoma; GJF, giant juvenile fibroadenoma; BMI, body mass index; BPT, Benign phyllodes tumor; NAC, nipple areolar complex; PASH, pseudoangiomatous stromal hyperplasia; FNA, fine-needle aspiration
Keywords: Giant fibroadenoma, Juvenile fibroadenoma, Sub-areolar excision, Benign breast lump
Abstract
Introduction
Fibroadenoma is the most common benign lesion of breast in young women, characterized by an aberrant proliferation of both epithelial and mesenchymal elements. It is termed giant fibroadenoma when it is larger than 5 cm or weighs more than 500 g with an incidence of 0.5–2% of all fibroadenomas.
Presentation of case
In this report, we discuss a case of a 13-year-old Pakistani girl who presented with a giant juvenile fibroadenoma in left breast and was treated by a subareolar lump excision through a periareolar incision with excellent cosmetic outcome. To the best of our literature search, this is the first case of giant juvenile fibroadenoma in an adolescent being reported from Pakistan.
Discussion
Surgical management of giant juvenile fibroadenoma in immature breast is challenging as it may either result in asymmetric defect or damage to developing breast tissue resulting in long term poor outcomes. Surgical decision should be carefully undertaken and reported for future reference in such cases.
Conclusion
The diagnosis and management of giant juvenile fibroadenoma can be challenging because these tumors clinically and histologically mimic phyllodes tumor due to their rapid growth and large size. Excision through a periareolar approach for fibroadenomas located in subareolar region provides good cosmetic results in these patients with minimal scar visibility.
1. Introduction
Fibroadenoma is the most common benign lesion of breast in young women [1]. Fibroadenoma is termed giant fibroadenoma (GF) when it is larger than 5 cm or weighs more than 500 g [1,2]. Juvenile fibroadenoma (JF) is a specialized variant of fibroadenoma which commonly occurs in adolescence and it is histologically characterized by stromal hypercellularity, frequent pericanalicular growth pattern and epithelial hyperplasia [1,2]. Increasing levels of estrogen during puberty is suspected to be the likely cause of JF in this age group, however, the exact mechanism is still unknown [[2], [3], [4]]. Depending upon the size of the lump, age of the patient and developmental stage of the breast, management can vary from simple excision to sub-cutaneous mastectomy [[2], [3], [4]]. However, it is particularly important to differentiate a giant juvenile fibroadenoma (GJF) from other benign breast lesions on the basis of clinical, radiological and histological features in order to plan its surgical excision in the most appropriate way, so as to maintain the cosmetic outcome in the immature developing breast [5,9]. We aim to report a case of “Giant Juvenile Fibroadenoma” in a 13-year-old girl who presented with a left breast mass and was treated through subareolar lump excision with excellent cosmetic outcome.
A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. The work has been reported in line with the SCARE criteria [10].
2. Presentation of case
A 13-years-old girl, with a body mass index (BMI) of 14.8, presented to the Breast Surgery Clinic with a rapidly enlarging left breast lump noticed around 15 days back. There was no associated pain, nipple discharge or weight loss. She attained menarche 2 months prior to this presentation. She denied any sexual history, history of chest irradiation or estrogen supplementation. There was no family history of breast or ovarian cancer.
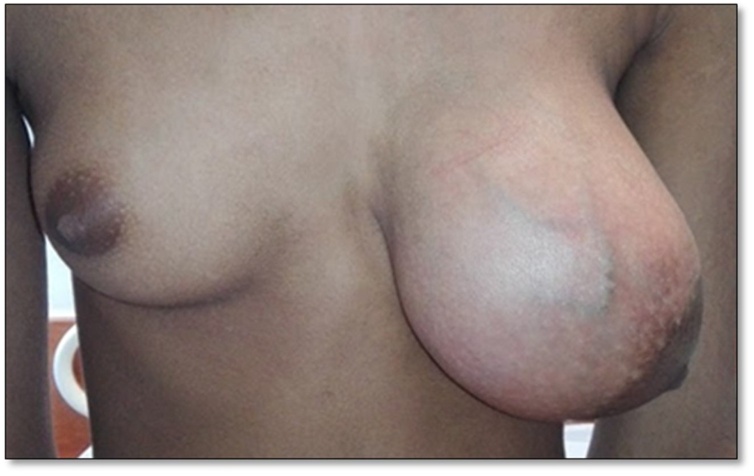
On examination, there was an obvious asymmetry of the breasts with left being significantly larger. There was a 13 × 13 cm, well-defined, firm to hard swelling occupying almost the entire left breast with stretched overlying skin and prominent engorged superficial veins. Posteriorly the mass was free from chest wall. There were no nipple abnormalities or peau d’orange (Fig. 1).
Fig. 1.
A large mass occupying the entire left breast causing its significant enlargement with stretching and outward displacement of nipple areola complex and prominent superficial veins.
Her ultrasound showed a large, predominantly hypoechoic solid mass occupying whole of the left breast with mild peripheral vascularity. No definite cystic spaces were seen. Right breast and both axillae were normal clinically and ultrasonographically.
Trucut biopsy of the mass revealed a fibroepithelial lesion with ductal and stromal elements. The predominant stromal component showed pericanalicular growth pattern and increased cellularity. Leaf-like architecture, nuclear atypia and increased mitoses were not appreciated. Based on histological features, the differential diagnoses on the trucut biopsy were JF and benign phyllodes tumor (BPT).
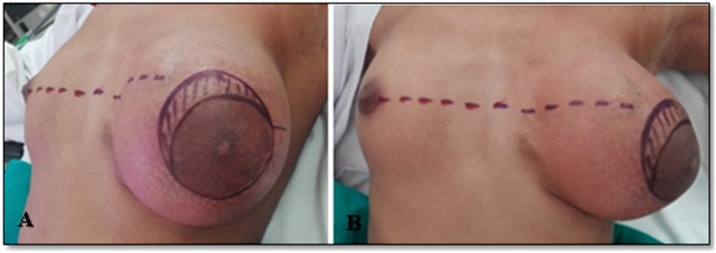
Patient and her family were counseled in detail regarding the need for complete excision of lump. With laxity of the skin and small contralateral breast, restoration of size, shape and symmetry of left breast and nipple areolar complex (NAC) was a major challenge. Also addressing the margin status in case of final histopathology turning out to be phyllodes tumor with scanty breast tissue on left side was also discussed. Possibility of mastectomy with or without reconstruction were kept in mind. Informed written consent was obtained for surgery and for photographs from her guardian in breast clinic. Pre-operative skin marking was done in standing position by the operating breast surgeon (Fig. 2A&B).
Fig. 2.
(A,B): Pre-operative skin markings comparing level of NAC along with the incision mark at upper medial aspect and a sliver of skin (oblique lines) which was removed in an attempt to centralize the NAC.
A periareolar skin incision was made along the medial half of areola. The lump was dissected from surrounding tissues and completely enucleated with ligation of dilated blood vessels. A large cavity and minimal breast tissue was found after removal of lump. A crescent shaped piece of skin was excised from the upper medial aspect of the incision line in order to symmetrize the NAC as compared to contralateral breast. A vacuum drain was placed and the wound was closed in layers using absorbable sutures. The patient had an uneventful postoperative recovery and was discharged home on the first postoperative day. Drain was removed after 6 days.
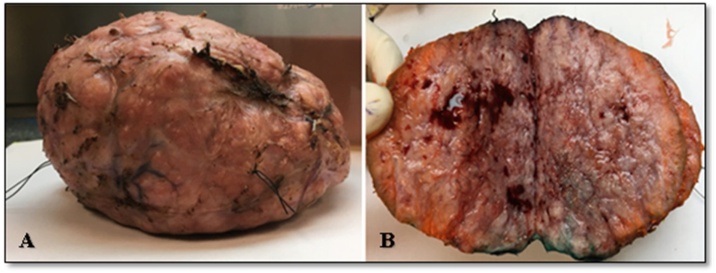
Gross size of the tumor was 13.5 × 11.5 × 6 cm and weight was 570 g. Tumor was well-circumscribed with smooth and slightly bosselated outer surface. Cut surface was tan white, firm and lobulated with frequent blood vessels. (Fig. 3A&B).
Fig. 3.
(A) Gross appearance of tumor showing well circumscribed, smooth and slightly bosselated outer surface and (B) tan white, firm, lobulated cut surface.
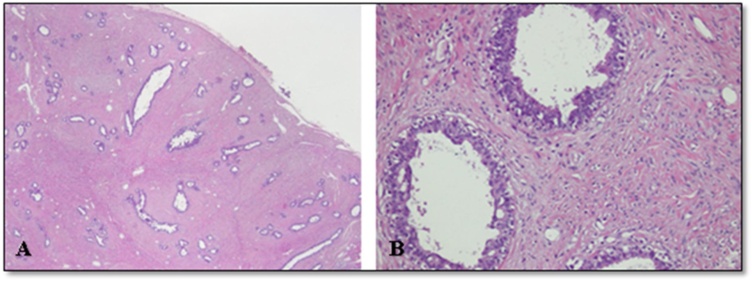
Microscopic examination revealed a well-circumscribed fibroepithelial lesion. The epithelial component comprised of compressed benign breast ducts lined by intact double layer of epithelium. These ducts frequently showed mild usual ductal hyperplasia. The predominant stromal component exhibited pericanalicular growth pattern and increased cellularity. The stromal component was arranged in bundles of spindle shaped cells with moderate amount of eosinophilic cytoplasm and bland, elongated and normochromatic nuclei with finely dispersed chromatin, inconspicuous nucleoli and didn’t exhibit increased mitotic activity. No leaf-like architecture was appreciated and no evidence of malignancy was seen. Hence, the case was diagnosed as GJF (Fig. 4A&B).
Fig. 4.
(A) Low power view of the tumor showing a fibroepithelial lesion surrounded by thin fibrous capsule at the periphery. The stromal component is dominant and it shows pericanalicular growth pattern. (B) High power view showing epithelial hyperplasia and stromal cells lacking nuclear atypia and increased mitoses.
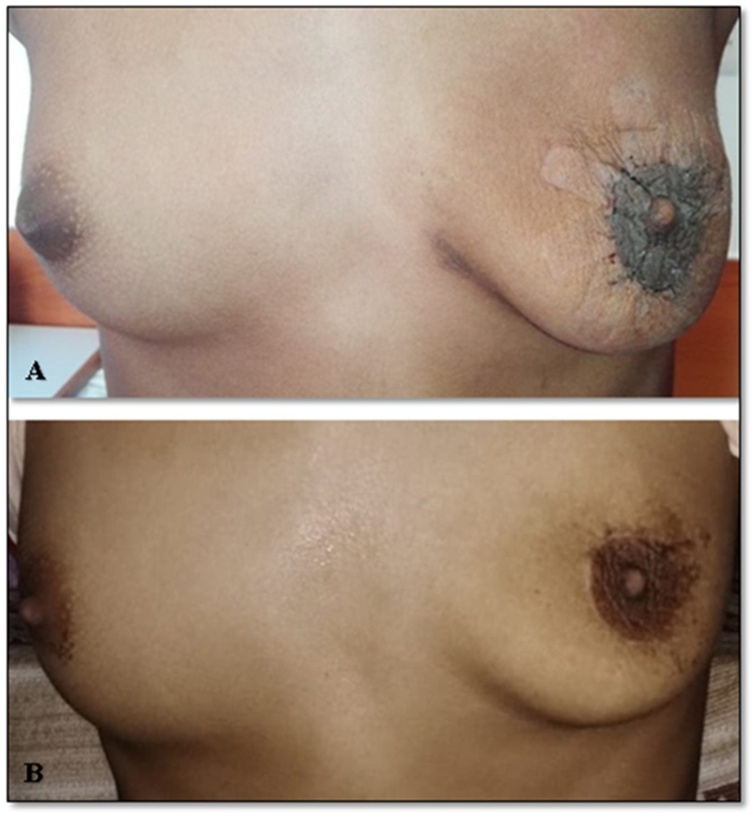
At 2 weeks, her wound had healed and she did not seem to need any further procedures with regard to the skin laxity. She had obtained a reasonable cosmetic outcome. At 1 year, the scar was barely visible at the limbus and both NACs were symmetrical and at the same level (Fig. 5A&B). The outcome considering her initial presentation was excellent and both the patient and guardians were satisfied. She will continue to receive follow-up until a final decision for her cosmetic outcome can be undertaken once her breasts are fully developed.
Fig. 5.
(A) Appearance of breasts at 2 weeks follow up showing regression of skin elasticity and a near normal appearance of left breast. (B) At 1 year follow-up with excellent cosmetic outcome.
3. Discussion
Fibroadenomas are common below 30 years of age [1]. They are divided into adult-type and juvenile-type. JF occurs between the ages of 10 and 18 years [2]. They are mostly solitary and can be unilateral or bilateral with 10–20% occurring in a multiple fashion [3]. It is usually well defined, mobile and encapsulated [2,3]. Unopposed estrogen stimulation, increased estrogen receptor sensitivity or diminished estrogen antagonist sensitivity are thought to be the chief causative factors [4].
A fibroadenoma within the breast which is more than 5 cm in any dimension, weighing more than 500 g, rapidly growing, or disproportionally large as compared to rest of the breast is termed as GF [5]. Both adult and juvenile-types of fibroadenoma can acquire the size and weight of GF. GJF comprises 0.5–2% of all fibroadenomas and is characterized by rapid growth, large size, stretching of the overlying skin, and dilatation of superficial veins [6]. Most cases of GJF are reported in African-American race, with only few case reports from Asia [7,8].
It is extremely important to differentiate GJF from other similar pathologies such as BPT, which usually occur in women between 35–55 years of age and are characterized by increased cellularity and increased propensity for recurrence and metastasis [9]. Histologically, BPT is differentiated by the presence of leaf-like structures and stromal cell atypia. The stromal cellularity is usually heterogenous in BPT while the hypercellular stroma is homogenously spread in JF. Subepithelial stromal condensation is also a feature of BPT which is usually not seen in JF. Mitotic figures are rare in JF occurring in adults while increased mitoses can be seen in younger patients [11,12].
Adult-type fibroadenoma usually shows low stromal cellularity and combination of pericanicular and intracanalicular growth patterns. Other mesenchymal lesions such as pseudoangiomatous stromal hyperplasia (PASH), myofibroblastoma and myoid hamartoma are seldom included in the differential diagnosis of JF. The ductal elements in these lesions are usually entrapped rather than integral component of the lesion intermingled with stromal component [12].
Ultrasound is the first step in evaluation of such lumps in young patients in order to rule out malignancy with a negative predictive value of 99.5% [13]. Tumors greater than 3 cm in young patients are more likely to be diagnosed as phyllodes tumor, therefore, tissue biopsy is necessary to establish the diagnosis. Fine-needle aspiration (FNA) biopsy does not differentiate BPT from GF due to lack of definite tissue for accurate characterization, hence, core needle biopsy is required [14].
The goal of treatment with such large tumors is not just its complete excision, but preservation of the NAC and achievement of symmetrical breasts also pose a bigger challenge. Avoidance of ugly or obvious scar also needs consideration, especially in young patients who may already have a psychological impact due to such large tumors. In our patient, we used a periareolar incision in order to minimize scarring. Axillary and sub-mammary incisions have also been used by various surgeons depending upon the size and tumor location [15]. Tumor to breast size ratio is an extremely important predictor of cosmetic outcomes. According to a surgical algorithm prepared by Lim et al., in large non-malignant tumors of breast, tumor to breast size ratio of up to 70% is considered to have a good to excellent cosmetic outcome [16]. In our patient, due to her young age, low BMI and under developed breasts, her tumor to breast size ratio approached almost 70%, hence a good cosmetic outcome was expected. Reconstructive methods were previously considered for resection of large breast lesions in order to achieve breast symmetrization [17]. However, a systematic review has shown that reconstructive surgery is not warranted for all GF [18]. Simple mastectomy may only be considered if the breast is too small or is almost totally occupied by the GF, in that case, an immediate reconstruction can be undertaken [18]. Long-term follow-up is required in such cases to address any cosmetic issues after the breasts are fully developed [15,19].
To the best of our knowledge and literature search, this is the first case of GJF in a 13-year-old girl reported from Pakistan, who has undergone a successful surgical excision with excellent cosmetic outcomes.
4. Conclusion
The diagnosis and management of GJF can be challenging because these tumors clinically and histologically mimic BPT due to their rapid growth and large size. In young females with developing breasts, the challenge gets tougher in order to maintain the breast shape and symmetry. Excision through a periareolar approach for fibroadenomas located in subareolar region provides good cosmetic results with minimal scar visibility.
5. Learning points
-
•
The diagnosis and management of giant juvenile fibroadenoma can be challenging because these tumors clinically and histologically mimic phyllodes tumor due to their rapid growth and large size.
-
•
In young females with developing breasts, the challenge gets tougher in order to maintain the breast shape and symmetry after complete excision of such large tumors.
-
•
Excision through a periareolar approach for fibroadenomas located in subareolar region provides good cosmetic results in these patients with minimal scar visibility.
-
•
An excellent cosmetic outcome can be achieved if the tumor to breast size ratio approaches 70% in non-malignant tumors of breast.
6. Patient’s perspective (patient’s maternal aunt)
We live in a village far away from the city where our niece’s breast surgery was done. Our niece was very shy to share her condition with anyone and kept her breasts hidden with her dupatta (a piece of cloth to cover the chest over clothes). It came to our attention when her mother noticed the huge size discrepancy and we brought her to Karachi immediately for her treatment. It has been 1 year since her surgery. Our niece feels relaxed now after removal of the heavy mass from her breast and we are satisfied with the result. We do not want to pursue any further corrective surgery. We are happy with her surgical outcome.
Declaration of Competing Interest
None.
Funding
None.
Ethical approval
Approval as exemption has been taken from Ethical Review Committee of The Aga Khan University with manuscript number: 2020-5472-13928.
Consent
Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Sana Zeeshan (corresponding author): Conception and design, Acquisition of data, Drafting the article, Critical revision of the article, Final approval of the version to be published.
Dr. Kulsoom Shaikh - Acquisition of data, Writing the paper, Critical revision of the article, Final approval of the version to be published.
Dr. Muhammad Usman Tariq - Acquisition of data, Critical revision of the article, Final approval of the version to be published.
Dr. Lubna Mushtaque Vohra - Critical revision of the article, Final approval of the version to be published.
Registration of research studies
Not applicable.
Guarantor
Dr. Sana Zeeshan.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
None.
Contributor Information
Sana Zeeshan, Email: sana.zeeshan@aku.edu.
Kulsoom Shaikh, Email: kulsoom.shaikh@aku.edu.
Muhammad Usman Tariq, Email: Mohammad.usman@aku.edu.
Lubna Mushtaque Vohra, Email: lubna.vohra@aku.edu.
References
- 1.Jayasinghe Y., Simmons P.S. Fibroadenomas in adolescence. Curr. Opin. Obstet. Gynecol. 2009;21(October (5)):402–406. doi: 10.1097/GCO.0b013e32832fa06b. [DOI] [PubMed] [Google Scholar]
- 2.Park C.A., David L.R., Argenta L.C. Breast asymmetry: presentation of a giant fibroadenoma. Breast J. 2006;12(September (5)):451–461. doi: 10.1111/j.1075-122X.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 3.Matz D., Kerivan L., Reintgen M., Akman K., Lozicki A., Causey T., Clynes C., Giuliano R., Acs G., Cox J., Cox C. Breast preservation in women with giant juvenile fibroadenoma. Clin. Breast Cancer. 2013;13(June (3)):219–222. doi: 10.1016/j.clbc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Musio F., Mozingo D., Otchy D.P. Multiple, giant fibroadenoma. Am. Surg. 1991;57(July (7)):438–441. PMID: 1647714. [PubMed] [Google Scholar]
- 5.Cyrlak D., Pahl M., Carpenter S.E. Breast imaging case of the day. Multiple giant fibroadenomas associated with cyclosporin A therapy. Radiographics. 1999;19(2):549–551. doi: 10.1148/radiographics.19.2.g99mr24549. [DOI] [PubMed] [Google Scholar]
- 6.Dolmans G.H., Hoogbergen M.M., van Rappard J.H. Giant fibroadenoma of one breast: immediate bilateral reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2007;60(October (10)):1156–1157. doi: 10.1016/j.bjps.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Song B.S., Kim E.K., Seol H., Seo J.H., Lee J.A., Kim D.H., Lim J.S. Giant juvenile fibroadenoma of the breast: a case report and brief literature review. Ann. Pediatr. Endocrinol. Metab. 2014;19:45–48. doi: 10.6065/apem.2014.19.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugburo A.O., Olajide T.O., Fadeyibi I.O., Mofikoya B.O., Lawal A.O., Osinowo A.O. Differential diagnosis and management of giant fibroadenoma: comparing excision with reduction mammoplasty incision and excision with inframammary incision. J. Plast. Surg. Hand Surg. 2012;46:354–358. doi: 10.3109/2000656X.2012.697066. [DOI] [PubMed] [Google Scholar]
- 9.Reinfuss M., Mituś J., Duda K., Stelmach A., Ryś J., Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer. 1996;77(March (5)):910–916. doi: 10.1002/(SICI)1097-0142(19960301)77:5<910::AID-CNCR16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 11.AlGhamdi S., Ali A.G., Ali S.N., Rasheed K., Yousef Y. Giant juvenile fibroadenoma of breast in adolescent girls. J. Pediatr. Surg. Case Rep. 2018;28(January):33–36. doi: 10.1016/j.epsc.2017.09.029. [DOI] [Google Scholar]
- 12.Brogi E. Fibroepithelial neoplasms. In: Hoda S.A., Brogi E., Koerner F.C., Rosen P.P., editors. Rosen’s Breast Pathology. 4th edition. Lippincott Williams & Wilkins; Philadelphia, PA (USA): 2014. pp. 213–270. [Google Scholar]
- 13.Gobbi D., Dall’Igna P., Alaggio R., Nitti D., Cecchetto G. Giant fibroadenoma of the breast in adolescents: report of 2 cases. J. Pediatr. Surg. 2009;44(February (2)):e39–41. doi: 10.1016/j.jpedsurg.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Sosin M., Pulcrano M., Feldman E.D., Patel K.M., Nahabedian M.Y., Weissler J.M., Rodriguez E.D. Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations. Gland Surg. 2015;4(August (4)):312. doi: 10.3978/j.issn.2227-684X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam S., Saroop S., Bheem V. Largest giant juvenile fibroadenoma of the breast. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-227277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim G.H., Ng R.P., Leong L.C.H. Development of a surgical algorithm by using preoperative imaging to predict mammoplasty cosmetic outcomes for large nonmalignant tumours. Gland Surg. 2017;6:649–653. doi: 10.21037/gs.2017.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang D.S., McGrath M.H. Management of benign tumors of the adolescent breast. Plast. Reconstr. Surg. 2007;120(July (1)):13e–19e. doi: 10.1097/01.prs.0000264396.03452.62. [DOI] [PubMed] [Google Scholar]
- 18.Sosin M., Pulcrano M., Feldman E.D., Patel K.M., Nahabedian M.Y., Weissler J.M., Rodriguez E.D. Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations. Gland Surg. 2015;4(August (4)):312. doi: 10.3978/j.issn.2227-684X.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang I.C., Li P.C., Ding D.C. Recurrent juvenile fibroadenoma of the breast in an adolescent: a case report. Medicine. 2018;97(May (20)) doi: 10.1097/MD.0000000000010765. [DOI] [PMC free article] [PubMed] [Google Scholar]