Abstract
Introduction Smoking is one of the most important causes of mortality and morbidity in the world, as it is related to the risk factor and etiology of respiratory-tract diseases. Long-term smoking causes both structural and functional damage in the respiratory airways, leading to changes in nasal mucociliary clearance (NMC).
Objectives The aim of the present study was to look systematically into the current literature and carefully collect and analyze results to explore NMC in smokers.
Data Synthesis Two independent reviewers conducted a literature search on some Electronic database: Pubmed, Medline, Ebsco, Springer Link, Science Direct, Scopus, and Proquest searching for articles fulfilling the inclusion and exclusion criteria. The lead author independently assessed the risk of bias of each of the included studies and discussed their assessments with the other two authors to achieve consensus. Of the 1,654 articles identified in the database search, 16 met the criteria for this review. Most of the articles (15 out of 16) showed the impairment of NMC in smokers.
Conclusion The present systematic review suggests that there is an impairment of NMC in smokers. The impairment is not only observed in cigarette smoking, but also in passive smoking, bidi smoking, electronic smoking, and hookah smoking. The impairment of NMC in chronic exposure to smoking is caused by the ciliotoxic effect, hypersecretion and viscoelastic change of mucous, airway surface liquid depletion, increased oxidative stress, and deteriorations in the inflammatory and immune systems.
Keywords: nasal mucociliary clearance, smokers, smoking
Introduction
Smoking is one of the most important causes of mortality and morbidity in the world, especially in developing countries. 1 2 3 4 According to the World Health Organization (WHO), there are around 1.1 billion smokers in the world, and half of them die every year (2015). 1 There are many types of smoking, such as cigarette smoking, bidi smoking, hookah smoking, electronic cigarette (EC) smoking, passive smoking, and many more. 2 3 4 5 6 7 All of these types of smoking are related to the risk factor and etiology of many health problems, especially in the respiratory tract. In the respiratory tract, smoking is related to upper respiratory tract infection, asthma, chronic obstructive pulmonary disease, nasopharyngeal cancer, and lung cancer. 2 8 9 10 Smoking is a significant risk factor for respiratory diseases, considering its ability to lead to an alteration of nasal mucociliary clearance (NMC). 2 11 12
Nasal mucociliary clearance is the primary innate defense mechanism of the nose and paranasal sinuses, and it consists of mucous layer, airway surface liquid layer, and ciliary epithelia. 2 13 14 15 Mucus produced by the goblet cells of the mucosa is required for binding of the airborne pathogens (such as inhaled microbes and irritans). Ciliated epithelial cells are expectorated or swallowed. Normal functioning of the NMC requires high frequency, coordinated, and directional ciliary beating (metachronal waves) as well as proper mucus secretion and airway surface liquid. Ciliary beat frequency (CBF) was shown to be the major determinant of NMC effciency. 2 13 14 15 Nasal mucociliary clearance is influenced by physiological factors, such as mucus production and CBF; anatomic factors, such as nasal airflow and patency of the sinus ostia in the prechambers; and biochemical factors, such as mucus composition. 2 13 14 15 16
Various factors, like aging, body temperature, drugs (like adrenaline, acetlycholine, corticosteroid, and intranasal drug), tobacco use and smoking, and environment factors (like pollutant, smoke, and dust) affect this system, besides pathological conditions such as rhinits allergy, acute or chronic rhinosinusitis, and deviated nasal septum. 2 17 18 19 Any dysfunction in this defense system increases the inflammatory process and stasis of airborne pathogens, and the respiratory system becomes prone to obstructive airway diseases and infections. 2 11 12 Long-term smoking causes both structural and functional damage in the respiratory airways, leading to changes in NMC. 2 4 11 12 20
The adverse effects of smoking on NMC have been reported and explained in various studies, but we could not find any systematic review about NMC in smokers. The aim of the present study was to look systematically into the current literature and carefully collect and analyze results to explore NMC in smokers.
Review of the Literature
Scope of the Review: Inclusion and Exclusion Criteria
Inclusion criteria:
-
Publication type:
full-text articles discussing NMC in smokers
primary studies of every design (case study, case series, cross sectional, case control, cohort, clinical trial)
Languange of publication: English
Time of publication: January 2000 to August 2019
Methodology: studies included must explain NMC in smokers
Population: human at any age
Exclusion criteria:
Population: in-vitro samples
Objective and outcome measures are not relevant (are not about NMC in smokers)
Confounding variables are related to outcome of NMC in smokers
Literature Search
The current systematic review was conducted in accordance with Cochrane handbook for systematic reviews and is reported by using the guidelines of the preferred reporting items for systematic review and meta-analysis (PRISMA). 21 22 A systematic search strategy was conducted in the following electronic databases: Pubmed, Medline, Ebsco, Springer Link, Science Direct, Scopus, and Proquest. The search was conducted using the following keywords for title and abstract: nasal mucociliary clearance OR nasal mucociliary transport AND smoke OR smoker OR smoking OR cigarette . In the Pubmed database, the keywords were searched through the [tiab] and [MeSH] tags. No limitation was applied during the search. The reference lists of the retrieved papers were also examined to avoid missing any published data.
Data Collection and Analysis
Studies were selected for retrieval after two independent reviewers (A. P. and U. S.) had collected titles and abstracts identified in the electronic search. The results of the two reviewers were compared by a third independent reviewer (J. B.), and any differences of opinion were resolved by discussion. Full papers from potential studies were independently assessed by the investigators (A. P. and U. S.).
All studies selected for this systematic review were screened by two reviewers indepently to validate the results (A. P. and U. S.). The data from all retrieved studies are presented in the summary table ( Table 1 ) featuring key points of each study. The following data were collected: first author and year, study design, sample, sample characteristic (age and gender), smoking characteristic (type, years of smoking, cigarettes-/day or packs/year), NMC measurement test, and result.
Table 1. Newcastle-Ottawa scale (prospective study).
| No. | First author, year | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | ||||
| 1. | Dülger et al, 2018 4 | * | * | * | * | * | 5 | |||
| 2. | Utiyama et al, 2016 11 | * | * | * | * | * | * | * | 7 | |
| 3 | Yadav et al, 2014 7 | * | * | ** | * | * | * | 7 | ||
| 4 | Ramos et al, 2011 31 | * | * | ** | * | * | * | 7 | ||
Maximum points for comparability were 2.
Quality Assesment
The lead author independently assessed the risk of bias of each of the included studies and discussed their assessments with other two authors to achieve consensus. The Newcastle-Ottawa scale adapted for cross-sectional studies, Newcastle-Ottawa scale cohort version, and the Cochrane risk of bias were used to assess the methodological quality of the studies. 21 23 24 25 The Newcastle-Ottawa scale adapted for cross-sectional studies was used to assess cross sectional studies; interpretation of the total score was: 9 to 10 points were considered very good studies, 7 to 8 points were considered good studies, 5 to 6 points were considered satisfactory studies, and 0 to 4 were considered unsatisfactory studies. 23 The Newcastle-Ottawa scale cohort version was used to asses prospective studies; interpretation of the total score was: ≥ 7 points were considered good studies, 5 to 6 points were considered fair studies, and < 5 points were considered poor studies. 24 25 26 27 RThe Cochrane risk of bias was used to assess randomized control trial studies, whose results were either high risk or some concerns or low risk. 21
Results
Selection of Articles for Review
Fig. 1 summarized the identified, screened, and included articles for review. Initially, 1,622 peer-reviewed articles were identified from electronic databases, and an additional 32 articles were identified through other sources (search engine). After removing duplicates, 384 articles remained for title and abstract screening. Articles that did not meet the inclusion and exclusion criteria were not further screened. Twenty-six articles were screened for eligibility, 16 of which met all the inclusion criteria.
Fig. 1.
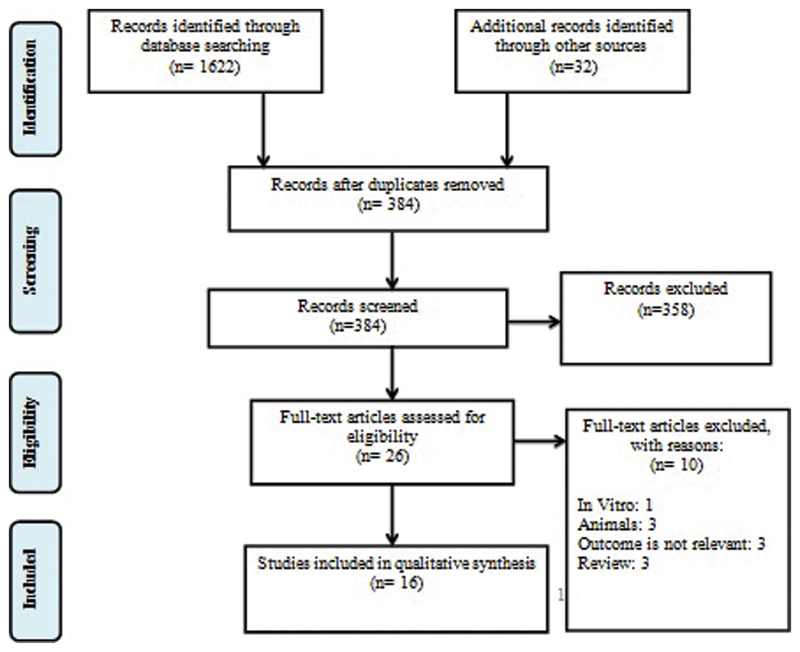
Preferred reporting items for systematic reviews and meta-analysis (PRISMA). 22
Assesment of Study Validity (Risk of Bias)
All eligible studies were associated with NMC in smokers. Table 2 provides quality scores for cross-sectional studies; all studies that got 6 to 8 points were included in the satisfactory and good studies category. Table 1 provides quality scores for prospective studies; all studies that gor 5 to 7 points were included in the fair and good studies category. Table 3 provides quality scores for randomized control trial studies; all studies presented results of some concerns or low risk.
Table 2. Newcastle-Ottawa scale adapted for cross-sectional studies.
| No. | First author, year | Selection | Comparability | Outcome | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | ||||
| 1. | Arıcıgil M and Arbağ, 2018 5 | * | ** | ** | * | 6 | |||
| 2. | Paul et al, 2018 3 | * | * | ** | ** | * | 7 | ||
| 3. | Solak et al, 2018 2 | * | ** | ** | * | 6 | |||
| 4. | Uzeloto et al, 2018 48 | * | ** | ** | * | 6 | |||
| 5. | Habesoglu et al, 2015 29 | * | ** | ** | * | 6 | |||
| 6. | Pagliuca et al, 2015 12 | * | ** | ** | * | 6 | |||
| 7. | Baby et al, 2014 17 | * | ** | ** | * | 6 | |||
| 8. | Nicola et al, 2014 28 | * | ** | ** | * | 6 | |||
| 9. | Xavier et al, 2013 20 | * | * | * | ** | * | 7 | ||
| 10. | Habesoglu et al, 2012 38 | * | ** | ** | ** | * | 8 | ||
| 11. | Proença et al, 2011 35 | * | ** | ** | ** | * | 8 | ||
Maximum points for selection number 4, comparability, and outcome number 1 were 2.
Table 3. Cochrane risk of bias: Kumral TL, 2016 6 .
| No. | Domain | Description of domain | Results |
|---|---|---|---|
| 1. | Domain 1 | risk of bias arising from the randomization process | some concerns |
| 2. | Domain 2 | risk of bias due to deviations from the intended interventions ( effect of adhering to intervention ) | some concerns |
| 3. | Domain 3 | missing outcome data | low risk |
| 4. | Domain 4 | risk of bias in measurement of the outcome | low risk |
| 5. | Domain 5 | risk of bias in selection of the reported result | low risk |
Study Characteristic
Study characteristics for the included studies could be seen in Table 4 . The majority of the studies followed the cross-sectional design (11 out of 16). Most of the samples were in productive age and reported cigarette smoking. Nasal mucociliary clearance measurement tests mostly used saccharin transfer/transit time test.
Table 4. Study characteristics.
| No. | First author, year | Study design | Sample (N) | Sample characteristic: age (year), gender (male, female) | Smoking characteristic: type, years of smoking, cigarettes/day and/or packs/year | NMC measurement test | Result |
|---|---|---|---|---|---|---|---|
| 1. | Arıcıgil and Arbağ , 2018 5 | Cross-sectional | Non-smokers: 40 Smokers I (once every week): 20 Smokers II (more than once a week/2–5 session-week): 18 |
Age: 18–41 years Non-smokers: 27.5 ± 6.4 Smokers I: 26.9 ± 6.8 Smokers II: 27.7 ± 6.3 Gender Non-smokers: 22,18 Smokers I: 11,9 Smokers II: 10,8 |
Hookah smoking | Saccharin transfer/transit time test |
• NMC value (STT) in smokers was significantly higher than in non smokers (
p
< 0.001).
• NMC value (STT) in the smokers-II group was significantly higher than in the smokers-I group and non-smokers (19.2 ± 2.5; 11.9 ± 2.8; 11.1 ± 3; p < 0.001). |
| 2. | Dulger et al, 2018 4 | Prospective study (2 years period) | Non-smokers: 35 Smokers: 50 |
Age: 18–65 years Non-smokers: 35.8 Smokers: 31.4 Gender Non-smokers: 23,12 Smokers: 30,20 |
Cigarette smoking Cigarettes/day: 20.6 Packs-year: ≤10 packs/year: 11 10–20 packs/year: 28 20–30 packs/year: 5 ≥ 30 packs/year: 6 |
Saccharin transfer/transit time test |
• NMC value (STT) in smokers was significantly higher than in non-smokers (12 minutes, 9 minutes;
p
< 0.001).
• No stastistically significant difference in nasal MCC value and packs/year ( p = 0.943). |
| 3. | Paul et al, 2018 3 | Cross-sectional | Non-smokers: 20 Cigarette smokers: 20 Bidi smokers: 20 |
Age: 20–40 years Non-smokers: 29.3 ± 6.25 Cigarette smokers: 31.2 ± 5.42 Bidi smokers: 30.2 ± 6.77 Gender: male |
Cigarette and Bidi smoking Packs/year: Cigarette smokers: 4.63 ± 2.74 Bidi smokers: 7.5 ± 5.1 |
Methylene blue dye test |
• NMC value was significantly decreased in bidi smokers as compared with cigarette smokers and non-smokers (59.25 ± 12.38 mm; 67 ± 5.48 mm; 67.89 ± 4.10 mm;
p
< 0.05).
• Multivariate analysis revealed a significant association between NMC and bidi smoking, number of cigarettes or bidis smoked per day, and packs/year ( p < 0.05). |
| 4. | Solak et al, 2018 2 | Cross-sectional | Non-smokers: 74 Smokers: 123 |
Age: 18–55 years Non-smokers: 38.79 ± 9.66 Smokers: 40.33 ± 8.94 Gender Non-smokers: 58,16 Smokers: 23,100 |
Cigarette smoking Years of smoking: 24.31 ± 9.66 Cigarettes/day: 18.45 ± 8.78 Packs/year: 28.49 ± 15.68 |
Saccharin transfer/transit time test |
• NMC value (STT) in smokers was significantly higher than in non-smokers (536.19 ± 254.81 seconds 320.43 ± 184.98 seconds
p
< 0.001).
• Positive correlation between STT and number of cigarettes/day (p: 0.012, r: 0.225), STT and packs/year (p:0.001, r:0.296), STT and years of smoking ( p = 0.027, r = 0.200). |
| 5. | Uzeloto et al, 2018 48 | Cross-sectional | Non-smokers: 69 Smokers: 70 |
Age: 30–50 years Non-smokers: 39.5* Smokers: 41* Gender Non-smokers: 32,37 Smokers: 33,37 *: median |
Cigarette smoking Years of smoking: 21.50* Cigarettes/day: 20.00* Packs/year: 22.00* *: median |
Saccharin transfer/transit time test | NMC value (STT) in smokers was insignificantly higher than in non-smokers (9.7 minutes; 9.145 minutes; p > 0.05). |
| 6. | Kumral et al, 2016 6 | Prospective randomized single-blind clinical trial | Non-smokers: 40 Smokers: 58 |
Age Non-smokers: 38.0 ± 8.2 Smokers: 33.9 ± 7.9 Gender Non-smokers: 16,14 Smokers: 18,24 |
EC smoking duration: 3 months |
Saccharin transfer/transit time test | NMC value (STT) in electronic cigarette was significantly higher than in non-smokers (11.93 ± 1.81; 10.36 ± 1.61; p = 0.0003). |
| 7. | Utiyama et al, 2016 11 | Prospective study (12 months duration) | Quitters: 20 Smokers: 13 |
Age Quitters: 51 ± 9 Smokers: 52 ± 10 Gender Quitters: 9,11 Smokers: 6,7 |
Cigarette smoking Packs/year: Quitters: 40 ± 27 Smokers: 45 ± 28 |
Saccharin transfer/transit time test | • NMC value (STT) in smokers showed increases of impairment after 12 months observation (±14 minutes; ± 15 minutes). • NMC value (STT) in quitter showed decreases of impairment after 12 months observation (±15 minutes, ± 10 minutes). |
| 8. | Habesoglu et al, 2015 29 | Cross-sectional | Group I (control): 18 Group II (living with at least one adult smoker outside the house): 15 Group III (living with at least one adult smoker inside the house): 17 |
Age 6–14 years Group I: 10.22 ± 2.39 Group II: 11.2 ± 1.97 Group III: 10.65 ± 2.09 Gender Group I: 9,9 Group II: 7,8 Group III: 9,8 |
Passive smoking | Saccharin transfer/transit time test |
• NMC value (STT) in group II was insignificantly higher than in group I (
p
= 0.067).
• NMC value (STT) in group III was significantly higher than in group I ( p < 0.001). • NMC value (STT) in group III was insignificantly higher than in group II ( p = 0.173). • NMC value (STT) in groups I, II, and III: 7.33 ± 2.91 minutes, 10.00 ± 4.78 minutes, 12.41 ± 3.44 minutes. |
| 9. | Pagliuca et al, 2015 12 | Cross-sectional | Non-smokers: 30 Ex-smokers:30 Smokers:30 |
Age Non-smokers: 53.17 ± 5.53 Ex-smokers: 50.73 ± 6.51 Smokers: 51.97 ± 6.02 Gender Non-smokers: 19,11 Ex-smokers: 23,7 Smokers: 24,6 |
Cigarette smoking Cigarettes/day: ex-smokers:25 ± 7.76 smokers: 24.67 ± 6.3 |
Saccharin transfer/transit time test | NMC value (STT) in smokers was significantly higher than in others (smokers: 15.6 minutes, ex-smokers: 11.77 minutes, non-smokers: 11.71 minutes, p < 0.0001). |
| 10. | Baby et al, 2014 17 | Cross-sectional | Non-smokers: 30 Smokers: 30 |
Age: 21–40 years Non-smokers: 26.8 ± 1.2 Smokers: 24.96 ± 1 Gender: male |
Cigarette smoking | Saccharin transfer/transit time test |
• NMC value (STT) in smokers was significantly higher than in the non-smokers group (481.2 ± 29.83 seconds 300.32 ± 17.42 seconds;
p
< 0.01).
• A statistically significant increase in the NMC value was observed with an increase in duration of smoking habit (NMC in smoking < 1 year: 492.25 ± 79.93 seconds; 1–5 years: 516.7 ± 34.01 seconds > 5 years: 637.5 ± 28.49 seconds p = 0.0000). |
| 11. | Nicola et al, 2014 28 | Cross-sectional | Non-smokers: 32 Smokers < 2.5 packs/year: 20 Smokers > 2.5 packs/year: 20 |
Age: 18–35 years Non-smokers: 21 Smokers <2.5 packs-year: 19 Smokers >2.5 packs-year: 24 Gender Non-smokers: 29, 3 Smokers <2.5 packs-year: 20,0 Smokers >2.5 packs-year: 17,3 |
Cigarette smoking Years of smoking Smokers < 2.5 packs/year: 3* Smokers > 2.5 packs/year: 7* Packs/year: Smokers < 2.5 packs/year: 0.7* Smokers > 2.5 packs/year: 6* *: median |
Saccharin transfer/transit time test | • NMC value (STT) in smokers was significantly lower than in non-smokers (5.9 ± 3.1 minutes; 7.7 ± 4.1 minutes; p = 0.033). |
| 12. | Yadav et al, 2014 7 | Prospective study (five years duration) | Non-smokers: 50 Active smokers: 50 Passive smokers: 50 |
Age: 20–50 years Non-smokers: 38.7 Active smokers: 39.1 Passive smokers: 35.1 Gender: Non-smokers: 44,6 Active smokers: 42,8 Passive smokers: 24,26 |
Cigarette smoking (active and passive) | Saccharin transfer/transit time test |
• NMC value (STT) in smokers (active and passive) was significantly higher than in non-smokers (23.08 ± 4.60; 20.31 ± 2.51; 8.57 ± 2.12;
p
< 0.0003).
• NMC value (STT) in active smokers was insignificantly higher than in passive smokers ( p = 0.03). |
| 13. | Xavier et al, 2013 20 | Cross-sectional | Non-smokers: 24 Light smokers: 14 Moderate smokers: 34 Heavy smokers: 27 |
Age: Non-smokers: 50 ± 11 Light smokers: 51 ± 15 Moderate smokers: 49 ± 7 Heavy smokers: 46 ± 8 Gender Non-smokers: 7,17 Light smokers: 5,9 Moderate smokers: 14, 20 Heavy smokers: 13, 14 |
Cigarette smoking Years of smoking Light smokers: 32 ± 17 Moderate smokers: 29 ± 9 Heavy smokers: 32 ± 8 Cigarettes/day: Light smokers: 9 ± 1 Moderate smokers: 18 ± 3 Heavy smokers: 39 ± 11 Packs/year index: Light smokers: 17 ± 10 Moderate smokers: 27 ± 11 Heavy smokers: 59 ± 44 |
Saccharin transfer/transit time test |
• NMC value (STT) in moderate and heavy smokers was significantly higher (
p
= 0.0001) than in light smokers and non-smokers.
• A positive correlation was observed between STT and cigarettes/day (r = 0.3; p = 0.02). |
| 14. | Habesoglu et al, 2012 38 | Cross-sectional | Non-smokers: 15 Passive smokers: 15 Active smokers: 17 |
Age: 17–47 years Active smokers: 28.12 ± 10.79 Passive smokers: 29.17 ± 12.18 Non-smokers: 27.92 ± 11.29 Gender Active smokers: 11,6 Passive smokers: 8,7 Non-smokers: 6,9 |
Cigarette smoking (active and passive) | Saccharin transfer/transit time test |
• NMC value (STT) in active smokers was significantly higher than in passive smokers and non-smokers (23.59 ± 12.41 minutes; 12.6 ± 4.67 minutes; 6.4 ± 1.55 minutes;
p
< 0.01).
• NMC value (STT) in the passive group was significantly higher than in the non-smokers group ( p < 0.01). |
| 15. | Proença et al, 2011 35 | Cross-sectional | Non-smokers: 19 Smokers: 19 |
Age Non-smokers: 47 ± 11 Smokers: 51 ± 16 Gender: Non-smokers: 10,9 Smokers: 11,8 |
Cigarette smoking Years of smoking: 33 ± 11 Cigarettes/day: 27 ± 11 Packs/year index: 44 ± 25 |
Saccharin transfer/transit time test |
• NMC value (STT) in smokers was significantly higher than in non-smokers 8 hours after smoking (16 ± 6 minutes; 10 ± 4 minutes,
p
= 0.005) and insignicantly higher immediately after smoking (11 ± 6 minutes; 10 ± 4 minutes;
p
= 0.87).
• NMC value (STT) 8 hours after smoking was correlated positively with age (r = 0.59; p = 0.007), cigarettes per day (r = 0.53; p = 0.02) and packs/year index (r = 0.74; p = 0.0003). |
| 16. | Ramos et al, 2011 31 | Prospective study (1 year-period) | Non-smokers: 33 Smokers: 33 |
Age Non-smokers: 52 ± 14 Smokers: 49 ± 12 Gender: Non-smokers: 18, 15 Smokers: 12,21 |
Cigarette smoking Years of smoking: 21 ± 8 Cigarettes/day: 20 ± 10 Packs/year index: 44 ± 25 |
Saccharin transfer/transit time test | • NMC value (STT) in smokers was significantly higher than in non-smokers (±14 minute, ± 8 minutes, p = 0.002). • NMC value (STT) decreased to within normal range on day 15 after smoking cessation (±10 minutes, p < 0.01), and remained in the normal range until the end of study period (±8 minutes). |
Abbreviations: min, minutes; mm, millimeters; NMC, nasal mucociliary clearance; s, seconds; STT, saccharin transfer time.
Most of the studies showed the impairment of NMC in smokers. Nasal mucociliary clearance in cigarette smokers was explained in 13 studies (11 studies reported significant impairment in smokers, 1 study reported insignificant impairment in smokers, 1 studies reported that the NMC value in smokers was significantly lower than in non-smokers). Nasal mucociliary clearance in passive smokers was explained in three studies, and all of the studies reported that the NMC value in passive smokers was significantly higher than in non-smokers. Nasal mucociliary clearance in bidi, hookah, and electronic smoking, respectively, was explained in only one study.
Discussion
Nasal Mucociliary Clearance in Smokers
Most of the studies (15 out of 16 studies) showed the impairment of NMC in smokers. One study (Nicola et al, 2014) 28 reported that the NMC value in smokers was lower than non-smokers. Nicola et al (2014) speculated that young smokers may have a protective response to cigarette smoking (increase of the CBF and transport system). 28 The impairment was not only observed in active cigarette smoking, but also in bidi, EC, hookah, and passive smoking. This happens because all of them contain harmful constituents that affect NMC. The saccharin transfer time test was used in many studies to evaluate NMC because it is easy, safe, and inexpensive. 4 5 29
Various chemicals present in cigarette smoke, including acrolein, formaldehyde, carbon monoxide, nicotine, cotinine, acetaldehyde, phenol, and potassium cyanide, have been identified as having high toxicity to NMC. 7 30 The gaseous phase of cigarette smoke contains high concentrations of free radicals (>10 15 molecules per puff), resulting in increased oxidative stress that, in turn, causes changes in the structure and function of the NMC. 31 32 The impairment of NMC in chronic exposure to cigarette smoke is caused by the ciliotoxic effect, hypersecretion and viscoelastic change of mucous (particularly of more viscous properties), airway surface liquid depletion, increased oxidative stress, and deteriorations in the inflammatory and immune systems (increased of macrophages, neutrophil, and proinflammatory cytokines), which cause elongation of the NMC time and stagnation of toxic substances. 4 11 17 20 The ciliotoxic effect of cigarette smoking reduces cilia genesis, paralyzes ciliary beating activity (reduces CBF), is related to abnormality in the cilia ultrastructure, and decreases the number of cilia. 4 11 Cigarette smoking also causes metaplastic changes of the respiratory mucosa, with increase in the number and size of the goblet cells that leads to increased production of respiratory airway secretions. 12 20 The acrolein in cigarette smoke reduces the cystic fibrosis transmembrane conductance regulator (CFTR) gene expression, resulting in airway surface liquid depletion and mucus stasis in the airway epithelium. 20 33 34 Other studies also showed that chronic exposure to cigarette smoke stimulates the parasymphatethic nervous system (glandular hypersecretion and vasodilatation), inhibits secretion of Cl − and K+ conductance in normal respiratory epithelium cells, alters epithelial salts and water transport, alters morphology of the epithelium in the entire of respiratory tract (metaplasia with keratinisation), reduces cells viability, and induces cell apoptosis (opposite mitogenic effect or proapoptotis depending on the concentration of smoke and impairment of cell regeneration in respiratory epithelium), induces matrix metaloproteinases (zinc dependent endopeptidase) that relates to the loss of NMC function and the epithelial disruption exposed to smoke via direct cell-to-cell or cell-to-basement membrane connections, and increases protein kinase-C (PKC) activity related to inhibitory effect on CBF. 7 30 35 36 37
Proença M (2011) reported that the NMC value in smokers was insignificantly higher immediately after smoking and significantly higher than in non-smokers 8 hours after smoking. The difference between them may be an effect of nicotine on the sympathetic nervous system, which accelerates CBF. However, the circulating nicotine is metabolized in 2 hours, indicating that, after this period, the stimulatory effect ceases, and the smoker's CBF (or the efficiency of transport and defense mechanism) returns to its “normal” (impaired) state. 35
The improvement of NMC due to smoking cessation is caused by normalization of the cilia structure, genesis, and also ciliary beating function; changes of the cytomorphological features, such as fewer columnar cells, less mucus and reduced epithelial-cell metaplasia; restoration of the ionic transport function and mucus hydration at the basolateral membrane (increased mucus clearability); and detoxification of hydrogen peroxide through the activity of the glutathione peroxidase (antioxidant enxyme). 11 31
The finding of increased NMC value in passive smokers indicates that passive smokers present impairment of the NMC just like active smokers. 7 29 38 It has been observed that chronic passive exposure to cigarette smoke in the household has slightly higher serum continine, which is a metabolite of nicotine that decreases ciliary activity. 7 Other studies also reported that passive smoking has a negative impact on cilia regeneration, as it impairs the structure of the nasal mucosa (patchy loss of cilia, generalized loss of cilia, squamous metaplasia, vascular congestion, hyperplasia of goblet cells and seromucinous acini), and impairs epithelial ion transport (inhibit chloride secretion and basolateral K+ conductance), which can negatively affect NMC. 7 29 38
Bidi is a hand-rolled tobacco product, and, unlike cigarettes, it is devoid of a filter (the filter of a cigarette functions as a barrier, by preventing the noxious smokes from reaching the nasal cavity). 3 39 Although a bidi contains a lower amount of tobacco compared with a cigarette, it produces higher levels of noxious susbtances like nicotine, carbon monoxide, tar, phenols, and ammonia and results in a higher degree of impairment and addiction. 3 40 It has been reported that bidi produces three times the amount of carbon monoxide and nicotine, and five times the amount of tar than cigarettes. Given the low combustibility of the tendu leaf wrapper, bidi smokers must take more frequent and deeper puffs, resulting in the inhalation of more smoke, which is disseminated deeper into the lungs. The decreased NMC detected in bidi smokers may be due to reduced CBF, decline in the number of cilia, and alterations in the viscoelastic properties of mucus. 3
The hookah, also known as a shisha or water pipe, is a traditional method of smoking tobacco. 5 41 Both hookah and cigarette contain nicotine, harmful gases such as carbon monoxide and volatile aldehydes, ultrafine particles, and carcinogenic polycyclic aromatic hydrocarbons (PAHs). 5 42 43 After comparing a 45-minute hookah session to smoking a single cigarette, it was found that hookah smokers had higher concentrations of nicotine, carbon monoxide, and PAHs than cigarette smokers. 5 44 Hookah smoking resulted in increased airway resistance, inflammation, immune cells (neutrophils and lymphocytes), oxidative stress, nitric oxide, and catalase activity in the lungs and NMC of animals. 5 45 46 In the study by Arıcıgil, the group that used hookahs more than once a week was shown to have impaired NMC. This predisposes them to upper respiratory tract inflammation and injury. 5
The electronic cigarette (EC) is a device that carries aerosolized nicotine to the respiratory tract. 6 The EC is marketed as a safer alternative to conventional cigarettes due to its defined composition and noncombustible nature. 6 36 47 A short-term study investigating the possible side-effects of EC use revealed that EC was safer than cigarettes but had more side-effects than nicotine replacement therapy. 6 Propylene glycol is the primary ingredient in the majority of EC cartridges on the marketplace today. 6 Nasal mucociliary clearance in EC smokers was impaired by oxidative stress induced by nicotine exposure via transient receptor potential ankyrin 1 (TRPA 1) receptors. 6 47 These receptors induce airway surface liquid volume loss and decrease mucus density. 6 47 Other studies also reported that formaldehyde in ECIG-generated aerosols related to DNA strand breaks and cell death and propylene glycol thicken the respiratory epithelium by increasing the number of goblet cells and increasing the content of mucin within the goblet cell. 6 36
Strength and Limitation of the Study
The present systematic review included studies that reported NMC in many types of smoking (cigarette, passive, bidi, hookah, and electronic smoking). In addition, a comprehensive literature search was followed as well as bias protection methods, such as using three independent reviewers.
The limitation of the study was related to the minimal sample of each study and the fact that most of the studies were cross sectional. There were only one randomized controlled trial (RCT) and three prospective studies.
Future Implication
The current systematic review is expected to be a scientific consideration to clinician-related NMC in smokers and general information related the dangers of smoking for the public society. Futher research is needed on each component of the NMC for development upon this systematic review.
Final Comments
Our findings suggest that there is an impairment of NMC in smokers. The impairment is not only seen in cigarette smokers but also in passive, bidi, electronic, and hookah smokers. The impairment of NMC in chronic exposure to smoking is caused by the ciliotoxic effect, hypersecretion and viscoelastic change of mucous, airway surface liquid depletion, increased oxidative stress, and deteriorations in the inflammatory and immune systems.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.World Health Organization . 2018. WHO global report on trends in prevalence of tobacco smoking 2000–2025, second edition. [Google Scholar]
- 2.Solak I, Marakoglu K, Pekgor S, Kargin N C, Alatas N, Eryilmas M A. Nasal mucociliary activity changes in smokers. Konuralp Tip Derg. 2018;10(03):269–275. [Google Scholar]
- 3.Paul B, Menon S S, Vasthare R, Balakrishnan R, Acharya S. Effect of bidi smoking on nasal mucociliary clearance: a comparative study. J Laryngol Otol. 2018;132(12):1077–1082. doi: 10.1017/S0022215118002049. [DOI] [PubMed] [Google Scholar]
- 4.Dülger S, Akdeniz Ö, Solmaz F, Şengören Dikiş Ö, Yildiz T. Evaluation of nasal mucociliary clearance using saccharin test in smokers: A prospective study. Clin Respir J. 2018;12(04):1706–1710. doi: 10.1111/crj.12733. [DOI] [PubMed] [Google Scholar]
- 5.Arıcıgil M, Arbağ H. Hookah smoking impairs nasal mucociliary clearance. Tob Induc Dis. 2018;16(06):6. doi: 10.18332/tid/85067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumral T L, Saltürk Z, Yildirim G. How does electronic cigarette smoking affect sinonasal symptoms and nasal mucociliary clearance? B-ENT. 2016;12(01):17–21. [PubMed] [Google Scholar]
- 7.Yadav J, Kaushik G, Ranga R K. Passive smoking affects nasal mucociliary clearance. JIACM. 2014;15(02):96–99. [Google Scholar]
- 8.Kim S Y, Sim S, Choi H G. Active and passive smoking impacts on asthma with quantitative and temporal relations: A Korean Community Health Survey. Sci Rep. 2018;8(01):8614. doi: 10.1038/s41598-018-26895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatkin J M, Dullius C R. The management of asthmatic smokers. Asthma Res Pract. 2016;2(10):10. doi: 10.1186/s40733-016-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long M, Fu Z, Li P, Nie Z. Cigarette smoking and the risk of nasopharyngeal carcinoma: a meta-analysis of epidemiological studies. BMJ Open. 2017;7(10):e016582. doi: 10.1136/bmjopen-2017-016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utiyama D MO, Yoshida C T, Goto D M. The effects of smoking and smoking cessation on nasal mucociliary clearance, mucus properties and inflammation. Clinics (São Paulo) 2016;71(06):344–350. doi: 10.6061/clinics/2016(06)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagliuca G, Rosato C, Martellucci S. Cytologic and functional alterations of nasal mucosa in smokers: temporary or permanent damage? Otolaryngol Head Neck Surg. 2015;152(04):740–745. doi: 10.1177/0194599814566598. [DOI] [PubMed] [Google Scholar]
- 13.Alekseenko S I, Skalny A V, Ajsuvakova O P, Skalnaya M G, Notova S V, Tinkov A A. Mucociliary transport as a link between chronic rhinosinusitis and trace element dysbalance. Med Hypotheses. 2019;127:5–10. doi: 10.1016/j.mehy.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Bustamante-Marin X M, Ostrowski L E. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9(04):1–17. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging. 2014;34(03):171–177. doi: 10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- 16.Paul P, Johnson P, Ramaswamy P, Ramadoss S, Geetha B, Subhashini A S. The Effect of ageing on nasal mucociliary clearance in women: A pilot study. ISRN Pulmonol. 2013;•••:1–5. [Google Scholar]
- 17.Baby M K, Muthu P K, Johnson P, Kannan S. Effect of cigarette smoking on nasal mucociliary clearance: A comparative analysis using saccharin test. Lung India. 2014;31(01):39–42. doi: 10.4103/0970-2113.125894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao J, Zhang L. Influence of intranasal drugs on human nasal mucociliary clearance and ciliary beat frequency. Allergy Asthma Immunol Res. 2019;11(03):306–319. doi: 10.4168/aair.2019.11.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarosław P, Agnieszka P, Jarosław M. Influence of environmental and clinical factors on nasal mucociliary clearance of the students of the medical university of silesia. Pol Przegląd Otorynolaryngol. 2015;4(02):45–52. [Google Scholar]
- 20.Xavier R F, Ramos D, Ito J T. Effects of cigarette smoking intensity on the mucociliary clearance of active smokers. Respiration. 2013;86(06):479–485. doi: 10.1159/000348398. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Green S. United Kingdom: John Wiley and Sons; 2017. Cochrane handbook for systematic reviews of intervention 5.2; pp. 1–50. [Google Scholar]
- 22.PRISMA Group . Moher D, Liberati A, Tetzlaff J, Altman D G, Group T P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(07):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog R, Álvarez-Pasquin M J, Díaz C, Del Barrio J L, Estrada J M, Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13(154):154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019.
- 25.Viswanathan M, Ansari M T, Berkman N D. USA: AHRQ Publication; 2008. Methods guide for effectiveness and comparative effectiveness reviews, assessing the risk of bias of individual studies in systematic reviews of health care interventions. [PubMed] [Google Scholar]
- 26.Islam M M, Iqbal U, Walther B.Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis Neuroepidemiology 201647(3-4):181–191. [DOI] [PubMed] [Google Scholar]
- 27.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the newcastle ottawa scale. World J Metaanal. 2017;5(04):80–84. [Google Scholar]
- 28.Nicola M L, Carvalho H B, Yoshida C T. Young “healthy” smokers have functional and inflammatory changes in the nasal and the lower airways. Chest. 2014;145(05):998–1005. doi: 10.1378/chest.13-1355. [DOI] [PubMed] [Google Scholar]
- 29.Habesoglu T E, Kule M, Kule Z G. How does parental smoking affect nasal mucociliary clearance in children? Eur Arch Otorhinolaryngol. 2015;272(03):607–611. doi: 10.1007/s00405-014-3110-7. [DOI] [PubMed] [Google Scholar]
- 30.Tamashiro E, Cohen N A, Palmer J N, Lima W T, Lima A. Effects of cigarette smoking on the respiratory epithelium and its role in the pathogenesis of chronic rhinosinusitis. Rev Bras Otorrinolaringol (Engl Ed) 2009;75(06):903–907. doi: 10.1016/S1808-8694(15)30557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos E M, De Toledo A C, Xavier R F. Reversibility of impaired nasal mucociliary clearance in smokers following a smoking cessation programme. Respirology. 2011;16(05):849–855. doi: 10.1111/j.1440-1843.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 32.Leopold P L, O'Mahony M J, Lian X J, Tilley A E, Harvey B G, Crystal R G. Smoking is associated with shortened airway cilia. PLoS One. 2009;4(12):e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander N S, Blount A, Zhang S. CFTR modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122(06):1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen N A, Zhang S, Sharp D B. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119(11):2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 35.Proença M, Fagundes Xavier R, Ramos D, Cavalheri V, Pitta F, Cipulo Ramos E M. [Immediate and short term effects of smoking on nasal mucociliary clearance in smokers] Rev Port Pneumol. 2011;17(04):172–176. doi: 10.1016/j.rppneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Palazzolo D L, Nelson J M, Ely E A, Crow A P, Distin J, Kunigelis S C.The effects of electronic cigarette (ECIG)-generated aerosol and conventional cigarette smoke on the mucociliary transport velocity (MTV) using the bullfrog (R. catesbiana) palate paradigm Front Physiol 20178(1023):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott M K, Sisson J H, Wyatt T A. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36(04):452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habesoglu M, Demir K, Yumusakhuylu A C, Yilmaz A S, Oysu C. Does passive smoking have an effect on nasal mucociliary clearance? Otolaryngol Head Neck Surg. 2012;147(01):152–156. doi: 10.1177/0194599812439004. [DOI] [PubMed] [Google Scholar]
- 39.Duong M, Rangarajan S, Zhang X. Effects of bidi smoking on all-cause mortality and cardiorespiratory outcomes in men from south Asia: an observational community-based substudy of the Prospective Urban Rural Epidemiology Study (PURE) Lancet Glob Health. 2017;5(02):e168–e176. doi: 10.1016/S2214-109X(17)30004-9. [DOI] [PubMed] [Google Scholar]
- 40.Amith H V, Agrawal D, Gupta A, Shrivastava T P, Purohit B M, Bhambhani G. Assessing the nicotine content of smoked and smokeless forms of Tobacco Available in Bhopal. Indian J Dent Res. 2018;29(03):341–346. doi: 10.4103/ijdr.IJDR_664_16. [DOI] [PubMed] [Google Scholar]
- 41.Yıldırım F, Çevik Y, Emektar E, Çorbacıoğlu ŞK, Katırcı Y. Evaluating ECG and carboxyhemoglobin changes due to smoking narghile. Inhal Toxicol. 2016;28(12):546–549. doi: 10.1080/08958378.2016.1224957. [DOI] [PubMed] [Google Scholar]
- 42.Daher N, Saleh R, Jaroudi E. Comparison of carcinogen, carbon monoxide, and ultrafine particle emissions from narghile waterpipe and cigarette smoking: Sidestream smoke measurements and assessment of second-hand smoke emission factors. Atmos Environ (1994) 2010;44(01):8–14. doi: 10.1016/j.atmosenv.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shihadeh A, Salman R, Jaroudi E. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “tar” and nicotine yields. Food Chem Toxicol. 2012;50(05):1494–1498. doi: 10.1016/j.fct.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob P, III, Abu Raddaha A H, Dempsey D. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2345–2353. doi: 10.1158/1055-9965.EPI-11-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Zaatari Z M, Chami H A, Zaatari G S. Health effects associated with waterpipe smoking. Tob Control. 2015;24 01:i31–i43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemmar A, Yuvaraju P, Beegam S, John A, Raza H, Ali B H. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am J Physiol Heart Circ Physiol. 2013;305(05):H740–H746. doi: 10.1152/ajpheart.00200.2013. [DOI] [PubMed] [Google Scholar]
- 47.Chung S, Baumlin N, Dennis J S. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med. 2019;200(09):1134–1145. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C F Freire AP, G D Christofaro D, Mara C Ramos E . Uzeloto J S, Ramos D. Nasal mucociliary transportability of male and female smokers. Rev Bras Otorrinolaringol (Engl Ed) 2018;84(03):311–317. doi: 10.1016/j.bjorl.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


