Abstract
The key link between renin–angiotensin system (RAS) and COVID-19 is ACE2 (angiotensin-converting enzyme 2), which acts as a double-edged sword, because ACE2 increases the tissue anti-inflammatory response but it is also the entry receptor for the virus. There is an important controversy on several drugs that regulate RAS activity and possibly ACE2, and are widely used, particularly by patients most vulnerable to severe COVID-19. In the lung of healthy rats, we observed that candesartan (an angiotensin type-1, AT1, receptor blocker; ARB) and captopril (an ACE inhibitor; ACEI) up-regulated expression of tissue ACE2 and RAS anti-inflammatory axis receptors (AT2 and Mas receptors). This effect was particularly pronounced in rats with metabolic syndrome (obesity, increased blood pressure and hyperglycemia) and aged rats. Treatment of cultures of human type-II pneumocytes with candesartan or captopril induced up-regulation of ACE2 expression in cells. Treatment with viral spike protein induced a decrease in full-length (i.e. transmembrane) ACE2, an increase in levels of a short intracellular ACE2 polypeptide and an increase in ADAM17 activity in cells, together with an increase in levels of soluble ACE2 and major proinflammatory cytokines in the culture medium. Spike protein-induced changes and levels of spike protein internalization in cells were inhibited by pretreatment with the above-mentioned drugs. The results suggest that these drugs increase ACE2 levels and promote the anti-inflammatory RAS axis in the lung. Furthermore, possible up-regulation of viral entry by the drug-induced increase in expression of transmembrane ACE2 is counteracted by additional mechanisms, particularly by drug-induced inhibition of ADAM17 activity.
Keywords: ACE2, ACEIs, ADAM17, angiotensin, antihypertensives, ARBs
Introduction
The renin–angiotensin system (RAS) was initially considered as a circulating humoral system, with functions in regulating blood pressure and sodium and water homeostasis, and its role in the hypertension and several cardiovascular and renal diseases is classically known. However, more recent studies have shown that RAS dysregulation is also involved in the inflammatory response associated with many other diseases [1], including the proinflammatory state associated with obesity and metabolic syndrome [2,3] and aging-related processes [4,5]. In addition to the classic or circulating (i.e. endocrine) RAS, there are tissue (i.e. paracrine) RAS in most organs [6,7], and also an intracellular or intracrine RAS [8]. The RAS is basically organized into two arms that counteract each other and must be correctly balanced [9,10]: a proinflammatory and pro-oxidative axis mainly constituted by angiotensin II (AngII) and angiotensin type 1 (AT1) receptors, and an anti-inflammatory antioxidative axis formed by AngII/AT2 receptors, and particularly Ang1-7 /Mas receptors (MasR) (see diagram in Supplementary Figure S1). Angiotensin-converting enzyme 2 (ACE2) plays a key role in the balance, because ACE2 transforms components of the pro-inflammatory axis (AngI and particularly AngII) into components of the anti-inflammatory axis (Ang1-9, and particularly Ang1-7). ACE inhibitors (ACEIs) and antagonists of AT1 receptors (ARBs) are two of the most widely used antihypertensive drugs. However, they have also been suggested for treatment of several of the above-mentioned diseases mediated by inflammatory processes [1]. The potential anti-inflammatory antioxidative mechanism involved in their effects is related to inhibition of the proinflammatory axis activity by inhibition of AngII synthesis (ACEIs) or blockage of AT1 receptors (see Supplementary Figure S1 for details).
Many recent studies have highlighted the major role of tissue RAS in severity of COVID-19 [11–13]. ACE2, the receptor for SARS-CoV-2, arises as the key link between RAS and COVID-19 [14]. ACE2 is a protease that plays its key role as a double-edged sword [15–17]. An increase in ACE2 activity is essential to balance the RAS against the anti-inflammatory response. Consistent with this, several previous studies have shown the protective effects of ACE2 and its product Ang1-7 against experimental lung injures [10,18,19]. However, ACE2 is also the entry receptor for the virus [20–22] and an increase in ACE2 expression may increase cell infection. Furthermore, viral binding decreases the levels of ACE2 at the cell surface [23,24], thus shifting the balance toward inflammation, fibrosis and progression of disease severity. Therefore, a major question that has been insistently raised is whether we should promote the increase in ACE2 levels in tissues to reduce inflammation, or to decrease in ACE2 levels to reduce viral entry and replication. The lung is obviously a major target of research. The controversy has been particularly focused on several drugs that regulate RAS activity and are used by millions of patients of chronic diseases such as cardiovascular diseases, hypertension or diabetes. These drugs are ARBs (i.e. sartans) and ACEIs. Moreover, groups of patients that appear particularly vulnerable to COVID-19 such as elders, diabetics, obese and cardiovascular and hypertensive patients [25,26] are the most frequent users of these drugs.
Over the last few months, a large number of articles have focused on whether these drugs increase or decrease tissue levels (particularly lung levels) of ACE2 and whether this may increase or decrease the severity of COVID-19. However, most of them are review or opinion articles reporting that experimental data on this question are scarce and controversial [27–29]. Furthermore, in addition to ACE2, other RAS components may play a major role in COVID-19 severity, and the above-mentioned RAS modulatory drugs may also affect viral entry mechanisms by acting on different factors [30].
In the work reported here, we studied the effects of candesartan (an AT1 receptor blocker) and captopril (an ACEI) on lung levels of ACE2 and major components of lung RAS in young adult rats and in rat models of individuals vulnerable to severe COVID-19, such as rats with metabolic syndrome (obesity, increased blood pressure, hyperglycemia) and aged rats. In vitro, we used human alveolar type-II pneumocyte cells to study the effects of these drugs on changes induced by viral spike protein on ACE2 levels, on levels of spike protein internalization, and on the release of major proinflammatory cytokines such as IL-6, TNF-α and CCL-2.
Materials and methods
Experimental design
In a first set of experiments, we tested the effects of the AT1 receptor blocker candesartan and the ACE inhibitor captopril on lung levels of ACE2 and major components of lung RAS (i.e. AT1, AT2 and Mas receptors) using RT-PCR in young adult rats (10 weeks old; n=30; n=6 per group/treatment). Protein expression of ACE2 and AT1, AT2, and Mas receptors were confirmed by WB. In addition, we compared ACE2 activity levels in lung homogenates of control and treated young adult rats. In a second set of experiments, we used models of vulnerable groups including rats with metabolic syndrome (obesity, increased blood pressure, hyperglycemia; n=20; n=5 per group/treatment) and aged rats (18–20 months old; n=18; n=6 per group/treatment). We studied the effects of candesartan and captopril on ACE2 and major RAS receptor expression in lung tissue, relative to young adult controls. In a third set of experiments, we used the human alveolar type-II pneumocyte A549 cell line to study the effects of the above-mentioned drugs on changes induced by SARS-CoV-2 Spike RBD-Fc protein. All data were obtained from at least three independent experiments with at least n=5. First, we measured the effect of drugs on ACE2 mRNA expression in A549 cells in culture. Then, we studied the changes of both membrane-bound or intracellular forms of ACE2 (cellular lysate) and soluble released ACE2 (culture medium) after treatment with the viral spike protein with or without 24 or 48 h of preincubation with candesartan or captopril. Next, we investigated the effects of candesartan and captopril on SARS-CoV-2 Spike RBD-Fc internalization rate by measuring cytoplasmic fluorescence intensities of spike protein using confocal laser microscopy. In order to minimize the possibility that changes in levels of internalized spike protein could be related to differences in cell levels of ACE2 receptor, we used ACE2-GFP transiently transfected A549 cells and values of intracellular spike were expressed relative ACE2 levels. Finally, we quantified the effect of viral spike protein, preincubated or not preincubated with candesartan or captopril, on the release of major proinflammatory cytokines such as IL-6, TNF-α and CCL-2 to culture medium.
Cell cultures
Human alveolar type-II pneumocyte cell line A549 (CSC-C6236X, Creative Bioarray) was cultured in DMEM/F12 medium supplemented with 10% FBS, 2 mM L-glutamine (Sigma), 100 U/ml penicillin and 100 μg/ml Streptomycin. Cultures were maintained at 37°C and 5% CO2 in humidified incubator. Cells were cultured until maximal confluence was reached in order to get high levels of ACE2 in cells [31]. In order to study the interaction of RAS modulators or anti-inflammatory compounds with SARS-CoV-2 Spike RBD-Fc protein, cells were pretreated or not pretreated with the AT1 receptor blocker Candesartan (1 μM; 4791, Tocris) or the ACE inhibitor Captopril (50 ng/ml; C4042, Sigma) for 24 h. Then, 1 µg/ml of SARS-CoV-2 Spike RBD-Fc protein (40592-V02H, Sino Biological) was added to the cells for 3 h at 37°C. Doses were stablished based on preliminary experiments and previous in vitro studies [32,33].
Animal models
Lung tissues from male young adult rats, aged rats and adult rats with Metabolic Syndrome (obesity, increased blood pressure and hyperglycemia) were used. Young adult rats used for metabolic syndrome received a high fat diet with 60% calories fat supplemented with 4% of NaCl (D18042603; Research Diets, U.S.A.) for a period of 18 weeks. In these rats, serum triglycerides (TG), cholesterol and glucose were measured using commercially colorimetric enzymatic kits (1001093; 1001314; 1001191; Spinreact, Spain), according to the manufacturer’s instructions. Blood pressure was measured using a noninvasive pressure system MRBP (IITC Life Science, California, U.S.A.). The rats were orally treated or not treated with the AT1 receptor blocker Candesartan (1 or 10 mg/Kg; AstraZeneca) or the ACE inhibitor Captopril (5 or 40 mg/Kg; C4042, Sigma) for 3 weeks. The powered drug was administered orally mixed with “Nocilla” hazelnut cream (Nutrexpa, Barcelona, Spain). Doses were stablished based on preliminary experiments and previous in vivo studies [34–37]. Animal handling was performed in accordance with the Directive 2010/63/EU, European Council Directive 86/609/EEC and the Spanish legislation (RD53/2013). Animal experiments were approved by the corresponding committee at the University of Santiago de Compostela (15005/15/002) and were carried out in the Experimental Biomedicine Centre (CEBEGA; University of Santiago de Compostela). Rats were housed at constant room temperature (RT) (21–22°C) and 12-h light/dark cycle. The animals were euthanised using an overdose (800 mg/Kg) of intraperitoneal sodium pentobarbital and then the tissue samples were obtained.
Western blot analysis
Homogenates from rat lung tissue or A549 cells were lysed in RIPA buffer containing PMSF (Sigma) and protease inhibitor cocktail (Sigma). Total proteins were quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific). An equal amount of protein lysates or cell culture medium was separated on 8–10% Bis-Tris polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were incubated overnight at 4°C with primary antibodies against the extracellular domain of ACE2 (ab108252; Abcam; 1:1000), AT1 (sc-31181; Santa Cruz; 1:200), AT2 (sc-9040; Santa Cruz; 1:200) and Mas (AAR-013; Alomone labs; 1:1000) receptors. Membranes were reincubated with loading controls: anti-α-tubulin (T5168; 1:50.000; Sigma) or GAPDH (G9545; 1:25.000; Sigma), or ponceau (Sigma, P7170-1L). The following horseradish peroxidase (HRP)-conjugated secondary antibodies were used: goat anti-rabbit-HRP and goat anti-mouse-HRP (Santa Cruz Biotechnology; 1:2500). Bound antibody was detected with an Immun-Star HRP Chemiluminescent Kit (Bio-Rad; 170-5044) and visualized with a chemiluminescence detection system (Bio-Rad; Molecular Imager ChemiDoc XRS System). The data were then expressed relative to the value obtained for the control to counteract possible variability among batches.
Specificity of antibodies
The specificity of antibodies against ACE2, AT1, AT2 and Mas receptors was assessed in our laboratory by WB analysis of lysates from HEK293 cells transiently transfected with the corresponding GPCR tagged to fusion tail DDK (i. e. a C-terminal DDK epitope tag DYKDDDDK) or GFP (green fluorescent protein), or preabsorption with the corresponding synthetic peptide antigen (see [9,38]).
RNA extraction and real-time quantitative polymerase chain reaction
Total RNA from rat lung tissues or A549 cell homogenate was extracted with TRIzol (Invitrogen, Paisley, U.K.) following the manufacturer’s protocol. Total RNA (2 µg) was reversed transcribed to complementary DNA (cDNA) using nucleoside triphosphate containing deoxyribose, random primers and Moloney murine leukaemia virus (MMLV; Invitrogen, Thermo Fisher Scientific; 200U) reverse transcriptase. The RT-PCR analysis was performed using a QuantStudio 3 platform (Applied Biosystems, Foster City, CA, U.S.A.), the EvaGreen qPCR MasterMix (Applied Biological Materials Inc., Vancouver, Canada), and the corresponding primer sequences (see below) were used to examine the relative levels of ACE2, AT1, AT2 and Mas receptors. β-Actin was used as a housekeeping gene and was amplified in parallel with the genes of interest. We used the comparative cycle threshold values (cycle threshold (Ct)) method (2−ΔΔCt) to examine the relative messenger RNA (mRNA) expression. A normalized value was obtained by subtracting the Ct of β-actin from the Ct of interest (ΔCt). As it is uncommon to use ΔCt as a relative expression data due to this logarithmic characteristic, the 2−ΔΔCt parameter was used to express the relative expression data. Primer sequences were as follows: for Mas receptor, forward 5′-CTTTGTGGAGAACGGGAT-3′; reverse 5′-GGAGATGTCAGCAATGGA-3′ (NM_012757.2 Rattus norvegicus Mas1 proto-oncogene, G protein-coupled receptor (Mas1), mRNA); for ACE2, forward 5′-GTGGAGGTGGATGGTCTTTCAGG-3′; reverse 5′CACCAACGATCTCCCGCTTCA-3′ (NM_001012006.1 Rattus norvegicus angiotensin I converting enzyme 2 (Ace2), mRNA); for AT1, forward 5′-TTGTCTGGATAAATCACACAACCC-3′; reverse 5′-GTTAAGGGCCATTTTGTTTTTCTGG-3′ (NM_030985.4 Rattus norvegicus angiotensin II receptor, type 1a (Agtr1a), mRNA); for AT2, forward 5′-AACATCTCGCTGAAGACCAATAG-3′; reverse 5′-AGAAGGTCAGAACATGGAAGG-3′ (NM_012494.3 Rattus norvegicus angiotensin II receptor, type 2 (Agtr2), mRNA); for human ACE2, forward 5′-TTCCATGCTAACGGACCCAGGA-3′; reverse 5′- TTTGTGCACATAAGGATCCTGAAGT-3′ (NM_021804.3 Homo sapiens angiotensin I converting enzyme 2 (ACE2), transcript variant 2, mRNA).
Transfection of ACE2
Human epithelial lung cell line A549 were seeded at a density of 0.35 × 106/well on to 12-well plates with glass cover and maintained at 37°C in a humidified CO2 incubator (5% CO2, 95% air). Cells were transiently transfected with 2 μg of ACE2 cDNA (ACE2 tGFP-tagged, RG208442, Origene) using a commercial transfection reagent, Turbofect (R0533, Thermo Scientific). Forty-eight hours after transfection, cells were treated with different compounds, fixed and processed for confocal studies.
SARS-CoV-2 Spike RBD-Fc protein internalization assay
Human alveolar type-II pneumocyte cell lines, A549, transiently transfected with tGFP ACE2 were treated or not treated with Candesartan or Captopril for 24 h. Then, 1 µg/ml of SARS-CoV-2 Spike RBD-Fc protein (40592-V02H, Sino Biological) was added to the cells for 3 h at 37°C. Cells were fixed and incubated overnight at 4°C with a mouse monoclonal antibody against human IgG-Fc (ab99757, abcam, 1:500) diluted in DPBS containing 1% BSA, 2% normal goat serum and 0.05% Triton X-100. Then, cells were incubated with the fluorescent secondary antibody Alexa Fluor 568- conjugated goat anti-mouse IgG (Molecular Probes; 1:200) for 2.5 h at RT. Finally, mounting was performed with Immu-mount (Thermo-Shandon). Internalization was measured using confocal laser microscopy (AOBS-SP5X; Leica Microsystems Heidelberg GmbH, Mannheim, Germany), performing sequential scan to avoid any potential overlap with the LAS AF software (Leica Microsystems GmbH). For each sample, over 25–30 optical fields were chosen randomly using a 63× objective. Images of cells at a unique plane were used for the analysis. RBD Spike protein internalization rate was expressed as the ratio of the fluorescence intensities measured at 568 nm (human Fc signal) and 488 nm (ACE2-GFP signal) excitation wavelengths. Fluorescence intensity was measured at the level of cellular cytoplasm and the background of each image was subtracted before calculating the ratio using the Software Leica LAS AF. The same conditions of laser intensities/exposure times were used for the entire experiment.
ACE2 activity
ACE2 activity from 20 μg of rat lung tissue or 5 μg of human A549 cells lysate was measured using a commercial ACE2 Activity Assay Kit (AnaSpec, AS-72086) following the manufacturer’s specifications. The kit is based on the Mca/Dnp fluorescence resonance energy transfer (FRET) peptide (10 μM). In the FRET peptide, the fluorescence of Mca is quenched by Dnp but a cleavage of the substrate produces a separation into two fragments by the enzyme so that the fluorescence of Mca is measured at excitation/emission = 330/390 nm using an Infinite M200 multiwell plate reader (TECAN). ACE2 activity was confirmed with the specific ACE2 inhibitor DX600 1 µM, included as a control in the same kit (AnaSpec, AS-72086).
ADAM17 activity assay
ADAM17 activity from 20 μg of human A549 cells lysate was measured using a commercial TACE activity assay kit (AnaSpec, AS-72085) following the manufacturer’s specifications. The kit is based on the 5-FAM/QXL® 520 based fluorescence resonance energy transfer (FRET) peptide (1 μM). In the FRET peptide, the fluorescence of 5-FAM is quenched by QXL® 520, but a cleavage of the substrate produces a separation into two fragments by the enzyme so that the fluorescence of 5-FAM is measured at excitation/emission = 490/520 nm using an Infinite M200 multiwell plate reader (TECAN). ADAM17 activity was confirmed with the TACE inhibitor TAPI-0 (10 μM), included as a control in the same kit (data not shown).
Enzyme immunoassays (EIA)
To analyze the release of proinflammatory markers, with or without the different treatments, A549 cell culture supernatants were collected and centrifuged at 2000 × g for 10 min to eliminate cell’s debris. Proinflammatory chemokine CCL-2 (MPC-1) (ab179886; Abcam) and proinflammatory cytokines IL-6 (BMS213HS, Invitrogen) and TNF-α (BMS223HS, Invitrogen) from A549 cells culture medium were measured using commercially available specific EIA Kits according to the manufacturer’s instructions. Proinflammatory cytokines concentration was quantified using specific Standard curve from each cytokine (4PL curve fit).
Statistical analysis
All statistical analyses were performed using SigmaPlot 11.0 (Systat Software, Inc., CA, U.S.A.). All datasets were tested for normality with the Kolmogorov–Smirnov test. If the dataset passed the normality test, parametric tests were used: Student’s t test for two group comparisons and one-way ANOVA followed by the Student–Newman–Keuls Method for multiple comparisons. For nonparametric data, two group comparisons were carried out by Mann–Whitney rank sum test and multiple comparisons by Kruskal–Wallis one-way analysis of variance on ranks test followed by Student–Newman–Keuls method or Dunn’s method were used. All data were expressed as means ± SEM. Differences were considered statistically significant at P<0.05. GraphPad Prism 8 software (GraphPad Inc., San Diego, CA, USA) was used to create scatter dot plot graphs.
Results
Effects of candesartan and captopril in young adult rats
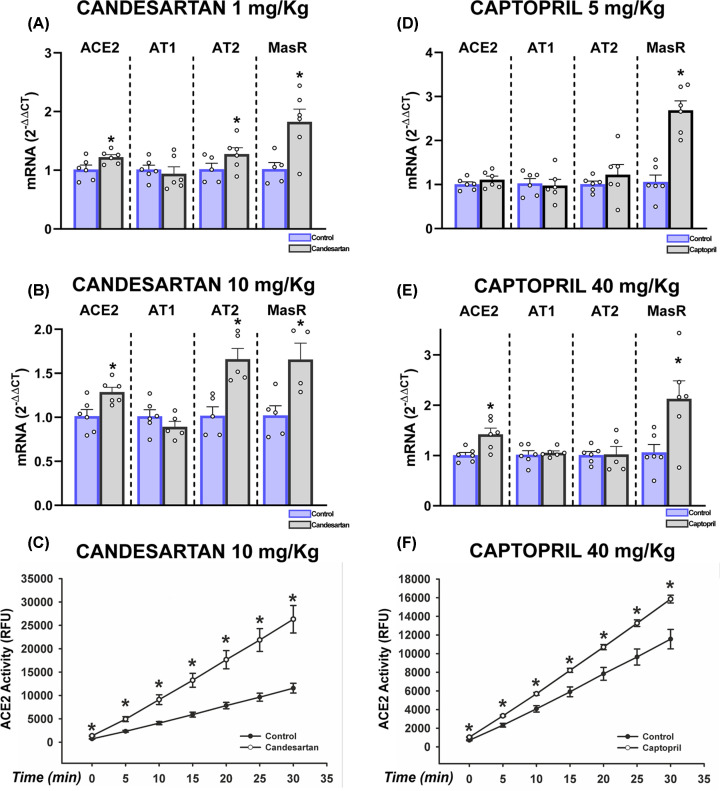
Candesartan induced a significant increase in lung levels of ACE2 both at low (1 mg/kg) and higher doses (10 mg/kg). RAS receptors of the anti-inflammatory axis were also up-regulated, particularly Mas receptors, while AT2 receptors were particularly up-regulated with the dose of 10 mg/kg (Figure 1A,B and Supplementary Figure S2a,b). Treatment with captopril (5 or 40 mg/kg) induced an increase in levels of ACE2 only with the higher dose, and a significant increase in Mas receptor expression was observed with both doses (Figure 1D,E and Supplementary Figure S2c,d). Candesartan and captopril also induced significant increases in lung ACE2 enzymatic activity (Figure 1C,F).
Figure 1. Effect of candesartan and captopril in the lung of young rats.
Effects of candesartan (A–C) and captopril (D–F) on mRNA expression of ACE2 and AT1, AT2 and Mas receptors (A,B,D,E) and ACE2 enzymatic activity (C,F) in the adult rat lung. Data are mean ± SEMs. *P<0.05 relative to control group (Student’s test and Mann–Whitney rank sum test).
Effects of treatments in rats with metabolic syndrome and aged rats
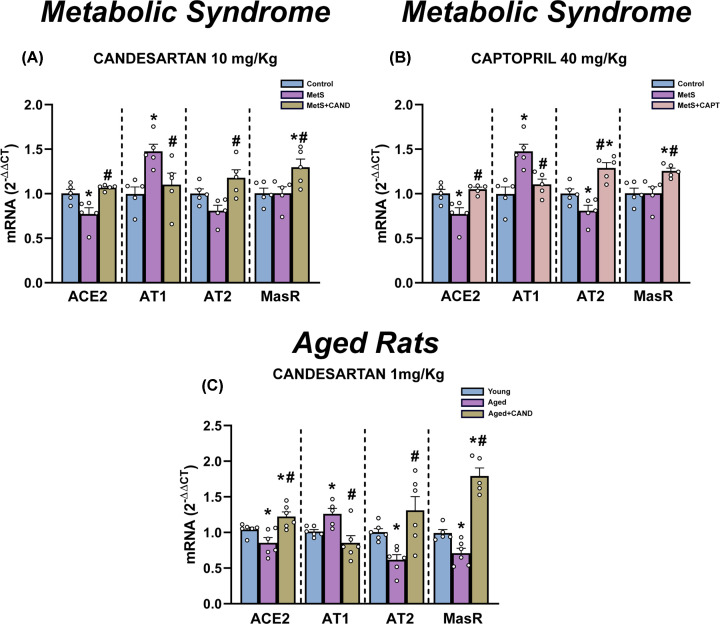
Rats with metabolic syndrome (MetS) showed a significant increase in weight relative to control rats, as well as significant increase in blood levels of cholesterol, triglycerides and glucose, and a significant increase in blood pressure (Supplementary Figure S3). Rats with MetS showed a decrease in ACE2 and AT2 receptor expression that were significantly increased by candesartan and captopril (Figure 2 and Supplementary Figure S4). Both drugs increased the expression of receptors of the anti-inflammatory axis (Mas and AT2). Interestingly, MetS rats also showed increased expression of receptors of the proinflammatory axis (i.e. AT1 receptors) that was significantly decreased by treatment with candesartan and captopril.
Figure 2. Effect of candesartan and captopril in the lung of rats with metabolic syndrome and aged rats.
Effects of candesartan (A and C) and captopril (B) on mRNA expression of ACE2 and AT1, AT2 and Mas receptors in the lung of rats with metabolic syndrome (A and B) and aged rats (C). Rats treated with candesartan (A) or captopril (B) were compared with the same group of untreated animals in (A) and (B). Data are mean ± SEMs. *P<0.05 relative to control adult rats; #P<0.05 relative to untreated rats with metabolic syndrome (A and B) or aged rats (C) (one-way ANOVA with Student–Newman–Keuls method post hoc test or Kruskal–Wallis one-way analysis of variance on ranks with Student–Newman–Keuls method post hoc test).
A similar picture was observed in aged rats, which showed a significant decrease in expression ACE2 and receptors of the RAS anti-inflammatory axis (Mas and AT2), and a significant increase in AT1 receptor levels. Candesartan significantly increased expression of ACE2, AT2 and Mas receptors, and decreased AT1 receptor expression (Figure 2C and Supplementary Figure S4e)
Effects on human alveolar type-II pneumocyte cultures
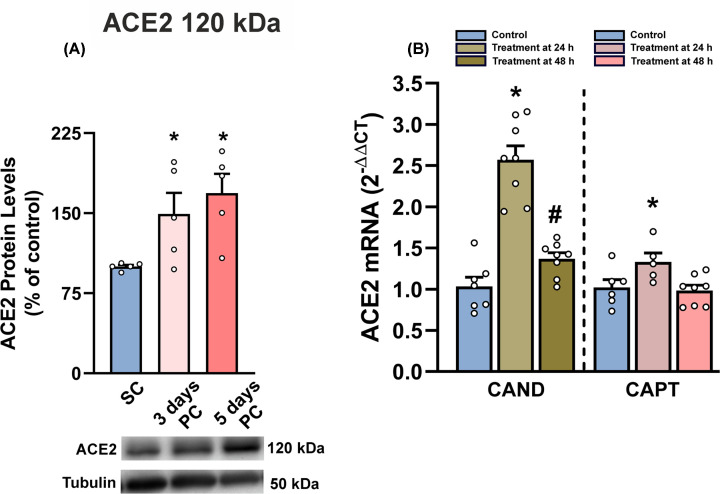
As indicated in methods, high level of cell confluence correlated with higher levels of ACE2 expression in cells (Figure 3A). First, we checked the effects of candesartan and captopril, which induced a significant increase in the expression of ACE2 in pneumocytes, particularly 24 h after treatment (Figure 3B). Treatment with viral spike protein (SARS-CoV-2 spike-RBD; 1 µg/ml) induced a significant decrease in cell levels of full-length ACE2 protein (120 kDa), which was inhibited by pretreatment with candesartan or captopril (Figure 4A). We also observed that treatment with spike protein induced a decrease in ACE2 activity in cells, which was inhibited by pretreatment with candesartan and captopril (Figure 4B,C). Interestingly, a short ACE2 isoform (60 kDa) increased in cells after treatment with spike protein and was significantly reduced by pretreatment with candesartan or captopril (Figure 4D). Treatment with spike protein induced an increase in levels of soluble ACE2 (105 kDa) in culture medium, which was reduced by pretreatment with the drugs (Figure 4F).
Figure 3. ACE2 levels in cultures of treated and untreated human alveolar type-II A549 cells.
Cell confluence increased cell levels of ACE2 protein (A). Effects of 24 or 48 h treatment with candesartan and captopril on mRNA expression of ACE2 enzyme (B). Data are mean ± SEMs. *P<0.05 relative to sub-confluence (A) or untreated controls (B), #P<0.05 relative to 24 h treatment (one-way ANOVA with Student–Newman–Keuls method post hoc test or Kruskal–Wallis one-way analysis of variance on ranks with Student–Newman–Keuls Method post hoc test); PC, post-confluence; SC, sub-confluence.
Figure 4. Levels of different ACE2 isoforms and ACE2 enzymatic activity in cells or culture medium after spike protein, candesartan and captopril treatments.
Levels of ACE2 protein (A,D and E) and enzymatic activity (B and C) in cells (A and D) and culture medium (E) in cultures of human alveolar type-II A549 cells treated with spike protein alone or spike protein together with candesartan or captopril. Laser confocal microscopy showing colocalization (H, yellow) of ACE2 (green, F) and spike protein (red, G) in cells after treatment of cultures with spike protein. *P<0.05 relative to untreated controls; #P<0.05 relative to cells treated with spike alone (one-way ANOVA with Student–Newman–Keuls Method post hoc test or Kruskal–Wallis one-way analysis of variance on ranks with Student–Newman–Keuls method post hoc test); scale bar (for F–H): 25 µm.
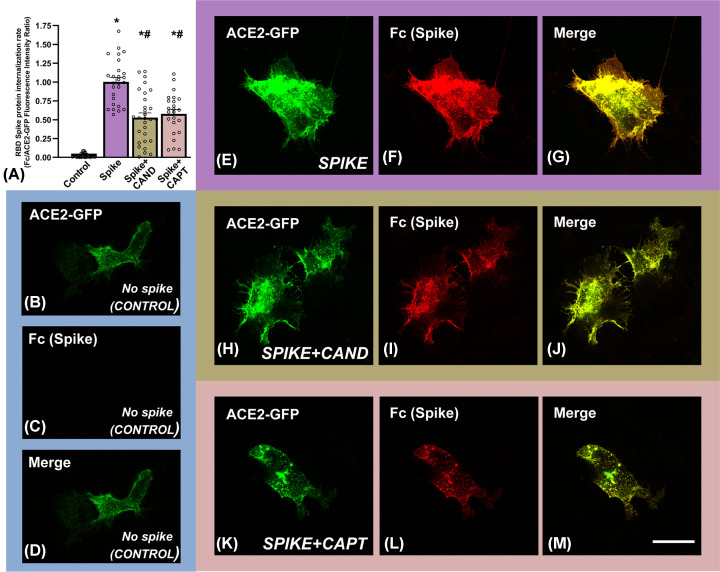
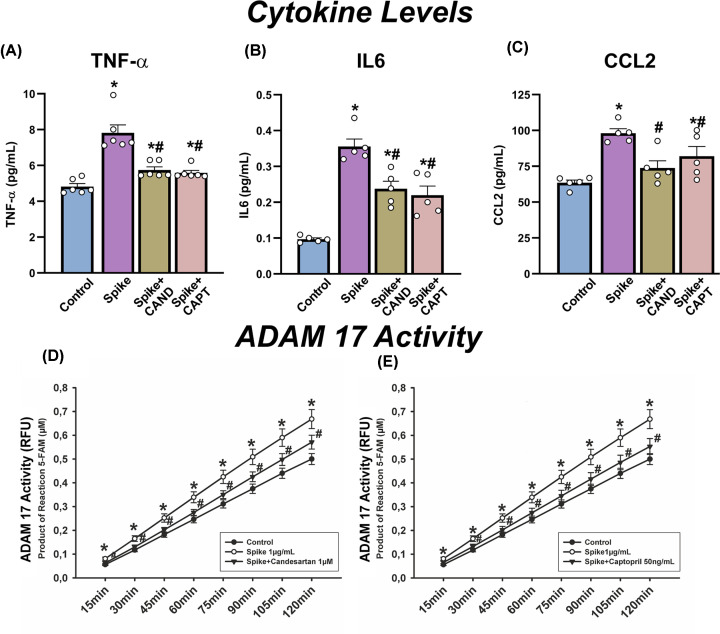
We also studied possible effects of treatments on spike protein internalization using confocal microscopy. First, we confirmed that spike protein was internalized by cultured pneumocytes expressing ACE2 (Figure 4F–H). Then, we investigated the effects of candesartan and captopril on SARS-CoV-2 Spike RBD-Fc internalization rate by measuring cytoplasmic fluorescence intensities of spike protein using confocal laser microscopy (Figure 5). To minimize the possibility that changes in levels of internalized spike protein could be related to differences in cell levels of ACE2 receptor, we used ACE2-GFP transiently transfected A549 cells and values of intracellular spike were expressed relative to ACE2 levels (Figure 5A). We observed high levels of spike protein internalization relative to controls not treated with spike protein (Figure 5A, and 5B–D relative to 5E–G). In cultures treated with spike protein and candesartan (Figure 5A,H–J) or captopril (Figure 5A,K–M), spike protein fluorescence in cells decreased around 50% relative to cells treated with spike protein alone (Figure 5A,E–G). Consistent with the above-mentioned observations, we also observed that treatment with spike protein induced a significant increase in levels of the pro-inflammatory cytokines IL-6, TNF-α, and CCL2 in the culture medium, which were significantly reduced in cultures treated candesartan or captopril prior to exposure to spike protein (Figure 6A–C)
Figure 5. Effects of candesartan and captopril on spike protein internalization in cultures of human alveolar type-II A549 cells.
Effects of candesartan and captopril on SARS-CoV-2 Spike RBD-Fc internalization rate by measuring cytoplasmic fluorescence intensities of spike protein using confocal laser microscopy (A). ACE2-GFP (green), spike protein (red) and merge (yellow) fluorescence in untreated controls (B–D), and cultures treated with spike protein alone (E–G) or spike protein and candesartan (H–J) or spike protein and captopril (K–M). *P<0.05 relative to untreated controls, #P<0.05 relative to cells treated with spike alone (Kruskal–Wallis one–way analysis of variance on ranks with Dunn’s method post hoc test); scale bar (for all photographs): 25 µm.
Figure 6. Levels of pro-inflammatory cytokines and ADAM17 enzymatic activity in cultures after treatments with spike protein, candesartan and captopril.
Levels of proinflammatory cytokines (TNF-α, IL-6, CCL-2; A–C) and ADAM17 enzymatic activity (D and E) in culture medium (A–C) and cells (D and E) in cultures of human alveolar type-II A549 cells treated with spike protein alone or spike protein together with candesartan or captopril. *P<0.05 relative to untreated controls; #P<0.05 relative to cells treated with spike alone (one-way ANOVA with Student–Newman–Keuls method post hoc test or Kruskal–Wallis one-way analysis of variance on ranks with Student–Newman–Keuls method post hoc test).
As several of the drug-induced effects cannot be explained by drug-induced increase in ACE2 expression, we investigated whether other mechanisms involved in spike protein internalization may contribute to the effects of drugs. After observing that drugs induced changes in the levels of soluble ACE2 in the culture medium, we investigated possible effects on ADAM17 activity. Interestingly, ADAM17 activity significantly increased in cells treated with spike protein relative to untreated control cells. However, the increase was inhibited by pretreatment with candesartan or captopril (Figure 6D,E)
Discussion
The effect of AT1 receptor blockers and ACEIs on lung levels of ACE2 is controversial, and several experimental or clinical trials have suggested that these drugs increase, decrease, or do not modify ACE2 levels [28,39–41]. In addition, the beneficial or detrimental effects of changes in ACE2 cell levels are also controversial, as an increase in cell-surface ACE2 may result in up-regulation of the anti-inflammatory and antioxidative RAS arm, but also in ACE2-mediated increase in viral entry [17,21,42]. The controversy appears particularly relevant for groups of patients vulnerable to severe COVID-19, such as aged people and people with hypertension, diabetes or obesity [24,25], many of them taking the above-mentioned drugs.
The present in vivo experiments show that an AT1 receptor blocker and an ACEI up-regulate ACE2 expression in the lung of control healthy rats, together with up-regulation of other major components of the RAS anti-inflammatory antioxidative axis such as AT2 and Mas receptors. Altogether suggests that the use of captopril, and probably other ACEIs, or candesartan, and probably other ARBs, enhances the anti-inflammatory RAS arm in the lung promoting anti-inflammatory, antifibrotic and antithrombotic responses (see Supplementary Figure S1) that may contribute to a better outcome of COVID-19 and other lung diseases. This effect was pronounced in rats with metabolic syndrome (obesity, hyperglycemia and increased blood pressure) and in aged rats. In these models of most vulnerable individuals, we observed a significant decrease in lung levels of ACE2 together with a decrease in the expression of receptors of the anti-inflammatory axis (AT2 in MetS rats and AT2 and Mas receptors in aged rats), and a significant increase in the expression of AT1 receptors, which revealed a clear unbalance toward the proinflammatory RAS in lungs. Interestingly, treatment with candesartan and captopril shifted the balance towards the anti-inflammatory axis by inducing a significant increase in lung levels of ACE2, AT2 and Mas receptors, and a decrease in AT1 receptor expression. Expression of receptors of the proinflammatory axis (AT1) was reduced to levels of young healthy controls, while the expression of receptors of the anti-inflammatory axis such as AT2 and, particularly Mas receptors was increased to levels higher than those of young healthy untreated rats. This suggests that treatment with these drugs may reduce vulnerability of aged patients and patients with MetS even below levels of healthy young patients, at least in that related to RAS dysregulation. It is frequently assumed that changes in ACE2 levels are responsible for the unbalance between both RAS axes, and this is the case for COVID-19. Further factors may impact on RAS unbalance in other diseases. In the case of MetS and aging, the decrease in levels of ACE2 may be the cause or, more probably, the consequence of dysregulation of other RAS components. For instance, in aged brains dysregulation of RAS components may be related to initial dysregulation of other factors such as sirtuins or IGF-1 [43,44]. In COVID-19, the dysregulation observed in MetS and aging is further increased (and probably more hardly compensated) by binding of SARS-CoV-2 to ACE2. In any case, the present results indicate that the dysregulation can be counteracted by the drugs here assayed.
Although the up-regulation of lung ACE2 and anti-inflammatory axis receptors appears beneficial for the outcome of COVID-19 patients, an up-regulation of the viral receptors may be deleterious so that ACE2 up-regulation has been considered as a double-edged sword [15–17]. However, the present in vitro observations treating human type-II pneumocytes with the viral spike protein and RAS modulators, suggest that the above-mentioned drugs may even decrease viral entry as suggested by our observations on spike protein inside cells using laser confocal microscopy. After SARS-CoV binds to ACE2, the virus enters the host cell using two possible mechanisms: endocytosis and cell membrane fusion. Receptor-mediated endocytosis is a major mechanism for viral entry [24,45,46]. Spike protein binding is the trigger for this entry pathway and induces translocation of ACE2 together with spike protein from the cell surface to endosomes [24,46]. This was observed both using spike protein and spike-bearing pseudoviruses [24], which suggests that treatment of cells with spike protein is a reliable model to study effects of drugs on SARS-CoV entry mechanisms.
Consistent with that observed in the rat model, treatment of cultures with candesartan or captopril induced up-regulation of ACE2 expression in lung cells. Treatment with spike protein induced a decrease in cell levels of full-length (i.e. transmembrane) ACE2, which was inhibited by pretreatment with candesartan or captopril. This may be explained by the observed drug-induced up-regulation of ACE2 expression. Additional mechanisms may also contribute to drug-induced increase in cell-surface ACE2. It has been suggested that ACE2 and AT1 receptors may be colocalized at the plasma membrane forming complexes, and that elevated AngII levels decrease ACE2 activity through AT1-dependent ACE2 internalization followed by lysosomal targeting for degradation, which may be prevented by AT1 receptor blockers [47]. In the present experiments, a captopril-induced reduction in AngII levels or candesartan blockage of AT1 receptors may reduce internalization of ACE2 together with virus/spike protein and increase cell-surface ACE2 activity. However, the results observed in the present experiments suggest that additional mechanisms of interaction between SARS-CoV-2/spike protein and RAS are also involved. We observed that spike protein treatment induced an increase in levels of soluble ACE2 in the culture medium. This is consistent with previous studies showing that binding the virus or spike protein to cell-surface ACE2 induces ACE2 ectodomain shedding, decreases cell-surface levels of ACE2 and promotes virus/spike protein internalization [30,48–50]. Interestingly we also detected a spike-induced increase in cell levels of a short ACE2 polypeptide (60 kDa), which may correspond to an internalized glycosylated ACE2 polypeptide.
ADAM17 is a metalloprotease and disintegrin located in the cell membrane and that can cleave a wide number of substrates and has been involved in several cardiovascular and neurological disorders [51]. Interestingly, ADAM17 also plays a major role in the entry mechanism of several viruses [52,53] and particularly SARS-CoV viruses. The mechanism used by ADAM17 to facilitate SARS-CoV entry has not been totally clarified; however, it is known that activation of ADAM17 plays a major role in ACE2 shedding [49–51] and enhances viral entry [30,48]. It has been shown that activation of ADAM17 by the viral spike protein depends on the cytoplasmatic domain of ACE2 and that this is positively involved in viral entry [30,48]. In addition, the ACE2 cytoplasmic domain is also required for SARS-spike-induced ACE2 shedding [30,48]. Consistent with this, ADAM17 inhibitors attenuated entry of both pseudotyped virus expressing the SARS-spike protein and infectious strains of SARS-CoV, and have been suggested as antiviral compounds [48]. Interestingly, the present experiments showed that the investigated drugs act as ADAM17 inhibitors. Candesartan and captopril reduce levels of soluble ACE2 in the culture medium suggesting a reduction of ACE2 shedding, and induce also a reduction in levels of intracellular short-length ACE2, which suggests reduction in spike/ACE2 internalization. This may be related with the observation of drug-induced decrease in ADAM17 activity in our cultures, which is consistent with previous observations in cardiomyocytes and neurons showing that AngII via AT1/Nox-derived ROS increases ADAM17 activity [54,55].
Our observations on proinflammatory cytokine release also suggest an overall beneficial effect of candesartan and captopril. Consistent with previous studies [56,57], we observed a spike-induced increase in the release to culture medium of major proinflammatory cytokines, which are also increased in SARS-CoV-infected patients [58,59]. We analyzed TNF-α, IL-6 and CCL2. TNF-α [60] and IL-6 [32,61] have been identified as major inducers of the SARS-CoV-2 hyperinflammatory response. CCL2 is considered a fibrosis-associated chemokine involved in several lung inflammatory disorders including SARS, asthma and pulmonary fibrosis. CCL2 is up-regulated in the sera of SARS-CoV patients and the supernatant of a SARS-CoV-infected culture systems [56,57,59,62]. Several mechanisms may be involved in the changes in proinflammatory cytokine release observed in the present study. First, we observed a spike-induced down-regulation of the anti-inflammatory RAS axis and up-regulation of the proinflammatory AT1/NOX2 axis, which may lead to an increase in cytokine release [32,63,64] mediated by NOX2-derived ROS [54,65]. Second, ADAM17 is not only involved in shedding of ACE2 [49–51], but in the release several cytokines [66]. It is well-known that ADAM17 or TACE (TNF-α -converting enzyme) releases TNF-α from cells [67], and that ADAM17 promotes IL-6/soluble IL-6 receptor release [32]. In addition, ADAM17 may induce CCL2 release via an indirect mechanism involving acidic mammalian chitinase (AMCase) [68]. In summary, both an increase in the anti-inflammatory axis activity (together with inhibition of the proinflammatory arm) and a decrease in ADAM17 activity may explain a decrease in spike-induced proinflammatory cytokine release in our cultures after treatment with candesartan or captopril.
Conclusions
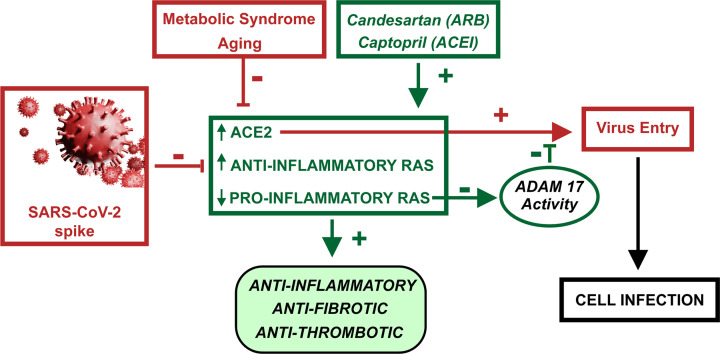
The present in vivo and in vitro studies show that candesartan, an AT1 receptor blocker, and captopril, an ACEI, up-regulate ACE2 expression and anti-inflammatory RAS activity in the lung. Furthermore, up-regulation of the SARS-CoV-2 main receptor, ACE2, is counteracted by drug-induced mechanisms that reduce SARS-CoV-2 spike protein entry, particularly by inhibition of ADAM17 activity (Figure 7).
Figure 7. Diagram summarizing major results and conclusions.
Candesartan and captopril increase ACE2 levels and anti-inflammatory RAS arm activity, and inhibit the proinflammatory RAS axis.
This leads to anti-inflammatory, anti-fibrotic and anti-thrombotic effects in the lung, and counteracts the opposite effects of aging and metabolic syndrome (obesity, hyperglycemia and hypertension) and SARS-CoV-2/spike protein on the same RAS components. The candesartan/captopril-induced increase in ACE2 levels leads to an increase in levels of viral receptors. However, a simultaneous candesartan/captopril-induced reduction in ADAM17 activity reduces viral entry and cell infection.
Clinical perspectives
There is an important controversy on effects of several drugs that regulate RAS activity, and possibly ACE2 levels, on COVID-19 outcome. This has been particularly focused on AT1 receptor blockers (ARBs) and ACE inhibitors that are widely used, particularly by patients most vulnerable to severe COVID-19.
Our results with candesartan and captopril suggest that these drugs up-regulate ACE2 expression and anti-inflammatory RAS activity in the lung. Furthermore, up-regulation of the SARS-CoV-2 main receptor, ACE2, is counteracted by drug-induced mechanisms that reduce SARS-CoV-2 spike protein entry, particularly by inhibition of ADAM17 activity.
Our observations indicate an overall beneficial effect of the here tested drugs in COVID-19 clinical outcome.
Supplementary Material
Acknowledgements
We thank Pilar Aldrey, Iria Novoa and Cristina Gianzo for their technical assistance.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ACEI
angiotensin-converting enzyme inhibitor
- AngII
angiotensin II
- AT1
angiotensin type 1
- AT2
angiotensin type 2
- CCL2
Chemokine CC motif ligand 2
- FRET
fluorescence resonance energy transfer
- IL-6
interleukin 6
- MasR
Mas receptor
- MetS
metabolic syndrome
- RAS
renin–angiotensin system
- TG
triglyceride
- TNF-α
tumor necrosis factor-α
Contributor Information
Jose L. Labandeira-Garcia, Email: joseluis.labandeira@usc.es.
Ana I. Rodriguez-Perez, Email: anai.rodriguez@usc.es.
Data Availability
Data are available from the corresponding author upon reasonable request
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
Axencia Galega de Innovación [grant number IN845D 2020/20]; Spanish Ministry of Economy and Competitiveness [grant numbers RTI2018-098830-B-I00 and RTI2018-094204-B-I00]; Spanish Ministry of Health [grant numbers PI17/00828, RD16/0011/0016 and CIBERNED]; and Galician Government [grant numbers XUGA, ED431C 2018/10 and ED431G/05]; and FEDER (Regional European Development Fund).
CRediT Author Contribution
Maria A. Pedrosa: Investigation, Methodology. Rita Valenzuela: Conceptualization, Investigation, Methodology. Pablo Garrido-Gil: Investigation, Methodology. Carmen M. Labandeira: Conceptualization, Writing—review and editing. Gemma Navarro: Investigation, Methodology. Rafael Franco: Conceptualization, Writing—review and editing. Jose L. Labandeira-Garcia: Conceptualization, Formal analysis, Funding acquisition, Writing—original draft, Writing—review and editing. Ana I. Rodriguez-Perez: Conceptualization, Formal analysis, Funding acquisition, Writing—original draft, Writing—review and editing.
Ethics Approval
Animal handling was conducted in accordance with the Directive 2010/63/EU, European Council Directive 86/609/EEC and the Spanish legislation (RD53/2013). Rodent experiments were approved by the corresponding committee at the University of Santiago de Compostela. Animals were housed at constant room temperature (RT) (21–22°C) and 12-h light/dark cycle.
References
- 1.Ranjbar R., Shafiee M., Hesari A., Ferns G.A., Ghasemi F. and Avan A. (2019) The potential therapeutic use of renin-angiotensin system inhibitors in the treatment of inflammatory diseases. J. Cell. Physiol. 234, 2277–2295 10.1002/jcp.27205 [DOI] [PubMed] [Google Scholar]
- 2.Cabandugama P.K., Gardner M.J. and Sowers J.R. (2017) The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med. Clin. North. Am. 101, 129–137 10.1016/j.mcna.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kloet A.D., Krause E.G. and Woods S.C. (2010) The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 100, 525–534 10.1016/j.physbeh.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benigni A., Cassis P. and Remuzzi G. (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol. Med. 2, 247–257 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Perez A.I., Valenzuela R., Villar-Cheda B., Guerra M.J. and Labandeira-Garcia J.L. (2012) Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: role of the brain angiotensin system. Brain 135, 124–138 10.1093/brain/awr320 [DOI] [PubMed] [Google Scholar]
- 6.Ganong W.F. (1994) Origin of the angiotensin II secreted by cells. Proc. Soc. Exp. Biol. Med. 205, 213–219 10.3181/00379727-205-43699A [DOI] [PubMed] [Google Scholar]
- 7.Re R.N. (2004) Tissue renin angiotensin systems. Med. Clin. North. Am. 88, 19–38 10.1016/S0025-7125(03)00124-X [DOI] [PubMed] [Google Scholar]
- 8.Labandeira-Garcia J.L., Valenzuela R., Costa-Besada M.A., Villar-Cheda B. and Rodriguez-Perez A.I. (2020) The intracellular renin-angiotensin system: Friend or foe. Some light from the dopaminergic neurons. Prog. Neurobiol. 8, 101919 10.1016/j.pneurobio.2020.101919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labandeira-Garcia J.L., Rodriguez-Perez A.I., Garrido-Gil P., Rodriguez-Pallares J., Lanciego J.L. and Guerra M.J. (2017) Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 9, 129 10.3389/fnagi.2017.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarzani R., Giulietti F., Di Pentima C., Giordano P. and Spannella F., 2020) Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L325–L336 10.1152/ajplung.00189.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brojakowska A., Narula J., Shimony R. and Bander J. (2020) Clinical Implications of SARS-CoV-2 Interaction With Renin Angiotensin System: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 75, 3085–3095 10.1016/j.jacc.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perico L., Benigni A. and Remuzzi G. (2020) Reply to the Comment by Dr. Cure on “Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade” Nephron 144, 253–254 10.1159/000507861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A. and Solomon S.D. (2020) Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 382, 1653–1659 10.1056/NEJMsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J.et al. (2020) Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 126, 1456–1474 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.South A.M., Tomlinson L., Edmonston D., Hiremath S. and Sparks M.A. (2020) Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 16, 305–307 10.1038/s41581-020-0279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K., Gheblawi M. and Oudit G.Y. (2020) Angiotensin Converting Enzyme 2: A Double-Edged Sword. Circulation 142, 426–428 10.1161/CIRCULATIONAHA.120.047049 [DOI] [PubMed] [Google Scholar]
- 17.Yan T., Xiao R. and Lin G. (2020) Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: A double-edged sword? FASEB J. 34, 6017–6026 10.1096/fj.202000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein N., Gembardt F., Supe S., Kaestle S.M., Nickles H., Erfinanda L.et al. (2013) Angiotensin-(1-7) protects from experimental acute lung injury. Crit. Care Med. 41, e334–e343 10.1097/CCM.0b013e31828a6688 [DOI] [PubMed] [Google Scholar]
- 19.Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M.et al. (2009) Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 83, 7062–7074 10.1128/JVI.00127-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H.et al. (2004) Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem. Biophys. Res. Commun. 319, 1216–1221 10.1016/j.bbrc.2004.05.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B.et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 11, 875–879 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan R., Zhang Y., Li Y., Xia L., Guo Y. and Zhou Q. (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367, 1444–1448 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdecchia P., Cavallini C., Spanevello A. and Angeli F. (2020) The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 76, 14–20 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G.et al. (2008) SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 18, 290–301 10.1038/cr.2008.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M.et al. (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 55,2000547 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J.et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danser A.H.J., Epstein M. and Batlle D. (2020) Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension 75, 1382–1385 10.1161/HYPERTENSIONAHA.120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A.et al. (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111, 2605–2610 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 29.Namsolleck P. and Moll G.N. (2020) Does activation of the protective Renin-Angiotensin System have therapeutic potential in COVID-19? Mol. Med. 26, 80 10.1186/s10020-020-00211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T.et al. (2008) Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U.S.A. 105, 7809–7814 10.1073/pnas.0711241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi C.K., Holmes R., Gewolb I.H. and Uhal B.D. (2019) Degradation of Lung Protective Angiotensin Converting Enzyme-2 by Meconium in Human Alveolar Epithelial Cells: A Potential Pathogenic Mechanism in Meconium Aspiration Syndrome. Lung 197, 227–233 10.1007/s00408-019-00201-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patra T., Meyer K., Geerling L., Isbell T.S., Hoft D.F., Brien J.et al. (2020) SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 16, e1009128 10.1371/journal.ppat.1009128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhal B.D., Gidea C., Bargout R., Bifero A., Ibarra-Sunga O., Papp M.et al. (1998) Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am. J. Physiol. 275, L1013–L1017 [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Wahab B.A., Metwally M.E., El-khawanki M.M. and Hashim A.M. (2014) Protective effect of captopril against clozapine-induced myocarditis in rats: role of oxidative stress, proinflammatory cytokines and DNA damage. Chem. Biol. Interact. 216, 43–52 10.1016/j.cbi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 35.Arumugam S., Thandavarayan R.A., Palaniyandi S.S., Giridharan V.V., Arozal W., Sari F.R.et al. (2012) Candesartan cilexetil protects from cardiac myosin induced cardiotoxicity via reduction of endoplasmic reticulum stress and apoptosis in rats: involvement of ACE2-Ang (1-7)-mas axis. Toxicology 291, 139–145 10.1016/j.tox.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 36.Inada Y., Ojima M., Kanagawa R., Misumi Y., Nishikawa K. and Naka T. (1999) Pharmacologic properties of candesartan cilexetil–possible mechanisms of long-acting antihypertensive action. J. Hum. Hypertens. 13, S75–S80 10.1038/sj.jhh.1000749 [DOI] [PubMed] [Google Scholar]
- 37.Zhu B., Sun Y., Sievers R.E., Browne A.E., Pulukurthy S., Sudhir K.et al. (2000) Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J. Am. Coll. Cardiol. 35, 787–795 10.1016/S0735-1097(99)00592-6 [DOI] [PubMed] [Google Scholar]
- 38.Valenzuela R., Costa-Besada M.A., Iglesias-Gonzalez J., Perez-Costas E., Villar-Cheda B., Garrido-Gil P.et al. (2016) Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 7, e2427 10.1038/cddis.2016.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancia G., Rea F., Ludergnani M., Apolone G. and Corrao G. (2020) Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N. Engl. J. Med. 382, 2431–2440 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommerstein R., Kochen M.M., Messerli F.H. and Grani C. (2020) Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect? J. Am. Heart Assoc. 9, e016509 10.1161/JAHA.120.016509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y.et al. (2020) Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 98, 219–227 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J., Deng W., Li S. and Yang X. (2020) Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol. Life Sci 10.1007/s00018-020-03611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munoz A., Correa C.L., Lopez-Lopez A., Costa-Besada M.A., Diaz-Ruiz C. and Labandeira-Garcia J.L. (2018) Physical Exercise Improves Aging-Related Changes in Angiotensin, IGF-1, SIRT1, SIRT3, and VEGF in the Substantia Nigra. J. Gerontol. A Biol. Sci. Med. Sci. 73, 1594–1601 10.1093/gerona/gly072 [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Perez A.I., Borrajo A., Diaz-Ruiz C., Garrido-Gil P. and Labandeira-Garcia J.L. (2016) Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget 7, 30049–30067 10.18632/oncotarget.9174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M.et al. (2007) Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 81, 8722–8729 10.1128/JVI.00253-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S., Guo F., Liu K., Wang H., Rao S., Yang P.et al. (2008) Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 136, 8–15 10.1016/j.virusres.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deshotels M.R., Xia H., Sriramula S., Lazartigues E. and Filipeanu C.M. (2014) Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 64, 1368–1375 10.1161/HYPERTENSIONAHA.114.03743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N.et al. (2010) TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 85, 551–525 10.1016/j.antiviral.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L.et al. (2009) Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L84–L96 10.1152/ajplung.00071.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I.et al. (2005) Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 280, 30113–30119 10.1074/jbc.M505111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J., Mukerjee S., Silva-Alves C.R., Carvalho-Galvao A., Cruz J.C., Balarini C.M.et al. (2016) A Disintegrin and Metalloprotease 17 in the Cardiovascular and Central Nervous Systems. Front. Physiol. 7, 469 10.3389/fphys.2016.00469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kononchik J., Ireland J., Zou Z., Segura J., Holzapfel G., Chastain A.et al. (2018) HIV-1 targets L-selectin for adhesion and induces its shedding for viral release. Nat. Commun. 9, 2825 10.1038/s41467-018-05197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikulicic S., Finke J., Boukhallouk F., Wustenhagen E., Sons D., Homsi Y.et al. (2019) ADAM17-dependent signaling is required for oncogenic human papillomavirus entry platform assembly. Elife 8, e44345 10.7554/eLife.44345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R.et al. (2014) Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the. RAS. J. Mol. Cell Cardiol. 66, 167–176 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 55.Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O.et al. (2017) Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 121, 43–55 10.1161/CIRCRESAHA.116.310509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen I.Y., Chang S.C., Wu H.Y., Yu T.C., Wei W.C., Lin S.et al. (2010) Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling pathway. J. Virol. 84, 7703–7712 10.1128/JVI.02560-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H.et al. (2005) Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79, 7819–7826 10.1128/JVI.79.12.7819-7826.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He L., Ding Y., Zhang Q., Che X., He Y., Shen H.et al. (2006) Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 210, 288–297 10.1002/path.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J.et al. (2005) Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 171, 850–857 10.1164/rccm.200407-857OC [DOI] [PubMed] [Google Scholar]
- 60.Robinson P.C., Liew D.F.L., Liew J.W., Monaco C., Richards D., Shivakumar S.et al. (2020) The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19. Med (N. Y.) 1, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aziz M., Fatima R. and Assaly R. (2020) Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 92, 2283–2285 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coperchini F., Chiovato L., Croce L., Magri F. and Rotondi M. (2020) The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 53, 25–32 10.1016/j.cytogfr.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M.et al. (2010) Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122, 717–728, 18 p following 728 10.1161/CIRCULATIONAHA.110.955369 [DOI] [PubMed] [Google Scholar]
- 64.Zhong J., Guo D., Chen C.B., Wang W., Schuster M., Loibner H.et al. (2011) Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension 57, 314–322 10.1161/HYPERTENSIONAHA.110.164244 [DOI] [PubMed] [Google Scholar]
- 65.Shang F., Wang J., Liu X., Li J., Zheng Q., Xue Y.et al. (2012) Involvement of reactive oxygen species and JNK in increased expression of MCP-1 and infiltration of inflammatory cells in pressure-overloaded rat hearts. Mol. Med. Rep. 5, 1491–1496 [DOI] [PubMed] [Google Scholar]
- 66.de Queiroz T.M., Lakkappa N. and Lazartigues E. (2020) ADAM17-Mediated Shedding of Inflammatory Cytokines in Hypertension. Front. Pharmacol. 11, 1154 10.3389/fphar.2020.01154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F.et al. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- 68.Hartl D., He C.H., Koller B., Da Silva C.A., Homer R., Lee C.G.et al. (2008) Acidic mammalian chitinase is secreted via an ADAM17/epidermal growth factor receptor-dependent pathway and stimulates chemokine production by pulmonary epithelial cells. J. Biol. Chem. 283, 33472–33482 10.1074/jbc.M805574200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon reasonable request