Abstract
Anaplastic thyroid carcinoma (ATC) is resistant to standard therapies and has no effective treatment. Eukaryotic translation initiation factor 5A2 (EIF5A2) has shown to be upregulated in many malignant tumors and proposed to be a critical gene involved in tumor metastasis. In this study, we aimed to investigate the expression status of EIF5A2 in human ATC tissues and to study the role and mechanisms of EIF5A2 in ATC tumorigenesis in vitro and in vivo. Expression of EIF5A2 protein was analyzed in paraffin-embedded human ATC tissues and adjacent nontumorous tissues (ANCT) (n = 24) by immunochemistry. Expressions of EIF5A2 mRNA and protein were analyzed in fresh-matched ATC and ANCT (n = 23) and ATC cell lines by real-time polymerase chain reaction (PCR) and Western blotting. The effect of targeting EIF5A2 with short hairpin RNA (shRNA) or EIF5A2 overexpression on the ATC tumorigenesis and TGF-β/Smad2/3 signals in vitro and in vivo was investigated. Expression of EIF5A2 was significantly upregulated in ATC tissues and cell lines compared with ANCT and normal follicular epithelial cell line. Functional studies found that targeting EIF5A2 induced SW1736 cell death in vitro and in vivo, followed by significantly downregulated phosphorylation of Smad2/3 (p-Smad2/3) in SW1736 cells at the protein level. Ectopic expression of EIF5A2 could promote 8505C cell growth in vitro and in vivo, followed by significantly upregulated p-Smad3 at the protein level. Recombinant human TGF-β1 (hTGF-β1) treatment decreased the antiproliferative activity of the EIF5A2 downexpressing 8505C cells through reversing pSmad2/3. Using the specific inhibitor SB431542 to block TGF-β pathway or Smad3 siRNA to knock down Smad3 increased the antiproliferative activity of the EIF5A2-overexpressing 8505C cells through inhibiting pSmad2/3. Our findings indicated that EIF5A2 controled cell growth in ATC cells, and EIF5A/TGF-β/Smad2/3 signal may be a potential therapeutic target for ATC treatment.
Key words: Anaplastic thyroid carcinoma (ATC), Eukaryotic translation initiation factor 5A2 (EIF5A2), Transforming growth factor-β (TGF-β)
INTRODUCTION
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive human malignancies. Currently, no effective therapies exist1. Thus, we need an understanding of the carcinogenesis to develop new modes of treatment for this carcinoma to improve the quality of life of patients with ATC.
Eukaryotic translation initiation factor 5A (EIF5A), a conserved lysine to hypusine, is strictly indispensable for the survival of eukaryotic cells2. There are two EIF5A isoforms in humans: EIF5A1 and EIF5A2. The former is expressed in most cells and tissues, and the latter is expressed rarely in most normal tissues, and high levels of expression only in parts of the brain and testis3–5. Both isoforms have a high degree of amino acid homology, which in humans is 84% identical and are 94% similar6. Accumulating evidence has been implicated that both isoforms are related to cell proliferation, apoptosis, cancer progression, and poor clinical prognosis7–10.
EIF5A2 has been reported to be overexpressed in gallbladder cancer7, upper urinary tract urothelial carcinoma11, gastric cancer12, prostate cancer13, nasopharyngeal carcinoma14, and cervical cancer15. EIF5A2 upregulation in cancer tissues is associated with poor prognosis in these patients. In addition, overexpression of EIF5A2 could promote cancer cell invasion and growth, and vice versa16,17, suggesting EIF5A2 might be a potential candidate target for diagnosis and therapeutics of cancers. To date, however, the function of EIF5A2 in ATC and its underlying molecular mechanisms are still unclear.
Transforming growth factor-β (TGF-β) emerges as one of the key regulators of tumor development and progression. TGF-β signaling pathway is deregulated in many diseases, including cancer. In healthy cells and early stage cancer cells, this pathway has tumor suppressor functions, including cell cycle arrest and apoptosis18. However, its activation in late stage cancer can promote metastasis and chemoresistance by inducing epithelial–mesenchymal transition (EMT)19,20. There is strong evidence that TGF-β signaling contributes to proliferation, growth, differentiation, and apoptosis of many kind of cells21–25. TGF-β1 also regulated apoptosis, proliferation, invasion, and migration in thyroid cancer cells in vitro and in vivo via different mechanisms26–29.
In patients with bladder cancer (BC), EIF5A2 overexpression was related with metastatic potential. Furthermore, EIF5A2 elevated TGF-β1 expression through STAT3 to induce EMT and promote aggressiveness in BC cells in vitro and in vivo30. In A549 cells in vitro, the expression of eIF5A-2 was upregulated following TGF-β1-induced EMT phenotype changes, which correlated with enhanced tumor invasion and metastatic capabilities. Furthermore, targeting EIF5A-2 could reverse the process of EMT phenotype change, resulting in weakening of both invasive and metastatic capabilities in the A549 cell line31. In non-small cell lung cancer (NSCLC) cells in vitro, targeting EIF5A-2 with GC7 (a novel EIF5A-2 inhibitor) or with siRNA interference to EIF5A-2 induced cell apoptosis and enhanced cisplatin cytotoxicity in NSCLC cells32.
In the present study, we first investigated the expression of EIF5A2 in human ATC and human ATC cell lines. We then investigated the role of EIF5A2 downregulation and upregulation on ATC cells in vitro and in vivo. We finally investigated its underlying molecular mechanisms.
MATERIALS AND METHODS
Tissue Samples
Paraffin-embedded ATC and adjacent nontumorous tissues (ANCT) (n = 24) or fresh-matched ATC and ANCT (n = 23) were collected from the Department of Thyroid Surgery and Pathology, the Affiliated Hospital of Qingdao University. All surgical specimens were obtained after patients signed informed consent. The study was approved by the ethical committee of the Affiliated Hospital of Qingdao University.
Cell Culture and Agents
The human ATC cell lines 8505C, SW1736, KAT18, and Cal-62 and primary human thyroid follicular epithelial cell Nthy-ori 3-1 were used in the present study; the information of the cells was published previoiusly33. The following antibodies were used: EIF5A2 rabbit mAb, TGF-β1, phospho-Smad2 (Ser465/467), phospho-Smad3 (Ser423/425) rabbit mAb, rabbit SMAD-3 antibody, GAPDH (14C10) rabbit mAb, and secondary antibody (anti-rabbit IgG or anti-mouse IgG) (Santa Cruz Biotechnology, Shanghai, China). All the antibodies were purchased from Cell Signaling Technology (Shanghai, China). Human TGF-β1 (hTGF-β1) (#8915) was obtained from Cell Signaling Technology, and specific TGF-β pathway inhibitor SB431542 (#14775; 10 μM) was obtained from TOCRIS Biosciences (Shanghai, China).
Short Hairpin RNA (shRNA) Transfection or Plasmid Construction and Establishment of Stable Cells Lines
To construct shRNAs targeting human EIF5A2, an shRNA sequence targeting EIF5A2 (EIF5A2 shRNA) was cloned into the pGV248-lentivirus vector (Shanghai GenePharma Co. Ltd., Shanghai, China). This vector expresses green fluorescent protein (GFP) under the control of the CMV promoter to allow monitoring of transfection efficiency. The control shRNA (CN shRNA) was used as control. Three different shRNAs were designed targeting different EIF5A2 mRNAs, as well as one nonsense sequence. All subcloned sequences were verified by DNA sequencing. The correct constructs were named as EIF5A2 shRNA1, EIF5A2 shRNA2, EIF5A2 shRNA3, and scrambled shRNA, respectively. To get the stably transfected cell clones, EIF5A2 shRNA1, EIF5A2 shRNA2, EIF5A2 shRNA3, and scrambled shRNA were transfected into the ATC cells with Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific Inc., Carlsbad, CA, USA) and selected with 200 mg/ml G418 for 2–3 weeks. GFP expression was checked in stable clones by flow cytometry.
For EIF5A2 overexpression construct, the human EIF5A2 cDNA was purchased from PROSPEC (Shanghai, China) and cloned into pCDNA3.1+ using EcoRI and NotI sites for the construction of pcDNA3.1-EIF5A2 plasmid. We transfected ATC cells using the Lipofectamine 2000 reagent in a serum containing medium according to the manufacturer’s guidelines. Transfection of the empty pcDNA3.1 vector served as the control. The resultant clones were subjected to a sequencing analysis. Stably transfected clones were selected with 400 mg/ml G418 for 2–3 weeks (Cellgro, Guangzhou, China).
siRNA Transfection
The stable pcDNA3.1-EIF5A2-transfected 8505C cells (3 × 104 cells/ml) were plated in six-well plates. Twenty-four hours later, 50 nmol/L of validated Smad3 specific and scrambled siRNAs (Santa Cruz Biotechnology) were mixed with the use of Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. Cells were collected 72 h later to assess the downregulation of each target.
Cell Survival, Colony Formation, and Apoptosis Assay
The effect of EIF5A2 expression on ATC cell viability by WST-1 assays was determined by incubating ATC cells [cells were plated into 96-well-plates (4 × 103 cells/well)] transfected with EIF5A2 shRNA2 or pcDNA3.1-EIF5A2 or its controls in medium containing 2% FBS for 2–72 h. All data were normalized to respective controls.
The effect of EIF5A2 overexpression on ATC cell growth was also observered by colony formation assay. Briefly, ATC cells (EIF5A2 shRNA2 or pcDNA3.1-EIF5A2 or its control-transfected cells) (200 cells/well) were seeded in a six-well plate and cultured for 2 weeks in medium containing 10% FBS. After removing the medium, cells were washed with PBS, fixed with pure methanol, and stained in crystal violet. Colony-forming unit of more than 50 cells was counted using the inverted microscope.
The effect of targeting EIF5A2 expression with EIF5A2 shRNA2 on ATC cell apoptosis was determined by performing annexin V/propidium iodide staining (BD BioScience Inc., San Jose, CA, USA), followed by flow cytometric analysis. Apoptotic cell was evaluated by counting the number of cells that stained positive for annexin V-fluorescein isothiocyanate (FITC) and positive for propidium iodide (PI).
The SW1736 cells were treated with hTGF-1 (5 ng/ml) for 6 h, and then transfected with EIF5A shRNA2 or control shRNA for 48 h; cell viability and cell apoptosis were detected as described above. The stable pcDNA3.1-EIF5A2-transfected 8505C cells were transfected with Smad3 siRNA or control siRNA for 72 h or treated with or without SB431542 (10 μM) 2 μl or 1% DMSO 2 μl for 48 h; cell viability and cell apoptosis were detected as described above.
Western Blot Assay
Cells and tissues were harvested and solubilized in radioimmunoprecipitation assay (RIPA) buffer, and the whole-cell lysates were prepared. Standard Western blotting was carried out using whole-cell protein lysates. The cell lysates resolved on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes. Primary antibodies were incubated overnight at 4°C. The antibodies used in the analysis were anti-eIF5A2, anti-pSmad2, anti-pSmad3, anti-Smad2, anti-Smad3, and anti-GAPDH. The proteins were detected using horseradish peroxidase (HRP)-labeled secondary antibodies and visualized using the Amersham ECL System and detection system analyzed (ChemiDoc Touch, Bio-Rad, Hercules, CA, USA).
Reverse Transcription Polymerase Chain Reaction (RT-PCR) for EIF5A
Total RNA was isolated from ATC cells and the frozen ATC tissues using RNeasy Protect Kit (Life Technologies, Shanghai, China) and transcribed into cDNA using Superscript II Reverse Transcriptase (Invitrogen) according to the manufacturer’s instruction. The primer pairs for RT-PCR were EIF5A2: 5′-CCCTGCTGACAGAAACTGGT-3′ and 5′-TTGCACACATGACAGACACC-3′; GAPDH: 5′-AATCCCATCACCATCTTCCAGGAG-3′ and 5′-GCATTGCTGATGATCTTGAGGCTG-3′. RT-PCR was performed using an Applied Biosystems 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Data were analyzed by the ΔΔ cycle threshold method.
Xenograft Model
The stable EIF5A shRNA2- or CN shRNA-transfected SW1736 cells or the stable pcDNA3.1-EIF5A2- or pcDNA3.1-transfected 8505C cells (1 × 106) were injected into the right flank of nude mice subcutaneously. Tumor xenografts were analyzed twice weekly. The tumor volume was calculated using tumor volume (mm3) = π/6 × a × b 2. The animals were observed for 28 days after the last injection. After 28 days, tumor xenografts were harvested and analyzed. Immunohistochemical analysis for EIF5A2, p-Smad3, cleaved caspase 3, and Ki-67 was detected in the tumor tissues. All the in vivo experiments were approved by the Animal Care Committee of the Affiliated Hospital of Qingdao University.
Immunohistochemistry
Human ATC tissues were obtained from the Department of Pathology, the Affiliated Hospital of Qingdao University. Paraffin-embedded biopsy material sections were stained for EIF5A using anti-EIF5A antibody following the manufacturer’s instruction; <25 % EIF5A2 cytoplasmic staining was negative EIF5A2 expression, and ≥25% EIF5A2 cytoplasmic staining was positive EIF5A2 expression34.
Xenograft tumors were excised, fixed, and paraffin embedded. Paraffin-embedded biopsy material sections were stained for EIF5A2, p-Smad3, cleaved caspase 3, and Ki-67 according to the recommendations of the manufacturer.
Statistical Analysis
Results were expressed as mean ± standard deviation (SD). The data analysis was performed using the SPSS statistical software package (SPSS 22; Chicago, IL, USA). The unpaired Student’s t-test was performed to analyze the statistical significance between two groups. More than two independent groups were compared using analysis of variance (ANOVA), and statistically significant difference was defined as a value of p < 0.05. All experiments were performed in triplicate, unless otherwise stated.
RESULTS
Expression of EIF5A2 mRNA and Protein in ATC Cell Lines and Human ATC Tissues
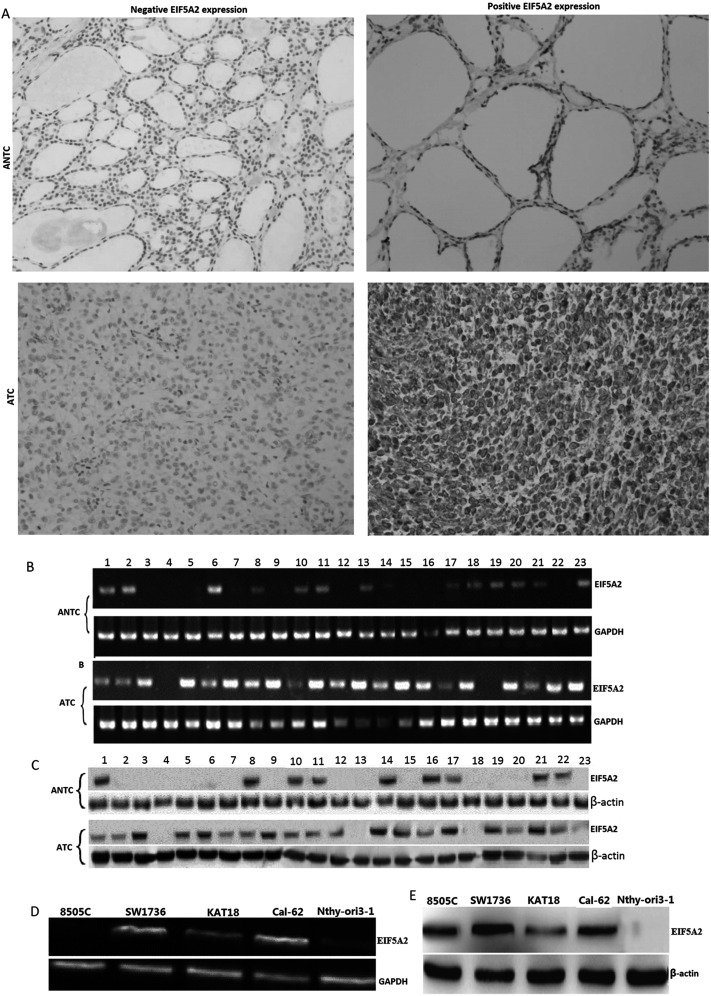
Expression of EIF5A2 protein was analyzed in paraffin-embedded 24 cases of human ATC tissues and ANCT by immunochemistry. The results showed that positive diffuse cytoplasmic staining of EIF5A2 was 68.3 ± 9.4% (median: 62.5%) (15 of 24) in ATC tissue specimens, which was higher than that in the ANCT tissues [20.4 ± 4.3% (median: 16.7%) (4 of 24)] (p = 0.013) (Fig. 1A). EIF5A2 mRNA and protein were detected by RT-PCR and Western blot in fresh ATC tissues (n = 23) and fresh-matched ANCT (n = 23). The relative EIF5A2 mRNA and protein were significantly upregulated in fresh ATC samples (0.94 ± 0.23 and 0.87 ± 0.19) compared with ANCT (0.24 ± 0.09 and 0.17 ± 0.07) (p < 0.01), respectively (Fig. 1B and C). EIF5A mRNA and protein were also detected by RT-PCR and Western blot in 8505C, SW1736, KAT18, Cal-62, and Nthy-ori 3-1 cells. EIF5A2 mRNA and protein were highly expressed in ATC cells compared with Nthy-ori 3-1 cells (Fig. 1D and E).
Figure 1.
EIF5A2 mRNA and protein expression in anaplastic thyroid carcinoma (ATC) tissues and ATC cells. (A) Immunohistochemistry assay for EIF5A2 protein in paraffin-embedded ATC and adjacent nontumorous tissues (ANCT) (n = 24) (original magnification: ×200). (B) EIF5A2 mRNA expression was detected by reverse transcription polymerase chain reaction (RT-PCR) in fresh ATC (n = 23) and fresh-matched ANCT. (C) EIF5A 2 protein expression was detected by Western blot assay in fresh ATC (n = 23) and fresh-matched ANCT. (D) EIF5A2 mRNA expression was detected by RT-PCR in ATC lines. (E) EIF5A2 protein expression was detected by Western blot assay in ATC lines.
Targeting EIF5A2 mRNA and Protein Expression in ATC Cells In Vitro
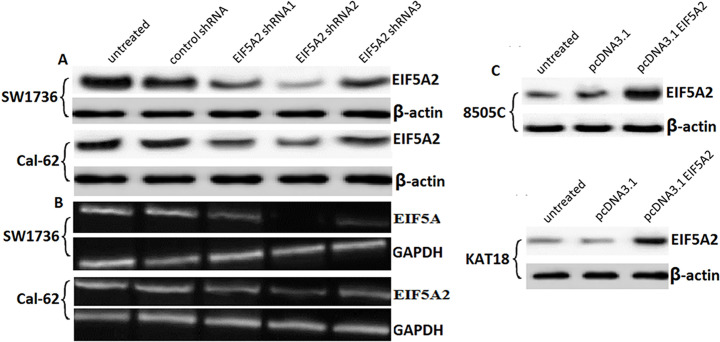
As shown in Figure 1D and E, SW1736 and Cal-62 cells expressed much EIF5A2 mRNA and protein, so we transfected three sets of EIF5A2-specific shRNA (EIF5A2 shRNA1, EIF5A2 shRNA2, and EIF5A2 shRNA3) or control shRNA in lentiviruses into the SW1736 and Cal-62 cells for 72 h. The results showed that EIF5A2 shRNA2 transfection resulted in 90% silencing of EIF5A2 in SW1736 cells and resulted in 60% silencing of EIF5A2 in Cal-62 cells as shown by RT-PCR (Fig. 2A). Control shRNA transfection did not affect EIF5A2 expression in both of the cells. Western blot analysis has the same results as that by RT-PCR assay (Fig. 2B). So we used the SW1736 cell line and EIF5A2 shRNA2 for further investigation.
Figure 2.
Targeting EIF5A2 in ATC cell lines. (A) Western blot analysis of EIF5A2 protein expression in SW1736 and Cal-62 cells after control shRNA, or EIF5A2 shRNA 1, 2, and 3 transfection for 48 h. (B) RT-PCR analysis of EIF5A2 mRNA expression in SW1736 and Cal-62 cells after control shRNA or EIF5A2 shRNA1, 2, and 3 transfection. (C) Western blot analysis of EIF5A2 protein expression in 8505C and KAT18 cells after pcDNA3.1-EIF5A2 or pcDNA3.1.
Expression of Endogenous EIF5A2 in ATC Cells In Vitro
As shown in Figure 1D and E, 8505C and KAT18 cells expressed less EIF5A2 mRNA and protein. So we transfected pcDNA3.1-EIF5A2 or pcDNA3.1 into 8505C and KAT18 cells for 72 h. The results showed that pcDNA3.1-EIF5A2 resulted in a 3.2-fold protein increase in 8505C cells and a 2.9-fold protein increase in KAT18 cells by Western blot analysis (Fig. 2C). pcDNA3.1 transfection did not affect EIF5A2 expression in both of the cells. We then used 8505C cells for further investigation.
Targeting EIF5A2 Inhibited Cell Growth and Induced Cell Apoptosis In Vitro
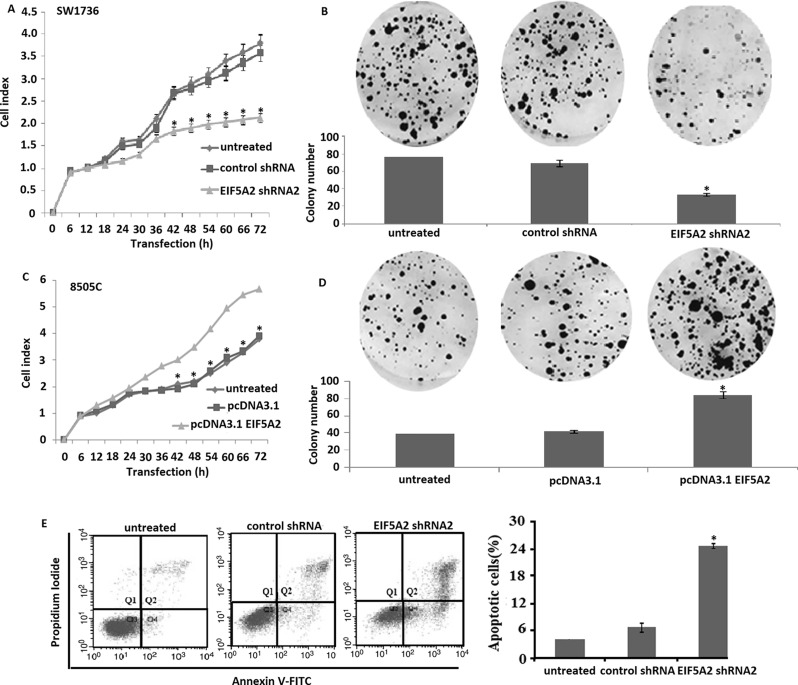
Following EIF5A2 downregulation in SW1736 for 24–72 h, we measured the viability in SW1736 cells. Compared to control shRNA, EIF5A2 shRNA2 transfection showed significantly decreased cell viability in the SW1736 (Fig. 3A), which was proved using a colony formation assay. Targeting EIF5A2 by EIF5A2 shRNA2 transfection reduced colony formation rate of SW1736 cells to 60% compared to the control shRNA-transfected SW1736 cells (Fig. 3B). However, compared to the controls, overexpression of EIF5A2 increased cell viability (Fig. 3C) and colony formation ability in the 8505C cells (Fig. 3D). We next detected the role of EIF5A2 downregulation on cell apoptosis in SW1736 cells in vitro. As shown in Figure 3E, targeting EIF5A2 significantly induced cell apoptosis in SW1736 cells compared to the controls (p < 0.01).
Figure 3.
Effects of EIF5A2 on cell viability and cell apoptosis in ATC cells in vitro. (A) Line graphs showing the survival of SW1736 cells after transfection with EIF5A2 shRNA2 or control shRNA and incubation for 72 h. EIF5A2 shRNA2 transfection significantly decreased cell survival compared to the controls using WST-1 assays (vs. untreated, *p < 0.01). (B) Colony-forming assay showed that SW1736 cells transfected with EIF5A2 shRNA2 decreased the colony number (columns, n = 3) (vs. untreated, *p < 0.01). (C) Line graphs showing the survival of 8505C cells after transfection with pcDNA3.1-EIF5A2 or control pcDNA3.1 and incubation for 72 h. pcDNA3.1-EIF5A2 transfection significantly increased survival compared to the controls using WST-1 assays (p < 0.001). (D) Colony-forming assay showed that 8505C cells transfected with pcDNA3.1-EIF5A2 increased the colony number (columns, n = 3) (vs. untreated, *p < 0.01). (E) SW1736 cells transfected with EIF5A2 shRNA2 for 72 h and followed cell apoptosis analysis by flow cytometry (vs. untreated, *p < 0. 01).
EIF5A2 Regulates TGF-β Signaling Pathway in ATC Cells In Vitro
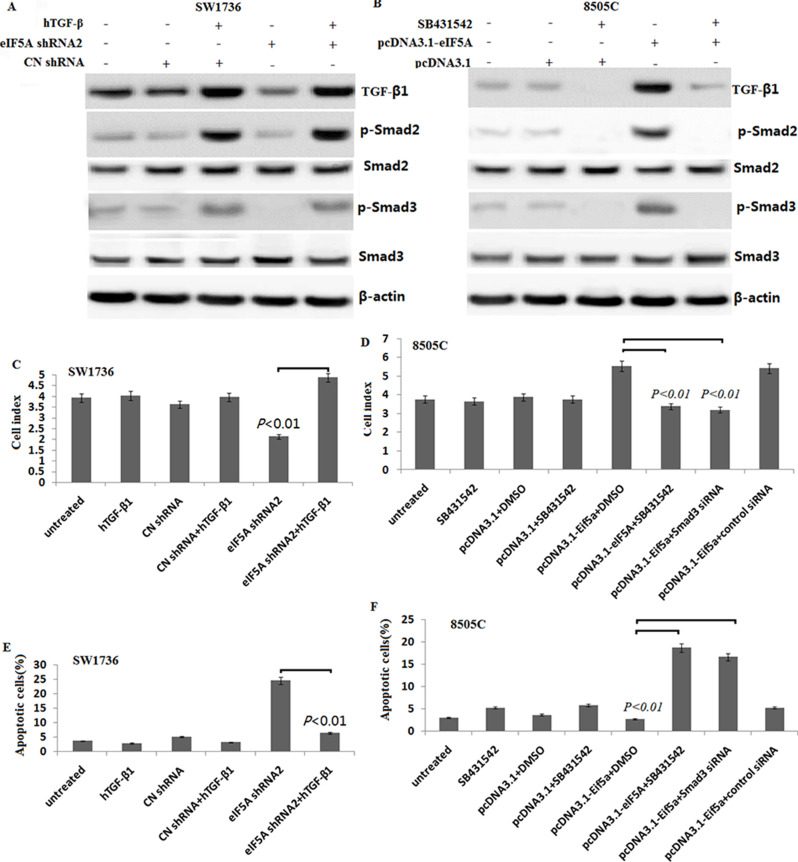
We detected the TGF-β signaling expression in the EIF5A downexpressing SW1736 cells in vitro. As shown in Figure 4A, the phosphorylated Smad-2 and -3 (pSmad2/3) expressed lower in the SW1736 cells, and 5 ng/ml hTGF-β treatment could activate phosphorylated Smad-2 and -3. However, targeting EIF5A reversed hTGF-β-induced pSmad2/3 activation. In the 8505C cells, EIF5A overexpression activated TGF-β signaling, resulting in upregulation of pSmad2/3 (Fig. 4B). However, the enhanced TGF-β signaling by EIF5A overexpression was blocked by the specific Smad-2 inhibitor SB431542 in the 8505C cells (Fig. 4B).
Figure 4.
EIF5A2 regulates cell viability and apoptosis via TGF-β-dependent Smad2/3 signals in vitro. (A) The pSmad2/3 and Smad2/3 was detected in the SW1736 cells transfected with EIF5A2 shRNA2, with or without hTGF-β1 (5 ng/ml) treatment by Western blot assay. (B) The pSmad2/3 and Smad2/3 were detected in the 8505C cells transfected with pcDNA3.1-EIF5A2, then with or without 2 μl of SB431542 (10 μM) treatment by Western blot assay. (C) Graph showing the cell viability of SW1736 cells transfected with EIF5A2 shRNA2 with or without hTGF-β1 (5 ng/ml) treatment using WST-1 assays. (D) SW1736 cells were transfected with EIF5A2 shRNA2, with or without hTGF-β1 (5 ng/ml) for 72 h followed by cell apoptosis analysis by flow cytometry. (E) Graph showing the cell viability of 8505C cells transfected with pcDNA3.1-EIF5A2, then with or without 2 μl of SB431542 (10 μM) treatment using WST-1 assays. (F) 8505C cells were transfected with pcDNA3.1-EIF5A2, then with or without 2 μl of SB431542 (10 μM) SB431542 or/and cotransfected with Smad3 siRNA followed cell apoptosis analysis by flow cytometry (vs. control, *p < 0.01).
EIF5A2 Regulates Cell Growth and Apoptosis via TGF-β-Dependent Smad2/3 Signals In Vitro
The SW1736 cells were treated with hTGF-β1 (5 ng/ml) for 6 h, and then transfected into EIF5A2 shRNA2 or control shRNA for 48 h; the cell viability and apoptosis were detected. As shown in Figure 4C and D, targeting EIF5A2 in combination with hTGF-β1 (5 ng/ml) treatment reversed cell viability and reduced cell apoptosis in the SW1736 cells compared to the targeting EIF5A2 alone (p < 0.01, respectively).
In the 8505C cells, pcDNA3.1-EIF5A2 transfection in combination with 2-μl SB431542 (10 μM) treatment significantly decreased cell viability (Fig. 4E) and increased cell apoptosis (Fig. 4F) compared to the controls. In addition, targeting Smad3 by Smad3 siRNA also significantly decreased pcDNA3.1-EIF5A2-induced 8505C cell viability (Fig. 4E) and increased cell apoptosis (Fig. 4F).
Targeting EIF5A2 Inhibits ATC Xenograft Growth In Vivo
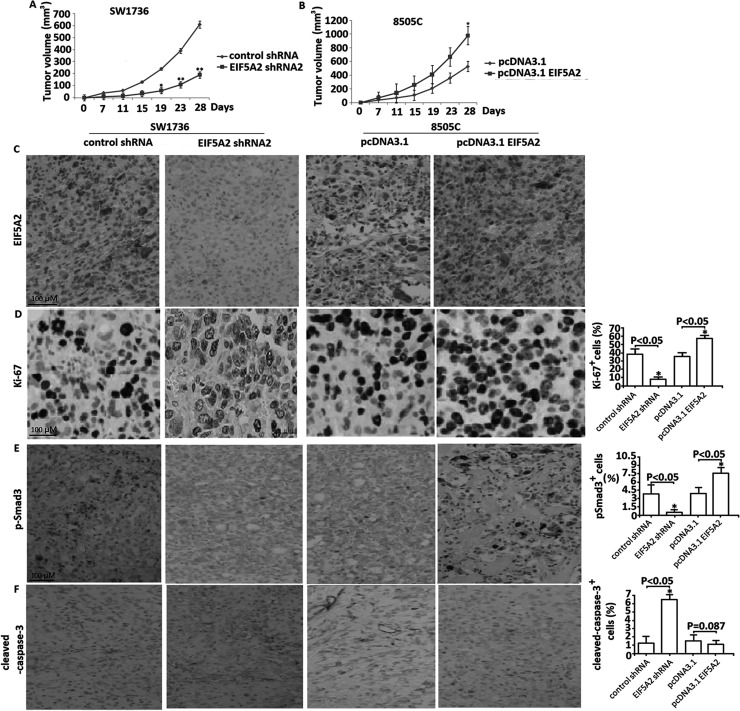
To determine the role of EIF5A2 downexpression on ATC growth in vivo, the stable EIF5A2 shRNA- or control shRNA-transfected SW1736 cells were subcutaneously injected into the right flank of the nude mice and its growth was observed for 28 days. As shown in Figure 5A, the mice injected with EIF5A2 shRNA-downxpressing SW1736 cells grew slowly in comparison with the control group mice. At the end of 4 weeks, the tumor volume of the EIF5A2 shRNA-transfected tumor was smaller than that in the control shRNA-transfected tumor (p < 0.01), suggesting that targeting EIF5A2 significantly inhibited xenografted tumor growth. By imunohistochemical analysis, targeting EIF5A2 inhibited EIF5A2 (Fig. 5C) and Ki-67 expression (Fig. 5D) and decreased Smad3 phosphorylation (Fig. 5E), as well as induced cleaved caspase 3 expression (Fig. 5F), suggesting that targeting EIF5A2 decreased cell proliferation and increased cell apoptosis, respectively.
Figure 5.
EIF5A2 regulated tumor growth in a nude murine model. (A) Sw1736 cells stably transfected with either EIF5A2 shRNA or control shRNA were injected subcutaneously into the left flanks of the mice; the animals were subsequently monitored by caliper measurement for tumor growth for 28 days. Measurement of tumor size over 28 days. *p < 0.05, **p < 0.01. (B) 8505C cells stably transfected with either pcDNA3.1-EIF5A2 or pcDNA3.1 were injected SC into the left flanks; the animals were subsequently monitored by caliper measurement for tumor growth for 28 days. Measurement of tumor size over 28 days. *p < 0.05. Tumor sections from nude mice in both groups were subjected to immunohistochemical analysis for EIF5A2 (C), Ki-67 (D), p-Smad-3 (E), and cleaved caspase 3 (F). Original magnification: 200×.
EIF5A2 Overexpression Promotes Xenograft Growth In Vivo
To determine the role of EIF5A2 overexpression on ATC growth in vivo, the stable pcDNA3.1-EIF5A2- or pcDNA3.1-transfected 8505C cells were subcutaneously injected into the mice and its growth was observed for 28 days. As shown in Figure 5B, EIF5A2 overexpression significantly promoted xenografted tumor growth compared with vector controls (p < 0.05). Immunohistochemical analysis showed that EIF5A2 overexpression upregulated EIF5A2 (Fig. 5C) and Ki-67 expression (Fig. 5D) and increased Smad3 phosphorylation (Fig. 5E), but did not affect cleaved caspase 3 expression (Fig. 5F), suggesting that EIF5A2 overexpression promoted tumor grow partly through enhancing cell proliferation, but not through inhibiting cell apoptosis.
DISCUSSION
ATC is an undifferentiated and aggressive type of cancer. It accounts for 1%–2% of all thyroid carcinomas20. The 5-year survival rate ranges from 0% to 14 %, with a median survival of 2–6 months35. ATC is not sensitive to any current systemic therapies, including chemotherapy, chemoradiotherapy, radiation, and experimental targeted therapies36. Therefore, novel treatments are desperately needed.
EIF5A2 is located on chromosome 3q26 and is weakly expressed in normal tissues but is overexpressed in a number of malignancies37. However, EIF5A2 is also poorly expressed in some malignant tumors, such as some lymphoma and leukemia cell lines and glioblastoma38–40. In the present study, we found that EIF5A2 mRNA and protein are overexpressed in human ATC tissues compared with normal thyroid tissues by RT-PCR, Western blotting, and immunohistochemical assay. EIF5A2 is also overexpressed in human ATC cell lines compared with normal follicular epithelial cell line by RT-PCR and Western blot assay.
Numerous studies have demonstrated that EIF5A2 overexpression stimulates growth of several types of tumors, and targeting EIF5A2 significantly induced malignant tumor cell death41–43. In this study, it was shown that EIF5A2 overexpression increases ATC cell viability in vitro by WST-1 assays and colony formation assay. In addition, pcDNA3.1-EIF5A2-mediated EIF5A2 overexpression in 8505C cells significantly promoted xenograft growth in vivo as measured by tumor volume and Ki-67-positive cells. But EIF5A2 overexpression did not significantly affect caspase 3 activity, indicating the cell apoptosis may not participate in the regulation of tumor growth by EIF5A2 overexpression. In addition, less activated caspase 3 expression was detected in the untransfected xenograft (data not shown). Therefore, it could not be true for EIF5A2 to inhibit cell apoptosis by blocking activated caspase 3 expression, resulting in increased tumor grow.
The SW1736 cells carried out the knockdown experiment. Targeting EIF5A2 expression in SW1736 cells significantly induced cell death and decreased cell growth in vitro as measured by WST-1 assays, colony formation assay, and flow cytometric analysis. In vivo, animals treated with EIF5A2 shRNA to knock down EIF5A2 had the lower tumor growth compared to the control. Tumor grow was significantly inhibited from day 19, and more pronounced in day 23 and day 28. In addition, more activated caspase 3 expression and decreased Ki-67 expression were detected in the EIF5A2 shRNA groups. We therefore suggested that targeting EIF5A2 inhibited tumor growth partly by inducing cell apoptosis and inhibiting cell proliferation, and targeting EIF5A2 may be a potential method for the treatment of ATC.
TGF-β has already been found to regulate the function of ATC cells in vitro and in vivo44,45. TGF-β activation could target Smad signals, resulting in nuclear translocation of p-Smad2/3, which increased metastatic ability and promoted cell proliferation46,47. In breast cancer cells, stimulation of breast cancer cell lines with the exogenous TGF-β1 triggered activation of the TGF-β/Smad pathway and stimulated the invasive potential of breast cancer cells48. Wei et al. reported that EIF5A2 might activate TGF-β1 expression to induce EMT and drive aggressiveness in BC cells30. In our study, EIF5A2 overexpression upregulated TGF-β1 and pSmad2/3, followed by increased cell survival, while treatment with specific TGF-β pathway inhibitor SB431542 or Smad3 silencing abrogated the effect of EIF5A2 overexpression on ATC cell growth and downstream signaling pathway activation. In addition, EIF5A2 silencing downregulated TGF-β1 and p-Smad2/3, followed by decreased cell survival and increased cell apoptosis, while restoration of TGF-β1 by hTGF-β1 stimulation abrogated the effect of EIF5A2 silencing-induced cell survival and downstream signaling activation. Thus, targeting EIF5A2 may inhibit ATC cell growth through TGF-β1/Smad2/3-dependent signals.
CONCLUSION
We found that EIF5A2 is overexpressed in ATC cell lines and human ATC tissues but is rare in normal thyroid tissues. Targeting EIF5A2 in ATC cells reduced ATC cell growth and increased ATC cell apoptosis in vitro and partly in vivo, and vice versa. Our experiments suggested that EIF5A2 enhances the activation of the TGF-β1/Smad2/3 pathway in ATC cells, providing the foundation for further investigation of EIF5A2 as a promising target for the ATC treatment.
ACKNOWLEDGMENT
This work was supported by the grants from the Shandong Natural Science Foundation (No. 2015ZRB01493).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr Relat Cancer 2009;16:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel PH, Costa-Mattioli M, Schulze KL, Bellen HJ. The Drosophila deoxyhypusine hydroxylase homologue nero and its target eIF5A are required for cell growth and the regulation of autophagy. J Cell Biol. 2009;185:1181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Fang Y, Sham JS. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197–200. [DOI] [PubMed] [Google Scholar]
- 4. Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology 2010;51:1255–63. [DOI] [PubMed] [Google Scholar]
- 5. Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, Kung HF, Guan XY. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39:80–6. [DOI] [PubMed] [Google Scholar]
- 6. Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, Park MH, Johansson HE. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254–63. [DOI] [PubMed] [Google Scholar]
- 7. Zheng X, Gao L, Wang BT, Shen P, Yuan XF, Zhang LQ, Yang L, Zhang DP, Zhang Q, Wang XM. Overexpression of EIF5A2 is associated with poor survival and aggressive tumor biology in gallbladder cancer. Histol Histopathol. 2019:18186. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Mo HQ, Tian FJ, Zeng WH, Liu XR, Ma XL, Li X, Qin S, Fan CF, Lin Y. EIF5A1 promotes trophoblast migration and invasion via ARAF-mediated activation of the integrin/ERK signaling pathway. Cell Death Dis. 2018;9:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turpaev KT. Translation factor eIF5A, Modification with hypusine and role in regulation of gene expression. eIF5A as a target for pharmacological interventions. Biochemistry 2018;83:863–73. [DOI] [PubMed] [Google Scholar]
- 10. Güth R, Adamian Y, Geller C, Molnar J, Maddela J, Kutscher L, Bhakta K, Meade K, Kim SL, Agajanian M, Kelber JA. DHPS-dependent hypusination of eIF5A1/2 is necessary for TGFβ/fibronectin-induced breast cancer metastasis and associates with prognostically unfavorable genomic alterations in TP53. Biochem Biophys Res Commun. 2019;519:838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang Y, Cen JJ, Cao JZ, Huang Y, Feng ZH, Lu J, Wei JH, Chen ZH, Liang YP, Liao B, Luo JH, Chen W. Overexpression of EIF5A2 in upper urinary tract urothelial carcinoma is a new independent prognostic marker of survival. Future Oncol. 2019;15:2009–18. [DOI] [PubMed] [Google Scholar]
- 12. Ba MC, Ba Z, Cui SZ, Gong YF, Chen C, Lin KP, Wu YB, Tu YN. Thermo-chemotherapy inhibits the proliferation and metastasis of gastric cancer cells via suppression of EIF5A2 expression. Onco Targets Ther. 2019;12:6275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu J, Zhao HW, Chen Y, Wei JH, Chen ZH, Feng ZH, Huang Y, Chen W, Luo JH, Fang Y. Eukaryotic translation initiation factor 5A2 is highly expressed in prostate cancer and predicts poor prognosis. Exp Ther Med. 2019;17:3741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang PY, Zeng TT, Ban X, Li MQ, Zhang BZ, Zhu YH, Hua WF, Mai HQ, Zhang L, Guan XY, Li Y. Expression of EIF5A2 associates with poor survival of nasopharyngeal carcinoma patients treated with induction chemotherapy. BMC Cancer 2016;16:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang SS, Gao Y, Wang DY, Xia BR, Liu YD, Qin Y, Ning XM, Li GY, Hao LX, Xiao M, Zhang YY. Overexpression of eukaryotic initiation factor 5A2 (EIF5A2) is associated with cancer progression and poor prognosis in patients with early-stage cervical cancer. Histopathology 2016;69:276–87. [DOI] [PubMed] [Google Scholar]
- 16. Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, Guan XY, Xie D. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial–mesenchymal transition. Gut 2012;61:562–75. [DOI] [PubMed] [Google Scholar]
- 17. Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology 2010;51:1255–63. [DOI] [PubMed] [Google Scholar]
- 18. Lee KY, Bae SC. TGF-beta-dependent cell growth arrest and apoptosis. J Biochem Mol Biol. 2002;35:47–53. [DOI] [PubMed] [Google Scholar]
- 19. Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2018;50:121–32. [DOI] [PubMed] [Google Scholar]
- 20. Fabregat I, Fernando J, Mainez J, Sancho P. TGF-beta signaling in cancer treatment. Curr Pharm Des. 2014;20:2934–47. [DOI] [PubMed] [Google Scholar]
- 21. Zhao Y, Xia S, Cao C, Du X. Effect of TGF-β1 on apoptosis of colon cancer cells via the ERK signaling pathway. J BUON. 2019;24:449–55. [PubMed] [Google Scholar]
- 22. Zhang Y, Alexander PB, Wang XF. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9:a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Di C, Zhang X, Wang J, Wang F, Yan JF, Xu C, Zhang J, Zhang Q, Li H, Yang H, Zhang H. Transforming growth factor β signaling pathway: A promising therapeutic target for cancer. J Cell Physiol. 2020;235:1903–14. [DOI] [PubMed] [Google Scholar]
- 24. Luo H, Guo Y, Liu Y, Wang Y, Zheng R, Ban Y, Peng L, Yuan Q, Liu W. Growth differentiation factor 11 inhibits adipogenic differentiation by activating TGF-beta/Smad signalling pathway. Cell Prolif. 2019;52:e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Q, Chen J, Zhu Y, Xu Z. Mesenchymal stem cells accelerate the remodeling of bladder VX2 tumor interstitial microenvironment by TGF-β1-Smad pathway. J Cancer 2019;10:4532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia-Rendueles AR, Rodrigues JS, Garcia-Rendueles ME, Suarez-Fariña M, Perez-Romero S, Barreiro F, Bernabeu I, Rodriguez-Garcia J, Fugazzola L, Sakai T, Liu F, Cameselle-Teijeiro J, Bravo SB, Alvarez CV. Rewiring of the apoptotic TGF-β-SMAD/NFκB pathway through an oncogenic function of p27 in human papillary thyroid cancer. Oncogene 2017;36:652–66. [DOI] [PubMed] [Google Scholar]
- 27. Li T, Zhao N, Lu J, Zhu Q, Liu X, Hao F, Jiao X. Epigallocatechin gallate (EGCG) suppresses epithelial–mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 2019;10:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y, Gao H, Li Y. Inhibition of LncRNA FOXD3-AS1 suppresses the aggressive biological behaviors of thyroid cancer via elevating miR-296-5p and inactivating TGF-β1/Smads signaling pathway. Mol Cell Endocrinol. 2020;500:110634. [DOI] [PubMed] [Google Scholar]
- 29. Bhatti MZ, Pan L, Wang T, Shi P, Li L. REGγ potentiates TGF-β/Smad signal dependent epithelial–mesenchymal transition in thyroid cancer cells. Cell Signal 2019;64:109412. [DOI] [PubMed] [Google Scholar]
- 30. Wei JG, Cao JZ, Zhang D, Liao B, Zhong WM, Lu J, Zhao WH, Zhang JX, Tong ZT, Fan S, Liang CZ, Liao YB, Pang J, Wu RH, Fang Y, Chen ZH, Li B, Xie D, Chen D, Luo JH. EIF5A2 predicts outcome in localised invasive bladder cancer and promotes bladder cancer cell aggressiveness in vitro and in vivo. Br J Cancer 2014;110:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu GD, Shi XB, Sun LB, Zhou QY, Zheng DW, Shi HS, Che YL, Wang ZS, Shao GF. Down-regulation of eIF5A-2 prevents epithelial–mesenchymal transition in non-small-cell lung cancer cells. J Zhejiang Univ Sci B 2013;14:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu G, Yu H, Shi X, Sun L, Zhou Q, Zheng D, Shi H, Li N, Zhang X, Shao G. Cisplatin sensitivity is enhanced in non-small cell lung cancer cells by regulating epithelial–mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2. BMC Pulm Med. 2014;14:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiao X, Zhang H, Xu X, Yu Y, Zhang H, Zhang J, Ning L, Hao F, Liu X, Niu M, Chen CT, Chen D, Zhang K. S100A4 knockout sensitizes anaplastic thyroid carcinoma cells harboring BRAFV600E/Mt to vemurafenib. Cell Physiol Biochem. 2018;49:1143–62. [DOI] [PubMed] [Google Scholar]
- 34. Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, Qian C, Cai Z, Xie D, Huang P, Guan XY. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology 2014;146:1701–13. [DOI] [PubMed] [Google Scholar]
- 35. Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR. Prospective multicenter study of thyroid carcinoma treatment: Initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 1998;83:1012–21. [DOI] [PubMed] [Google Scholar]
- 36. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg. 2001;25:617–22. [DOI] [PubMed] [Google Scholar]
- 37. Vaysburd M. Anaplastic thyroid carcinoma. In: Braunstein GD, editor. Thyroid cancer. New York: Springer; 2012. p. 189–200. [Google Scholar]
- 38. Mathews MB, Hershey JW. The Translation factor eIF5A and human cancer. Biochim Biophys Acta 2015;1849:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenkins ZA, Haag PG, Johansson HE. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics 2001;71:101–9. [DOI] [PubMed] [Google Scholar]
- 40. Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, Park MH, Johansson HE. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254–63. [DOI] [PubMed] [Google Scholar]
- 41. Preukschas M, Hagel C, Schulte A, Weber K, Lamszus K, Sievert H, Pallmann N, Bokemeyer C, Hauber J, Braig M, Balabanov S. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: Implications for new targeted therapies. PLoS One 2012;7:e43468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tu C, Chen W, Wang S, Tan W, Guo J, Shao C, Wang W. MicroRNA-383 inhibits doxorubicin resistance in hepatocellular carcinoma by targeting eukaryotic translation initiation factor 5A2. J Cell Mol Med. 2019;23:7190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C, Zhang B, Wu S, Song Y, Li J. Knockdown of EIF5A2 inhibits the malignant potential of non-small cell lung cancer cells. Oncol Lett. 2018;15:4541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Gao H, Li Y. Inhibition of LncRNA FOXD3-AS1 suppresses the aggressive biological behaviors of thyroid cancer via elevating miR-296-5p and inactivating TGF-β1/Smads signaling pathway. Mol Cell Endocrinol. 2020;500:110634. [DOI] [PubMed] [Google Scholar]
- 45. Sun W, Xu Y, Zhao C, Hao F, Chen D, Guan J, Zhang K. Targeting TGF-β1 suppresses survival of and invasion by anaplastic thyroid carcinoma cells. Am J Transl Res. 2017;9:1418–25. [PMC free article] [PubMed] [Google Scholar]
- 46. Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial–mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine 2019;56:126–35. [DOI] [PubMed] [Google Scholar]
- 47. Laverty HG, Wakefield LM, Occleston NL, O’Kane S, Ferguson MW. TGF-beta3 and cancer: A review. Cytokine Growth Factor Rev. 2009;20:305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fanayan S, Firth SM, Butt AJ, Baxter RC. Growth inhibition by insulin-like growth factor-binding protein-3 in T47D breast cancer cells requires transforming growth factor-beta (TGF-beta) and the type II TGF-beta receptor. J Biol Chem. 2000;275:39146–51. [DOI] [PubMed] [Google Scholar]