The following was originally published in Volume 24, No. 2, pages 99–108, 2016 (doi: http://dx.doi.org/10.3727/096504016X14611963142173). The images of Figure 3E and Figure 4E were incorrect in the published article. The corrected version of both figures are provided here. The figures have also been replaced with the correct versions in the original published version in the online site (https://www.ingentaconnect.com/contentone/cog/or/2016/00000024/00000002/art00004). The original versions of the figures did not affect any results or conclusions in the article.
Abstract
It has been demonstrated that microRNAs (miRNAs) act as oncogenes or tumor suppressors in a variety of cancers. Our previous work suggested that miR-10a/b functioned as a tumor suppressor in gastric cancer, and miR-10b was also reported to be significantly downregulated in advanced stage cervical cancer tissues. However, the aberrant expression of miR-10b in cervical cancer and its possible role in cervical carcinogenesis was largely unknown. In this study, we investigated the expression of miR-10b in cervical cancer tissues, carcinoma in situ tissues, mild dysplasia, moderate dysplasia, severe dysplasia tissues, and normal controls. We found that miR-10b was significantly downregulated during cervical cancer progression, and the lower level of miR-10b in cervical cancer was significantly associated with a more aggressive tumor phenotype. Moreover, overexpression of miR-10b in cervical cancer cells could inhibit the cell proliferation and invasion, and the further mechanism study suggested that its role was possibly through directly targeting HOXA1. These results suggested that the downregulation of miR-10b and the resulting elevated HOXA1 level in cervical cancer tissues might play critical roles in cervical cancer progression.
Key words: Cervical cancer, miR-10b, HOXA1, Tumor suppressor
Figure 3.
miR-10b can inhibit cervical cancer cell growth and invasion. (A) Enforced expression of miR-10b in Hela cells was validated by qRT-PCR. (B) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of Hela cells. (C) Enforced expression of miR-10b in SiHa cells was validated by qRT-PCR. (D) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of SiHa cells. (E) The invaded Hela cells in the Matrigel Transwell invasion assay. (F) The invaded SiHa cells in the Matrigel Transwell invasion assay. Data are shown as mean ± SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001.
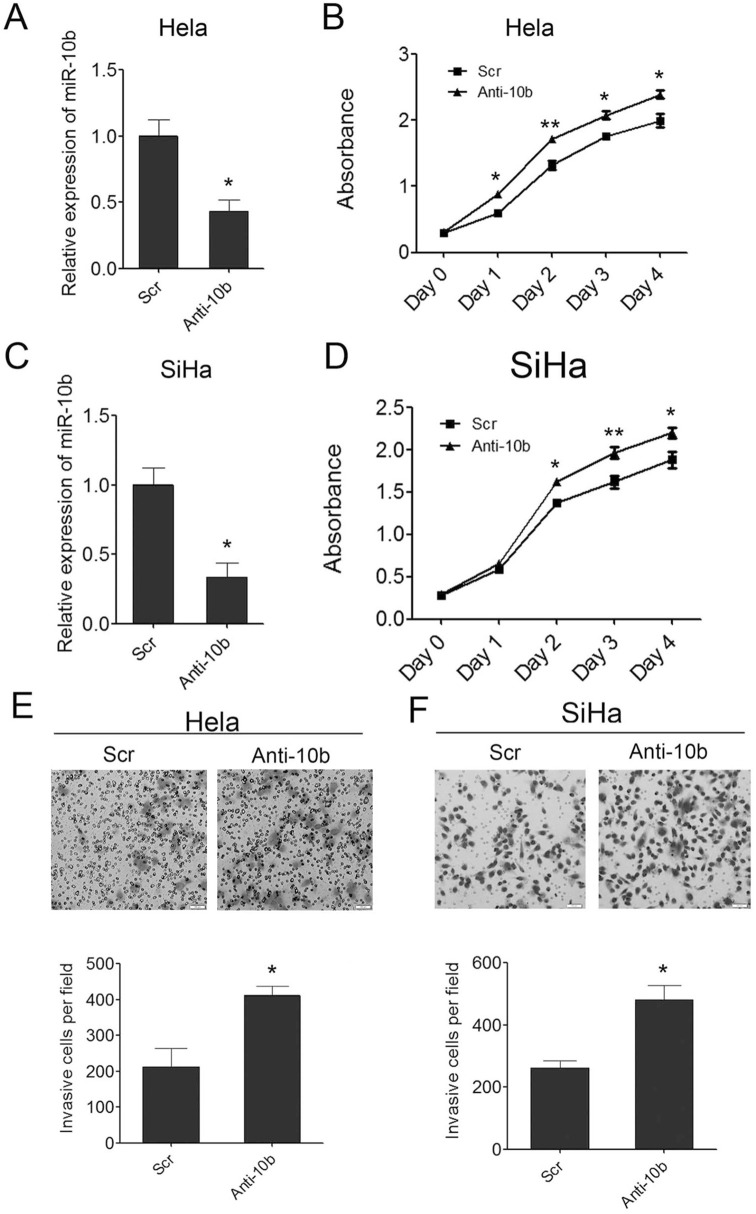
Figure 4.
Inhibition of miR-10b in cervical cancer cells can promote cell growth and invasion. (A) Inhibition of miR-10b in Hela cells was confirmed by qRT-PCR. (B) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of Hela cells. (C) Inhibition of miR-10b in SiHa cells was confirmed by qRT-PCR. (D) Proliferation assays by CCK-8 at days 0, 1, 2, 3, and 4 posttransfection of SiHa cells. (E) The invaded Hela cells in the Matrigel Transwell invasion assay. (F) The invaded SiHa cells in the Matrigel Transwell invasion assay. Data are shown as mean ± SD (n = 3). *p < 0.05; **p < 0.01.