Abstract
Vasohibin2 (VASH2), a proangiogenic factor, has been demonstrated to play an oncogenic role in some common human cancers. However, the detailed function of VASH2 in non-small cell lung cancer (NSCLC) has not previously been studied. In this study, we found that VASH2 was significantly upregulated in NSCLC tissues and cell lines, and its increased expression was associated with NSCLC progression and poor prognosis of patients. Knockdown of VASH2 markedly inhibited cell proliferation and P-glycoprotein expression in NSCLC cells. Overexpression of VASH2 enhanced cell proliferation, P-glycoprotein expression, as well as doxorubicin resistance in NSCLC cells. Moreover, the expression levels of VASH2 were significantly increased in newly established doxorubicin-resistant NSCLC cells. Molecular mechanism investigation revealed that inhibition of VASH2 expression in NSCLC cells suppressed the activity of AKT signaling, and overexpression of VASH2 enhanced the activity of AKT signaling. We further showed that downregulation of AKT signaling activity using AKT inhibitor LY294002 markedly inhibited NSCLC cell proliferation and resistance to doxorubicin induced by VASH2. In conclusion, the findings in the present study indicate that VASH2 promotes NSCLC cell proliferation and resistance to doxorubicin via modulation of AKT signaling. Thus, we suggest that VASH2 may become a potential therapeutic target for the treatment of NSCLC.
Key words: Non-small cell lung cancer (NSCLC), Vasohibin2 (VASH2), Doxorubicin, Chemoresistance
INTRODUCTION
Non-small cell lung cancer (NSCLC) is the most common human cancer in both males and females worldwide, accounting for about 85% of all lung cancers1,2. Despite improvements of surgery combined with radiotherapy and chemotherapy, the prognosis for NSCLC patients is still unsatisfactory because of metastasis, recurrence, and chemoresistance1,3,4. Therefore, revealing the molecular mechanism of chemoresistance may be beneficial for developing novel therapeutic strategy for NSCLC treatment. Doxorubicin (also called adriamycin) is one of the most important anticancer drugs and is effective in treating multiple types of human cancers including NSCLC through inhibiting DNA transcription and replication5,6. However, acquired resistance is a major limitation to the clinical application of doxorubicin in NSCLC treatment7,8. Therefore, uncovering the molecular mechanisms underlying doxorubicin resistance is urgently needed for the improvement of NSCLC treatment.
The vasohibin 2 (VASH2) gene, located on human chromosome 1q32.3, encodes the VASH2 protein containing 355 amino acid residues9. As an important member of the vasohibin family, VASH2 has been identified as a proangiogenesis factor and plays a key role in some physiological and pathological processes10,11. For instance, VASH2 is expressed in cells other than endothelial cells to promote angiogenesis and is also involved in neuron differentiation via its tubulin carboxypeptidases activity12,13. Moreover, the oncogenic role of VASH2 in tumor growth has been reported in some common human cancers14–17. For instance, VASH2 is significantly upregulated in hepatocellular carcinoma and promotes the malignant transformation of tumor by inducing epithelial–mesenchymal transition (EMT) and angiogenesis14,17. Knockdown of VASH2 significantly suppresses tumor growth of endometrial cancer cells and ovarian cancer cells by inhibition of angiogenesis15,16. However, the detailed function of VASH2 in NSCLC still remains unknown.
Recently, VASH2 has been found to be associated with chemoresistance in several cancer types18–20. For instance, Tu et al. showed that VASH2 induced gemcitabine resistance of pancreatic cancer cells via Jun-dependent transactivation of ribonucleotide reductase regulatory subunit M218. Li et al. reported that VASH2 decreased cisplatin sensitivity of hepatocarcinoma cells by inhibiting the expression of p5320. Furthermore, VASH2 was found to enhance doxorubicin resistance of breast cancer cells through regulating ABCG2 via AKT signaling pathway19. However, whether VASH2 is involved in doxorubicin resistance in NSCLC still remains unclear.
Thus, in the present study, we aimed to explore the potential function of VASH2 in the regulation of cell proliferation and chemotherapy resistance in NSCLC, as well as the underlying molecular mechanism.
MATERIALS AND METHODS
Clinical Tissues
This study was approved by the Research Ethics Committee of No. 180 Hospital of People’s Liberation Army. Primary NSCLC tissues and adjacent nontumor tissues were collected from 200 NSCLC patients in the Department of Thoracic Surgery, No. 180 Hospital of People’s Liberation Army between June 2011 and March 2013. Written informed consent was obtained from all patients. The clinicopathological information of these patients was obtained from medical records. The tissues were immediately snap frozen in liquid nitrogen and stored at −80°C until use.
Cell Culture
Normal human lung epithelial cell line BEAS-2B and NSCLC cell lines (A549 and H358) were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, P.R. China). Cells were cultured in DMEM (Life Technologies, Carlsbad, CA, USA) added with 10% FBS (Life Technologies) at 37°C with 5% CO2.
Establishment of Doxorubicin Resistance in NSCLC Cell Lines
A549 and H358 cells were cultured in DMEM with 10% FBS containing doxorubicin [half maximal inhibitory concentration (IC50)]. After incubation at 37°C for 48 h, the culture system was replaced by DMEM with 10% FBS. Cells were passaged cultured when reaching 100% confluence. When cells could grow steadily in the medium containing doxorubicin (IC50), they were cultured in DMEM with 10% FBS and doxorubicin (IC50). When they could grow steadily, the doxorubicin resistance A549 and H358 cells were established.
Cell Transfection
Lipofectamine™ 2000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to conduct cell transfection, according to the manufacturer’s instructions. In brief, A549 and H358 cells (3 × 105 cells/well) were seeded in six-well plates. After incubation at 37°C with 5% CO2 for 24 h, cells were VASH2 siRNA, negative control (NC) siRNA, pEZM61-VASH2 plasmid (Gene Copoeia, Guangzhou, P.R. China), or blank pEZM61 vector, respectively. After transfection for 48 h, the following experiments were conducted.
CCK-8 Assay
Cell proliferation was studied using the CCK-8 assay. The transfected cells (2 × 103 cells/well) were seeded in 96-well plates. After culturing at 37°C with 5% CO2 for 0, 24, 48, and 72 h, the cell proliferation was measured using Cell Counting Kit-8 (Thermo Fisher Scientific, Inc.). The OD value was read at 450-nm wavelength on a microplate reader (Bio-Rad, Hercules, CA, USA).
MTT Assay
The transfected cells (10,000 cells/well) were seeded in 96-well plates. After incubation at 37°C for 24 h, DMEM with doxorubicin (2, 4, 8, 16, or 32 μmol/L) was added into each well. After incubation at 37°C for 48 h, the medium was removed, and 50 μl of DMSO (Sigma-Aldrich, St. Louis, MO, USA) was added. After incubation at 37°C for 10 min, the OD value was read at 570-nm wavelength on a microplate reader (Bio-Rad). The IC50 was calculated.
RT-qPCR
Total RNA was extracted from tissues and cells using TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.). Then RNA (1 μg) was converted into cDNA using a SuperScript® One-Step RT-PCR kit (Thermo Fisher Scientific, Inc.), and qPCR was performed to examine the mRNA expression using a SuperScript® One-Step RT-PCR kit (Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol. The PCR reaction conditions were as follows: 95°C for 3 min, followed by 40 cycles at 95°C for 15 s and 60°C for 30 s. The relative expression levels were analyzed using the 2−ΔΔCq method21.
Western Blot
Western blot was used to examine the protein expression. In brief, protein was extracted from cell lines using RIPA lysis (Beyotime Biotechnology, Hangzhou, P.R. China), according to the manufacturer’s protocol. The protein concentration was examined using the BCA kit (Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol. The protein (50 μg per lane) was separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes (Thermo Fisher Scientific, Inc.). The membranes were incubated with primary antibodies (Abcam, Cambridge, MA, USA) at 37°C for 4 h, followed by incubation with HRP-conjugated goat anti-rabbit secondary antibodies (Abcam) at room temperature for 1 h. Signaling was examined using an Enhanced Chemiluminescence Western Blotting Kit (Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol. The protein expression was analyzed using Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA), according to the manufacturer’s protocol.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). The statistical analysis was performed using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA, USA). The overall survival rate estimates over time were calculated using the Kaplan–Meier method with log-rank test. Chi-square test was applied to analyze the association between VASH2 expression and clinicopathological features in NSCLC. Differences were analyzed using Student’s t-test between two groups, or one-way ANOVA among more than two groups followed by the Tukey’s post hoc test. A value of p < 0.05 was considered statistically significant.
RESULTS
Upregulation of VASH2 Is Associated With NSCLC Progression and Poor Prognosis
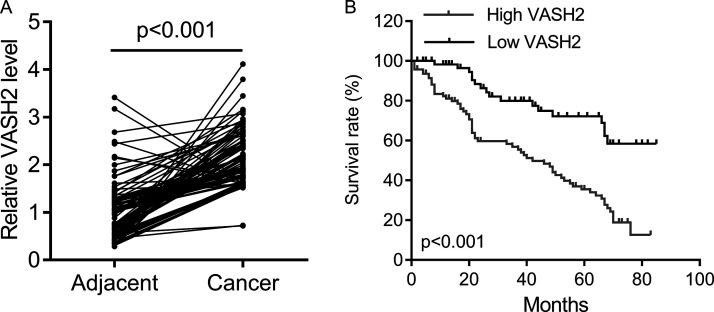
To reveal the role of VASH2 in NSCLC, we first examined its expression levels in NSCLC tissues and cell lines. As shown in Figure 1A, the expression levels of VASH2 were significantly increased in NSCLC tissues when compared with those in adjacent normal tissues. After that, we studied the clinical significance of VASH2 expression in NSCLC, and our data showed that high expression of VASH2 was significantly associated with tumor size, lymph node/distant metastasis, and advanced clinical stage in NSCLC (Table 1). In addition, we investigated the factors that could predicate the prognosis of NSCLC patients using univariate and multivariate analyses. Univariate analysis indicated that the VASH2 level (HR = 4.64, p = 0.01), as well as the tumor size (HR = 3.23, p = 0.03), lymph node metastasis (HR = 4.06, p = 0.01), distant metastasis (HR = 5.14, p = 0.01), and TNM stage (HR = 3.74, p = 0.02) were significantly associated with patients’ prognosis (Table 2). Multivariate analysis revealed that the VASH2 level (HR = 4.12, p = 0.01), tumor size (HR = 3.56, p = 0.01), lymph node metastasis (HR = 4.86, p = 0.02), distant metastasis (HR = 5.18, p = 0.01), and TNM stage (HR = 3.87, p = 0.01) were found to be independent factors for predicating the prognosis of NSCLC patients (Table 3). Moreover, the NSCLC patients with high VASH2 expression showed shorter overall survival time (Fig. 1B). Therefore, upregulation of VASH2 is associated with NSCLC progression and poor prognosis.
Figure 1.
The vasohibin2 (VASH2) expression in non-small cell lung cancer (NSCLC) tissues. (A) qPCR was performed to measure the expression of VASH2 in NSCLC tissues and the matched adjacent tissues (n = 200). (B) The Kaplan–Meier survival curve showed the survival rate in patients with high VASH2 expression (n = 116) and those with low VASH2 expression (n = 84).
Table 1.
Clinical Association Between Vasohibin2 (VASH2) Levels and Clinicopathological Variables of Patients With Non-Small Cell Lung Cancer (NSCLC)
| Variable | VASH2 | Chi-Square p Value | |
|---|---|---|---|
| Low Expression (n = 84) | High Expression (n = 116) | ||
| Age | 0.666 | ||
| <60 | 36 | 54 | |
| ≥60 | 48 | 62 | |
| Gender | 0.886 | ||
| Male | 44 | 63 | |
| Female | 40 | 53 | |
| Tumor size | <0.001 | ||
| <3 cm | 58 | 41 | |
| ≥3 cm | 26 | 75 | |
| Lymph node metastasis | <0.001 | ||
| N0–1 | 52 | 38 | |
| N2–4 | 32 | 78 | |
| Distant metastasis | 0.006 | ||
| No | 59 | 58 | |
| Yes | 25 | 58 | |
| TNM stage | 0.006 | ||
| I–II | 54 | 51 | |
| III–IV | 30 | 65 | |
Table 2.
Univariate Analysis of Prognostic Factors of NSCLC
| Variable | Hazard Ratio | p Value |
|---|---|---|
| Age (≥50/<50) | 1.14 | 0.45 |
| Gender (male/female) | 1.27 | 0.09 |
| Tumor size (≥3 cm/<3 cm) | 3.23 | 0.03 |
| Lymph node metastasis (N2–4/N0–1) | 4.06 | 0.01 |
| Distant metastasis (yes/no) | 5.14 | 0.01 |
| TNM stage (III–IV/I–II) | 3.74 | 0.02 |
| VASH2 (high/low) | 4.64 | 0.01 |
Table 3.
Multivariate Analysis of Independent Prognostic Factors of NSCLC
| Variable | Hazard Ratio | p Value |
|---|---|---|
| Tumor size | 3.56 | 0.01 |
| Lymph node metastasis | 4.86 | 0.02 |
| Distant metastasis | 5.18 | 0.01 |
| TNM stage | 3.87 | 0.01 |
| VASH2 levels | 4.12 | 0.01 |
Promoting Effects of VASH2 on NSCLC Cell Proliferation and Resistance to Doxorubicin
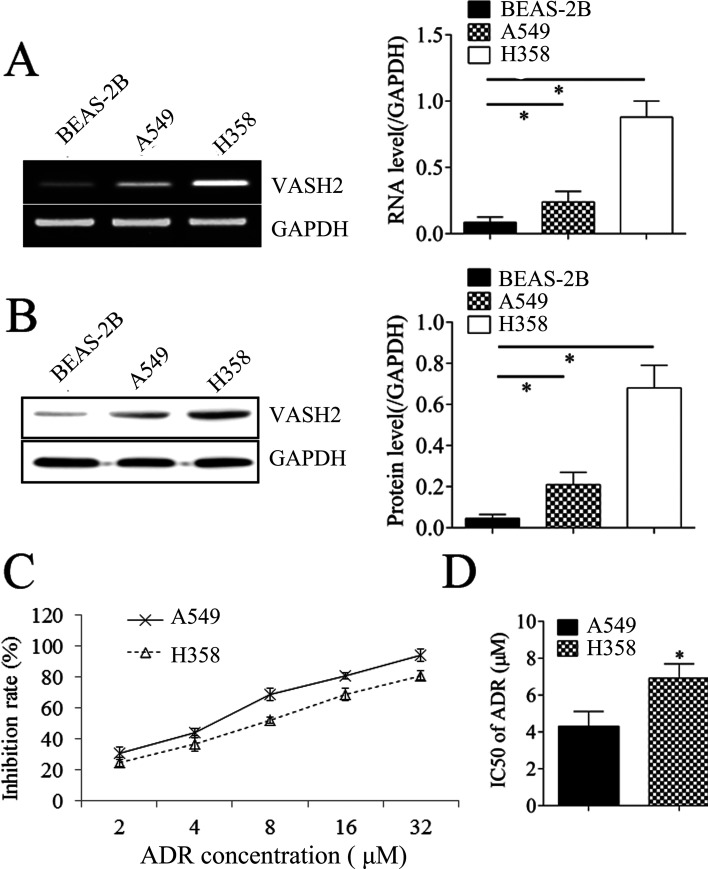
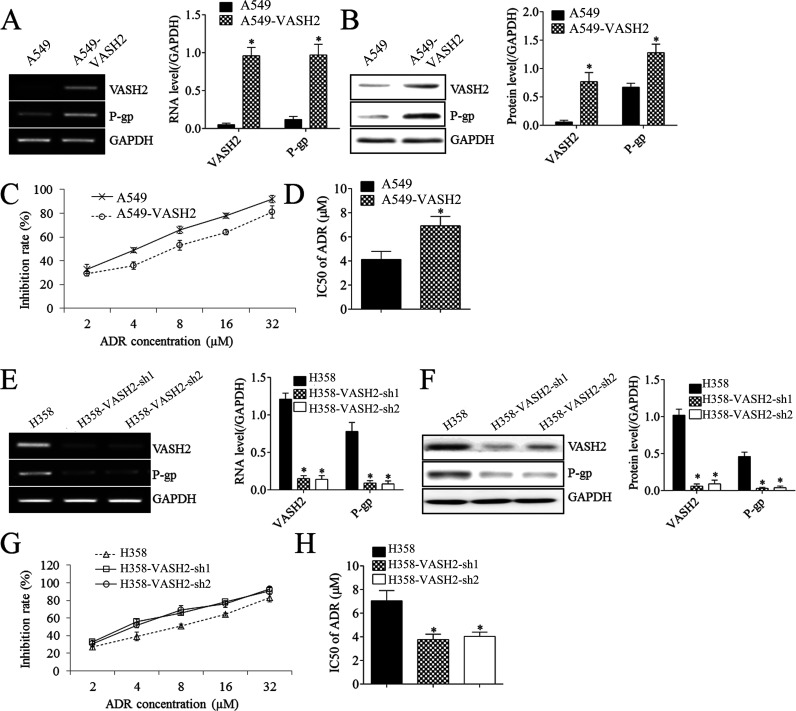
We then studied the effects of VASH2 on NSCLC cell proliferation and resistance to doxorubicin. We found that the expression levels of VASH2 were significantly increased in NSCLC cells (A549 and H358) compared with normal cell line BEAS-2B (Fig. 2A and B). The expression levels of VASH2 in H358 were higher than in A549 cells (Fig. 2A and B). As expected, the inhibition rates in H358 were lower than in A549 when the cells were treated with ADR; thus, the IC50 of H358 was higher than that of A549 (Fig. 2C and D). A549 cells were transfected with VASH2 expression plasmid to upregulate its expression. After transfection, the VASH2 levels were upregulated in the VASH2 group compared to the blank group (Fig. 3A and B). We then found that overexpression of VASH2 enhanced the protein expression of P-glycoprotein (Fig. 3B) in NSCLC cells. Moreover, VASH2 upregulation significantly reduced the inhibition rate (IR) of cells in doxorubicin (Fig. 3C), and the IC50 of doxorubicin was significantly increased (Fig. 3D). These findings suggest that VASH2 is associated with doxorubicin resistance in NSCLC cells.
Figure 2.
The VASH2 expression in NSCLC cell lines. (A) RT-PCR was performed to measure the expression of VASH2 in NSCLC cell lines (A549 and H358) and the normal cell line BEAS-2B. (B) Western blot was performed to measure the expression of VASH2 in NSCLC cell lines (A549 and H358) and the normal cell line BEAS-2B. (C) MTT assay was performed to measure the inhibition rate in A549 and H358 cells after doxorubicin treatment (2, 4, 8, 16, or 32 μmol/L). (D) The half maximal inhibitory concentration (IC50) was calculated from (C). *p < 0.05.
Figure 3.
The effects of VASH2 on cell proliferation and doxorubicin chemotherapy in A549 and H358 cells. (A) RT-PCR was performed to measure the expression of VASH2 and P-glycoprotein (P-gp) in A549 cells after VASH2 plasmid transfection. (B) Western blot was performed to measure the expression of VASH2 and P-gp in A549 cells after VASH2 plasmid transfection. (C) MTT assay was performed to measure the inhibition rate in A549 cells after VASH2 plasmid transfection under doxorubicin treatment (2, 4, 8, 16, or 32 μmol/L). (D) The IC50 was calculated from (C). (E) RT-PCR was performed to measure the expression of VASH2 and P-gp in H358 cells after VASH2 shRNA (sh-1, sh-2) transfection. (F) Western blot was performed to measure the expression of VASH2 and P-gp in H358 cells after VASH2 shRNA (sh-1, sh-2) transfection. (G) MTT assay was performed to measure the inhibition rate in H358 cells after VASH2 shRNA (sh-1, sh-2) transfection under doxorubicin treatment (2, 4, 8, 16, or 32 μmol/L). (H) The IC50 was calculated from (G). ADR, doxorubicin. *p < 0.05.
To further confirm these findings, VASH2 siRNA was used to transfect H358 cells to downregulate its expression. After transfection, the expression levels of VASH2 were markedly decreased in the shVASH2 group compared to the shNC group (Fig. 3E and F). We then observed that knockdown of VASH2 caused a significant reduction in protein expression of P-glycoprotein (Fig. 3F). Moreover, we showed that silencing of VASH2 increased the IR of NSCLC cells in doxorubicin (Fig. 3G), and the IC50 of doxorubicin was significantly declined (Fig. 3H). These above findings demonstrate that VASH2 has promoting effects on NSCLC cell proliferation and resistance to doxorubicin.
VASH2 Is Upregulated in Established Doxorubicin-Resistant NSCLC Cells
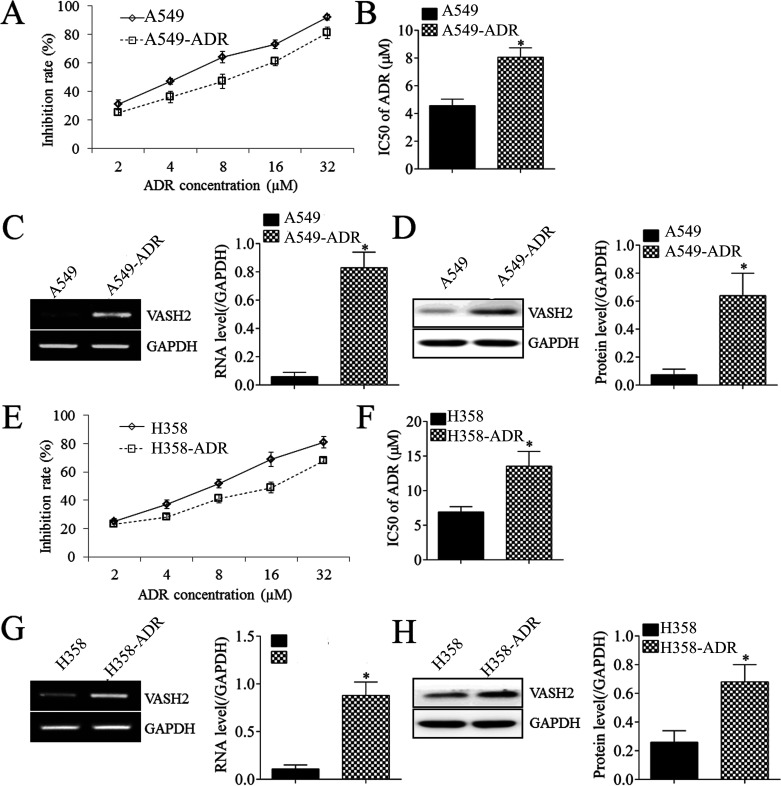
To further confirm that VASH2 is involved in the resistance of NSCLC cells to doxorubicin, two doxorubicin-resistant NSCLC cell lines were established. The established A549-doxorubicin (Fig. 4A and B) and H358-doxorubicin cells (Fig. 4E and F) showed reduced IR and increased IC50 in doxorubicin. Moreover, the mRNA and protein levels of VASH2 were significantly increased in doxorubicin-resistant NSCLC cells (Fig. 4C, D, G, and H). These findings further suggest that VASH2 plays a key role in doxorubicin resistance in NSCLC cells.
Figure 4.
The expression of VASH2 in doxorubicin-resistant A549 and H358 cells. (A) MTT assay was performed to measure the inhibition rate in A549 and doxorubicin-resistant A549 cells. (B) The IC50 was calculated from (A). (C) RT-PCR was performed to measure the expression of VASH2 in A549 and doxorubicin-resistant A549 cells. (D) Western blot was performed to measure the expression of VASH2 in A549 and doxorubicin-resistant A549 cells. (E) MTT assay was performed to measure the inhibition rate in H358 and doxorubicin-resistant H358 cells. (F) The IC50 was calculated from (E). (G) RT-PCR was performed to measure the expression of VASH2 in H358 and doxorubicin-resistant H358 cells. (H) Western blot was performed to measure the expression of VASH2 in H358 and doxorubicin-resistant H358 cells. *p < 0.05.
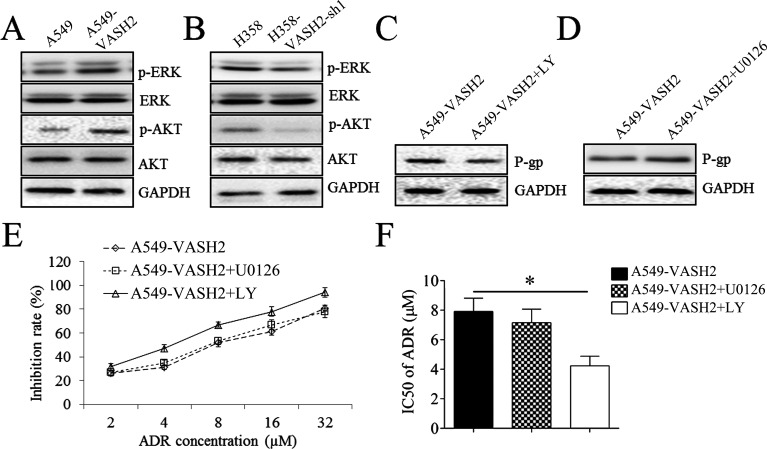
Inhibition of AKT Signaling Reduces NSCLC Cell Proliferation and Resistance to Doxorubicin Induced by VASH2
We then studied the underlying molecular mechanism and found that VASH2 overexpression enhanced the phosphorylation of ERK and AKT (Fig. 5A), and knockdown of VASH2 reduced the phosphorylation of ERK and AKT in NSCLC cells (Fig. 5B). These findings suggest that the ERK or AKT signaling may be involved in the VASH2-mediated NSCLC cell proliferation and resistance to doxorubicin. To confirm this speculation, the AKT inhibitor LY294002 and ERK inhibitor U0126 were used. Our findings showed that treatment with LY294002, but not U0126, decreased P-glycoprotein protein of A549-VASH2 cells (Fig. 5C and D), accompanied with increased doxorubicin IR and reduced IC50 of doxorubicin (Fig. 5E and F).
Figure 5.
The effects of VASH2 on AKT signaling in A549 and H358 cells. (A) Western blot was performed to measure the expression of ERK and AKT and their phosphorylated form in A549 cells after VASH2 plasmid transfection. (B) Western blot was performed to measure the expression of ERK and AKT and their phosphorylated form in H358 cells after VASH2 shRNA-1 transfection. (C) Western blot was performed to measure the expression of P-gp in A549 cells after VASH2 plasmid transfection plus AKT inhibitor LY294002. (D) Western blot was performed to measure the expression of P-gp in A549 cells after VASH2 plasmid transfection plus ERK inhibitor U0126. (E) MTT assay was performed to measure the inhibition rate in A549 cells after VASH2 plasmid transfection plus either ERK inhibitor U0126 or AKT inhibitor LY294002. (F) The IC50 was calculated from (E). *p < 0.05.
DISCUSSION
The function of VASH2 in NSCLC has not previously been explored. In this study, we found that VASH2 was significantly upregulated in NSCLC tissues and cell lines, and its increased expression was associated with NSCLC progression and poor prognosis of patients. Knockdown of VASH2 markedly inhibited cell proliferation and P-glycoprotein expression in NSCLC cells. Overexpression of VASH2 enhanced cell proliferation, P-glycoprotein expression, as well as doxorubicin resistance in NSCLC cells. Moreover, the expression levels of VASH2 were significantly increased in newly established doxorubicin-resistant NSCLC cells. Molecular mechanism investigation revealed that inhibition of VASH2 expression in NSCLC cells suppressed the activity of AKT signaling, and overexpression of VASH2 enhanced the activity of AKT signaling. We further showed that downregulation of AKT signaling activity using AKT inhibitor LY294002 markedly inhibited NSCLC cell proliferation and resistance to doxorubicin induced by VASH2.
The promoting role of VASH2 has been reported in several cancer types including gastric cancer, liver cancer, pancreatic ductal adenocarcinoma, and breast cancer. For instance, Kitahara et al. suggested that VASH2 participated in the onset of tumors in the gastrointestinal tract by regulating tumor angiogenesis 21. Tu et al. reported that VASH2 could promote breast cancer cell proliferation via increasing the expression of FGF2 and GDF1522. VASH2 is upregulated in pancreatic ductal adenocarcinoma, which is associated with tumor progression as well as poor prognosis of patients23. However, the expression pattern of VASH2 in NSCLC as well as the clinical significance has not previously been studied. In this study, we found that the expression levels of VASH2 were significantly increased in NSCLC tissues compared with those in adjacent nontumor tissues, and high expression of VASH2 was significantly associated with tumor size, lymph node metastasis, and advanced clinical stage, suggesting that upregulation of VASH2 is involved in NSCLC progression. In addition, we found that the NSCLC patients with high expression of VASH2 showed shorter survival time when compared with those with low VASH2 expression. These findings further confirm the importance of VASH2 in NSCLC. To study the function of VASH2 in NSCLC, we upregulated or downregulated the expression of VASH2 in NSCLC cells and then examined cell proliferation. Our data showed that VASH2 overexpression significantly enhanced NSCLC cell proliferation, and knockdown of VASH2 suppressed cell proliferation.
P-glycoprotein is an ATP-binding cassette (ABC) drug efflux pump and could reduce the concentration of drugs in cells. P-glycoprotein has been demonstrated to be frequently upregulated in chemoresistant tumor cells and participate in the chemotherapy resistance of various human cancers. In our study, we found that overexpression of VASH2 caused a significant increase in the expression of P-glycoprotein in NSCLC cells and induced doxorubicin resistance. On the contrary, knockdown of VASH2 significantly reduced P-glycoprotein expression and increased the IR of NSCLC cells in doxorubicin. To further confirm these findings, we then established doxorubicin-resistant NSCLC cell lines and found that VASH2 was significantly upregulated in these doxorubicin-resistant cells. Therefore, targeting VASH2 may be used as a potential strategy for the treatment of doxorubicin-resistant NSCLC.
The activity of AKT signaling has been demonstrated to be frequently upregulated in various human cancers including NSCLC and play promoting roles during tumor progression24–26. Inhibition of AKT signaling activity has been found to suppress tumor growth as well as chemoresistance26. A previous study has shown that VASH2 can enhance the doxorubicin resistance of breast cancer cells via AKT signaling pathway19. Thus, we studied the relationship between VASH2 and AKT signaling in NSCLC cells. We found that VASH2 overexpression significantly upregulated the activity of AKT signaling and that knockdown of VASH2 downregulated the AKT signaling activity in NSCLC cells, suggesting that the function of VASH2 in NSCLC may be through modulating AKT signaling. Further investigation showed that inhibition of AKT signaling using LY294002 suppressed the VASH2-induced NSCLC cell proliferation and doxorubicin resistance, which confirms our speculation.
In conclusion, to our knowledge, this is the first study demonstrating that VASH2 can promote tumor cell proliferation and induce doxorubicin resistance in NSCLC, suggesting that VASH2 may become a promising therapeutic target for the treatment of NSCLC.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 4. Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: The prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer 2006;51(2):143–58. [DOI] [PubMed] [Google Scholar]
- 5. Baxter-Holland M, Dass CR. Doxorubicin, mesenchymal stem cell toxicity and antitumour activity: Implications for clinical use. J Pharm Pharmacol. 2018;70(3):320–7. [DOI] [PubMed] [Google Scholar]
- 6. Lipinska N, Romaniuk A, Paszel-Jaworska A, Toton E, Kopczynski P, Rubis B. Telomerase and drug resistance in cancer. Cell Mol Life Sci. 2017;74(22):4121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dragoj M, Milosevic Z, Bankovic J, Tanic N, Pesic M, Stankovic T. Targeting CXCR4 and FAK reverses doxorubicin resistance and suppresses invasion in non-small cell lung carcinoma. Cell Oncol. (Dordr) 2017;40(1):47–62. [DOI] [PubMed] [Google Scholar]
- 8. Chen YL, Yang TY, Wu CL, Chen KC, Hsu SL, Hsueh CM. Mechanisms underlying lung resistance-related protein (LRP)-mediated doxorubicin resistance of non-small cell lung cancer cells. Chin J Physiol. 2016;59(6):331–47. [DOI] [PubMed] [Google Scholar]
- 9. Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M, Moriya T, Ohta H, Sonoda H, Shimosegawa T. Isolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler Thromb Vasc Biol. 2006;26(5):1051–7. [DOI] [PubMed] [Google Scholar]
- 10. Masuda K, Tanabe K, Ujike H, Hinamoto N, Miyake H, Tanimura S, Sugiyama H, Sato Y, Maeshima Y, Wada J. Deletion of pro-angiogenic factor vasohibin-2 ameliorates glomerular alterations in a mouse diabetic nephropathy model. PLoS One 2018;13(4):e0195779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, Altelaar M, Knipscheer P, Perrakis A, Blomen VA. Vasohibins encode tubulin detyrosinating activity. Science 2017;358(6369):1453–6. [DOI] [PubMed] [Google Scholar]
- 12. Sato Y. The vasohibin family: A novel family for angiogenesis regulation. J Biochem. 2013;153(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 2017;358(6369):1448–53. [DOI] [PubMed] [Google Scholar]
- 14. Xue X, Zhang Y, Zhi Q, Tu M, Xu Y, Sun J, Wei J, Lu Z, Miao Y, Gao W. MiR200-upregulated Vasohibin 2 promotes the malignant transformation of tumors by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. Cell Commun Signal. 2014;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koyanagi T, Saga Y, Takahashi Y, Suzuki Y, Suzuki M, Sato Y. Downregulation of vasohibin-2, a novel angiogenesis regulator, suppresses tumor growth by inhibiting angiogenesis in endometrial cancer cells. Oncol Lett. 2013;5(3):1058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koyanagi T, Suzuki Y, Saga Y, Machida S, Takei Y, Fujiwara H, Suzuki M, Sato Y. In vivo delivery of siRNA targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer Sci. 2013;104(12):1705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue X, Gao W, Sun B, Xu Y, Han B, Wang F, Zhang Y, Sun J, Wei J, Lu Z. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 2013;32(13):1724–34. [DOI] [PubMed] [Google Scholar]
- 18. Tu M, Li H, Lv N, Xi C, Lu Z, Wei J, Chen J, Guo F, Jiang K, Song G. Vasohibin 2 reduces chemosensitivity to gemcitabine in pancreatic cancer cells via Jun proto-oncogene dependent transactivation of ribonucleotide reductase regulatory subunit M2. Mol Cancer 2017;16(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma D, Wu L, Li S, Sun Z, Wang K. Vasohibin2 promotes adriamycin resistance of breast cancer cells through regulating ABCG2 via AKT signaling pathway. Mol Med Rep. 2017;16(6):9729–34. [DOI] [PubMed] [Google Scholar]
- 20. Li Z, Tu M, Han B, Gu Y, Xue X, Sun J, Ge Q, Miao Y, Qian Z, Gao W. Vasohibin 2 decreases the cisplatin sensitivity of hepatocarcinoma cell line by downregulating p53. PLoS One 2014;9(3):e90358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kitahara S, Suzuki Y, Morishima M, Yoshii A, Kikuta S, Shimizu K, Morikawa S, Sato Y, Ezaki T. Vasohibin-2 modulates tumor onset in the gastrointestinal tract by normalizing tumor angiogenesis. Mol Cancer 2014;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tu M, Liu X, Han B, Ge Q, Li Z, Lu Z, Wei J, Song G, Cai B, Lv N. Vasohibin2 promotes proliferation in human breast cancer cells via upregulation of fibroblast growth factor2 and growth/differentiation factor15 expression. Mol Med Rep. 2014;10(2):663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JC, Kim KT, Park JT, Kim HJ, Sato Y, Kim HS. Expression of vasohibin-2 in pancreatic ductal adenocarcinoma promotes tumor progression and is associated with a poor clinical outcome. Hepatogastroenterology 2015;62(138):251–6. [PubMed] [Google Scholar]
- 24. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335–50. [DOI] [PubMed] [Google Scholar]
- 25. Zhang B, Ma L, Wei J, Hu J, Zhao Z, Wang Y, Chen Y, Zhao F. miR-137 suppresses the phosphorylation of AKT and improves the dexamethasone sensitivity in multiple myeloma cells via targeting MITF. Curr Cancer Drug Targets 2016;16(9):807–17. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta 2015;444:182–92. [DOI] [PubMed] [Google Scholar]