Abstract
Natural products are becoming increasingly popular in a variety of traditional, complementary, and alternative systems due to their potency and slight side effects. Natural compounds have been shown to be effective against many human diseases, especially cancers. Sulforaphane (SFE) is a traditional Chinese herbal medicine. In recent years, an increasing number of studies have been conducted to evaluate the antitumor effect of SFE. The roles of SFE in cancers are mainly through the regulation of potential biomarkers to activate or inhibit related signaling pathways. SFE has exhibited promising inhibitory effects on breast cancer, lung cancer, liver cancer, and other malignant tumors. In this review, we summarized the reports on the activity and functional mechanisms of SFE in cancer treatment and explored the efficacy and toxicity of SFE.
Key words: Sulforaphane, Malignant tumor, Antitumor effects
INTRODUCTION
Cancers have become a major public health threat, representing one of the leading causes of deaths worldwide1,2. Radiation, surgery, and drugs are currently effective treatments for malignant tumors. However, they all have different risks, especially with chemotherapy. Although chemical drugs are effective in treating cancer, their resistance and serious side effects, such as damage to liver function, bone marrow suppression, and neurotoxicity, often lead to treatment failure3,4. Therefore, we still need to find new drugs for treating cancer that are more effective and have fewer side effects than existing drugs. In this regard, plant-derived products, such as triptolide5, have received considerable attention due to their lower levels of side effects and effective inhibition of various signaling-mediated prosurvival roles. Many antitumor natural compounds have been shown to be highly effective against a variety of solid tumors6,7. It has been reported that angelica blood-enriching decoction can induce autophagic death of colorectal cancer cells by upregulating autophagy-related protein Atg78. Diosmetin, a flavone found in legumes and olive leaves, enhances the radiosensitivity of radioresistant non-small cell lung cancer (NSCLC) cells by attenuating phosphatidylinositol 3′ phosphokinase/protein kinase B (PKB/Akt) activation9. Therefore, the extraction and identification of new compounds from Chinese herbal medicine have gained great potential for the development novel anticancer drugs.
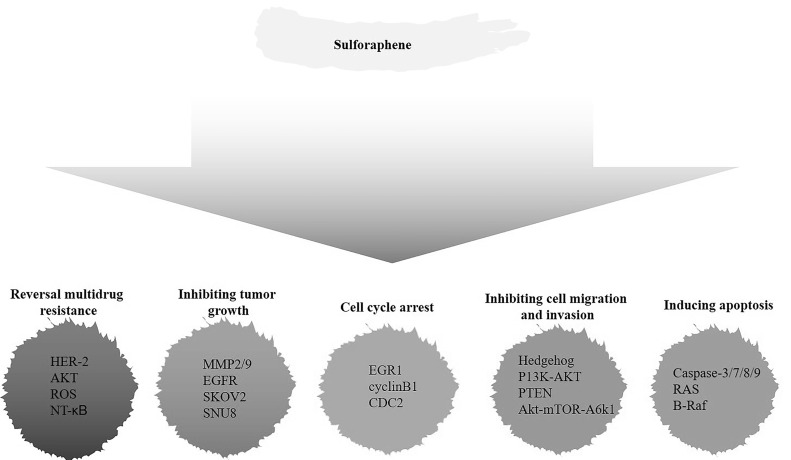
Sulforaphane (4-methylsufinyl-3-butenyl isothiocyanate; SFE), a member of the isothiocyanate family (ITCs), is derived from Raphanus sativus seeds. Raphanus sativus is a cultivated radish, which is a common cruciferous vegetable10. Given that the extracts derived from the roots of Raphanus sativus L. can significantly induce cell apoptosis and inhibit cell proliferation in a variety of human cancer cells by the induction of apoptosis-associated signaling pathways11, these isolated compounds have long been used to treat a variety of human malignant diseases. Modern pharmacological studies have shown that ITCs have great potential as anticancer agents12. It has been reported that ITCs are capable of inhibiting cell proliferation in a dose-dependent manner and inducing apoptosis in the HCT-116, LoVo, and HT-29 colon cancer cell lines13. ITCs can also reduce the cell proliferation of human erythroleukemic cells, T-lymphoid cells, and cervical carcinoma cells14. Under the guidance of biological experiments, Kim et al. isolated and identified seven ITC derivatives by extraction and chemical methods from Raphanus sativus seeds, which included SFE15. The chemical structure of SFE is highly similar to that of sulforaphane (SFN), another ITC derivative that is mainly extracted from broccoli16. Compared to SFN, SFE has an additional double bond in its chemical structure. In order to better separate and extract SFE, Sangthong et al., for the first time, used high-performance liquid chromatography (HPLC) to simultaneously determine the content of SFN and SFE in Raphanus sativus extract, and separated them effectively17. The anticancer effect of SFN has been demonstrated: (a) blocking the initiation state by inhibiting phase I enzymes to convert original carcinogen to proximate or final carcinogens; and (b) inducing phase II enzymes that detoxify carcinogens and promote their excretion from the body18. Because of the similarity of chemical structures to SFN, SFE has the potential to be an effective chemical preventive agent for cancer as well. In this review, we mainly discuss the antitumor activity of SFE and the related mechanisms in detail (Fig. 1, Table 1). At the same time, as a promising natural antitumor product that could be widely used in the future, its toxic side effects and clinical application value are also discussed.
Figure 1.
Overview of the natural compound sulforaphane (SFE) and the aberrant signaling pathways for human malignant cancer research and therapy.
Table 1.
Mechanism of Action of Sulforaphene (SFE) in Human Tumors
| Tumors | Action | Outcome | Model Used | Ref. |
|---|---|---|---|---|
| Breast cancer | Akt–mTOR–S6K kinase pathway↓ | Reversal multidrug resistance, apoptosis↑ | SKBR-3, BT-474 | 26 |
| Triple-negative breast cancer | Hedgehog↓, MMP-2↓, MMP-9↓ | Migration and invasion↓, apoptosis↑, proliferation↓ | MCF7, T47D, MCF10A, MCF10AT1, MCF10CA1a, SUM159 | 33 |
| Triple-negative breast cancer | EGR1 ↑, cyclinB1↓, Cdc2↓ | Apoptosis↑, cell cycle G2/M phase arrest | MDA-MB-231, MDA-MB-453, MDA-MB-436, MDA-MB-468 | 35 |
| Hepatocellular carcinoma | caspases -3/7 and -9↑, caspase-8↓ | Apoptosis↑, cell cycle G0/G1 phase arrest | MFC-7, HT-29 | 38 |
| Hepatocellular carcinoma | ROS↑, microtubule polymerization↑ | Apoptosis↑, radiation-induced cell death↑ | HB-8065 | 40 |
| Hepatocellular carcinoma | NF-κB↓ | Apoptosis↑, proliferation↓ | HepG2, Hep3B | 42 |
| Lung cancer | PI3K-Akt↓, PTEN↓ | Apoptosis↑, migration and invasion↓, proliferation↑ | A549, H460, H446, HCC827, H1975, H1299 | 48 |
| Non-small cell lung carcinoma | ROS↑, Bcl-2↓, Bax↓, cytochrome C↑, caspase 9/3↑ | Apoptosis↑, proliferation↓ | A549 | 49 |
| Cervical cancer | Caspase 3↑, caspase 9↑, EGFR↑ | Apoptosis↑, proliferation↓ | HeLa | 53 |
| Ovarian cancer | ROS↑, mitochondrial membrane depolarization | Apoptosis↑, proliferation↓ | SKOV 3, SNU 8 | 56 |
| Colon cancer | p38, CDK1, CDC25B | Apoptosis↑, cell cycle G2/M phase arrest | HCT116, HT-29, DLD1, KM12 | 58 |
| Gastric cancer | ROS↑, cytochrome c↑, Casp-3↑, Casp-8↑, PARP-1↑ | Apoptosis↑, migration and invasion↓ | AGS | 57 |
| Lymphoma | CRM1, p62↑, AMPK↑ | Apoptosis↑ | U937, HUT78, Raji, JeKo-1, U2932 | 59 |
| Thyroid cancer | Ras↑, MEK↑, ERK↑, B-Raf↑ | Apoptosis↑, proliferation↓ | FRO | 60, 61 |
MMP, matrix metalloproteinases; EGR1, early growth response 1; Cdc2, cell division cycle gene 2; ROS, reactive oxygen species; PTEN, phosphatase and tensin homolog; Bcl-2, B-cell lymphoma 2; CDK1, cyclin dependent kinase 1; CDC25B, cell division cycle 25 B; CRM1, chromosome-region-maintenance-1; AMPK, AMP-activated protein kinase; MEK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase. ↑: activation/upregulation; ↓: suppression/downregulation.
ACTION OF SULFORAPHANE IN BREAST CANCER
Breast cancer is one of the most common malignant tumors in the world, especially in women19,20. According to statistics, among women younger than 45, breast cancer is undoubtedly the leading cause of cancer-related death21. At present, the treatment of breast cancer mainly includes surgical resection, radiotherapy, and chemotherapy. However, the therapy responses are often disappointing22. Previous research found that pretreatment with SFE, at as low concentration as 5 μM, inhibited cell clonogenicity by nearly 70% in breast cancer cells, when compared to untreated cells. However, SFN administration could inhibit the clonogenic potential of breast cancer cells only by about 30% at the similar dose. This indicates that SFE might be considered as a more effective anticancer drug than SFN23,24. Human epidermal growth factor receptor 2 (HER-2) is known to be involved in the proliferation and division of breast cancer cells25, specifically through the Akt–mTOR–S6K kinase pathway26,27. The anti-HER2-targeted drug lapatinib is often used in breast cancer patients with HER2 overexpression28. Studies have found that the combination of SFE (2.5 μM) and lapatinib (100 nM) could effectively induce cell apoptosis and decrease cell viability mainly by inhibiting the Akt–mTOR–S6K pathway in breast cancer cells, thus improving the therapeutic effect of lapatinib29. Triple-negative breast cancer (TNBC) is a common subtype of breast cancer lacking estrogen receptor, progesterone receptor, and HER2 gene overexpression30–32. SFE also has the significant therapeutic potential against TNBC. In recent years, the Hedgehog (Hh) pathway has been identified as a key signaling pathway that drives tumorigenesis in TNBC33. Downregulation of the Hh signaling pathway by inhibitors can reduce cell migration and invasion34,35. SFE can significantly inhibit the Hh pathway, thereby reducing the activity of the downstream signal modulators matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) and inhibiting the invasion of human TNBC cells36. Early growth response 1 (EGR1) is an immediate early gene induced by estrogen, growth factor, or stress signal that can exert both cancer-suppressive and -promoter activities37. At the same time, EGR1 was successfully verified as a uniformly activated marker after SFE treatment in TNBC cell lines MDA-MB453 and MDA-MB-436. The data indicated that SFE could inhibit the expression of cyclin B1 and phosphorylated Cdc2 by mediating tumor suppressor EGR1, thus inducing G2/M phase arrest of TNBC cells38.
ACTION OF SULFORAPHANE IN HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is one of the deadliest and most common cancers in humans. The treatment of liver cancer mostly involves surgical resection, transplantation, and ablation, but the therapeutic effect is not good39,40. Some researchers have found that SFE can promote apoptosis of HCC cells, which is morphologically manifested as cell contraction, blistering, chromatin condensation, and nuclear fragmentation. They also found that SFE was most toxic in HepG2 cells. SFE exhibited an IC50 value of 33.8 μM when incubated with HepG2 cells for 72 h. An annexin V assay found that the same treatment increased caspases 3/7 and 9 activities, while caspase 8 activity decreased41. Oxidative reactive oxygen species (ROS), which are responsible for killing cancer cells, also affect secondary signaling networks42. SFE can induce the generation of intracellular ROS and inhibit the polymerization of microtubules, leading to the apoptosis and necrosis of HCC cells43. The transcription factor nuclear factor-κB (NF-κB) is a key transcriptional regulator in the inflammatory response. The NF-κB pathway is one of the important pathways activated during liver injury and inflammation and has been widely studied in the development of liver cancer44. SFE can inhibit NF-κB activity and downstream gene expression of the NF-κB pathway in HCC cells. SFE can increase the radiation sensitivity of HCC by blocking the NF-κB pathway45.
ACTION OF SULFORAPHANE IN LUNG CANCER
As we all know, lung cancer is the leading cause of cancer death in the world46. NSCLC, the most frequent subtype of lung cancer, has increased in both incidence and mortality47. At present, research advancement in the field has revealed the tumor promotion roles of PI3K–Akt overactivation in NSCLC48. The PI3K–Akt pathway promotes proliferation, migration, invasion, and resistance to treatment by activating a variety of mechanisms, including the loss of the negative regulator phosphatase and tensin homolog (PTEN) and/or Akt1 itself49,50. SFE-treated NSCLC cells have significant inhibitory effects on the PI3K–Akt signaling pathway, including inhibition of PTEN expression and inhibition of Akt phosphorylation51. SFE (7.5 μM) combined with the chemotherapy drug carboplatin (20 μM) can significantly induce mitochondrial membrane potential and intracellular ROS depolarization. By activating caspases, destroying MMPs, and arresting the cell cycle, combination treatment with SFE and carboplatin synergistically promotes the apoptosis and antiproliferative effects of human NSCLC cells A549d and enhances the tumor toxicity effect of conventional therapy alone52.
ACTION OF SULFORAPHANE IN CERVICAL CANCER
Cervical cancer remains the third most common cancer in developing countries, despite a wide range of screening procedures53. The therapeutic effects of photodynamic therapy in cervical intraepithelial neoplasia (CIN) and cervical cancer have been extensively studied54,55. Effects of photodynamic therapy with a very low dose of SFE (2.0 μg/ml) and radachlorin (0.5 μg/ml) at a fluence of 27 J/cm2 (30 mW/cm2, λmax ∼ 670 ± 3 nm) on human cervical cancer cells HeLa has shown a synergistic effect in inducing cell apoptosis. This combination therapy activates the mitochondrial apoptotic pathway primarily through upregulating the levels of caspase 3 and caspase 9. This therapeutic strategy also activates the caspase 8-dependent death receptor pathway and inhibits cell proliferation by downregulating EGFR56.
ACTION OF SULFORAPHANE IN OTHER TUMORS
SFE obviously also has cytotoxic effects on other human malignant tumor models. For example, cisplatin is a first-line chemotherapy drug for a variety of cancers, including ovarian cancer57,58. SFE can sensitize cisplatin by enhancing ROS and mitochondrial membrane depolarization and can activate multiple apoptotic pathways to synergistically inhibit the proliferation of ovarian cancer SKOV3 and SNU8 cells and induce apoptosis. Therefore, SFE could be used as a promising chemotherapy sensitizer to improve the efficacy of cisplatin in ovarian cancer59. SFE can also reduce the viability of gastric cancer cells and induce apoptosis60. In addition, SFE can induce cell cycle arrest and apoptosis in the G2/M phase of colon cancer cells, accompanied by the phosphorylation of CDK1 and CDC25B inhibitory sites and the upregulation of the p38 and JNK pathways61. Surprisingly, SFE can selectively clear lymphoma cells via CRM1-mediated SQSTM1/p62 overexpression and AMPK activation. At the same time, SFE protects normal lymphocytes by inducing cophage and apoptosis62. SFE and photosensitive fiber-mediated photodynamic therapy can induce the apoptosis of thyroid cancer cells via significantly upregulating Ras, mitogen-activated protein kinase (MEK), extracellular signal-regulated kinase (ERK), and B-Raf protein expression levels. After combined treatment, their proapoptosis and antiproliferative effects were both significantly enhanced to a much higher level than the single dose63,64.
SAFETY AND EFFICACY
SFE is often used as an anticancer and/or anti-inflammatory drug in traditional medicine15. SFE is unstable in aqueous medium and at high temperature; thus, the stability of SFE during storage is the focus of its biological activity research. Studies have shown that −20°C and 4°C are the best storage temperatures for SFE12. As a potential antitumor drug, SFE exhibits a wide range of activities in vivo and in vitro against most tumors10. Because of its certain cytotoxicity, it is of great significance to evaluate the clinical safety of SFE65. Some researchers have tested SFE in acute toxicity analyses. After fasting overnight, 48 mice were given five different doses of SFE at 400, 300, 225, 168.8, and 126.6 mg/kg (8 in each group), and any serious effects or mortality were carefully observed after administration. After 14 days, all eight mice treated with 126.6 mg/kg SFE survived during treatment. However, eight, seven, four, or two animals treated with 400, 300, 225, or 168.8 mg/kg SFE died within 24 h of dosing. In addition, one mouse treated with 225 or 168.8 mg/kg SFE died within 48 h. For the 126.6 mg/kg SFE group, no physical or abnormal changes were observed in sleep patterns, behavior patterns, fur, skin, eyes, mucous membranes, tremors, or salivation51. In another study, scientists implanted lymphoma cells in nude mouse xenografts and administered SFE to them twice a week, 100 mg/kg each time. After 10 days, there was no significant change in body weight compared with the control group, indicating that SFE is less toxic62. Thus, the dose-associated superiority of SFE in reducing adverse reactions is obvious in current preclinic research. In addition, the findings from Li et al.66 have shown that SFE could be able to evidently restrain the pathological process of diseases in C57BL/6J mice associated with increased intestinal inflammatory factors. They demonstrated no apparent toxicity to animals induced by SFE administration.
Currently, it is well known that evaluation of the bioavailability of natural compounds is one challenge in the design of clinical trials for studying their biological activity. Recently, Fahey et al. identified that changes of inflammatory-related genes in peripheral blood mononuclear cells have significant influence on the SFE bioavailability in 20 healthy participants67. Similarly, another research has been carried out to evaluate the bioavailability of SFE in 14 women and found that repeated dosing of SFE could not result in the accumulation of toxic metabolites in urine over time68. Moreover, SFN-loaded nanostructured lipid carriers (NLCs) were developed and optimized to effectively improve its bioavailability and cytotoxicity efficacy against cancers69. These findings provide valuable recommendation to better design the clinical trials to study the SFE functionality in the future. To date, a preliminary randomized controlled trial was performed to demonstrate that pretreatment with broccoli sprout extract could improve the bioavailability and chemopreventive activity of SFE, together with downregulation of several prostate cancer development-associated genes in the biopsy from 98 men70. However, unfortunately, there are no clinical trials for direct evaluation of SFE on its antitumor effect. Therefore, further additional investigation, mainly well-designed clinical trials, are required to establish correlations and allow to further verify the efficacy, safety, and possible adverse reactions of SFE products.
DISCUSSION
SFE extracted from Raphanus sativus is unstable in aqueous solutions and at high temperatures11. This instability undermines many useful applications of SFE. Generally, the degradation rate of SFE increases with increasing of temperature. Some researchers have found that the optimal storage temperature of SFE is −20°C and 4°C by electrospray ionization (ESI)/mass spectrometry (MS), nuclear magnetic resonance (NMR), and other research methods. After 5 weeks of storage, the residual rates remained around 96.56 ± 0.15% and 95.18 ± 0.20%, respectively12. To overcome the instability of SFE at high temperatures, some researchers have developed hydroxypropyl-β-cyclodextrin (HP-β-CD) and maltodextrin (MD) microcapsules loaded with SFE71. As ROS-induced oxidative stress has been shown to be involved in the pathogenesis of many diseases, a recent study showed that MD microcapsules can increase the antioxidant capacity of natural compound anthocyanins and reduce ROS levels72. This suggests that HP-β-CD and MD microcapsules containing SFE might also have similar potential and need to be further clarified in other clinical applications.
Increasing numbers of studies have shown that SFE has potential as an effective cancer chemopreventive agent. For example, SFE can reduce cell proliferation in human and murine erythroleukemia cells, human T lymphocytes, human cervical cancer cells, and H3-T1-1 cells12. Studies have identified SFE, and its analog, SFN, as ITC derivatives extracted from dextran73. SFN and SFE belong to the same family and exhibit similar effects through various mechanisms. Studies have found that low concentrations of ITCs can induce apoptosis in human malignant melanoma (A375) cells74. It is well known that pSTAT3 is a key carcinogen in head and neck squamous cell carcinoma (HNSCC)75. SFN promotes non-NRF2-dependent dephosphorylation/inactivation of pSTAT376,77. A high level of aldehyde dehydrogenase (ALDH) enzyme activity in breast cancer cells results in breast cancer stem cell (BCSC) properties by upregulating Notch-1 and epithelial–mesenchymal markers78. Studies have shown that SFN can reduce the number of ALDH+ cells in human breast cancer cells by 65% to 80%. At the same time, SFN downregulated the Wnt/β-catenin signaling pathway79, an important regulator for the stem cell self-renewal. In addition, miR-616-5p was identified as a carcinogenic marker associated with the risk of recurrence and metastasis in patients with NSCLC80. Epithelial–mesenchymal transition (EMT) is an important mechanism leading to cancer metastasis81–83. SFN inhibits miR-616-5p expression and abrogates EMT processes in NSCLC cells, thereby inhibiting lung cancer metastasis80. These results further suggest the indirect antitumor effect of SFE. At the same time, the abovementioned findings can provide clues to finding more active substances to enrich our clinical drug classes.
CONCLUSION
In recent years, identifying active ingredients in plants that can be used to treat diseases has been the research approach for creating new drugs both at home and abroad. As shown in previous studies, SFE has significant antitumor effects and exhibits enormous clinical potential due to its undiscovered activity. However, studies on the mechanism of SFE antitumor activity have not been comprehensive, and there is a lack of available information for evidence-based medicine. In addition, the safety and toxic side effects of SFE have yet to be further studied. In conclusion, with continuous research and increasing understanding of the cancer prevention and anticancer mechanisms, SFE has emerged as a very promising new drug in antitumor clinical treatment.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (81703036, 81803035, and 81572946), the China Postdoctoral Science Foundation (2017M610510), and the Natural Science Foundation of Hunan Province, China (2019JJ50932). We also thank Elsevier’s Language Editing for assistance with English language polishing. All data generated or analyzed during this study are included in this published article. G.W., Y.Y., J.Z., and Z.X. were the main authors of the manuscript; G.W., Y.Z., X.C., S.Z., X.W., J.W., W.L., and C.O. contributed to the design and format of figures and tables; Y.Y. and Z.X. revised the manuscript; and G.W., Y.Y., J.Z., and Z.X. were responsible for the manuscript writing. All authors read and approved the final manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 2. Ganz PA. Current US cancer statistics: Alarming trends in young adults? J Natl Cancer Inst. 2019;111(12):1241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Yan Y, Chen X, Zeng S, Qian L, Ren X, Wei J, Yang X, Zhou Y, Gong Z, Xu Z. The antitumor activities of Marsdenia tenacissima. Front Oncol. 2018;8:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan Y, Xu Z, Dai S, Qian L, Sun L, Gong Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res. 2016;35:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei J, Yan Y, Chen X, Qian L, Zeng S, Li Z, Dai S, Gong Z, Xu Z. The roles of plant-derived triptolide on non-small cell lung cancer. Oncol Res. 2019;27(7):849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Issa S, Prandina A, Bedel N, Rongved P, Yous S, Le Borgne M, Bouaziz Z. Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J Enzyme Inhib Med Chem. 2019;34(1):1321–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharadwaj G, Nhan V, Yang S, Li X, Narayanan A, Macarenco AC, Shi Y, Yang D, Vieira LS, Xiao W, Li Y, Lam KS. Cholic acid-based novel micellar nanoplatform for delivering FDA-approved taxanes. Nanomedicine (Lond) 2017;12(10):1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen ST, Lee TY, Tsai TH, Huang YC, Lin YC, Lin CP, Shieh HR, Hsu ML, Chi CW, Lee MC, Chang HH, Chen YJ. Traditional Chinese medicine Danggui Buxue Tang inhibits colorectal cancer growth through induction of autophagic cell death. Oncotarget 2017;8(51):88563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Z, Yan Y, Xiao L, Dai S, Zeng S, Qian L, Wang L, Yang X, Xiao Y, Gong Z. Radiosensitizing effect of diosmetin on radioresistant lung cancer cells via Akt signaling pathway. PLoS One 2017;12(4):e0175977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, Conrads TP, Veenstra TD, Chung FL. Identification of potential protein targets of isothiocyanates by proteomics. Chem Res Toxicol. 2011;24(10):1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beevi SS, Mangamoori LN, Subathra M, Edula JR. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods Hum Nutr. 2010;65(3):200–9. [DOI] [PubMed] [Google Scholar]
- 12. Tian G, Li Y, Cheng L, Yuan Q, Tang P, Kuang P, Hu J. The mechanism of sulforaphane degradation to different water contents. Food Chem. 2016;194:1022–7. [DOI] [PubMed] [Google Scholar]
- 13. Papi A, Orlandi M, Bartolini G, Barillari J, Iori R, Paolini M, Ferroni F, Grazia Fumo M, Pedulli GF, Valgimigli L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J Agric Food Chem. 2008;56(3):875–83. [DOI] [PubMed] [Google Scholar]
- 14. Nastruzzi C, Cortesi R, Esposito E, Menegatti E, Leoni O, Iori R, Palmieri S. In vitro antiproliferative activity of isothiocyanates and nitriles generated by myrosinase-mediated hydrolysis of glucosinolates from seeds of cruciferous vegetables. J Agric Food Chem. 2000;48(8):3572–5. [DOI] [PubMed] [Google Scholar]
- 15. Kim KH, Moon E, Kim SY, Choi SU, Lee JH, Lee KR. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J Ethnopharmacol. 2014;151(1):503–8. [DOI] [PubMed] [Google Scholar]
- 16. Sivapalan T, Melchini A, Saha S, Needs PW, Traka MH, Tapp H, Dainty JR, Mithen RF. Bioavailability of glucoraphanin and sulforaphane from high-glucoraphanin broccoli. Mol Nutr Food Res. 2018;62(18):e1700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sangthong S, Weerapreeyakul N. Simultaneous quantification of sulforaphane and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016;201:139–44. [DOI] [PubMed] [Google Scholar]
- 18. Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269(2):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51. [DOI] [PubMed] [Google Scholar]
- 20. Huang SQ, Sun B, Xiong ZP, Shu Y, Zhou HH, Zhang W, Xiong J, Li Q. The dysregulation of tRNAs and tRNA derivatives in cancer. J Exp Clin Cancer Res. 2018;37(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: An overview. Updates Surg. 2017;69(3):313–7. [DOI] [PubMed] [Google Scholar]
- 22. Merino Bonilla JA, Torres Tabanera M, Ros Mendoza LH. Breast cancer in the 21st century: From early detection to new therapies. Radiologia 2017;59(5):368–79. [DOI] [PubMed] [Google Scholar]
- 23. Lopes CM, Dourado A, Oliveira R. Phytotherapy and nutritional supplements on breast cancer. Biomed Res Int. 2017;2017:7207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pawlik A, Wala M, Hac A, Felczykowska A, Herman-Antosiewicz A. Sulforaphane, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine 2017;29:1–10. [DOI] [PubMed] [Google Scholar]
- 25. Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 positive breast cancer—A mini-review. Asian Pac J Cancer Prev. 2016;17(4):1609–15. [DOI] [PubMed] [Google Scholar]
- 26. Cadona FC, Rosa JL, Schneider T, Cubillos-Rojas M, Sanchez-Tena S, Azzolin VF, Assmann CE, Machado AK, Ribeiro EE, da Cruz IBM. Guarana, a highly caffeinated food, presents in vitro antitumor activity in colorectal and breast cancer cell lines by inhibiting AKT/mTOR/S6K and MAPKs pathways. Nutr Cancer 2017;69(5):800–10. [DOI] [PubMed] [Google Scholar]
- 27. Leroy C, Ramos P, Cornille K, Bonenfant D, Fritsch C, Voshol H, Bentires-Alj M. Activation of IGF1R/p110beta/AKT/mTOR confers resistance to alpha-specific PI3K inhibition. Breast Cancer Res. 2016;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oh DY, Bang YJ. HER2-targeted therapies—A role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48. [DOI] [PubMed] [Google Scholar]
- 29. Kaczynska A, Swierczynska J, Herman-Antosiewicz A. Sensitization of HER2 positive breast cancer cells to lapatinib using plants-derived isothiocyanates. Nutr Cancer 2015;67(6):976–86. [DOI] [PubMed] [Google Scholar]
- 30. Navratil J, Fabian P, Palacova M, Petrakova K, Vyzula R, Svoboda M. [Triple negative breast cancer]. Klin Onkol 2015;28(6):405–15. [PubMed] [Google Scholar]
- 31. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–69. [DOI] [PubMed] [Google Scholar]
- 32. Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist 2016;21(9):1050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habib JG, O’Shaughnessy JA. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016;5(10):2989–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miele E, Po A, Begalli F, Antonucci L, Mastronuzzi A, Marras CE, Carai A, Cucchi D, Abballe L, Besharat ZM, Catanzaro G, Infante P, Di Marcotullio L, Canettieri G, De Smaele E, Screpanti I, Locatelli F, Ferretti E. β-Arrestin1-mediated acetylation of Gli1 regulates Hedgehog/Gli signaling and modulates self-renewal of SHH medulloblastoma cancer stem cells. BMC Cancer 2017;17(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, Bonanno E, Di Stefano E, Frajese GV, Orlandi A, Screpanti I, Gulino A, Modesti A, Bei R. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget 2016;7(8):9250–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao C, Kim MC, Chen J, Song J, Ko HW, Lee HJ. Sulforaphane interferes with human breast cancer cell migration and invasion through inhibition of hedgehog signaling. J Agric Food Chem. 2016;64(27):5515–24. [DOI] [PubMed] [Google Scholar]
- 37. Shajahan-Haq AN, Boca SM, Jin L, Bhuvaneshwar K, Gusev Y, Cheema AK, Demas DD, Raghavan KS, Michalek R, Madhavan S, Clarke R. EGR1 regulates cellular metabolism and survival in endocrine resistant breast cancer. Oncotarget 2017;8(57):96865–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang M, Teng W, Qu Y, Wang H, Yuan Q. Sulforaphane inhibits triple negative breast cancer through activating tumor suppressor Egr1. Breast Cancer Res Treat. 2016;158(2):277–86. [DOI] [PubMed] [Google Scholar]
- 39. Li S, Yang F, Ren X. Immunotherapy for hepatocellular carcinoma. Drug Discov Ther. 2015;9(5):363–71. [DOI] [PubMed] [Google Scholar]
- 40. Tahmasebi Birgani M, Carloni V. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. Int J Mol Sci. 2017;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kntayya SB, Ibrahim MD, Mohd Ain N, Iori R, Ioannides C, Abdull Razis AF. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphane in human hepatocarcinoma HepG2 cells. Nutrients 2018;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015;100:170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pocasap P, Weerapreeyakul N, Thumanu K. Structures of isothiocyanates attributed to reactive oxygen species generation and microtubule depolymerization in HepG2 cells. Biomed Pharmacother. 2018;101:698–709. [DOI] [PubMed] [Google Scholar]
- 44. He G, Karin M. NF-kappaB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ren K, Li Z, Li Y, Zhang W, Han X. Sulforaphane enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-kappaB pathway. J Biochem Mol Toxicol. 2017;31(8). [DOI] [PubMed] [Google Scholar]
- 46. Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103(3):463–73. [DOI] [PubMed] [Google Scholar]
- 47. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Marco C, Laudanna C, Rinaldo N, Oliveira DM, Ravo M, Weisz A, Ceccarelli M, Caira E, Rizzuto A, Zoppoli P, Malanga D, Viglietto G. Specific gene expression signatures induced by the multiple oncogenic alterations that occur within the PTEN/PI3K/AKT pathway in lung cancer. PLoS One 2017;12(6):e0178865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao ZQ, Yu ZY, Li J, Ouyang XN. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol Lett. 2016;12(1):63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yao W, Yue P, Zhang G, Owonikoko TK, Khuri FR, Sun SY. Enhancing therapeutic efficacy of the MEK inhibitor, MEK162, by blocking autophagy or inhibiting PI3K/Akt signaling in human lung cancer cells. Cancer Lett. 2015;364(1):70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang M, Wang H, Zhou M, Liu W, Kuang P, Liang H, Yuan Q. The natural compound sulforaphane, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget 2016;7(47):76656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chatterjee S, Rhee YH, Ahn JC. Sulforaphane–carboplatin combination synergistically enhances apoptosis by disruption of mitochondrial membrane potential and cell cycle arrest in human non-small cell lung carcinoma. J Med Food 2016;19(9):860–9. [DOI] [PubMed] [Google Scholar]
- 53. Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, Bothou A, Galazios G. Cervical cancer: Screening, diagnosis and staging. J BUON. 2016;21(2):320–5. [PubMed] [Google Scholar]
- 54. Chizenga EP, Chandran R, Abrahamse H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019;10(43):4380–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park YK, Park CH. Clinical efficacy of photodynamic therapy. Obstet Gynecol Sci. 2016;59(6):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Biswas R, Mondal A, Chatterjee S, Ahn JC. Evaluation of synergistic effects of sulforaphane with photodynamic therapy in human cervical cancer cell line. Lasers Med Sci. 2016;31(8):1675–82. [DOI] [PubMed] [Google Scholar]
- 57. Samuel P, Pink RC, Brooks SA, Carter DR. miRNAs and ovarian cancer: A miRiad of mechanisms to induce cisplatin drug resistance. Expert Rev Anticancer Ther. 2016;16(1):57–70. [DOI] [PubMed] [Google Scholar]
- 58. Choi BY, Joo JC, Lee YK, Jang IS, Park SJ, Park YJ. Anti-cancer effect of Scutellaria baicalensis in combination with cisplatin in human ovarian cancer cell. BMC Complement Altern Med. 2017;17(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Biswas R, Ahn JC, Kim JS. Sulforaphane synergistically sensitizes cisplatin via enhanced mitochondrial dysfunction and PI3K/PTEN modulation in ovarian cancer cells. Anticancer Res. 2015;35(7):3901–8. [PubMed] [Google Scholar]
- 60. Mondal A, Biswas R, Rhee YH, Kim J, Ahn JC. Sulforaphane promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 down-regulation. Gen Physiol Biophys. 2016;35(1):25–34. [DOI] [PubMed] [Google Scholar]
- 61. Byun S, Shin SH, Park J, Lim S, Lee E, Lee C, Sung D, Farrand L, Lee SR, Kim KH, Dong Z, Lee SW, Lee KW. Sulforaphane suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol Nutr Food Res. 2016;60(5):1068–78. [DOI] [PubMed] [Google Scholar]
- 62. Wang H, Wang F, Wu S, Liu Z, Li T, Mao L, Zhang J, Li C, Liu C, Yang Y. Traditional herbal medicine-derived sulforaphane promotes mitophagic cell death in lymphoma cells through CRM1-mediated p62/SQSTM1 accumulation and AMPK activation. Chem Biol Interact. 2018;281:11–23. [DOI] [PubMed] [Google Scholar]
- 63. Biswas R, Mondal A, Ahn JC. Deregulation of EGFR/PI3K and activation of PTEN by photodynamic therapy combined with carboplatin in human anaplastic thyroid cancer cells and xenograft tumors in nude mice. J Photochem Photobiol B 2015;148:118–27. [DOI] [PubMed] [Google Scholar]
- 64. Chatterjee S, Rhee Y, Chung PS, Ge RF, Ahn JC. Sulforaphane enhances the efficacy of photodynamic therapy in anaplastic thyroid cancer through Ras/RAF/MEK/ERK pathway suppression. J Photochem Photobiol B 2018;179:46–53. [DOI] [PubMed] [Google Scholar]
- 65. Liu X, Wang Z, Xie R, Tang P, Yuan Q. Design, synthesis and biological evaluation of novel carbamodithioates as anti-proliferative agents against human cancer cells. Eur J Med Chem. 2018;157:1526–40. [DOI] [PubMed] [Google Scholar]
- 66. Li M, Gao J, Tang Y, Liu M, Wu S, Qu K, Long X, Li H, Liu M, Liu Y, Yuan J, Mao L, Liu Y, Zheng X, Wang E, Wang J, Yang Y. Traditional herbal medicine-derived sulforaphane LFS-01 reverses colitis in mice by selectively altering the gut microbiota and promoting intestinal gamma-delta T cells. Front Pharmacol. 2017;8:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fahey JW, Wade KL, Stephenson KK, Panjwani AA, Liu H, Cornblatt G, Cornblatt BS, Ownby SL, Fuchs E, Holtzclaw WD, Cheskin LJ. Bioavailability of sulforaphane following ingestion of glucoraphanin-rich broccoli sprout and seed extracts with active myrosinase: A pilot study of the effects of proton pump inhibitor administration. Nutrients 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baenas N, Suarez-Martinez C, Garcia-Viguera C, Moreno DA. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res Int. 2017;100(Pt 1):497–503. [DOI] [PubMed] [Google Scholar]
- 69. Soni K, Rizwanullah M, Kohli K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif Cells Nanomed Biotechnol. 2018;46(Supp1):15–31. [DOI] [PubMed] [Google Scholar]
- 70. Zhang Z, Garzotto M, Davis EW 2nd, Mori M, Stoller WA, Farris PE, Wong CP, Beaver LM, Thomas GV, Williams DE, Dashwood RH, Hendrix DA, Ho E, Shannon J. Sulforaphane bioavailability and chemopreventive activity in men presenting for biopsy of the prostate gland: A randomized controlled trial. Nutr Cancer 2020;72(1):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tian G, Li Y, Yuan Q, Cheng L, Kuang P, Tang P. The stability and degradation kinetics of sulforaphane in microcapsules based on several biopolymers via spray drying. Carbohydr Polym. 2015;122:5–10. [DOI] [PubMed] [Google Scholar]
- 72. Ma Y, Feng Y, Zeng W, Luo H. Anthocyanin encapsulated by ferulic acid-grafted-maltodextrin (FA-g-MD) microcapsules potentially improved its free radical scavenging capabilities against H2O2-induced oxidative stress. Molecules 2019;24(8):E1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Russo M, Spagnuolo C, Russo GL, Skalicka-Wozniak K, Daglia M, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit Rev Food Sci Nutr. 2018;58(8):1391–405. [DOI] [PubMed] [Google Scholar]
- 74. Mantso T, Sfakianos AP, Atkinson A, Anestopoulos I, Mitsiogianni M, Botaitis S, Perente S, Simopoulos C, Vasileiadis S, Franco R, Pappa A, Panayiotidis MI. Development of a novel experimental in vitro model of isothiocyanate-induced apoptosis in human malignant melanoma cells. Anticancer Res. 2016;36(12):6303–9. [DOI] [PubMed] [Google Scholar]
- 75. Bharadwaj U, Eckols TK, Xu X, Kasembeli MM, Chen Y, Adachi M, Song Y, Mo Q, Lai SY, Tweardy DJ. Small-molecule inhibition of STAT3 in radioresistant head and neck squamous cell carcinoma. Oncotarget 2016;7(18):26307–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bauman JE, Zang Y, Sen M, Li C, Wang L, Egner PA, Fahey JW, Normolle DP, Grandis JR, Kensler TW, Johnson DE. Prevention of carcinogen-induced oral cancer by sulforaphane. Cancer Prev Res (Phila) 2016;9(7):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu CM, Peng CY, Liao YW, Lu MY, Tsai ML, Yeh JC, Yu CH, Yu CC. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J Formos Med Assoc. 2017;116(1):41–8. [DOI] [PubMed] [Google Scholar]
- 78. Pal D, Kolluru V, Chandrasekaran B, Baby BV, Aman M, Suman S, Sirimulla S, Sanders MA, Alatassi H, Ankem MK, Damodaran C. Targeting aberrant expression of Notch-1 in ALDH(+) cancer stem cells in breast cancer. Mol Carcinog. 2017;56(3):1127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16(9):2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang DX, Zou YJ, Zhuang XB, Chen SX, Lin Y, Li WL, Lin JJ, Lin ZQ. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3beta/beta-catenin signaling pathways. Acta Pharmacol Sin. 2017;38(2):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ye X, Weinberg RA. Epithelial–mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015;25(11):675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen X, Bode AM, Dong Z, Cao Y. The epithelial–mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB J. 2016;30(9):3001–10. [DOI] [PubMed] [Google Scholar]