Abstract
To evaluate the cost–utility of pembrolizumab versus chemotherapy as the first-line setting for metastatic non-small cell lung cancer (NSCLC) from the US health care system perspective, a Markov model was developed to compare the lifetime cost and effectiveness of pembrolizumab versus chemotherapy for untreated metastatic NSCLC, based on the clinical data derived from phase III randomized controlled trial (KEYNOTE-042; ClinicalTrials.gov; NCT02220894). Weibull distribution was fitted to simulate the parametric survival functions. Drug costs were collected from official websites, and utility values were obtained from published literature. Total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were computed as primary output indicators. The impact of different PD-L1 expression levels on ICER was also evaluated. One-way and probabilistic sensitivity analyses were performed to assess the model uncertainty. Compared with chemotherapy, patients treated with pembrolizumab provided an additional 1.13, 1.01, and 0.59 QALYs in patients with PD-L1 expression levels of ≥50%, ≥20%, and ≥1%, with corresponding incremental cost of $53,784, $47,479, and $39,827, respectively. The resultant ICERs of pembrolizumab versus chemotherapy were $47,596, $47,184, and $68,061/QALY, in three expression levels of PD-L1, respectively, all of which did not exceed the WTP threshold of 180,000/QALY. Probability sensitivity analysis outcome supported that pembrolizumab exhibited evident advantage over chemotherapy to be cost-effective. One-way sensitivity analysis found that ICERs were most sensitive to utility value of pembrolizumab in progression survival state. All the adjustment of parameters did not qualitatively change the result. For treatment-naive, metastatic NSCLC patients with PD-L1+, pembrolizumab was estimated to be cost-effective compared with chemotherapy for all PD-L1 expression levels at a WTP threshold of $180,000/QALY in the context of the US health care system.
Key words: Programmed cell death ligand 1 (PD-L1), Pembrolizumab, Non-small cell lung cancer (NSCLC), Cost–utility, Chemotherapy
INTRODUCTION
Lung cancer is the neoplasm with the highest incidence worldwide and is considered to be responsible for nearly 20% and ranked first in cancer-related deaths1. Non-small cell lung cancer (NSCLC) accounts for approximately 80%–85% of all lung cancers2. Over the past decade, platinum-based chemotherapy has remained the traditional mainstay of treatment for metastasis in NSCLC patients3, which is associated with modest efficacy and has reached a plateau. Fortunately, the tremendous efforts in developing new drugs and profound research on potential biomarkers have promoted the emergency of immune checkpoint inhibitors (ICIs) that block the programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) pathway to treat NSCLC, showing considerable advantages4–6.
Pembrolizumab (Keytruda), a humanized monoclonal antibody designed to block the PD-1 receptor, was approved by the Food and Drug Administration (FDA) as the first-line treatment for metastatic NSCLC patients without an EGFR mutation or ALK translocation7–9. The use of pembrolizumab brings hopes to the treatment of metastatic NSCLC patients. New clinical outcomes have further proved that pembrolizumab survival benefits statistically compared to chemotherapy5. Taking the drug price into account, this could result in a significant rise of financial burden on the health care system, so it is necessary to conduct an economic analysis.
On the basis of survival data from a series of KEYNOTE trials, numerous economic appraisals of pembrolizumab have been carried out from different countries’ perspective10–12. However, there are still no relevant reports based on the KEYNOTE 042, which investigated the benefits of pembrolizumab monotherapy compared with chemotherapy in PD-L1 ≥50%, ≥20%, and ≥1% metastatic NSCLC patients, respectively, to evaluate the cost–utility of pembrolizumab. By reason of patients with a higher expression level of PD-L1 could achieve longer median survival time than that lower PD-L1 expression, the possibility of pembrolizumab to be cost saving might vary from different PD-L1 levels. Therefore, in this analysis, we sought to evaluate the cost–utility of pembrolizumab in metastatic NSCLC patients with different expression levels of PD-L1 in the context of the US health care system.
MATERIALS AND METHODS
Model Structure
A Markov model was developed to project expected costs and efficacy of pembrolizumab compared with chemotherapy, within three mutually exclusive health states: “progression-free survival (PFS)” (initial state of patient until progression), “progression survival (PS)” (state after disease progression), and “death” (absorbing state) (Fig. 1). Eligible population in this model was based on the enrollment criterion of KEYNOTE-42 trial6, which was patients with locally advanced or metastatic NSCLC, previously untreated, and without EGFR mutation or ALK translocation. Pembrolizumab was administrated up to a maximum of 35 cycles, and platinum-based chemotherapy was continued for 4–6 cycles or until disease progression. As disease progressed, patients were allowed to receive subsequent treatment including chemotherapy, target therapy, immunotherapy, or two treatment options mentioned above simultaneously. The parametric survival curve fitting was performed in R software (version 3.5.1), and the Markov model was developed and run in TreeAge 2017.
Figure 1.
Model structure.
The analysis was conducted from the perspective of the US health care system. The cycle was as 21 days, and the model was run until 99% of the patients enter death state with a time horizon of lifetime. The primary outputs of this model were lifetime health care costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). ICERs were compared with the willingness-to-pay (WTP) threshold of $180,000/QALY in the US13 to estimate the cost–utility. Costs were discounted at an annual rate of 3%10.
Effectiveness Parameters
The model effectiveness parameters were obtained from KEYNOTE-0426. The survival functions, which were fitted according to the PFS and OS data from Kaplan–Meier (KM) curves, were used to calculate the transfer probability among the states. Both the Weibull and the log-logistic distributions were fitted, and the optimal survival function was employed. The fitting was conducted following UK National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) guidelines14. In adjusted R 2 of fit tests, the matching degree of extrapolation outcome and clinical data were considered in selecting the final distribution functions for the model. The background mortality rate for each age group was derived from published literature and adopted to estimate the transfer probability of PFS to death15. Finally, we fitted three series curves for patients with PD-L1 expressions of ≥50%, ≥20% and ≥1% according to KEYNOTE-042 setting (Table 1).
Table 1.
Clinical Inputs: Weibull Survival Curve-Fitting Parameters
| PD-L1 Expression | Regimen | Scale (λ) | Shape (γ) | Adjusted R 2 |
|---|---|---|---|---|
| Overall survival | ||||
| ≥50% | Pembrolizumab | 0.063985 | 0.796046 | 0.999902 |
| Chemotherapy | 0.041061 | 1.089799 | 0.999146 | |
| ≥20% | Pembrolizumab | 0.065791 | 0.816173 | 0.999862 |
| Chemotherapy | 0.041653 | 1.066223 | 0.999218 | |
| ≥1% | Pembrolizumab | 0.072141 | 0.8074439 | 0.999866 |
| Chemotherapy | 0.04438 | 1.059809 | 0.999284 | |
| Progression-free survival | ||||
| ≥50% | Pembrolizumab | 0.144153 | 0.769625 | 0.998946 |
| Chemotherapy | 0.062074 | 1.232194 | 0.998621 | |
| ≥20% | Pembrolizumab | 0.156777 | 0.770046 | 0.998626 |
| Chemotherapy | 0.059433 | 1.226069 | 0.999018 | |
| ≥1% | Pembrolizumab | 0.16432 | 0.79536 | 0.998187 |
| Chemotherapy | 0.065666 | 1.197265 | 0.999148 | |
PD-L1, programmed cell death ligand 1.
Utility value, which measures health-related quality of life (HR-QoL) in a range of 0–1, was obtained from published literature16 (Table 2). QALYs were computed by multiplying life-years by utility values.
Table 2.
Base Parameter Input to Model and Ranges of Sensitivity Analyses
| Parameters | Base Value | Lower | Upper | Distribution | Source |
|---|---|---|---|---|---|
| Cost ($) | |||||
| Pembrolizumab (200 mg) | 9,180.80 | 7,344.64 | 11,016.96 | Log-normal | 15, www.drugs.com |
| Carboplatin (50 mg) | 12.79 | 10.23 | 15.35 | Log-normal | 15, www.drugs.com |
| Paclitaxel (30 mg) | 15.57 | 72.66 | 109.00 | Log-normal | 15, www.drugs.com |
| Pemetrexed (100 mg) | 728.91 | 583.13 | 874.69 | Log-normal | 15, www.drugs.com |
| Nivolumab (40 mg) | 1,119.90 | 895.92 | 1,343.88 | Log-normal | 15, www.drugs.com |
| Docetaxel (20 mg) | 609.43 | 487.54 | 731.32 | Log-normal | 15, www.drugs.com |
| Gemcitabine (1000 mg) | 782.50 | 626.00 | 939.00 | Log-normal | 15, www.drugs.com |
| Gefitinib (250 mg) | 271.34 | 217.07 | 325.61 | Log-normal | 15, www.drugs.com |
| Crizotinib (250 mg) | 293.48 | 234.78 | 352.18 | Log-normal | 15, www.drugs.com |
| Bevacizumab (100 mg) | 840.51 | 672.41 | 1,008.61 | Log-normal | 15, www.drugs.com |
| Pneumonitis | 6,491.17 | 5,192.94 | 7,789.40 | Log-normal | 15,23 |
| Anemia | 6,461.96 | 5,169.57 | 7,754.35 | Log-normal | 15,23 |
| Neutropenia | 104.48 | 83.58 | 125.38 | Log-normal | 15,23 |
| Thrombocytopenia | 232.55 | 186.04 | 279.06 | Log-normal | 15,23 |
| Follow-up | 3,785.00 | 3,028.00 | 4,542.00 | Log-normal | 15,17 |
| Best support care | 124.95 | 99.96 | 149.94 | Log-normal | 15,21 |
| Terminal care | 5,546.18 | 4,436.94 | 6,655.42 | Log-normal | 15,20 |
| Utility values | |||||
| Pembrolizumab of PFS | 0.71 | 0.47 | 0.95 | gamma | 16 |
| Pembrolizumab of PS | 0.67 | 0.47 | 0.87 | gamma | 16 |
| Chemotherapy of PFS | 0.68 | 0.44 | 0.92 | gamma | 16 |
| Chemotherapy of PS | 0.67 | 0.47 | 0.87 | gamma | 16 |
| Body surface area (m2) | 1.82 | 1.52 | 1.92 | Normal | 17 |
| Body weight (kg) | 71.4 | 29 | 112 | gamma | 18 |
| Discount rate (%) | 3 | 0 | 5 | Fixed | 10,17 |
PFS, progression-free survival; PS, progression survival.
Cost Inputs
Only direct medical costs were considered in the model. Cost components associated with cancer treatment included drug acquisition, follow-up, best support care (BSC), treatment of serious adverse events (SAEs), and terminal care.
Drug acquisition cost was based on the dosing schedule reported in KEYNOTE-0426. Pembrolizumab was administered at a dose of 200 mg every 3 weeks (q3w). The chemotherapy group regimens, namely, pemetrexed/paclitaxel + carboplatin, were administered q3w for 4 to 6 cycles depending on specific regimen choice, which was calculated based on the proportion of patients on each regimen according to the trial. As disease progressed, both groups of patients received one or more treatment regimens, so the drug cost for PS state was calculated and adjusted according KEYNOTE-042 trail. The patients’ body surface area (BSA) and typical weigh were assumed to be 1.82 m2 ( 17 ) and 71.4 kg18, respectively, to estimate the doses of chemotherapy agents as well as second-line drug therapy (nivolumab and bevacizumab). Unit prices of drugs in the US were obtained from the website www.drugs.com.
Follow-up cost was counted from PFS state and throughout the treatment process, while BSC occurred in PS state after 5-month subsequent therapy on the basis of several articles about second-line treatment of NSCLC patients4,19. Terminal care costs were also included as a one-time cost in the final state in the US20. The above resource costs were obtained from previously published studies17,21,22.
SAE-related costs were computed by multiplying the estimated incidence rates of per AEs by the corresponding unit treatment cost. Four SAEs with high incidence rates (>1%) and expensive treatment expenditure were enrolled in this model, which were neutropenia, anemia, thrombocytopenia, and pneumonitis (Table 3). AE unit treatment costs were obtained from an analysis conducted in developed countries23. All the unit costs used in the base analysis were presented as US dollars and are listed in Table 2.
Table 3.
Treatment-Related Adverse Events
| Adverse Event Rates6 | Pembrolizumab (%) | Chemotherapy (%) |
|---|---|---|
| Pneumonitis | 20 (3%) | 0 |
| Anemia | 4 (<1%) | 80 (13%) |
| Neutropenia | 1 (<1%) | 46 (7%) |
| Thrombocytopenia | 1 (<1%) | 10 (2%) |
Sensitivity Analyses
One-way deterministic sensitivity analysis (DSA) was conducted to evaluate the variation of the model result by changes in key parameters within the plausible ranges. To be specific, parameters were imposed lower and upper limits with mean ± standard deviation obtained from the literature16 or a range of ±20% of the base case value15. Probabilistic sensitivity analysis (PSA) was carried out to test the alteration of the model with respect to uncertainty in model input parameters, performing Monte Carlo simulation with 1,000 iterations using different distributions such as log-normal for cost values and gamma for utility values. The ranges and distributions of parameters for sensitivity analyses are given in Table 2.
RESULTS
Base Case Results
Compared with chemotherapy, pembrolizumab yielded a gain of QALYs, along with an increase in total costs for treatment-naive, metastatic NSCLC patients (Table 4). In this analysis, patients treated with pembrolizumab produced an additional 1.13, 1.01, and 0.59 QALYs more than chemotherapy in the cohorts with PD-L1 expression levels of ≥50%, ≥20%, and ≥1%, respectively. From the US health care system perspective, the corresponding incremental costs over lifetime horizon spent for pembrolizumab in comparison to chemotherapy were $53,784, $47,479, and $39,827. Consequently, ICERs were estimated to be $47,596/QALY, $47,184/QALY, and $68,061/QALY in PD-L1 expression levels of ≥50%, ≥20%, and ≥1%, respectively, all of which were within the WTP threshold of $180,000/QALY.
Table 4.
Base Case Results
| Pembrolizumab | Chemotherapy | Incremental | |
|---|---|---|---|
| PD-L1 expression≥50% | |||
| QALY | 1.87 | 0.74 | 1.13 |
| Total cost | 117,390 | 63,605 | 53,784 |
| ICER | 47,596 | ||
| PD-L1 expression≥20% | |||
| QALY | 1.78 | 0.77 | 1.01 |
| Total cost | 112,341 | 64,862 | 47,479 |
| ICER | 47,184 | ||
| PD-L1 expression≥1% | |||
| QALY | 1.37 | 0.78 | 0.59 |
| Total cost | 104,747 | 64,919 | 39,827 |
| ICER | 68,061 | ||
PD-L1, programmed cell death ligand 1; QALY, quality-adjust life years; ICER, incremental cost-effectiveness ratio.
Sensitivity Analyses
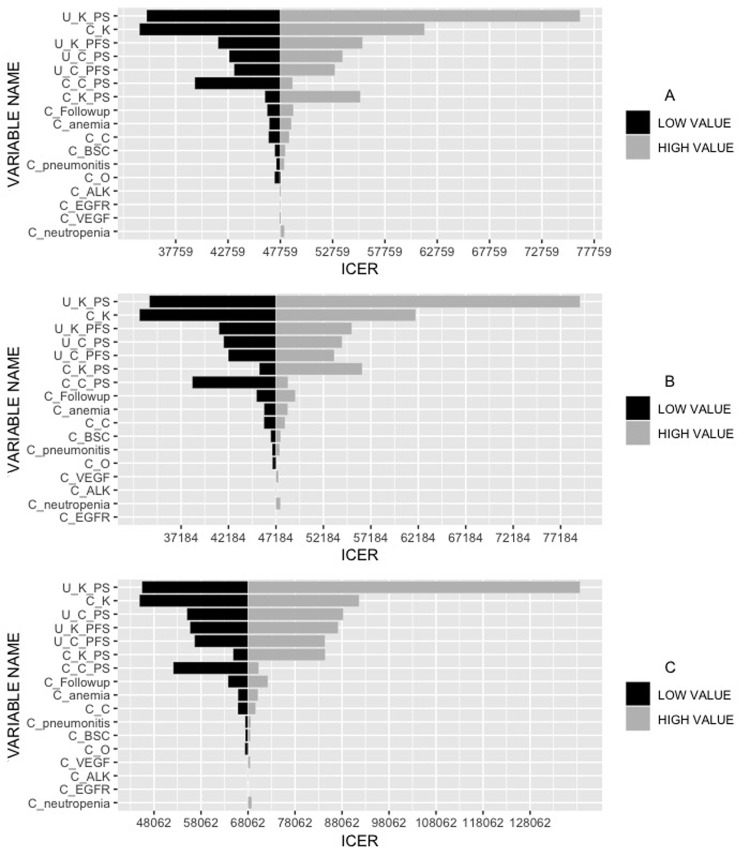
The results of one-way DSA are shown as the tornado diagrams (Fig. 2). It manifested that the utility value of pembrolizumab in PS state had the greatest impact on ICERs regardless of PD-L1 expression levels. The ICER varied from $34,985 to $76,420/QALY, $32,804 to $79,243/QALY, and $45,498 to $138,720/QALY in patients of PD-L1 ≥50%, ≥20%, and ≥1%, respectively, both of which were far below the WTP (Fig. 2A–C). Other variables that considerably impacted the ICERs were the price of pembrolizumab, utility value of pembrolizumab in PFS state, and utility value of chemotherapy in the PS state. Taken together, varying the key parameters in a sensible range had limited impact on the results.
Figure 2.
Tornado diagrams for one-way deterministic sensitivity analysis. U_K_PS, utility of pembrolizumab in progression survival state; C_K, cost of pembrolizumab; U_K_PFS, utility of pembrolizumab in progression-free survival state; U_C_PS, utility of chemotherapy in progression survival state; U_C_PFS, utility of chemotherapy in progression-free survival state; C_C_PS, cost of chemotherapy in progression survival state; C_K_PS, cost of pembrolizumab in progression survival state; C-BSC, cost of best support care; C_C, cost of chemotherapy; C_pneumonitis, cost of treatment in pneumonitis; C_O, cost of nivolumab; C_Followup, cost of follow-up; C_anemia, cost of treatment in anemia; C_VEGF, cost of bevacizumab; C_ALK, cost of crizotinib; C_neutropenia, cost of treatment in neutropenia; C_EGFR, cost of epidermal growth factor receptor inhibitors.
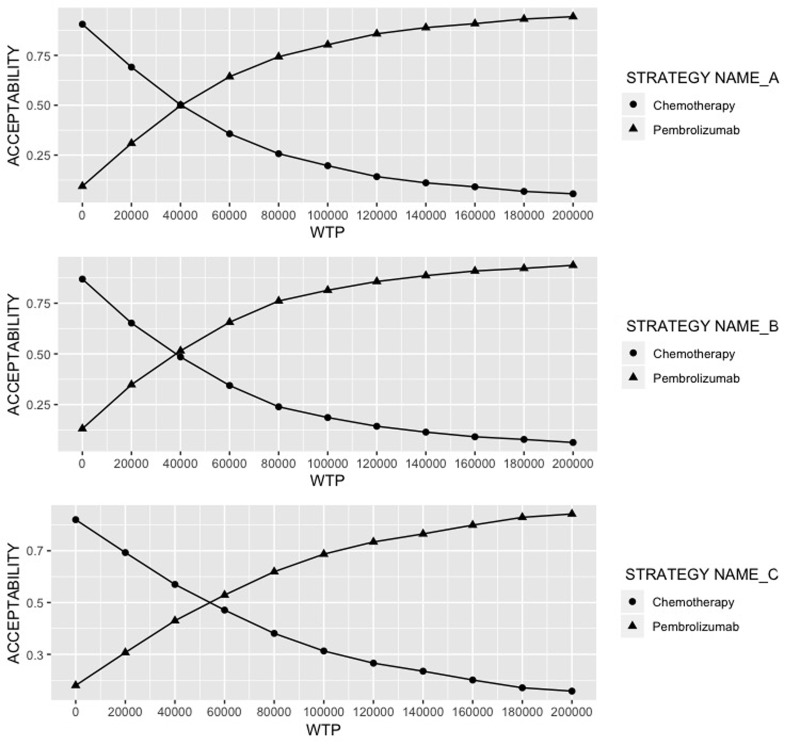
The PSA results are exhibited as the cost-effectiveness acceptability curve (Fig. 3). At the WTP of $180,000/QALY, pembrolizumab showed an obvious advantage over chemotherapy to be cost-effective, with 92.1%, 92.4%, and 82.3% of the 1,000 Monte Carlo simulations favored the outcome in patients of PD-L1 ≥50%, ≥20%, and ≥1%, respectively.
Figure 3.
Cost-effectiveness acceptability curve. WTP, willingness-to-pay.
DISCUSSION
The sharp rise in the cost of ICIs is a worldwide puzzle, and recently a lot of research has focused on their cost-effectiveness. Pembrolizumab, as a first-line therapy for metastatic NSCLC with PD-L1 expression level ≥50%, was previously evaluated in the US, France, and the UK11,16,24. The analyses results revealed that in the US and France, pembrolizumab was considered a cost-effective strategy for treatment-naive NSCLC patients with PD-L1 expression ≥50%11,24, yet it was not a cost-saving regimen in the UK16. However, this research only covered the population with PD-L1 expression ≥50% due to the clinical data limitation in KEYNOTE-02425. KEYNOTE-0426, a further and enlarged research of KEYNOTE-024, revealed the different survival benefits for three levels of PD-L1 expression (≥50%, ≥20%, and ≥1%). Given the different PFS and OS benefits associated with different PD-L1 expression levels, we specifically constructed a Markov model to evaluate the cost-effectiveness of pembrolizumab as first-line treatment for metastatic NSCLC patients in three levels of PD-L1 expression.
Base case results indicated that, compared to platinum-based chemotherapy, pembrolizumab provided the ICERs of $47,596/QALY, $47,184/QALY, and $68,061/QALY at the PD-L1 expression levels of ≥50%, ≥20%, and ≥1%, respectively. All ICERs were below the WTP threshold of $180,000/QALY, which means the additional benefits associated with pembrolizumab is worth the extra costs in the context of the US health care system. Generally, the ICER decreased with the increase in PD-L1 expression along with more therapy efficiency. The higher the PD-L1 expression, the better the cost-effectiveness. We found that the ICER of expression level ≥50% close to that of expression ≥20% may be due to the fact that in KEYNOTE-042 trial, the numbers of patients with expression level ≥50% accounted for nearly 75% in the expression level ≥20% group.
One-way DSA revealed that the main driver of the incremental cost was the utility value of pembrolizumab in the PS state. Because PS state occupies a longer duration in patients’ overall survival time, a slight change in the utility value in the PS state could cause a significant impact on ICERs. However, the relationship between the ICERs and WTP remained unchanged in either lower or upper values of key parameters. According to the PSA results, the possibility of pembrolizumab over chemotherapy to be cost-effective at a WTP of $180,000/QALY was 92.1%, 92.4%, and 82.3% in patients with PD-L1 ≥50%, ≥20%, ≥1%, respectively. Even if the WTP was lowered to $100,100/QALY, pembrolizumab also surpasses chemotherapy to be cost saving. It signifies that pembrolizumab monotherapy is worth being widely used in clinics when both price and efficacy are taken into account simultaneously.
There are some strengths in our study. One major strength is that we used the most novel survival data from a randomized controlled trial, which directly compared pembrolizumab monotherapy with chemotherapy as first-line treatment for metastatic NSCLC patients with a median follow-up time of 12.8 months, especially 25.2 month for patients with PD-L1 expression level ≥50%. Nevertheless, the earlier pharmacoeconomic evaluations of pembrolizumab simulated patient survival based on the data with a shorter follow-up time of 11.2 months11 and thus the model assumptions would have an impact on the results to a greater extent. These long-term data are likely to confirm robustness of extrapolations and reduce uncertainty around results. Another strength is that we evaluated the cost-effectiveness of pembrolizumab with different expression levels of PD-L1, and the outcomes suggest that the acceptability among patients with three levels of expression of PD-L1 presented a similar result. Furthermore, our analysis chose two survival functions, Weibull and log-logistics distributions, that agree well with clinical data in previous publications15. By comparison of two fitted curves we selected Weibull distribution due to its higher adjusted R 2, and the predicted data were in agreement with the trial results.
Some limitations also need to be pointed out, which are mainly governed by data availability and model assumptions. First, intangible cost such as pain and fear were included in utility weights; hence, they were not computed separately. Indirect costs, which were wage losses caused by suspension of school, work, early death, etc., are difficult to estimate in most cases, so we did not incorporate this into the model. Although there was little difference between the regimens in intangible and indirect costs, further cost components need to be considered for an adequate assessment. Second, pseudoindividual patient data that could improve estimate accuracy of mean survival time were unavailable, and thus we adopted a Markov model that relied on the aggregate survival data reported from the clinical trial. We compared the overall survival curves simulated by the model with the KM curves of clinical trial using R software and confirmed that two groups of curves coincided. The cohort study results showed that the median overall survival of three PD-L1 expression levels predicted by this model, which was 20.5, 17.2, and 18.0 months for pembrolizumab and 12.7, 13.5, and 12.7 months for chemotherapy, did accord with the data of clinical trial KEYNOTE-042. Furthermore, utility value reflects the HR-QoL, which is a subjective experience and may vary greatly among individuals, so it is difficult to provide an accurate value. In our study, utility values were derived from published literature16, which directly evaluate the utility of patients using pembrolizumab from the real world. The utility values were suitable for our study, and one-way DSA demonstrated that the variation of utility values did not qualitatively change the result.
CONCLUSION
From the perspective of US health care system, pembrolizumab is estimated to be cost-effective compared to chemotherapy for previously untreated NSCLC patients with different expression levels of PD-L1 at a WTP threshold of $180,000/QALY.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support of the Natural Science Foundation of Fujian Province (2019J01446) and Startup Fund for Scientific Research, Fujian Medical University (Grant No.: 2018QH1091). All authors contributed to data analysis, drafting, and revising the article, and provided final approval of the version to be published.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Fitzmaurice C, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, Aremu O. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon H, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW, Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(4):504–35. [DOI] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY-S, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 6. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee M, Turner DC, Felip E, Lena H, Cappuzzo F, Horn L, Garon EB, Hui R, Arkenau H-T, Gubens MA, Hellmann MD, Dong D, Li C, Mayawala K, Freshwater T, Ahamadi M, Stone J, Lubiniecki GM, Zhang J, Im E, De Alwis DP, Kondic AG, Fløtten Ø. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(7):1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 9. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016;387(10027):1540–50. [DOI] [PubMed] [Google Scholar]
- 10. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Ejzykowicz F, Burke T. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin. 2019;35(7):1–16. [DOI] [PubMed] [Google Scholar]
- 11. Chouaid C, Bensimon L, Clay E, Millier A, Levy-Bachelot L, Huang M, Levy P. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer Amst Neth. 2019;127:44–52. [DOI] [PubMed] [Google Scholar]
- 12. Georgieva M, da Silveira Nogueira Lima JP, Aguiar P, de Lima Lopes G, Haaland B. Cost-effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer 2018;124:248–54. [DOI] [PubMed] [Google Scholar]
- 13. Aguiar PN, Haaland B, Park W, San Tan P, Del Giglio A, de Lima Lopes G. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 2018;4(8):1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latimer N. NICE DSU technical support document 14: Survival analysis for economic evaluations alongside clinical trials—Extrapolation with patient-level data. 2011. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf [PubMed]
- 15. Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: A cost-effectiveness analysis. JAMA Oncol. 2019;5(4):E1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu X, Hay JW. First-line pembrolizumab in PD-L1 positive non-small-cell lung cancer: A cost-effectiveness analysis from the UK health care perspective. Lung Cancer Amst Neth. 2018;123:166–71. [DOI] [PubMed] [Google Scholar]
- 17. Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Burke T. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21(12):1191–205. [DOI] [PubMed] [Google Scholar]
- 18. Sarfaty M, Leshno M, Gordon N, Moore A, Neiman V, Rosenbaum E, Goldstein DA. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol. 2018;73(4):628–34. [DOI] [PubMed] [Google Scholar]
- 19. Ellis PM, Leighl NB, Hirsh V, Reaume MN, Blais N, Wierzbicki R, Sadrolhefazi B, Gu Y, Liu D, Pilz K, Chu Q. A randomized, open-label phase ii trial of volasertib as monotherapy and in combination with standard-dose pemetrexed compared with pemetrexed monotherapy in second-line treatment for non–small-cell lung cancer. Clin Lung Cancer 2015;16(6):457–65. [DOI] [PubMed] [Google Scholar]
- 20. Department of Health, NHS Reference Costs 2015–2016. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016. 2017.
- 21. Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: A modelling study. Palliat Med. 2015;29(10):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu B, Chen H, Shen J, Ye M. Cost-effectiveness of adding Rh-endostatin to first-line chemotherapy in patients with advanced non-small-cell lung cancer in China. Clin Ther. 2011;33(10):1446–55. [DOI] [PubMed] [Google Scholar]
- 23. Durand J-P, Madelaine I, Scotté F. Recommandations pour la prévention et le traitement des nausées et vomissements induits par la chimiothérapie. Bull Cancer 2009;96:951–60. [DOI] [PubMed] [Google Scholar]
- 24. Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, Velcheti V. Cost effectiveness of Pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. PharmacoEconomics 2017;35(8):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]