Abstract
This study aimed to investigate the efficacy, safety, and prognostic factors of drug-eluting beads transarterial chemoembolization (DEB-TACE) in treating Chinese patients with liver cancer. A total of 367 liver cancer patients from 24 medical centers were consecutively enrolled in this multiple-center, prospective cohort study, including 275 hepatocellular carcinoma (HCC) cases, 37 intrahepatic cholangiocarcinoma (ICC) cases, and 55 secondary liver cancer cases. All the patients received CalliSpheres® DEB-TACE treatment. Treatment response, overall survival (OS), change of liver function, and adverse events (AEs) were assessed. DEB-TACE treatment achieved 19.9% complete response (CR) and 79.6% objective response rate (ORR), with mean OS of 384 days [95% confidence interval (CI): 375–393 days]. CR and ORR were both higher in HCC patients compared with primary ICC patients and secondary liver cancer patients, while no difference was discovered in OS. Portal vein invasion was an independent risk factor for CR, while portal vein invasion, previous conventional TACE (cTACE) treatment, and abnormal blood creatinine (BCr) were independent risk factors for ORR. In addition, largest nodule size ≥5.0 cm, abnormal albumin (ALB), and abnormal total bilirubin (TBIL) independently correlated with unfavorable OS. Most liver function indexes were recovered to baseline levels at 1–3 months after DEB-TACE. Common AEs were pain, fever, vomiting, and nausea; most of them were at mild grade. CalliSpheres® DEB-TACE is efficient and well tolerated in Chinese liver cancer patients. Portal vein invasion, previous cTACE treatment, largest nodule size, abnormal BCr, ALB, and TBIL correlate with worse prognosis independently.
Key words: Drug-eluting beads transarterial chemoembolization (DEB-TACE), Liver cancer, Efficacy, Safety, Prognosis
INTRODUCTION
Liver cancer, as the second leading cause of cancer-related deaths in men worldwide, is a critical life-threatening disease to public health, which mainly consists of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). A Global Cancer Statistics report discloses that an estimated 782,500 new liver cancer cases and 745,500 deaths occurred all over the world during 2012. Eastern Asia, Southeastern Asia, and Northern African rank as the top 3 in both incidence and mortality of liver cancer1. As in China, a previous report illuminated that China alone contributes to nearly 50% of total liver cancer cases (360,000) and related deaths (350,000)2.
Despite the progress in the field of early diagnosis of liver cancer such as novel tumor biomarkers and advanced imaging technology, more than 50% of patients are still diagnosed at the intermediate to advanced stage, which makes curative therapies inaccessible (including surgery, transplantation, and radiofrequency ablation)3,4. The prognosis of these patients is poor: it is reported that the 1-, 3-, and 5-year overall survival (OS) of patients at the intermediate stage is 80%, 65%, and 50%, respectively, while that of OS patients at the advanced stage is 29%, 16%, and 8%, respectively5. In addition, up to 70% of patients who have undergone curative therapies relapse within 5 years, and a great amount of these patients are not candidates for secondary surgery therapy6–8. Thus, exploration of novel and more effective treatment options is of great need for a number of liver cancer patients.
Transarterial chemoembolization (TACE), as an interventional therapy that selectively embolizes arteries feeding the tumors and releases chemotherapeutic drugs, is recommended as the first-line treatment for intermediate stage HCC by various guidelines such as the Barcelona Clinic Liver Cancer (BCLC) tumor staging and management guidelines and the European Association for the Study of the Liver (EASL) guidelines9–11. In ICC patients who are ineligible to receive curative treatments, TACE treatment is also increasingly accepted as a good option12,13. Although conventional TACE (cTACE) has been used to treat liver cancer patients for over 20 years, its severe systematic toxicity due to iodipin diffusion, lack of calibrated operative techniques, and diversified embolization agents limit its application in recent years. Thus, drug-eluting beads (DEB)-TACE, as a novel drug delivery embolization system, is introduced to further improve the patients’ outcomes and reduce the systemic toxicity due to its higher intratumoral chemotherapeutic drug concentration and reduced drug infiltration into systemic circulation14,15.
In Europe and North America, DEB-TACE has been increasingly explored and a great deal of reports have been published, although the results are controversial and the sample sizes are small. Some studies disclose that DEB-TACE does not improve efficacy or safety compared with cTACE, while some recent meta-analysis reports reveal that DEB-TACE illustrates a better treatment response, progression-free survival (PFS), OS, and less common adverse events (AEs)5,16–18. However, in Asia, only a few studies with small sample size population have been revealed, and the investigation of DEB-TACE treatment for liver cancer with a large sample size population in China is needed.
Thus, we conducted this multiple-center, prospective cohort study that enrolled 367 liver cancer patients to investigate the efficacy, safety, and prognostic factors of DEB-TACE treatment in Chinese patients with liver cancer.
MATERIALS AND METHODS
Study Design
The CTILC study (Chinese CalliSpheres® Transarterial chemoembolization In Liver Cancer) was a multicenter, prospective cohort study that aimed to investigate the efficacy and safety of DEB-TACE treatment by CalliSpheres® in Chinese patients and to improve the prognosis and patients’ satisfaction, which included 24 medical centers in China, and it was registered on clinicaltrials.gov with registry No. NCT03317483. This study was approved by the ethics committee of Zhejiang Cancer Hospital. All the patients or their legal guardians provided written informed consent. This study was conducted according to the Declaration of Helsinki.
Patients
A total of 367 liver cancer patients who were about to receive DEB-TACE treatment from November 12, 2015 to November 4, 2016 were included in the CTILC study. The inclusion criteria were as follows: (1) diagnosed as primary HCC, primary ICC, or secondary liver cancer confirmed by pathological findings, clinical features, and radiographic examinations according to the American Association for the Study of the Liver Diseases (AASLD) guidelines; (2) age above 18 years; (3) about to receive DEB-TACE treatment with CalliSpheres® according to clinical needs and patients’ willingness; (4) able to be followed up regularly; and (5) life expectancy above 12 months. The exclusions were as follows: (1) history of liver transplantation; (2) history of hematological malignances; (3) severe hepatic failure or renal failure; (4) contraindication for angiography, embolization procedure, or artery puncture; (5) patients with cognitive impairment or unable to understand the study consents; and (6) women in the gestation or lactation period. The inclusion and exclusion criteria were also available on clinicaltrials.gov with registry No. NCT03317483.
Baseline Data Collection
Detailed baseline characteristics of 367 patients were recorded including demographic features, treatment history, clinical features, and biochemical indexes. The detailed information included the following: (1) demographic features: age and gender; (2) previous history: hepatitis B (HB), hepatitis C (HC), and drinking history; (3) clinical features: histology (primary HCC, primary ICC, and secondary liver cancer), tumor distribution (multifocal disease or unifocal disease), tumor location (left liver, right liver, and bilobar), largest nodule size, portal vein invasion, hepatic vein invasion, Eastern Cooperative Oncology Group (ECOG) performance status, Child–Pugh stage, BCLC stage, and cycles of DEB-TACE treatment (one cycle and two or more cycles); (4) blood routine indexes: white blood cell (WBC), red blood cell (RBC), absolute neutrophil count (ANC), hemoglobin (Hb), and platelet (PLT); (5) liver function indexes: albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP); (6) kidney function indexes: blood creatinine (BCr) and blood urea nitrogen (BUN); (7) tumor markers: alpha fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen199 (CA199); (8) chemoembolization reagents and combination of ordinary embolization agent; and (9) DEB size and loaded drug dosage.
Procedure of DEB-TACE Treatment
DEB-TACE was performed in all patients according to the clinical conditions and patients’ willingness. The beads used in DEB-TACE were CalliSpheres® Beads (CB) (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) in our study, the diameter of which ranges from 100 to 300 μm or from 300 to 500 μm. Before the initiation of the operation, the beads were loaded with anthracycline (60–80 mg) for patients with primary liver cancer, and irinotecan (50–100 mg) for patients with secondary liver cancer. Anthracycline 80 mg or irinotecan 100 mg were defined as normal drug dosage, while anthracycline <80 mg or irinotecan <100 mg was defined as low drug dosage. First, the chemoembolization reagent was dissolved in a solution with concentration of 20 mg/ml and extracted into a 10-ml injector for further use. Second, the CB was prepared using the method as follows: one bottle of CB was shaken up and then the bead suspension was extracted into a 20-ml injector, which stood at room temperature (RT) for 5 min, and the liquid supernatant was pushed out and thus the beads were left in the injector. Third, the chemotherapy reagent solution was mixed with the beads using a tee joint, after which the nonionic contrast agent was administered at a ratio of 1:1, and the mixture was placed for 30 min at RT (23–28°C) for further application. If the embolization point was not reached after a bottle of CB was emptied, ordinary embolization agents were used.
The DEB-TACE operation was conducted in the digital subtraction angiography (DSA) room. Under local anesthesia, the hepatic angiography was conducted to detect the tumor-supplying vessels, and a 2.4F microcatheter (Merit Maestro, Merit Medical System Inc., South Jordan, UT, USA) was used for the embolization. The embolization was then started; the microcatheter was inserted to the tumor-supplying vascular led by a microwire, and was stopped after the flow of contrast agent stagnated. Subsequently, 5 min after embolization, a second angiography was conducted to detect whether there were remaining blushed nodules, after which embolization was performed until there were no more blushed tumors. If embolization was no longer needed, the microcatheter was pulled out and hemostasis by compression was conducted.
The punctured wound of the patient was bound up, and all patients postembolization were told to lie on one side and extend the punctured leg for 6 to 12 h.
Patients with postoperative nausea and vomiting were treated with intravenous injection of tropisetron and ondansetron. Pethidine, dexamethasone, and lidocaine were given as analgesic treatment for pain.
Treatment Response Assessment
Treatment response was assessed at 1–3 months after DEB-TACE treatment according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) by enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) examination. The detailed response assessment criteria of mRECIST were as follows: (1) complete response (CR): no existence of arterial enhancement of targeted tumors; (2) partial response (PR): the decrease in diameter of targeted tumor (with arterial enhancement) ≤30%; (3) stable disease (SD): the decrease in diameter of targeted tumor (with arterial enhancement) did not achieved PR or less than progressive disease (PD); and (4) PD: the increase in diameter of the targeted tumor (with arterial enhancement) ≥20% or new tumor existed. Objective response rate (ORR) was calculated and defined as the value of CR plus PR.
OS Assessment
OS was calculated from the time of DEB-TACE operation to the time of patient’s death from any cause. The median follow-up duration was 171 days (range: 38–404), and the last follow-up date was December 28, 2016.
Liver Function Evaluation
Liver function indexes including ALB, TP, TBIL, TBA, ALT, AST, and ALP were recorded before the first DEB-TACE operation, at 1 week after the first DEB-TACE operation, and at 1–3 months after the first DEB-TACE operation, so as to evaluate the influence of DEB-TACE on liver function. It is noted that the analysis of liver function evaluation was based on the treatment records of DEB-TACE (N = 440).
Adverse Events
AEs were recorded during DEB-TACE operation and 1 month after DEB-TACE operation, and the analysis of AEs was based on the treatment records of DEB-TACE (N = 440).
Statistics
Statistical analysis was performed using SPSS 21.0 software (IBM, Armonk, NY, USA) and Microsoft OFFICE 2015 (Microsoft, Redmond, WA, USA). Data were mainly presented as count (%), mean ± standard deviation, or median (25th–75th). Comparison between two groups was determined by chi-square test; comparison between each visit was determined by the McNemar test; and comparison of OS between/among groups was analyzed by the Kaplan–Meier (K-M) curves and log-rank test. Factors affecting CR/ORR achievement were determined by univariate logistic regression analysis, while all factors with p value no more than 0.1 were further detected by multivariate logistic regression analysis. Factors affecting OS were determined by univariate Cox’s proportional hazards regression model analysis, while all factors with p value no more than 0.1 were further detected by multivariate Cox’s proportional hazards regression analysis. A value of p < 0.05 was considered significant.
RESULTS
Study Flow
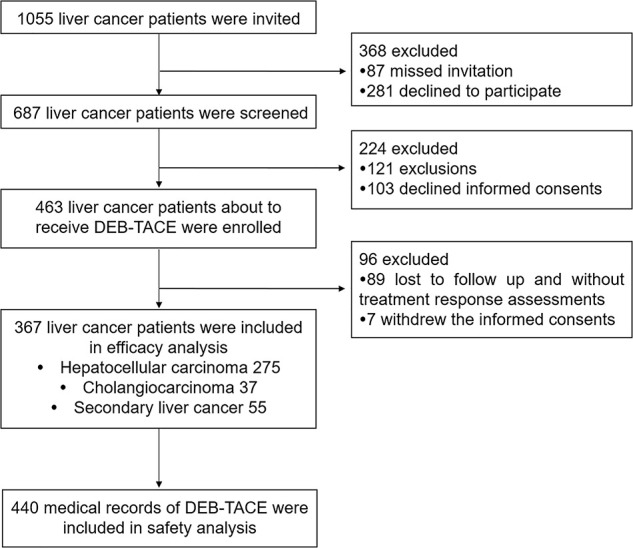
As presented in Figure 1, 1,055 liver cancer patients were invited to participate in this study, among which 368 cases were excluded due to missed invitation (n = 87) or declined to participate (n = 281); the remaining 687 liver cancer patients were screened for eligibility, while 224 cases were excluded including 121 exclusions and 103 who declined informed consent. Thus, 463 liver cancer patients about to receive DEB-TACE treatment were enrolled, while among them 96 were excluded who were lost to follow-up, without treatment response assessment records (n = 89), or withdrawal of informed consent (n = 7). The remaining 367 liver cancer patients were subsequently included in the final efficacy analysis, including 275 HCC cases, 37 ICC cases, and 55 secondary liver cancer cases, and 440 corresponding medical records of DEB-TACE were included in the safety analysis.
Figure 1.
Study flow.
Baseline Characteristics
The mean age of the 367 liver cancer patients was 59.95 ± 11.60 years. There were 286 males (77.9%) and 81 (22.1%) females, and 275 (74.9%), 37 (10.1%), and 55 (15.0%) cases were HCC, ICC, and secondary liver cancer, respectively. There were 244 (66.5%), 103 (28.1%), and 48 (13.1%) cases with multifocal disease, portal vein invasion, and hepatic vein invasion, respectively. There were 200 (54.5%), 127 (34.6%), 30 (8.2%), and 10 (2.7%) cases with ECOG performance scores of 0, 1, 2, and 3; 62 (16.9%) cases received two or more cycles of DEB-TACE treatment, while 305 (83.1%) cases received only one cycle of DEB-TACE treatment. Among the HCC and ICC patients, 261 (83.7%), 49 (15.7%), and 2 (0.6%) cases were at Child–Pugh stages A, B, and C, and 1 (0.3%), 76 (24.4%), 119 (38.1%), 115 (36.9%), and 1 (0.3%) cases were at BCLC 0, A, B, C, and D stages. The other detailed demographic, clinical, biochemical, and treatment history are presented in Table 1.
Table 1.
Baseline Characteristics of 367 Patients With Liver Cancers Who Underwent DEB-TACE Treatment
| Parameters | Patients (N = 367) |
|---|---|
| Age (years) | 59.95 ± 11.60 |
| Gender (female/male) | 286/81 |
| History of HB [n (%)] | 244 (66.5) |
| History of HC [n (%)] | 5 (1.4) |
| History of drink [n (%)] | 153 (41.7) |
| History of cirrhosis [n (%)] | 176 (48.0) |
| Histology | |
| Primary HCC [n (%)] | 275 (74.9) |
| Primary ICC [n (%)] | 37 (10.1) |
| Secondary liver cancer [n (%)] | 55 (15.0) |
| Tumor distribution | |
| Multifocal [n (%)] | 244 (66.5) |
| Unifocal [n (%)] | 123 (33.5) |
| Tumor location | |
| Left liver [n (%)] | 56 (15.3) |
| Right liver [n (%)] | 183 (49.9) |
| Bilobar [n (%)] | 128 (34.9) |
| Largest nodule size (cm) | 4.80 (2.70–8.30) |
| Portal vein invasion [n (%)] | 103 (28.1) |
| Hepatic vein invasion [n (%)] | 48 (13.1) |
| ECOG performance status | |
| 0 [n (%)] | 200 (54.5) |
| 1 [n (%)] | 127 (34.6) |
| 2 [n (%)] | 30 (8.2) |
| 3 [n (%)] | 10 (2.7) |
| Child–Pugh stage (for HCC and ICC, n = 312) | |
| A [n (%)] | 261 (83.7) |
| B [n (%)] | 49 (15.7) |
| C [n (%)] | 2 (0.6) |
| BCLC stage (for HCC and ICC, n = 312) | |
| 0 [n (%)] | 1 (0.3) |
| A [n (%)] | 76 (24.4) |
| B [n (%)] | 119 (38.1) |
| C [n (%)] | 115 (36.9) |
| D [n (%)] | 1 (0.3) |
| Cycles of DEB-TACE treatment | |
| 1 cycle [n (%)] | 305 (83.1) |
| 2 or more cycles [n (%)] | 62 (16.9) |
| Blood routine | |
| WBC (×109 cell/L) | 5.16 (4.00–6.60) |
| RBC (×1012 cell/L) | 4.27 (3.80–4.69) |
| ANC (%) | 61.2 (52.5–69.4) |
| Hb (g/L) | 127 (109–142) |
| PLT (×109 cell/L) | 124 (76–178) |
| Liver function | |
| ALB (g/L) | 39.6 (36.2–43.7) |
| TP (g/L) | 69.4 (64.6–73.8) |
| TBIL (μmol/L) | 14.5 (11.0–22.0) |
| TBA (I/L) | 10.0 (6.0–22.2) |
| ALT (U/L) | 27.0 (19.0–40.0) |
| AST (U/L) | 35.0 (25.0–53.0) |
| ALP (U/L) | 117 (85–164) |
| Kidney function | |
| BCr (μmol/L) | 70.0 (60.0–80.0) |
| BUN (mmol/L) | 4.90 (3.94–6.10) |
| Tumor markers | |
| AFP (μg/L) | 21.5 (3.6–562.2) |
| CEA (μg/L) | 2.9 (1.9–5.1) |
| CA199 (ku/L) | 15.4 (6.6–40.5) |
| Previous treatments | |
| cTACE [n (%)] | 138 (37.6) |
| Surgery [n (%)] | 109 (29.7) |
| Systematic chemotherapy [n (%)] | 46 (12.5) |
| Radiofrequency ablation [n (%)] | 53 (14.4) |
| Targeted therapy [n (%)] | 12 (3.3) |
| DEBs size | |
| 100–300 μm [n (%)] | 354 (96.5) |
| 300–500 μm [n (%)] | 13 (3.5) |
| Drug dosage | |
| Low dose [n (%)] | 34 (9.3) |
| Normal dose [n (%)] | 333 (90.7) |
| Chemoembolization reagents (440 DEB-TACE records) | |
| Anthracyclines [n (%)] | 387 (88.0) |
| Irinotecan [n (%)] | 53 (12.0) |
| Combination of ordinary embolization agent [n (%)] | 116 (31.6) |
Data are presented as mean ± standard deviation, median (25th–75th), or count (%). HB, hepatitis B; HC, hepatic C; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; DEB-TACE, drug-eluting bead transarterial chemoembolization; WBC, white blood cell; RBC, red blood cell; ANC, absolute neutrophil count; Hb, hemoglobin; ALB, albumin; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen199; cTACE, conventional transarterial chemoembolization.
Treatment Response
In 367 DEB-TACE-treated patients, 73 (19.9%) and 219 (59.7%) cases realized CR and PR, respectively, resulting in 292 cases (79.6%) with ORR. Besides, 53 (14.4%) and 22 (6.0%) patients were SD and PD, respectively (Fig. 2A). In patients with PR, 35.2%, 48.4%, and 16.4% cases had necrosis rate ≥80%, 50%–80%, and <50%, respectively (Fig. 2B).
Figure 2.
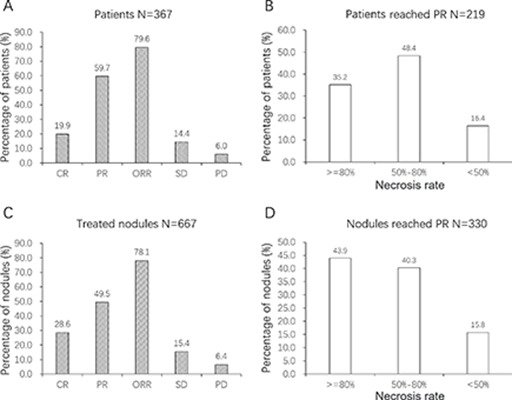
Treatment response of drug-eluting beads transarterial chemoembolization (DEB-TACE) treatment. (A) The complete response (CR), partial response (PR), objective response rate (ORR), stable disease (SD), and progressive disease (PD) within 1–3 months after treatments were 19.9%, 59.7%, 79.6%, 14.4%, and 6.0% in 367 patients. (B) In 219 patients with PR, 35.2%, 48.4%, and 16.4% patients had necrosis rates of ≥80%, 50%–80%, and <50%. (C) In terms of the treated nodules, 28.6%, 49.5%, 78.1%, 15.4%, and 6.4% nodules were CR, PR, ORR, SD, and PD, respectively. (D) In nodules achieving PR, 43.9%, 40.3%, and 15.8% had necrosis rates of ≥80%, 50%–80% and <50%.
As to the corresponding 667 treated nodules, 191 (28.6%) and 330 (49.5%) nodules realized CR and PR, respectively, resulting in 521 nodules (78.1%) with ORR. Besides, 103 (15.4%) and 43 (6.4%) nodules were SD and PD, respectively (Fig. 2C). In nodules achieving PR, 43.9%, 40.3%, and 15.8% had necrosis rates of ≥80%, 50%–80%, and <50% (Fig. 2D).
OS Evaluation
The K-M curve revealed that the mean OS of all patients was 384 days (95% CI: 375–393 days), and 6-month OS was estimated to be 94.6% ± 1.4% (Fig. 3). However, because of the short follow-up duration, the long-term OS rate was not able to be calculated.
Figure 3.
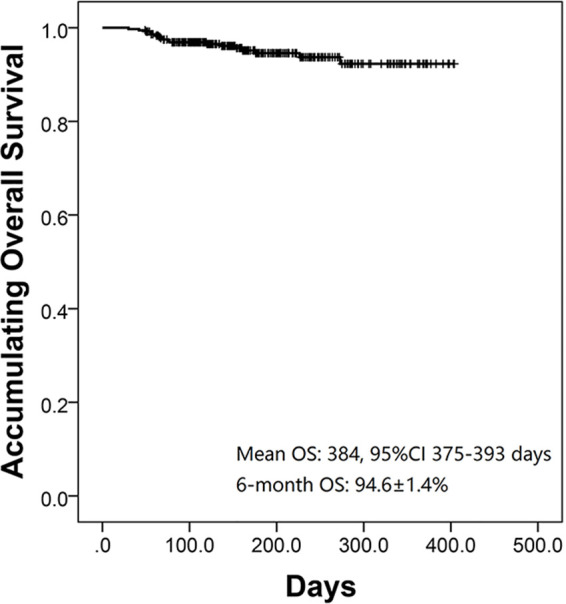
Overall survival (OS) of DEB-TACE treatment. Mean OS of all patients was 384 days [95% confidence interval (CI): 375–393 days], and estimated 6-month OS was 94.6% ± 1.4%.
Comparison of CR by Subgroup Analysis
Subgroup analysis by demographic and clinical characteristics illustrated that primary HCC patients (22.9%) achieved a higher CR rate compared with secondary liver cancer patients (12.7%) and primary ICC patients (8.1%) (p = 0.037) (Table 2). In addition, patients with multifocal disease (p = 0.008), ≥5 cm largest nodule size (p = 0.002), portal vein invasion (p < 0.001), or higher BCLC stage (p = 0.021) had worse CR rate, while patients with history of HB (p = 0.001) achieved better CR rate (Table 2). As to subgroup analysis divided by chemical indexes, we found that patients with abnormal ALB (p = 0.022) or abnormal AST (p = 0.029) realized worse CR rate (Table 3).
Table 2.
Comparison of CR in Subgroups Divided by Demographic and Clinical Characteristics
| Parameters | N | Not CR | CR | p Value |
|---|---|---|---|---|
| Age | 0.091 | |||
| ≥65 years | 121 | 103 (85.1) | 18 (14.9) | |
| <65 years | 246 | 191 (77.6) | 55 (22.4) | |
| Gender | 0.506 | |||
| Male | 286 | 227 (79.4) | 59 (20.6) | |
| Female | 81 | 67 (82.7) | 14 (17.3) | |
| History of HB | 0.001 | |||
| Yes | 244 | 184 (75.4) | 60 (24.6) | |
| No | 123 | 110 (89.4) | 13 (10.6) | |
| History of HC | 0.995 | |||
| Yes | 5 | 4 (80.0) | 1 (20.0) | |
| No | 362 | 290 (80.1) | 72 (19.9) | |
| History of drink | 0.496 | |||
| Yes | 153 | 120 (78.4) | 33 (21.6) | |
| No | 214 | 174 (81.3) | 40 (18.7) | |
| History of cirrhosis | 0.795 | |||
| Yes | 176 | 140 (79.5) | 36 (20.5) | |
| No | 191 | 154 (80.6) | 37 (19.4) | |
| Histology | 0.037 | |||
| Primary HCC | 275 | 212 (77.1) | 63 (22.9) | |
| Primary ICC | 37 | 34 (91.9) | 3 (8.1) | |
| Secondary liver cancer | 55 | 48 (87.3) | 7 (12.7) | |
| Tumor distribution | 0.008 | |||
| Multifocal | 244 | 205 (84.0) | 39 (16.0) | |
| Unifocal | 123 | 89 (72.4) | 34 (27.6) | |
| Tumor location | 0.052 | |||
| Left liver | 56 | 41 (73.2) | 15 (26.8) | |
| Right liver | 183 | 142 (77.6) | 41 (22.4) | |
| Bilobar | 128 | 111 (86.7) | 17 (13.3) | |
| Largest nodule size | 0.002 | |||
| ≥5 cm | 184 | 159 (86.4) | 25 (13.6) | |
| <5 cm | 183 | 135 (73.8) | 48 (26.2) | |
| Portal vein invasion | <0.001 | |||
| Yes | 103 | 95 (92.2) | 8 (7.8) | |
| No | 264 | 199 (75.4) | 65 (24.6) | |
| Hepatic vein invasion | 0.078 | |||
| Yes | 48 | 43 (89.6) | 5 (10.4) | |
| No | 319 | 251 (78.7) | 68 (21.3) | |
| ECOG performance status | 0.058 | |||
| 0 | 200 | 153 (76.5) | 47 (23.5) | |
| 1 | 127 | 103 (81.1) | 24 (18.9) | |
| 2 | 30 | 28 (93.3) | 2 (6.7) | |
| 3 | 10 | 10 (100.0) | 0 (0.0) | |
| Child–Pugh Stage | 0.314 | |||
| A | 261 | 203 (77.8) | 58 (22.2) | |
| B | 49 | 41 (83.7) | 8 (16.3) | |
| C | 2 | 2 (100.0) | 0 (0.0) | |
| BCLC Stage | 0.021 | |||
| 0 | 1 | 1 (100.0) | 0 (0.0) | |
| A | 76 | 50 (65.8) | 26 (34.2) | |
| B | 119 | 98 (82.4) | 21 (17.6) | |
| C | 115 | 96 (83.5) | 19 (16.5) | |
| D | 1 | 1 (100.0) | 0 (0.0) | |
| Cycles of DEB-TACE treatment | 0.908 | |||
| 1 cycle | 305 | 244 (80.0) | 61 (20.0) | |
| 2 or more cycles | 62 | 50 (80.6) | 12 (19.4) | |
| Previous cTACE treatment | 0.491 | |||
| Yes | 138 | 108 (78.3) | 30 (21.7) | |
| No | 229 | 186 (81.2) | 43 (18.8) | |
| Previous surgery | 0.845 | |||
| Yes | 109 | 88 (80.7) | 21 (19.3) | |
| No | 258 | 206 (79.8) | 52 (20.2) | |
| Previous systematic chemotherapy | 0.396 | |||
| Yes | 46 | 39 (84.8) | 7 (15.2) | |
| No | 321 | 255 (79.4) | 66 (20.6) | |
| Previous radiofrequency ablation | 0.091 | |||
| Yes | 53 | 47 (88.7) | 6 (11.3) | |
| No | 314 | 247 (78.7) | 67 (21.3) | |
| Previous targeted therapy | 0.776 | |||
| Yes | 12 | 10 (83.3) | 2 (16.7) | |
| No | 355 | 284 (80.0) | 71 (20.0) | |
| DEBs size | 0.262 | |||
| 100–300 μm | 354 | 282 (79.7) | 72 (20.3) | |
| 300–500 μm | 13 | 12 (92.3) | 1 (7.7) | |
| Drug dosage | 0.427 | |||
| Low dose | 34 | 29 (85.3) | 5 (14.7) | |
| Normal dose | 333 | 265 (79.6) | 68 (20.4) | |
| Combination of ordinary embolization agent | 0.252 | |||
| Yes | 116 | 97 (83.6) | 19 (16.4) | |
| No | 251 | 197 (78.5) | 54 (21.5) |
Data are presented as count with percentage in parentheses. Comparison between two groups was determined by chi-square test. A value of p < 0.05 was considered significant (bold). CR, complete response.
Table 3.
Comparison of CR in Subgroups Divided by Biochemical Indexes
| Parameters | N | Not CR | CR | p Value |
|---|---|---|---|---|
| Blood routine | ||||
| WBC | 0.965 | |||
| Abnormal | 96 | 77 (80.2) | 19 (19.8) | |
| Normal | 270 | 216 (80.0) | 54 (20.0) | |
| RBC | 0.331 | |||
| Abnormal | 138 | 114 (82.6) | 24 (17.4) | |
| Normal | 227 | 178 (78.4) | 49 (21.6) | |
| ANC | 0.070 | |||
| Abnormal | 101 | 87 (86.1) | 14 (13.9) | |
| Normal | 264 | 205 (77.7) | 59 (22.3) | |
| Hb | 0.186 | |||
| Abnormal | 155 | 129 (83.2) | 26 (16.8) | |
| Normal | 210 | 163 (77.6) | 47 (22.4) | |
| PLT | 0.593 | |||
| Abnormal | 145 | 114 (78.6) | 31 (21.4) | |
| Normal | 220 | 178 (80.9) | 42 (19.1) | |
| Liver function | ||||
| ALB | 0.022 | |||
| Abnormal | 143 | 123 (86.0) | 20 (14.0) | |
| Normal | 223 | 170 (76.2) | 53 (23.8) | |
| TP | 0.330 | |||
| Abnormal | 94 | 72 (76.6) | 22 (23.4) | |
| Normal | 272 | 221 (81.3) | 51 (18.8) | |
| TBIL | 0.338 | |||
| Abnormal | 94 | 72 (76.6) | 22 (23.4) | |
| Normal | 271 | 220 (81.2) | 51 (18.8) | |
| TBA | 0.753 | |||
| Abnormal | 126 | 99 (78.6) | 27 (21.4) | |
| Normal | 215 | 172 (80.0) | 43 (20.0) | |
| ALT | 0.762 | |||
| Abnormal | 80 | 65 (81.3) | 15 (18.8) | |
| Normal | 286 | 228 (79.7) | 58 (20.3) | |
| AST | 0.029 | |||
| Abnormal | 145 | 124 (85.5) | 21 (14.5) | |
| Normal | 218 | 166 (76.1) | 52 (23.9) | |
| ALP | 0.140 | |||
| Abnormal | 135 | 113 (83.7) | 22 (16.3) | |
| Normal | 224 | 173 (77.2) | 51 (22.8) | |
| Kidney function | ||||
| BCr | 0.756 | |||
| Abnormal | 43 | 35 (81.4) | 8 (18.6) | |
| Normal | 322 | 257 (79.8) | 65 (20.2) | |
| BUN | 0.069 | |||
| Abnormal | 45 | 41 (91.1) | 4 (8.9) | |
| Normal | 317 | 248 (78.2) | 69 (21.8) | |
| Tumor markers | ||||
| AFP | 0.266 | |||
| Abnormal | 179 | 146 (81.6) | 33 (18.4) | |
| Normal | 172 | 132 (76.7) | 40 (23.3) | |
| CEA | 0.176 | |||
| Abnormal | 87 | 73 (83.9) | 14 (16.1) | |
| Normal | 248 | 191 (77.0) | 57 (23.0) | |
| CA199 | 0.558 | |||
| Abnormal | 103 | 83 (80.6) | 20 (19.4) | |
| Normal | 229 | 178 (77.7) | 51 (22.3) | |
Data are presented as count with percentage in parentheses. Comparison between two groups was determined by chi-square test. A value of p < 0.05 was considered significant (bold).
Analysis of Factors Affecting CR Achievement
Univariate logistic regression analysis showed that history of HB (p = 0.002) and primary HCC (p = 0.015) were correlated with higher possibility of CR achievement, while multifocal disease (p = 0.009), bilobar disease (p = 0.022), largest nodule size ≥5.0 cm (p = 0.003), portal vein invasion (p = 0.001), higher ECOG performance status (p = 0.010), higher BCLC stage (p = 0.008), abnormal ALB (p = 0.024), and abnormal AST (p = 0.031) were associated with lower CR rate (Table 4). All factors with p values below 0.1 in univariate analysis were further included in the multivariate logistic regression analysis, and we found portal vein invasion was an only independent risk factor for CR achievement (p = 0.010) (Table 4).
Table 4.
Factors Affecting CR Achievement by Logistic Regression Model Analysis
| Parameters | Univariate Logistic Regression | Multivariate Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p Value | OR | 95% CI | p Value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥65 years | 0.094 | 0.607 | 0.339 | 1.088 | 0.221 | 0.643 | 0.316 | 1.305 |
| Male | 0.506 | 1.244 | 0.654 | 2.367 | – | – | – | – |
| History of HB | 0.002 | 2.759 | 1.448 | 5.256 | 0.148 | 2.004 | 0.781 | 5.144 |
| History of HC | 0.995 | 1.007 | 0.111 | 9.146 | – | – | – | – |
| History of drink | 0.496 | 1.196 | 0.714 | 2.005 | – | – | – | – |
| History of cirrhosis | 0.795 | 1.070 | 0.641 | 1.787 | – | – | – | – |
| Primary HCC | 0.015 | 2.437 | 1.193 | 4.977 | 0.325 | 1.958 | 0.513 | 7.465 |
| Primary ICC | 0.071 | 0.328 | 0.098 | 1.099 | – | – | – | – |
| Secondary liver cancer | 0.154 | 0.544 | 0.235 | 1.257 | – | – | – | – |
| Multifocal disease | 0.009 | 0.498 | 0.295 | 0.840 | 0.151 | 0.595 | 0.293 | 1.208 |
| Tumor location: left liver | 0.163 | 1.596 | 0.828 | 3.077 | – | – | – | – |
| Tumor location: right liver | 0.230 | 1.371 | 0.819 | 2.297 | – | – | – | – |
| Tumor location: bilobar | 0.022 | 0.500 | 0.277 | 0.904 | 0.316 | 0.686 | 0.328 | 1.434 |
| Largest nodule size ≥5 cm | 0.003 | 0.442 | 0.259 | 0.755 | 0.111 | 0.574 | 0.291 | 1.135 |
| Portal vein invasion | 0.001 | 0.258 | 0.119 | 0.559 | 0.010 | 0.253 | 0.088 | 0.722 |
| Hepatic vein invasion | 0.086 | 0.429 | 0.164 | 1.126 | 0.934 | 0.948 | 0.271 | 3.315 |
| Higher ECOG performance status | 0.010 | 0.581 | 0.385 | 0.877 | 0.489 | 0.836 | 0.503 | 1.390 |
| Higher Child–Pugh Stage | 0.244 | 0.637 | 0.298 | 1.360 | – | – | – | – |
| Higher BCLC Stage | 0.008 | 0.625 | 0.441 | 0.885 | 0.774 | 1.068 | 0.683 | 1.668 |
| Two or more cycles of DEB-TACE treatment | 0.908 | 0.960 | 0.482 | 1.913 | – | – | – | – |
| Previous cTACE treatment | 0.491 | 1.202 | 0.712 | 2.027 | – | – | – | – |
| Previous surgery | 0.845 | 0.945 | 0.537 | 1.663 | – | – | – | – |
| Previous systematic chemotherapy | 0.398 | 0.693 | 0.297 | 1.621 | – | – | – | – |
| Previous radiofrequency ablation | 0.098 | 0.471 | 0.193 | 1.148 | 0.121 | 0.462 | 0.174 | 1.227 |
| Previous targeted therapy | 0.776 | 0.800 | 0.171 | 3.733 | – | – | – | – |
| Higher DEBs size | 0.286 | 0.326 | 0.042 | 2.551 | – | – | – | – |
| Higher drug dosage | 0.424 | 1.488 | 0.555 | 3.988 | – | – | – | – |
| Combination of ordinary embolization agent | 0.253 | 0.715 | 0.401 | 1.272 | – | – | – | – |
| WBC abnormal | 0.965 | 0.987 | 0.550 | 1.770 | – | – | – | – |
| RBC abnormal | 0.332 | 0.765 | 0.445 | 1.315 | – | – | – | – |
| ANC abnormal | 0.072 | 0.559 | 0.296 | 1.054 | 0.379 | 0.722 | 0.349 | 1.492 |
| Hb abnormal | 0.187 | 0.699 | 0.411 | 1.190 | – | – | – | – |
| PLT abnormal | 0.593 | 1.152 | 0.685 | 1.939 | – | – | – | – |
| ALB abnormal | 0.024 | 0.522 | 0.297 | 0.917 | 0.466 | 0.773 | 0.387 | 1.545 |
| TP abnormal | 0.331 | 1.324 | 0.752 | 2.333 | – | – | – | – |
| TBIL abnormal | 0.339 | 1.318 | 0.748 | 2.322 | – | – | – | – |
| TBA abnormal | 0.753 | 1.091 | 0.635 | 1.874 | – | – | – | – |
| ALT abnormal | 0.762 | 0.907 | 0.483 | 1.705 | – | – | – | – |
| AST abnormal | 0.031 | 0.541 | 0.310 | 0.944 | 0.725 | 0.884 | 0.445 | 1.755 |
| ALP abnormal | 0.142 | 0.660 | 0.380 | 1.148 | – | – | – | – |
| BCr abnormal | 0.951 | 0.976 | 0.449 | 2.121 | – | – | – | – |
| BUN abnormal | 0.146 | 0.545 | 0.241 | 1.234 | – | – | – | – |
| AFP abnormal | 0.267 | 0.746 | 0.445 | 1.252 | – | – | – | – |
| CEA abnormal | 0.178 | 0.643 | 0.338 | 1.223 | – | – | – | – |
| CA199 abnormal | 0.558 | 0.841 | 0.471 | 1.501 | – | – | – | – |
Data are presented as p value, OR (odds ratio), and 95% CI (confidence interval). Factors affecting CR achievement were determined by univariate logistic regression analysis, while all factors with p value no more than 0.1 were further detected by multivariate logistic regression analysis. A value of p < 0.05 was considered significant (bold). Child–Pugh stage was scored as 0—A, 1—B, 2—C; BCLC stage was scored as 0—stage 0, 1—stage A, 2—stage B, 3—stage C, 4—stage D. The logistic analysis was performed based on these definitions.
Comparison of ORR by Subgroup Analysis
Subgroup analysis by demographic and clinical characteristics disclosed that primary HCC patients (83.6%) achieved an elevated ORR compared with primary ICC patients (67.6%) and secondary liver cancer patients (67.3%) (p = 0.004) (Table 5). In addition, patients with multifocal disease (p = 0.005), portal vein invasion (p = 0.010), or previous systematic chemotherapy (p = 0.010) had worse ORR, while patients with history of HB (p = 0.001) achieved better ORR (Table 5).
Table 5.
Comparison of ORR in Subgroups Divided by Baseline Characteristics
| Parameters | N | Not ORR | ORR | p Value |
|---|---|---|---|---|
| Age | 0.368 | |||
| ≥65 years | 121 | 28 (23.1) | 93 (76.9) | |
| <65 years | 246 | 47 (19.1) | 199 (80.9) | |
| Gender | 0.863 | |||
| Male | 286 | 59 (20.6) | 227 (79.4) | |
| Female | 81 | 16 (19.8) | 65 (80.2) | |
| History of HB | 0.001 | |||
| Yes | 244 | 38 (15.6) | 206 (84.4) | |
| No | 123 | 37 (30.1) | 86 (69.9) | |
| History of HC | 0.981 | |||
| Yes | 5 | 1 (20.0) | 4 (80.0) | |
| No | 362 | 74 (20.4) | 288 (79.6) | |
| History of drink | 0.649 | |||
| Yes | 153 | 33 (21.6) | 120 (78.4) | |
| No | 214 | 42 (19.6) | 172 (80.4) | |
| History of cirrhosis | 0.071 | |||
| Yes | 176 | 29 (16.5) | 147 (83.5) | |
| No | 191 | 145 (75.9) | 46 (24.1) | |
| Histology | 0.004 | |||
| Primary HCC | 275 | 45 (16.4) | 230 (83.6) | |
| Primary ICC | 37 | 12 (32.4) | 25 (67.6) | |
| Secondary liver cancer | 55 | 18 (32.7) | 37 (67.3) | |
| Tumor distribution | 0.005 | |||
| Multifocal | 244 | 60 (24.6) | 184 (75.4) | |
| Unifocal | 123 | 15 (12.2) | 108 (87.8) | |
| Tumor location | 0.267 | |||
| Left liver | 56 | 11 (19.6) | 45 (80.4) | |
| Right liver | 183 | 32 (17.5) | 151 (82.5) | |
| Bilobar | 128 | 32 (25.0) | 96 (75.0) | |
| Largest nodule size | 0.918 | |||
| ≥5 cm | 184 | 38 (20.7) | 146 (79.3) | |
| <5 cm | 183 | 37 (20.2) | 146 (79.8) | |
| Portal vein invasion | 0.010 | |||
| Yes | 103 | 30 (29.1) | 73 (70.9) | |
| No | 264 | 45 (17.0) | 219 (83.0) | |
| Hepatic vein invasion | 0.221 | |||
| Yes | 48 | 13 (27.1) | 35 (72.9) | |
| No | 319 | 62 (19.4) | 257 (80.6) | |
| ECOG performance status | 0.489 | |||
| 0 | 200 | 40 (20.0) | 160 (80.0) | |
| 1 | 127 | 25 (19.7) | 102 (80.3) | |
| 2 | 30 | 6 (20.0) | 24 (80.0) | |
| 3 | 10 | 4 (40.0) | 6 (60.0) | |
| Child–Pugh Stage | 0.087 | |||
| A | 261 | 47 (18.0) | 214 (82.0) | |
| B | 49 | 10 (20.4) | 39 (79.6) | |
| C | 2 | 0 (0.0) | 2 (100.0) | |
| BCLC Stage | 0.173 | |||
| 0 | 1 | 0 (0.0) | 1 (100.0) | |
| A | 76 | 12 (15.8) | 64 (84.2) | |
| B | 119 | 20 (16.8) | 99 (83.2) | |
| C | 115 | 25 (21.7) | 90 (78.3) | |
| D | 1 | 0 (0.0) | 1 (100.0) | |
| Cycles of DEB-TACE treatment | 0.356 | |||
| 1 cycle | 305 | 65 (21.3) | 240 (78.7) | |
| 2 or more cycles | 62 | 10 (16.1) | 52 (83.9) | |
| Previous cTACE treatment | 0.069 | |||
| Yes | 138 | 35 (25.4) | 103 (74.6) | |
| No | 229 | 40 (17.5) | 189 (82.5) | |
| Previous surgery | 0.181 | |||
| Yes | 109 | 27 (24.8) | 82 (75.2) | |
| No | 258 | 48 (18.6) | 210 (81.4) | |
| Previous systematic chemotherapy | 0.010 | |||
| Yes | 46 | 16 (34.8) | 30 (65.2) | |
| No | 321 | 59 (18.4) | 262 (81.6) | |
| Previous radiofrequency ablation | 0.424 | |||
| Yes | 53 | 13 (24.5) | 40 (75.5) | |
| No | 314 | 62 (19.7) | 252 (80.3) | |
| Previous targeted therapy | 0.074 | |||
| Yes | 12 | 0 (0.0) | 12 (100.0) | |
| No | 355 | 75 (21.1) | 280 (78.9) | |
| DEBs size | 0.101 | |||
| 100–300 μm | 354 | 70 (19.8) | 284 (80.2) | |
| 300–500 μm | 13 | 5 (38.5) | 8 (61.5) | |
| Drug dosage | 0.360 | |||
| Low dose | 34 | 9 (26.5) | 25 (73.5) | |
| Normal dose | 333 | 66 (19.8) | 267 (80.2) | |
| Combination of ordinary embolization agent | 0.190 | |||
| Yes | 116 | 19 (16.4) | 97 (83.6) | |
| No | 251 | 56 (22.3) | 195 (77.7) |
Data are presented as count with percentage in parentheses. Comparison between two groups was determined by chi-square test. A value of p < 0.05 was considered significant (bold). ORR, objective response rate.
As to subgroup analysis divided by chemical indexes, only patients with abnormal CA199 presented with worse ORR (p = 0.015), while no difference between/among other subgroups was observed (Table 6).
Table 6.
Comparison of ORR in Subgroups Divided by Biochemical Indexes
| Parameters | N | Not ORR | ORR | p Value |
|---|---|---|---|---|
| Blood routine | ||||
| WBC | 0.280 | |||
| Abnormal | 96 | 16 (16.7) | 80 (83.3) | |
| Normal | 270 | 59 (21.9) | 211 (78.1) | |
| RBC | 0.132 | |||
| Abnormal | 138 | 34 (24.6) | 104 (75.4) | |
| Normal | 227 | 41 (18.1) | 186 (81.9) | |
| ANC | 0.515 | |||
| Abnormal | 101 | 23 (22.8) | 78 (77.2) | |
| Normal | 264 | 52 (19.7) | 212 (80.3) | |
| Hb | 0.628 | |||
| Abnormal | 155 | 30 (19.4) | 125 (80.6) | |
| Normal | 210 | 45 (21.4) | 165 (78.6) | |
| PLT | 0.459 | |||
| Abnormal | 145 | 27 (18.6) | 118 (81.4) | |
| Normal | 220 | 48 (21.8) | 172 (78.2) | |
| Liver function | ||||
| ALB | 0.729 | |||
| Abnormal | 143 | 28 (19.6) | 115 (80.4) | |
| Normal | 223 | 47 (21.1) | 176 (78.9) | |
| TP | 0.502 | |||
| Abnormal | 94 | 17 (18.1) | 77 (81.9) | |
| Normal | 272 | 58 (21.3) | 214 (78.7) | |
| TBIL | 0.926 | |||
| Abnormal | 94 | 19 (20.2) | 75 (79.8) | |
| Normal | 271 | 56 (20.7) | 215 (79.3) | |
| TBA | 0.852 | |||
| Abnormal | 126 | 23 (18.3) | 103 (81.7) | |
| Normal | 215 | 41 (19.1) | 174 (80.9) | |
| ALT | 0.453 | |||
| Abnormal | 80 | 14 (17.5) | 66 (82.5) | |
| Normal | 286 | 61 (21.3) | 225 (78.7) | |
| AST | 0.991 | |||
| Abnormal | 145 | 30 (20.7) | 115 (79.3) | |
| Normal | 218 | 45 (20.6) | 173 (79.4) | |
| ALP | 0.261 | |||
| Abnormal | 135 | 32 (23.7) | 103 (76.3) | |
| Normal | 224 | 42 (18.8) | 182 (81.3) | |
| Kidney function | ||||
| BCr | 0.089 | |||
| Abnormal | 43 | 14 (32.6) | 29 (67.4) | |
| Normal | 322 | 61 (18.9) | 261 (81.1) | |
| BUN | 0.063 | |||
| Abnormal | 45 | 11 (24.4) | 34 (75.6) | |
| Normal | 317 | 61 (19.2) | 256 (80.8) | |
| Tumor markers | ||||
| AFP | 0.163 | |||
| Abnormal | 179 | 30 (16.8) | 149 (83.2) | |
| Normal | 172 | 39 (22.7) | 133 (77.3) | |
| CEA | 0.152 | |||
| Abnormal | 87 | 22 (25.3) | 65 (74.7) | |
| Normal | 248 | 45 (18.1) | 203 (81.9) | |
| CA199 | 0.015 | |||
| Abnormal | 103 | 29 (28.2) | 74 (71.8) | |
| Normal | 229 | 38 (16.6) | 191 (83.4) | |
Data are presented as count with percentage in parentheses. Comparison between two groups was determined by chi-square test. A value of p < 0.05 was considered significant.
Analysis of Factors Affecting ORR Achievement
Univariate and multivariate regression analyses were also performed to analyze the factors affecting ORR, which revealed that history of HB (p = 0.001) and primary HCC (p = 0.001) were correlated with increased ORR, while secondary liver cancer (p = 0.016), multifocal disease (p = 0.006), portal vein invasion (p = 0.011), previous systematic chemotherapy (p = 0.012), abnormal BCr (p = 0.031), and abnormal CA199 (p = 0.016) were correlated with decreased ORR (Table 7). Furthermore, portal vein invasion (p = 0.011), previous cTACE treatment (p = 0.006), and abnormal BCr (p = 0.038) were independent risk factors for ORR achievement (Table 7).
Table 7.
Factors Affecting ORR Achievement by Logistic Regression Model Analysis
| Parameters | Univariate Logistic Regression | Multivariate Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p Value | ORR | 95% CI | p Value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥65 years | 0.378 | 0.788 | 0.465 | 1.338 | – | – | – | – |
| Male | 0.863 | 0.947 | 0.511 | 1.756 | – | – | – | – |
| History of HB | 0.001 | 2.332 | 1.390 | 3.915 | 0.083 | 1.948 | 0.917 | 4.137 |
| History of HC | 0.981 | 1.028 | 0.113 | 9.333 | – | – | – | – |
| History of drink | 0.649 | 0.888 | 0.532 | 1.482 | – | – | – | – |
| History of cirrhosis | 0.112 | 1.522 | 0.907 | 2.556 | – | – | – | – |
| Primary HCC | 0.001 | 2.473 | 1.441 | 4.426 | 0.312 | 1.747 | 0.592 | 5.161 |
| Primary ICC | 0.060 | 0.492 | 0.234 | 1.031 | 0.888 | 1.085 | 0.349 | 3.377 |
| Secondary liver cancer | 0.016 | 0.459 | 0.244 | 0.865 | – | – | – | – |
| Multifocal disease | 0.006 | 0.426 | 0.231 | 0.787 | 0.104 | 0.563 | 0.282 | 1.126 |
| Tumor location: left liver | 0.873 | 1.060 | 0.519 | 2.165 | – | – | – | – |
| Tumor location: right liver | 0.163 | 1.439 | 0.862 | 2.431 | – | – | – | – |
| Tumor location: bilobar | 0.114 | 0.658 | 0.392 | 1.106 | – | – | – | – |
| Largest nodule size ≥5 cm | 0.918 | 0.974 | 0.586 | 1.617 | – | – | – | – |
| Portal vein invasion | 0.011 | 0.500 | 0.294 | 0.852 | 0.011 | 0.444 | 0.238 | 0.828 |
| Hepatic vein invasion | 0.223 | 0.650 | 0.324 | 1.301 | – | – | – | – |
| Higher ECOG performance status | 0.425 | 0.876 | 0.632 | 1.213 | – | – | – | – |
| Higher Child–Pugh stage | 0.987 | 1.006 | 0.514 | 1.968 | – | – | – | – |
| Higher BCLC stage | 0.276 | 0.814 | 0.562 | 1.179 | – | – | – | – |
| Two or more cycles of DEB-TACE treatment | 0.358 | 1.408 | 0.679 | 2.923 | – | – | – | – |
| Previous cTACE treatment | 0.071 | 0.623 | 0.373 | 1.041 | 0.006 | 0.411 | 0.217 | 0.776 |
| Previous surgery | 0.182 | 0.694 | 0.406 | 1.187 | – | – | – | – |
| Previous systematic chemotherapy | 0.012 | 0.422 | 0.216 | 0.825 | 0.748 | 0.855 | 0.330 | 2.219 |
| Previous radiofrequency ablation | 0.426 | 0.757 | 0.382 | 1.501 | – | – | – | – |
| Previous targeted therapy | 0.999 | – | – | – | – | – | – | – |
| Higher DEBs size | 0.112 | 0.394 | 0.125 | 1.242 | – | – | – | – |
| Higher drug dosage | 0.362 | 1.456 | 0.649 | 3.268 | – | – | – | – |
| Combination of ordinary embolization agent | 0.192 | 1.466 | 0.825 | 2.604 | – | – | – | – |
| WBC abnormal | 0.281 | 1.398 | 0.760 | 2.572 | – | – | – | – |
| RBC abnormal | 0.133 | 0.674 | 0.403 | 1.127 | – | – | – | – |
| ANC abnormal | 0.516 | 0.832 | 0.477 | 1.449 | – | – | – | – |
| Hb abnormal | 0.628 | 1.136 | .678 | 1.906 | – | – | – | – |
| PLT abnormal | 0.460 | 1.220 | 0.720 | 2.065 | – | – | – | – |
| ALB abnormal | 0.729 | 1.097 | 0.650 | 1.851 | – | – | – | – |
| TP abnormal | 0.503 | 1.228 | 0.674 | 2.237 | – | – | – | – |
| TBIL abnormal | 0.926 | 1.028 | 0.574 | 1.842 | – | – | – | – |
| TBA abnormal | 0.852 | 1.055 | 0.599 | 1.858 | – | – | – | – |
| ALT abnormal | 0.454 | 1.278 | 0.672 | 2.430 | – | – | – | – |
| AST abnormal | 0.991 | 0.997 | 0.594 | 1.675 | – | – | – | – |
| ALP abnormal | 0.262 | 0.743 | 0.442 | 1.249 | – | – | – | – |
| BCr abnormal | 0.031 | 0.470 | 0.237 | 0.932 | 0.038 | 0.429 | 0.193 | 0.954 |
| BUN abnormal | 0.949 | 1.024 | 0.497 | 2.108 | – | – | – | – |
| AFP abnormal | 0.165 | 1.456 | 0.857 | 2.475 | – | – | – | – |
| CEA abnormal | 0.154 | 0.655 | 0.366 | 1.171 | – | – | – | – |
| CA199 abnormal | 0.016 | 0.508 | 0.292 | 0.882 | 0.101 | 0.599 | 0.324 | 1.106 |
Data are presented as p value, OR, and 95% CI. Factors affecting ORR achievement were determined by univariate logistic regression analysis. All factors with p value no more than 0.1 were further detected by multivariate logistic regression analysis. A value of p < 0.05 was considered significant (bold). Child–Pugh stage was scored as 0—A, 1—B, 2—C; BCLC stage was scored as 0—stage 0, 1—stage A, 2—stage B, 3—stage C, 4—stage D. The logistic analysis was performed based on these definitions.
Comparison of OS by Subgroup Analysis Through K-M Curves
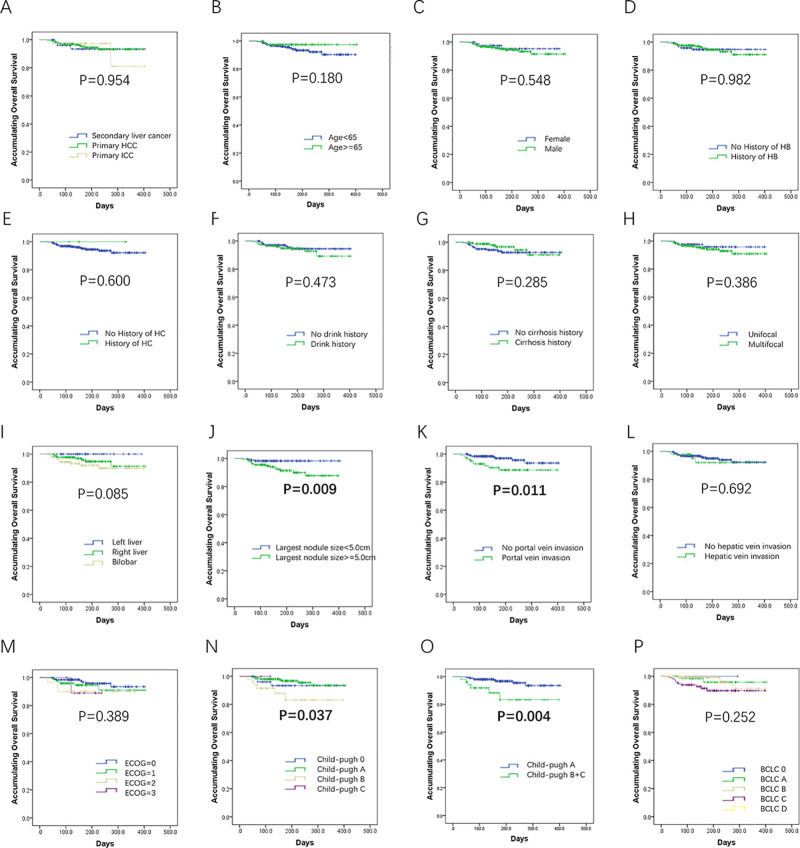
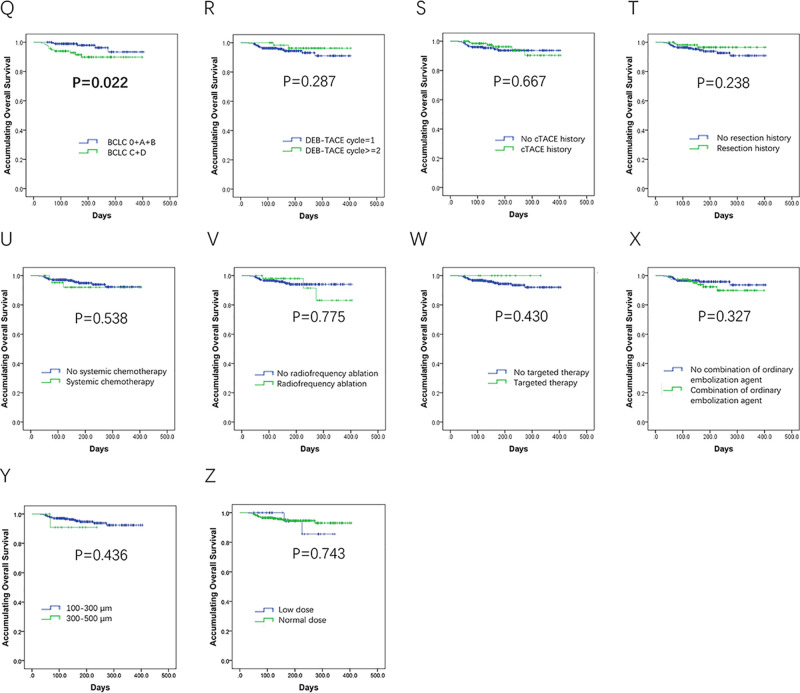
The K-M curves and log-rank test were used to compare the OS between/among subgroups, and we found no difference of OS among primary HCC patients, primary ICC patients, and secondary liver cancer patients (p = 0.954) (Fig. 4A). Patients with largest nodule size ≥5 cm (p = 0.009) (Fig. 4J), portal vein invasion (p = 0.011) (Fig. 4K), Child–Pugh stage B + C (p = 0.004) (Fig. 4O), or BCLC stage C + D (p = 0.022) (Fig. 4Q) presented worse OS. No other difference was discovered between/among the remaining subgroups (Fig. 4B–I, L, M, and R–X).
Figure 4.
Above and facing page. Comparison of OS by demographic and clinical parameters. No difference of OS among primary hepatocellular carcinoma (HCC) patients, primary intrahepatic cholangiocarcinoma (ICC) patients, and secondary liver cancer patients was observed (A), and patients with largest nodule size ≥5 cm (J), portal vein invasion (K), Child–Pugh stage B + C (O), or Barcelona Clinic Liver Cancer (BCLC) stage C + D (Q) had worse OS. No other difference was discovered between/among the remaining subgroups (B–I, L–N, P, R–Z). Comparison of OS between/among groups was determined by Kaplan–Meier (K-M) curves and log-rank test. A value of p < 0.05 was considered significant.
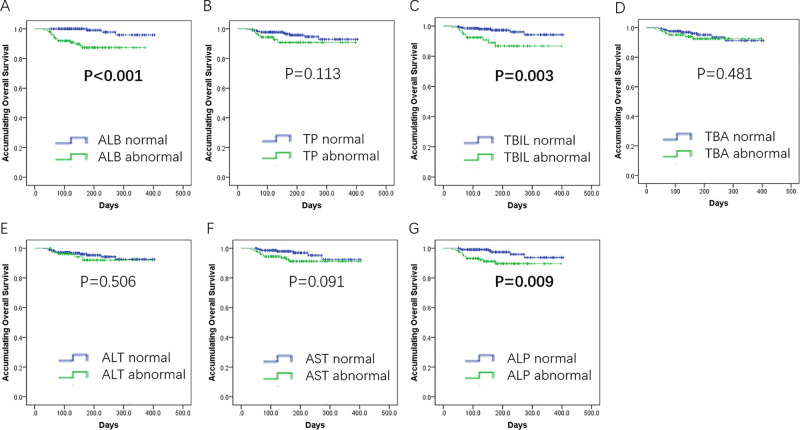
As to subgroup analysis of OS divided by baseline liver function indexes, patients with abnormal ALB (p < 0.001) (Fig. 5A), abnormal TBIL (p = 0.003) (Fig. 5C), or abnormal ALT (p = 0.009) (Fig. 5G) were observed to have unfavorable OS. No difference was discovered between subgroups divided by other liver function indexes (Fig. 5B and D–F).
Figure 5.
Subgroup analysis of OS by baseline liver function indexes. Patients with albumin (ALB) abnormal (A), total bilirubin (TBIL) abnormal (C), or alanine aminotransferase (ALT) abnormal (G) were observed to have unfavorable OS. No difference was discovered between subgroups divided by other liver function indexes (B, D–F). Comparison of OS between/among groups was analyzed by K-M curves and log-rank test. A value of p < 0.05 was considered significant.
Analysis of Factors Affecting OS
Univariate Cox’s proportional hazards regression model was performed to analyze the factors affecting OS, which disclosed that largest nodule size ≥5.0 cm (p = 0.017), portal vein invasion (p = 0.016), higher Child–Pugh stage (p = 0.001), abnormal RBC (p = 0.021), abnormal ALB (p = 0.001), abnormal TBIL (p = 0.005), abnormal ALP (p = 0.014), and abnormal BCr (p = 0.004) were correlated with shorter OS (Table 8). All factors with p value no more than 0.1 were further detected by multivariate Cox’s proportional hazards regression analysis, and we found largest nodule size ≥5.0 cm (p = 0.048), abnormal ALB (p = 0.044), and abnormal TBIL (p = 0.048) were independent predictive factors for unfavorable OS (Table 8).
Table 8.
Cox’s Proportional Hazards Regression Model Analysis of Factors Affecting OS
| Parameters | Univariate Cox’s Regression | Multivariate Cox’s Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥65 years | 0.191 | 0.437 | 0.126 | 1.512 | – | – | – | – |
| Male | 0.550 | 1.459 | 0.422 | 5.042 | – | – | – | – |
| History of HB | 0.982 | 1.012 | 0.380 | 2.696 | – | – | – | – |
| History of HC | 0.727 | 0.049 | 0.000 | 1156342 | – | – | – | – |
| History of drink | 0.475 | 1.400 | 0.556 | 3.528 | – | – | – | – |
| History of cirrhosis | 0.516 | 0.730 | 0.283 | 1.886 | – | – | – | – |
| Primary HCC | 0.772 | 0.858 | 0.306 | 2.409 | – | – | – | – |
| Primary ICC | 0.928 | 1.070 | 0.246 | 4.658 | – | – | – | – |
| Secondary liver cancer | 0.781 | 1.192 | 0.345 | 4.119 | – | – | – | – |
| Multifocal disease | 0.391 | 1.628 | 0.535 | 4.951 | – | – | – | – |
| Tumor location: left liver | 0.248 | 0.038 | 0.000 | 9.696 | – | – | – | – |
| Tumor location: right liver | 0.702 | 0.834 | 0.329 | 2.115 | – | – | – | – |
| Tumor location: bilobar | 0.083 | 2.280 | 0.898 | 5.788 | 0.683 | 1.301 | 0.367 | 4.609 |
| Largest nodule size ≥5 cm | 0.017 | 4.538 | 1.311 | 15.703 | 0.048 | 6.003 | 1.015 | 35.500 |
| Portal vein invasion | 0.016 | 3.146 | 1.241 | 7.975 | 0.684 | 1.304 | 0.363 | 4.686 |
| Hepatic vein invasion | 0.693 | 1.284 | 0.371 | 4.437 | – | – | – | – |
| Higher ECOG performance status | 0.088 | 1.551 | 0.936 | 2.570 | 0.741 | 1.125 | 0.558 | 2.269 |
| Higher Child–Pugh Stage | 0.001 | 1.688 | 1.226 | 2.324 | 0.318 | 1.603 | 0.635 | 4.047 |
| Higher BCLC stage | 0.058 | 2.081 | 0.975 | 4.442 | 0.644 | 1.256 | 0.479 | 3.292 |
| Two or more cycles of DEB-TACE treatment | 0.299 | 0.457 | 0.104 | 2.002 | – | – | – | – |
| Previous cTACE treatment | 0.668 | 0.807 | 0.303 | 2.151 | – | – | – | – |
| Previous Surgery | 0.248 | 0.481 | 0.139 | 1.665 | – | – | – | – |
| Previous systematic chemotherapy | 0.541 | 1.472 | 0.426 | 5.089 | – | – | – | – |
| Previous radiofrequency ablation | 0.775 | 1.198 | 0.347 | 4.140 | – | – | – | – |
| Previous targeted therapy | 0.601 | 0.047 | 0.000 | 4443.2 | – | – | – | – |
| Higher DEBs size | 0.448 | 2.190 | 0.289 | 16.587 | – | – | – | – |
| Higher drug dosage | 0.744 | 0.783 | 0.180 | 3.406 | – | – | – | – |
| Combination of ordinary embolization agent | 0.331 | 1.586 | 0.626 | 4.021 | – | – | – | – |
| WBC abnormal | 0.079 | 2.300 | 0.907 | 5.833 | 0.680 | 1.311 | 0.361 | 4.758 |
| RBC abnormal | 0.021 | 3.232 | 1.194 | 8.748 | 0.559 | 1.423 | 0.435 | 4.651 |
| ANC abnormal | 0.076 | 2.367 | 0.913 | 6.135 | 0.784 | 0.809 | 0.178 | 3.668 |
| Hb abnormal | 0.358 | 1.563 | 0.603 | 4.055 | – | – | – | – |
| PLT abnormal | 0.906 | 1.059 | 0.410 | 2.738 | – | – | – | – |
| ALB abnormal | 0.001 | 9.077 | 2.620 | 31.442 | 0.044 | 4.667 | 1.046 | 20.829 |
| TP abnormal | 0.122 | 2.119 | 0.819 | 5.481 | – | – | – | – |
| TBIL abnormal | 0.005 | 3.783 | 1.491 | 9.597 | 0.048 | 3.891 | 1.010 | 14.985 |
| TBA abnormal | 0.483 | 1.395 | 0.550 | 3.536 | – | – | – | – |
| ALT abnormal | 0.508 | 1.417 | 0.505 | 3.979 | – | – | – | – |
| AST abnormal | 0.099 | 2.220 | 0.860 | 5.733 | 0.743 | 0.786 | 0.187 | 3.313 |
| ALP abnormal | 0.014 | 3.424 | 1.284 | 9.128 | 0.545 | 0.998 | 0.992 | 1.004 |
| BCr abnormal | 0.004 | 4.174 | 1.573 | 11.076 | 0.086 | 3.225 | 0.848 | 12.257 |
| BUN abnormal | 0.056 | 2.701 | 0.976 | 7.474 | 0.470 | 1.604 | 0.444 | 5.790 |
| AFP abnormal | 0.591 | 1.303 | 0.496 | 3.425 | – | – | – | – |
| CEA abnormal | 0.601 | 1.326 | 0.461 | 3.817 | – | – | – | – |
| CA199 abnormal | 0.116 | 2.196 | 0.824 | 5.855 | – | – | – | – |
Data are presented as p value, HR (hazards ratio), and 95% CI. Factors affecting overall survival (OS) were determined by univariate Cox’s proportional hazards regression model analysis. All factors with p value no more than 0.1 were further detected by multivariate Cox’s proportional hazards regression analysis. A value of p < 0.05 was considered significant (bold). Child–Pugh stage was scored as 0—A, 1—B, 2—C; BCLC stage was scored as 0—stage 0, 1—stage A, 2—stage B, 3—stage C, 4—stage D. The Cox’s proportional hazards analysis was performed based on these definitions.
Change of Liver Function Before and After DEB-TACE Treatment
There were a total of 440 DEB-TACE procedure records included in the liver function analysis. As presented in Table 9, the percentage of patients with abnormal ALB, abnormal TP, abnormal TBIL, abnormal ALT, and abnormal AST were increased at 1 week after the DEB-TACE procedure compared to baseline (all p < 0.001), while all recovered at 1–3 months after the DEB-TACE procedure (all p > 0.05). The percentage of patients with abnormal TBA did not change at 1 week (p = 0.124) or 1–3 months (p = 0.433) after DEB-TACE procedure compared with baseline. As to percentage of patients with abnormal ALP, it remained the same at 1 week (p = 0.088), while it increased at 1–3 months (p < 0.001) after the DEB-TACE procedure compared with baseline.
Table 9.
Liver Function Before and After DEB-TACE Treatment (440 DEB-TACE Records)
| Baseline | 1 Week After DEB-TACE | 1–3 Months After DEB-TACE | p Value* | p Value† | |
|---|---|---|---|---|---|
| ALB abnormal | 177/438 (40.4) | 208/379 (54.9) | 168/399 (42.1) | <0.001 | 0.619 |
| TP abnormal | 114/438 (26.0) | 192/379 (50.7) | 94/399 (23.6) | <0.001 | 0.409 |
| TBIL abnormal | 104/437 (23.8) | 179/379 (47.2) | 100/399 (25.1) | <0.001 | 0.671 |
| TBA abnormal | 145/407 (35.6) | 118/352 (33.5) | 143/373 (38.3) | 0.124 | 0.433 |
| ALT abnormal | 93/438 (21.2) | 232/379 (61.2) | 93/399 (23.3) | <0.001 | 0.471 |
| AST abnormal | 166/435 (38.2) | 243/373 (65.1) | 162/397 (40.8) | <0.001 | 0.435 |
| ALP abnormal | 169/431 (39.2) | 168/372 (45.2) | 211/396 (53.3) | 0.088 | <0.001 |
Data are presented as count/total with percent in parentheses. Comparison among groups was determined by chi-square test. A value of p < 0.05 was considered significant. Analysis was based on 440 DEB-TACE records. *p Value of liver function-related biochemical indexes of patients from baseline to 1 week posttreatment. #p Value of liver function-related biochemical indexes of patients from baseline to 1–3 months posttreatment.
For patients with abnormal liver function indexes at baseline, we also analyzed the values of liver function indexes at each visit, which elucidated that most of the liver function indexes were improved at 1–3 months after DEB-TACE treatment (Table 10).
Table 10.
Liver Function Indexes Before and After DEB-TACE Treatment in Patients With Abnormal Liver Function-Related Indexes at Baseline
| Baseline | 1 Week After DEB-TACE | 1–3 Months After DEB-TACE | p Value* | p Value† | |
|---|---|---|---|---|---|
| ALB (g/L) (n = 177) | 34.3 (32.1–37.0) | 31.4 (28.2–34.1) | 35.1 (31.5–38.0) | <0.001 | 0.128 |
| TP (g/L) (n = 114) | 61.2 (58.6–63.9) | 58.6 (52.5–63.6) | 66.3 (60.4–72.6) | <0.001 | <0.001 |
| TBIL (μmol/L) (n = 104) | 29.0 (24.5–40.0) | 37.0 (26.9–55.2) | 27.0 (17.0–42.0) | <0.001 | 0.073 |
| TBA (I/L) (n = 145) | 29.0 (15.8–49.4) | 18.9 (9.3–33.4) | 21.9 (12.0–43.0) | <0.001 | 0.017 |
| ALT (U/L) (n = 93) | 59.5 (48.0–84.8) | 82.0 (49.0–175.8) | 34.0 (23.3–58.3) | <0.001 | <0.001 |
| AST (U/L) (n = 166) | 59.0 (48.0–88.0) | 84.5 (44.3–188.3) | 54.5 (36.6–84.5) | <0.001 | 0.005 |
| ALP (U/L) (n = 169) | 195 (157–272) | 212 (145–298) | 228 (149–334) | 0.818 | 0.055 |
Data are presented as median (25th–75th). Comparison between each visit was determined by signed Wilcoxon rank sum test. A value of p < 0.05 was considered significant (bold). Analysis was based on DEB-TACE records.
p Value of liver function-related biochemical indexes of patients from baseline to 1 week posttreatment.
p Value of liver function-related biochemical indexes of patients from baseline to 1–3 months posttreatment.
AE Evaluation
The most common AEs during the DEB-TACE operation were pain (58.9%), fever (36.6%), vomiting (17.0%), and nausea (13.6%), as shown in Table 11. One month after the operation, 30.0%, 21.1%, 10.5%, and 9.5% cases had pain, fever, vomiting, and nausea, respectively; 1.4% patients presented with bone marrow toxicity; and 0.9% patients had epichrosis (Table 11). In addition, most of the AEs were at mild grade.
Table 11.
Safety Profiles of DEB-TACE Treatment (440 DEB-TACE Records)
| Parameters | N (%) |
|---|---|
| During DEB-TACE operation | |
| Pain | 259 (58.9) |
| Fever | 161 (36.6) |
| Vomiting | 75 (17.0) |
| Nausea | 60 (13.6) |
| Others | 33 (7.5) |
| 1 month after DEB-TACE operation | |
| Pain | 132 (30.0) |
| Fever | 93 (21.1) |
| Vomiting | 46 (10.5) |
| Nausea | 42 (9.5) |
| Bone marrow toxicity | 6 (1.4) |
| Epichrosis | 4 (0.9) |
| Others | 8 (1.8) |
Data are presented as count with percent in parentheses. Description was based on 440 DEB-TACE records.
DISCUSSION
The CTILC study was a multiple-center, prospective cohort study investigating DEB-TACE treatment in liver cancer patients, with the largest sample size in China as well as in the world. Through the analysis of 367 liver cancer patients who underwent DEB-TACE, we found the following. (1) DEB-TACE treatment achieved 19.9% CR and 79.6% ORR, with a mean OS of 384 days (95% CI: 375–393 days) totally. (2) CR and ORR were increased in primary HCC patients (22.9% and 83.6%) compared with primary ICC patients (8.1% and 67.6%) and secondary liver cancer patients (12.7% and 67.3%), while no difference was discovered in OS among the three groups. (3) Portal vein invasion was observed to be an independent risk factor for CR achievement, while abnormal portal vein invasion, previous cTACE treatment, and BCr were independent risk factors for ORR achievement. In addition, largest nodule size ≥5.0 cm and abnormal ALB and TBIL were independently correlated with unfavorable OS. (4) Liver function indexes were worsened at 1 week after the procedure but recovered at 1–3 months after the procedure. (5) Common AEs were pain, fever, vomiting, and nausea, most of which were at mild grade.
Liver cancer, as one of the most severe cancers worldwide due to its poor prognosis on account of late diagnosis and heterogeneity, is a life-threatening malignance, especially in China19,20. Although the incidence and mortality rates are both declining in historically high-rate areas, including China, benefiting from the reduction in HBV infection through improved hygiene and sanitation, there are still 360,000 new liver cancer cases and 350,000 liver cancer-related deaths every year in China1,2. In order to further improve the prognosis of liver cancer, several prognostic factors are proposed such as BCLC tumor staging, TNM staging, and Okuda staging by various organizations worldwide, and all of them recommend the TACE treatment as the first-line option for intermediate stage liver cancer patients who are not suitable for curative therapies including surgery7,20–22. In the real-world condition, TACE is also applied in early stage liver cancer patients as bridge therapy for surgery or transplantation, and for some patients who are unsuitable for curative treatment due to physical condition, surgical contraindication, and so on22,23. As to advanced stage liver cancer patients, several studies also disclose that TACE could improve patients’ OS combined with sorafenib24. This evidence suggests TACE is a popular treatment option for most liver cancer patients.
As a new-generation technology for TACE, DEB-TACE is designed to load chemotherapeutic drugs on microspheres; when injected into the tumor, it will release the drug sustainably for more than 2 weeks25. Several studies have reported that the application of DEB-TACE increases the intratumor drug concentration and reduces systemic drug diffusion, which results in better treatment efficacy and less AEs14,26. A randomized controlled trial (RCT) conducted in Italy found that DEB-TACE (n = 89) did not improve treatment response (according to CR and ORR) compared with cTACE (n = 88), and the OS was similar between the two groups16. Another retrospective cohort study in Germany analyzed 76 DEB-TACE-treated and 174 cTACE-treated HCC patients, which revealed no difference in OS between the two groups17. In Asia, a retrospective cohort study in Korea disclosed that DEB-TACE treatment (n = 60) increased treatment response and improved time to progression compared with cTACE (n = 69)27. Another retrospective cohort performed in Malaysia also disclosed that DEB-TACE (n = 45) increased both treatment response and OS compared with cTACE (n = 34)28. These suggest the superiority of DEB-TACE compared to cTACE is still controversial based on different populations, varied spheres, and distinct patients’ conditions. However, several recent meta-analysis reviews illuminated that DEB-TACE is superior to cTACE in liver cancer patients with regard to treatment response, OS, and AEs5,18,29.
A single-center, phase II trial enrolled 20 unresectable HCC patients from 2005 to 2007 in the US to initially investigate the efficacy of DEB-TACE treatment, which discovered that no patients achieved CR and only 10% patients realized PR by DEB-TACE treatment, respectively. This result might be due to the lack of experience in the early days30. Until recently, DEB-TACE was observed to realize CR ranging from 17% to 71%, respectively, and ORR ranging from 50% to 90% in treating liver cancer patients, respectively16,17,27–29,31–33. A previous RCT study in Italy disclosed that DEB-TACE (n = 89) achieved 51.7% CR and 92.1% ORR at 1 month posttreatment, respectively, and 58.2% CR and 74.7% ORR at 3 months posttreatment in HCC patients, respectively16. A retrospective study in Korea revealed that 55.0% and 81.6% of HCC patients achieve CR and ORR by DEB-TACE treatment (n = 60), respectively27. Another retrospective cohort conducted in Malaysia illuminated that 17% and 41% patients realized CR and ORR by DEB-TACE treatment, respectively (n = 45). However, these studies were with small samples, and a study investigating efficacy of DEB-TACE treatment with a large sample size exceeding 200 patients has not been reported. Partially in line with these studies, we enrolled 367 liver cancer patients and found that DEB-TACE achieved 19.9% CR and 79.6% ORR in Chinese patients, respectively. The mild difference in the clinical outcomes among studies may mainly result from different eligibility, bead application, chemotherapeutic drug, population, sample size, technical skills, and so on. For example, in the present study, we not only enrolled primary HCC patients but also ICC patients and secondary liver cancer patients, which would reduce the response rate. In addition, previous studies mostly enrolled patients at the early to intermediate stage16,27, while we did not restrict the stage of patients; thus early, intermediate, and advanced stage patients were all included.
As to OS, a previous cohort study in Germany discovered that DEB-TACE treatment (n = 76) attained a median OS of 369 days (95% CI: 310–589 days) in unresectable HCC patients17. Another cohort study carried out in Italy presented that DEB-TACE treatment achieved a median OS of 39 months (95% CI: 32–47 months) in early/intermediate stage HCC patients (n = 145)31. As to studies in Asia, a study conducted in Korea observed that mean OS is 32.2 ± 1.9 months in DEB-TACE-treated patients (n = 60)27. Another study in Hong Kong enrolled HCC patients (n = 143) who underwent DEB-TACE with 9-year follow-up, which disclosed that DEB-TACE treatment realized a median OS of 12.53 months34. These suggest that DEB-TACE could achieve a good OS, although the exact OS differs according to the different designs of different studies. In this present study, we found that mean OS of all liver cancer patients was 384 days (95% CI: 375–393 days), and 6-month OS was estimated to be 94.6% ± 1.4%. However, because of the short follow-up duration (median follow-up duration was 171 days), the long-term OS was not able to be calculated, which was essential to further assess the efficacy of DEB-TACE treatment in Chinese patients.
Most of the previous studies mainly investigated the efficacy of DEB-TACE in primary HCC patients17,31,34, while in our study we also included patients with primary ICC and secondary liver cancer patients, which illuminated that HCC patients benefited more than ICC patients as well as secondary liver cancer patients according to CR and ORR by DEB-TACE treatment. This might result from the worse disease condition and less responsiveness to anticancer treatment in ICC patients and secondary liver cancer patients compared with HCC patients. However, we found 8.1% patients achieved CR and 67.6% patients achieved ORR in DEB-TACE-treated ICC patients, which was an exciting result since the treatment response of ICC is low by various kinds of treatments. TACE (as a palliative option) in treating ICC patients who are ineligible to receive curative treatments has become increasingly accepted, and there is growing evidence for the ability of TACE to achieve high tumor response rate12,13. As to secondary liver cancer, few studies have been reported about the efficacy of DEB-TACE application, and we found 12.7% and 67.3% secondary liver cancer patients reached CR and ORR, respectively, by DEB-TACE, which provided another therapeutic option for secondary liver patients, especially for colorectal cancer, gastric cancer, and pancreatic cancer with a high rate of liver metastasis35–37. However, no difference of OS was discovered, and the mean OS of all liver cancer patients was 384 days (95% CI: 375–393 days); this might result from the short follow-up duration that the difference of OS had not yielded yet.
On account of diversified patients’ conditions, clinicopathological features, and biochemical properties, the prognosis of liver cancer patients who underwent DEB-TACE differs greatly among each study16,17,27–33. Thus, it is of great necessity to seek novel and convincing biomarkers to predict treatment benefits both in treatment response and survival in liver cancer patients treated by DEB-TACE, so as to better optimize the efficacy of DEB-TACE treatment and improve the prognosis of liver cancer patients. A prospective historical cohort study in France analyzed 172 HCC patients with 315 tumor nodules treated by DEB-TACE, which revealed that tumor size ≥5 cm and location in segment 1 or 4 independently correlated with higher CR38. Another retrospective cohort study in America with 33 nodules in 32 HCC patients treated by DEB-TACE illustrated that tumor heterogeneity and tumor enhancement greater than 50% predicted better CR39. In addition, a phase II trial initially investigating the efficacy of DEB-TACE in America (patients between 2005 and 2007) illuminated that patients with more advanced disease condition such as Child–Pugh B, ECOG performance status 1, bilobar disease, and recurrent disease could enforce a lower objective response to DEB-TACE compared to cTACE30. However, the comprehensive analysis of various factors affecting treatment response to DEB-TACE treatment has not been reported yet. In our study, we included 45 parameters consisting of demographic features, previous history, clinical features, blood routine indexes, liver function indexes, kidney function indexes, and tumor markers into the univariate and multivariate logistic regression analysis, which disclosed that portal vein invasion was an independent risk factor for CR achievement, while portal vein invasion, previous cTACE treatment, and abnormal BCr were independent risk factors for ORR achievement. These might result from the following reasons. (1) Patients with portal vein invasion present with severe disease conditions that lack response to not only DEB-TACE treatment but also cTACE, systemic chemotherapy, and so on. Previous studies have indicated that DEB-TACE combined with sorafenib improves patients’ prognosis with portal vein invasion compared to sorafenib alone24. (2) Previous cTACE history increases refractory possibility of DEB-TACE and causes sustainable liver damage, which reduces the possibility of treatment response40. (3) Acute kidney injury (AKI) is a critical issue related to TACE treatment that is correlated with worse prognosis in patients, and baseline renal dysfunction (BCr abnormal) increases the risk of renal injury and, thus, decreases the prognosis41.
As to factors affecting survival profiles in DEB-TACE-treated patients, a previous cohort study in Germany suggested that higher Child–Pugh stage and portal vein invasion are independent factors for predicting unfavorable OS17. Another RCT study in Italy disclosed that higher ECOG performance score, elevated serum ALB, and multifocal disease correlated with worse OS independently in DEB-TACE- and cTACE-treated HCC patients16. A retrospective cohort study in Italy revealed that multifocal disease and maximum tumor diameter above 3.5 cm are associated with shorter OS31. In line with previous findings16,17,31, we found that largest nodule size ≥5.0 cm, abnormal ALB, and abnormal TBIL were independently correlated with unfavorable OS, which might result from the following: (1) largest nodule size has been demonstrated to be a critical risk factor for patients’ survival due to its correlation with severe disease condition, less treatment options, and reduced treatment response to various treatments, and (2) baseline liver dysfunction (ALB abnormal and TBIL abnormal) correlates with worse residual liver function after DEB-TACE treatment, which greatly influences the followed treatment, physical recovery, and the overall prognosis in liver cancer patients.
Notably, previous studies have suggested that within a certain range, the smaller size of DEBs and drug dosage yield better tumor response and fewer AEs10,14. However, the conclusion is not widely accepted because of limited samples. Thus, we further analyzed these issues and observed that higher DEB size showed a weak trend to be correlated with poor treatment CR, ORR, and OS, but without statistical analysis; higher drug dosage did not correlate with CR, ORR, or OS (p = 0.743) (Fig. 4Z). Furthermore, we added these two factors in the regression analysis as well, which showed that they did not correlate with CR, ORR, or OS either. The possible explanations were as follows: (1) a great majority of patients received 100- to 300-μm DEBs with normal drug dosage, while only very few patients received 300- to 500-μm DEBs or lower dosage, so this unbalance greatly decreased the statistical power; (2) higher drug dosage represented that the patients were with worse tumor burden, which objectively led to worse outcomes.
Liver function of patients with liver cancers is assessed as standard process before the initiation of resections, transplantation, chemotherapy, radiotherapy, or TACE treatments. Accordingly, it is also examined posttreatment as a method to evaluate the treatment-related liver damage, including the DEB-TACE treatment. Until now, the literature indicated a controversy in liver deterioration of patients after DEB-TACE compared with cTACE or other treatments. For instance, more hepatic artery injuries were observed in patients after DEB-TACE, while some other studies revealed that the incidence of liver dysfunction do not vary between DEB-TACE and cTACE treatments42,43. To further establish whether DEB-TACE is acceptable regarding postprocedure liver damage, we performed the assessment of liver function by evaluating the related laboratory indexes in this large-scale population study and found that most indexes aggravated rapidly but recovered at 1–3 months after the treatment, and one index did not worsen at 1 week or at 1–3 months. Those results suggest the liver function may not be permanently damaged by DEB-TACE, on account of the long-term recovery observed in our study. Part of the technique in DEB-TACE may contribute to relatively favorable outcome of liver function postoperation. It is reported that the selective arterial approach, which is often applied in the DEB-TACE procedure, is beneficial for reducing the injury of tumor-free liver tissue and correlates with decreased incidence of cholecystitis, which associates with worse liver function44,45. However, among all the seven indexes that were evaluated in our study, only the percentage of abnormal ALP increased at 1–3 months. The elevation of ALP level indicates liver diseases in clinical practice, such as bile duct obstruction46. In a prospective cohort study, the ALP level increased in patients with less favorable treatment responses after TACE47. In our study, the explanation of the elevated ALP level could be (1) the ALP level was high at baseline, which might cause the recovery to be more difficult compared with those who had lower level of ALP at baseline; (2) the ALP level could be increased due to the utilization of adriamycin drug, which is illuminated to be able to cause liver damage48,49.
DEB-TACE was designed and developed not only for optimizing the efficacy of TACE treatment but also for eliminating the toxicity and AEs50. Emerging reports revealed a relatively satisfactory safety profile of DEB-TACE, which demonstrates a similar or even decreased incidence of treatment-related toxicity compared with cTACE18,27,51. A prospective randomized study illustrated that the doxorubicin-induced toxicity in patients treated by DEB-TACE was notably less compared with cTACE51. The AEs during and postprocedure could be caused by both the operation itself and the chemotherapeutics, for instance, the embolization syndrome caused by the procedure and bone marrow toxicity as well as alopecia caused by doxorubicin5. In our study, the most common AEs were pain, fever, vomiting, and nausea, which were all light and manageable. In addition, cases presented with chemotherapy-related severe AEs in our study were relatively rare, indicating DEB-TACE is tolerable in Chinese liver cancer patients with mostly light AEs and mild chemotherapy drug toxicity.
There were some limitations in this study. First, the follow-up duration was relatively short with median follow-up time of 171 days (range: 38–404), while it is essential to investigate the long-term benefit of DEB-TACE treatment in liver cancer patients. Second, because of lack of compliance in the rigorous follow-up schedule, the PFS was not able to be evaluated, which was very important for prognosis assessment. Third, the patients enrolled in this study mainly came from east and middle China, which cannot stand for all the Chinese patients across different areas; thus, more medical centers in different areas of China should be invited into the CTILC study in the future. Fourth, the CTILC study was a multicenter, prospective cohort study that aimed to investigate the efficacy and safety of DEB-TACE treatment by CalliSpheres® in Chinese patients. Although it possessed the largest sample size of DEB-TACE-treated liver cancer to date, it was a single-armed design study and thus we could not explore the efficacy between DEB-TACE and cTACE.
In conclusion, CalliSpheres® DEB-TACE was efficient and well tolerated in Chinese liver cancer patients, and portal vein invasion, previous cTACE treatment, largest nodule size, abnormal BCr, ALB, and TBIL were correlated with worse prognosis independently.
ACKNOWLEDGMENTS
This work was supported by the National Nature Science Foundation of China (81371658) and Zhejiang Provincial Natural Science Foundation of China (LZ18H180001).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Chen JG, Zhang SW. Liver cancer epidemic in China: Past, present and future. Semin Cancer Biol. 2011;21(1):59–69. [DOI] [PubMed] [Google Scholar]
- 3. Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology 2018;67(1):401–21. [DOI] [PubMed] [Google Scholar]
- 4. Della Corte C, Triolo M, Iavarone M, Sangiovanni A. Early diagnosis of liver cancer: An appraisal of international recommendations and future perspectives. Liver Int. 2016;36(2):166–76. [DOI] [PubMed] [Google Scholar]
- 5. Chen P, Yuan P, Chen B, Sun J, Shen H, Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41(1):75–85. [DOI] [PubMed] [Google Scholar]
- 6. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525–35. [DOI] [PubMed] [Google Scholar]
- 7. Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg. 2016;263(6):1112–25. [DOI] [PubMed] [Google Scholar]
- 8. van der Geest LG, van Meer S, Schrier JG, Ijzermans JN, Klumpen HJ, van Erpecum KJ, de Man RA. Survival in relation to hospital type after resection or sorafenib treatment for hepatocellular carcinoma in The Netherlands. Clin Res Hepatol Gastroenterol. 2015;39(6):725–35. [DOI] [PubMed] [Google Scholar]
- 9. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014;63(5):844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. [DOI] [PubMed] [Google Scholar]
- 12. Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H, British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012;61(12):1657–69. [DOI] [PubMed] [Google Scholar]
- 13. Currie BM, Soulen MC. Decision making: Intra-arterial therapies for cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol. 2017;34(2):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future. Clin Mol Hepatol. 2015;21(4):344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jang JH, Lee JW, Hong JT, Jin YJ. Transarterial chemoembolization for hepatocellular carcinoma: An evidence-based review of its place in therapy. J Hepatocell Carcinoma 2015;2:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F. Precision Italia Study Group. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111(2):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C, Dueber C, Pitton MB. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015;15:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis. 2016;48(6):571–7. [DOI] [PubMed] [Google Scholar]
- 19. Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9):a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 2017;152(4):745–61. [DOI] [PubMed] [Google Scholar]
- 21. Sastre J, Diaz-Beveridge R, Garcia-Foncillas J, Guardeno R, Lopez C, Pazo R, Rodriguez-Salas N, Salgado M, Salud A, Feliu J. Clinical guideline SEOM: Hepatocellular carcinoma. Clin Transl Oncol. 2015;17(12):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adhoute X, Penaranda G, Castellani P, Perrier H, Bourliere M. Recommendations for the use of chemoembolization in patients with hepatocellular carcinoma: Usefulness of scoring system? World J Hepatol. 2015;7(3):521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu CY, Ou HY, Weng CC, Huang TL, Chen TY, Leung-Chit L, Hsu HW, Chen CL, Cheng YF. Drug-eluting bead transarterial chemoembolization as bridge therapy for hepatocellular carcinoma before living-donor liver transplantation. Transplant Proc. 2016;48(4):1045–8. [DOI] [PubMed] [Google Scholar]
- 24. Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: A meta-analysis. BMC Gastroenterol. 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montana X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–81. [DOI] [PubMed] [Google Scholar]
- 26. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–8. [DOI] [PubMed] [Google Scholar]
- 27. Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57(6):1244–50. [DOI] [PubMed] [Google Scholar]
- 28. Rahman FA, Naidu J, Ngiu CS, Yaakob Y, Mohamed Z, Othman H, Jarmin R, Elias MH, Hamid NA, Mokhtar NM, Ali RR. Conventional versus doxorubicin-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma: A tertiary medical centre experience in Malaysia. Asian Pac J Cancer Prev. 2016;17(8):4037–41. [PubMed] [Google Scholar]
- 29. Ni JY, Xu LF, Wang WD, Sun HL, Chen YT. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2014;20(45):17206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, Torbenson MS, Choti MA, Geschwind JF. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: Initial experience in the United States. Cancer J. 2009;15(6):526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchiano A, Camerini T, Bhoori S, Brunero F, Barone M, Mazzaferro V. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(3):645–53. [DOI] [PubMed] [Google Scholar]
- 32. Arabi M, BenMousa A, Bzeizi K, Garad F, Ahmed I, Al-Otaibi M. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015;21(3):175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Megias Vericat JE, Garcia Marcos R, Lopez Briz E, Gomez Munoz F, Ramos Ruiz J, Martinez Rodrigo JJ, Poveda Andres JL. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: A study of effectiveness, safety and costs. Radiologia 2015;57(6):496–504. [DOI] [PubMed] [Google Scholar]
- 34. Cheung AH, Lam CS, Tam HS, Cheung TT, Pang R, Poon RT. Nine-year experience of doxorubicin-eluting beads chemoembolization for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2016;15(5):493–8. [DOI] [PubMed] [Google Scholar]
- 35. De Greef K, Rolfo C, Russo A, Chapelle T, Bronte G, Passiglia F, Coelho A, Papadimitriou K, Peeters M. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J Gastroenterol. 2016;22(32):7215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Negoi I, Runcanu A, Paun S, Negoi RI, Beuran M. Resection of large metachronous liver metastasis with gastric origin: Case report and review of the literature. Cureus 2016;8(10):e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukumitsu N, Okumura T, Numajiri H, Takizawa D, Ohnishi K, Mizumoto M, Aihara T, Ishikawa H, Tsuboi K, Sakurai H. Follow-up study of liver metastasis from breast cancer treated by proton beam therapy. Mol Clin Oncol. 2017;7(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, Ingrand P, Tasu JP. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26(6):1640–8. [DOI] [PubMed] [Google Scholar]
- 39. Reis SP, Sutphin PD, Singal AG, Grzybowski R, Fisher S, Ball C, Xi Y, Grewal S, Kalva SP. Tumor enhancement and heterogeneity are associated with treatment response to drug-eluting bead chemoembolization for hepatocellular carcinoma. J Comput Assist Tomogr. 2017;41(2):289–93. [DOI] [PubMed] [Google Scholar]
- 40. Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Muller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: Retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013;57(6):2261–73. [DOI] [PubMed] [Google Scholar]
- 41. Hong CX, Lv LW, Hua LZ, Bo S, Sen CX, Xin NY, Wei YJ, Rui XJ, Qiang DX, Zhou ZJ. Epidemiology and management of acute kidney injury in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Curr Protein Pept Sci. 2017;18(12):1218–23. [DOI] [PubMed] [Google Scholar]
- 42. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(11):1545–52. [DOI] [PubMed] [Google Scholar]
- 43. Lee S, Kim KM, Lee SJ, Lee KH, Lee DY, Kim MD, Kim DY, Kim SU, Won JY. Hepatic arterial damage after transarterial chemoembolization for the treatment of hepatocellular carcinoma: Comparison of drug-eluting bead and conventional chemoembolization in a retrospective controlled study. Acta Radiol. 2017;58(2):131–9. [DOI] [PubMed] [Google Scholar]
- 44. Wagnetz U, Jaskolka J, Yang P, Jhaveri KS. Acute ischemic cholecystitis after transarterial chemoembolization of hepatocellular carcinoma: Incidence and clinical outcome. J Comput Assist Tomogr. 2010;34(3):348–53. [DOI] [PubMed] [Google Scholar]
- 45. Nishimine K, Uchida H, Matsuo N, Sakaguchi H, Hirohashi S, Nishimura Y, Guo Q, Ohishi H, Nagano N, Yoshioka T, Segmental transarterial chemoembolization with lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: Follow-up CT and therapeutic results. Cancer Chemother Pharmacol. 1994;33(Suppl):S60–8. [DOI] [PubMed] [Google Scholar]
- 46. Sharma U, Pal D, Prasad R. Alkaline phosphatase: An overview. Indian J Clin Biochem. 2014;29(3):269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kohles N, Nagel D, Jungst D, Durner J, Stieber P, Holdenrieder S. Relevance of circulating nucleosomes and oncological biomarkers for predicting response to transarterial chemoembolization therapy in liver cancer patients. BMC Cancer 2011;11:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamgang R, Foyet AF, Essame JL, Ngogang JY. Effect of methanolic fraction of Kalanchoe crenata on metabolic parameters in adriamycin-induced renal impairment in rats. Indian J Pharmacol. 2012;44(5):566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prahalathan C, Selvakumar E, Varalakshmi P. Protective effect of lipoic acid on adriamycin-induced testicular toxicity. Clin Chim Acta 2005;360(1–2):160–6. [DOI] [PubMed] [Google Scholar]
- 50. Imai N, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Hirooka Y, Goto H. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol. 2014;6(12):844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R, Investigators PV. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]