Abstract
The genomic landscape of breast cancer (BC) is complex. The purpose of this study was to decipher the mutational profiles of Taiwanese patients with BC using next-generation sequencing. We performed whole-exome sequencing on DNA from 24 tumor tissue specimens from BC patients. Sanger sequencing was used to validate the identified variants. Sanger sequencing was also performed on paired adjacent nontumor tissues. After genotype calling and algorithmic annotations, we identified 49 deleterious variants in canonical cancer-related genes in our BC cohort. The most frequently mutated genes were PIK3CA (16.67%), FKBP9 (12.5%), TP53 (12.5%), ATM (8.33%), CHEK2 (8.33%), FOXO3 (8.33%), NTRK1 (8.33%), and NUTM2B (8.33%). Seven mutated variants (ATR p.V1581fs, CSF1R p.R579Q, GATA3 p.T356delinsTMKS, LRP5 p.W389*, MAP3K1 p.T918fs, MET p.K1161fs, and MTR p.P1178S) were novel variants that are not present in any gene mutation database. After grouping the samples according to molecular subtype, we found that the cell cycle, MAPK, and chemokine signaling pathways in the luminal A subtype of BC; the focal adhesion, axon guidance, and endocytosis pathways in the luminal B subtype; and amyotrophic lateral sclerosis in the basal-like subtype were exclusively altered. Survival curve analysis showed that the presence of the MAPK signaling pathway and endocytosis mutations were correlated with a poor prognosis. These survival data were consistent with cBioPortal analyses of 2,051 BC cases. We discovered novel mutations in patients with BC. These results have implications for developing strategic, adjuvant, and gene-targeted therapies.
Key words: Breast cancer (BC), Whole-exome sequencing, Gene mutation, Pathway mutation
INTRODUCTION
Breast cancer (BC) is the leading cause of cancer-related mortality worldwide. Every year, over 1 million new cases are diagnosed, and over 500,000 deaths occur1. Risk factors for BC include age, obesity, family history of BC, genetics, hormonal and reproductive factors, dense breast tissue, and lifestyle factors including cigarette smoking, alcohol consumption, vitamin D deficiency, and physical inactivity2. High-risk women may be advised to undergo genetic testing or both regular mammography and magnetic resonance imaging screening.
BC is a complex disease; most cases are sporadic, but some are inherited. The two most important BC susceptibility genes involved in DNA repair are BRCA1 and BRCA2. Germline mutations in BRCA1 and BRCA2 can explain ∼25% of the familial aggregation of BC risk3 and thus 5%–10% of all BC cases4. Among carriers of BRCA1 and BRCA2 pathogenic mutations, approximately 72% and 69% of women will develop BC during their lives, respectively5. In addition to BRCA1 and BRCA2, high-penetrance genes include TP53, PTEN, STK11, and CDH1, whereas moderate-penetrance genes include CHEK2, ATM, BRIP1, PALB2, RAD51C, RAD50, and NBN 6. More recently, a genome-wide association study identified 65 loci significantly associated with BC7.
There are five intrinsic or molecular subtypes of BC defined by immunohistochemical expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2): luminal A, luminal B, HER2 overexpression, basal-like, and normal breast-like subtypes, which account for 23.7%, 52.8%, 11.2%, 12.3%, and 7.8% of cases, respectively. These BC subtypes have been correlated with treatment response and clinical outcome, with luminal A BC patients showing the best survival8.
Next-generation sequencing (NGS) can play an important role in diagnosis, prognosis, and treatment. A number of NGS platforms are available and have different properties, such as throughput (Gb), maximum read length (bp), reads, running time, and error profile. NGS platforms use two types of sequencing mechanisms: ligation or synthesis9. NGS of DNA includes whole-genome sequencing, whole-exome sequencing (WES), and targeted sequencing. WES can cover almost the entirety of protein-coding regions in the human genome, which contain approximately 85% of disease-causing variants. The exome comprises approximately 1% of the total human genome, so WES is considered an outstandingly powerful tool for medical genetic research. WES is often available at lower costs, allowing for more individuals to be sequenced, which will provide more powerful case/control and family-based NGS studies in the future. WES has been applied previously to analyze the BC genomic landscape10.
The aim of our study was to assess the genes implicated in BC. We grouped the BC patients according to molecular subtype and explored the differences in the affected pathways among the BC subtypes. We performed WES using fresh frozen tissues from 24 Taiwanese patients with BC.
MATERIALS AND Methods
Patients and DNA Extraction
Our study cohort comprised 24 tumor specimens from BC excisions collected at the time of surgery between 2003 and 2009 at China Medical University Hospital, Taiwan. The tissues were frozen at −80°C until DNA extraction. Genomic DNA was extracted using the illustra Tissue and Cells GenomicPrep Mini Spin kit (GE Healthcare, Chicago, IL, USA) according to the protocol provided by the manufacturer. DNA quantification was performed using the NanoDrop 1000 (Thermo Scientific, Wilmington, DE, USA) and Qubit 3.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). This protocol was approved by the Institutional Review Board of China Medical University Hospital.
Whole-Exome Sequencing
A total of 100 ng DNA (based on Qubit quantification) was mechanically fragmented on the Covaris M220 Focused-ultrasonicator (Covaris, Woburn, MA, USA). Quality control was performed using the Agilent Bioanalyzer 4200 (Agilent Technologies, Santa Clara, CA, USA) to ensure an average fragment size of 150–200 bp. End repair, A-tailing, adaptor ligation, and enrichment of DNA fragments were then performed. A 200- to 400-bp band was selected, and exome capture was performed using the TruSeq Exome Library Preparation kit (Illumina, San Diego, CA, USA). The DNA library was quantified using the Qubit 3.0 Fluorometer (Invitrogen) and Agilent 4200 Bioanalyzer (Agilent Technologies). Samples were subjected to paired-end sequencing using the Illumina NextSeq 500 platform with a 150-bp read length. The metadata were deposited in the NCBI Sequence Read Archive under accession No. SRP217293.
Data Analysis
Base calling and quality scoring were performed using an updated implementation of real-time analysis on the NextSeq 500 system. The Bcl2fastq Conversion Software was used to demultiplex the data and convert the BCL files to FASTQ files. The sequenced reads were trimmed to remove low-quality sequences and then aligned to the human reference genome (hg19) using the Burrows–Wheeler alignment tool11. Finally, single-nucleotide polymorphisms and small insertions/deletions were identified in individual samples using the Genome Analysis Toolkit and VarScan with the default settings12,13. ANNOVAR was then used to annotate the VCF files by gene, region, and several filters from other databases14. Finally, we annotated the mutations using several databases and tools, including dbSNP (build 147), ClinVar, COSMIC (ver. 70), TCGA, Polyphen-2, SIFT, and CADD15–21. We used the Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resource 6.7 (https://david-d.ncifcrf.gov) to aid the identification of significantly altered biological processes and pathways in the 24 BC patients.
Mutation Validation
For validation of mutations, we used polymerase chain reaction (PCR) and Sanger sequencing. The specific PCR primers were designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/) (see Supplemental Table 1, available at https://github.com/JanGowthChang/Breast-cancer.git). The products were directly sequenced using the ABI PRISM BigDye Kit on the ABI 3130 DNA sequencer (Applied Biosystems, Foster City, CA, USA). Sequencing results were analyzed using Chromas version 2.23 (Technelysium, Tewantin, Australia).
Statistical Analysis
Statistical analysis was performed using SPSS Version 22.0. For survival analysis, we used the Kaplan–Meier survival curve with Cox analysis for determination of p values and the log rank test for determination of hazard ratios. The mutations associated with survival pathways were compared with those reported in the cBioportal database.
RESULTS
Patient Characteristics
The clinical and pathological data of all patients included in this study are summarized in Table 1. The clinical indices include mean age, age range, stage, tumor size, lymph nodes, distant metastasis, and ER, PR, and HER2 statuses.
Table 1.
Clinicopathologic Characteristics of Patients With Breast Cancer (BC)
| Variable | No. of Patients N = 24 (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 66.46 ± 10.62 |
| Range | 47–90 |
| Clinical stage | |
| I | 2 (8.33) |
| IIA | 9 (37.5) |
| IIB | 4 (16.67) |
| IIIA | 4 (16.67) |
| IIIC | 1 (4.17) |
| IV | 2 (8.33) |
| NA | 2 (8.33) |
| Tumor size | |
| T1 | 5 (20.83) |
| T2 | 14 (58.33) |
| T3 | 3 (12.5) |
| NA | 2 (8.33) |
| Lymph nodes status | |
| N0 | 10 (41.67) |
| N1 | 7 (29.17) |
| N2 | 1 (4.17) |
| N3 | 2 (8.33) |
| NA | 4 (16.67) |
| Distant metastasis | |
| M0 | 16 (66.67) |
| M1 | 2 (8.33) |
| NA | 6 (25) |
| ER status | |
| Positive | 14 (58.33) |
| Negative | 10 (41.67) |
| PR status | |
| Positive | 17 (70.83) |
| Negative | 7 (29.17) |
| HER2 | |
| Positive | 11 (45.83) |
| Negative | 13 (54.17) |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; NA, data not available.
Mutation Landscape of the 769 Canonical Cancer-Related Genes
We identified 49 nonsynonymous mutations, which occurred in 43 genes and included 34 missense mutations, 7 stop-gain mutations, 4 frameshift deletion mutations, 3 nonframeshift deletion mutations, and 1 nonframeshift insertion mutation (see Supplemental Table 2, available at https://github.com/JanGowthChang/Breast-cancer.git). The most frequently mutated genes were PIK3CA (16.67%; 4/24), FKBP9 and TP53 (12.5%; 3/24), and ATM, CHEK2, FOXO3, NTRK1, and NUTM2B (8.33%; 2/24).
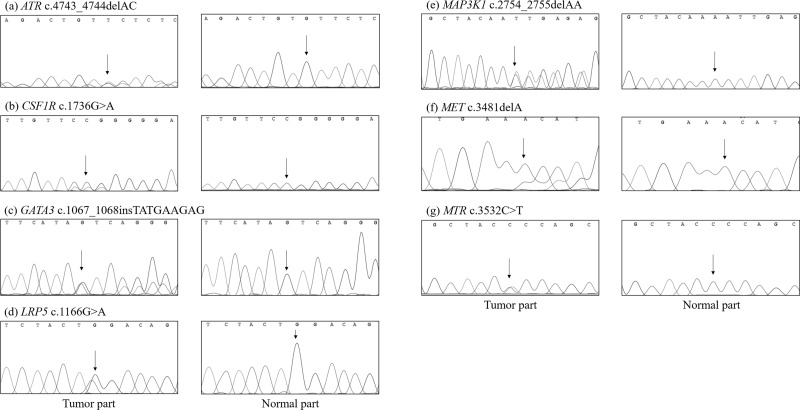
Of the 49 variants, 42 already exist in the dbSNP, COSMIC, or TCGA databases, whereas 7 do not. In addition, we found five somatic mutations in the EPHB1, FGFR4, FKBP9, PIK3CG, and SMAD4 genes that have been identified in other tumors but not in BC. We selected the following seven novel mutations for Sanger sequencing: ATR p.V1581fs, CSF1R p.R579Q, GATA3 p.T356delinsTMKS, LRP5 p.W389*, MAP3K1 p.T918fs, MET p.K1161fs, and MTR p.P1178S (Fig. 1).
Figure 1.
Sanger sequencing confirmation of canonical cancer-related genes identified by WES: (a) ATR, (b) CSF1R, (c) GATA3, (d) LRP5, (e) MAP3K1, (f) MET, and (g) MTR.
Mutation Landscape of the Noncanonical Cancer-Related Genes
The 960 nonsynonymous mutations identified in this study, including 581 missense mutations, 96 frameshift deletion mutations, 42 frameshift insertion mutations, 68 nonframeshift deletion mutations, 29 nonframeshift insertion mutations, 143 stop-gain mutations, and one stop-loss mutation, were located in 758 genes (see Supplemental Table 3, available at https://github.com/JanGowthChang/Breast-cancer.git). In total, 648 variants have previously been reported in the dbSNP, COSMIC, or TCGA databases, whereas 312 variants in 243 genes have not.
We found somatic mutations in ANKRD45, ATP1A4, BRINP2, CHML, DLG2, EXO1, KIAA1109, KRTAP5-8, LRIG1, MUC2, MYOT, OS9, PLA2G15, SMPD1, SREBF2, SUPT20HL1, UBXN11, and UNC80; mutations in these genes have been detected in other tumors previously, but not in BC. In addition, we found somatic mutations in ARHGAP24, C1orf198, CHD1L, FAM200B, GOLGA6L10, OR5P2, ROM1, and UFL1; mutations in these gene have been identified in BC previously in the dbSNP, COSMIC, or TCGA databases.
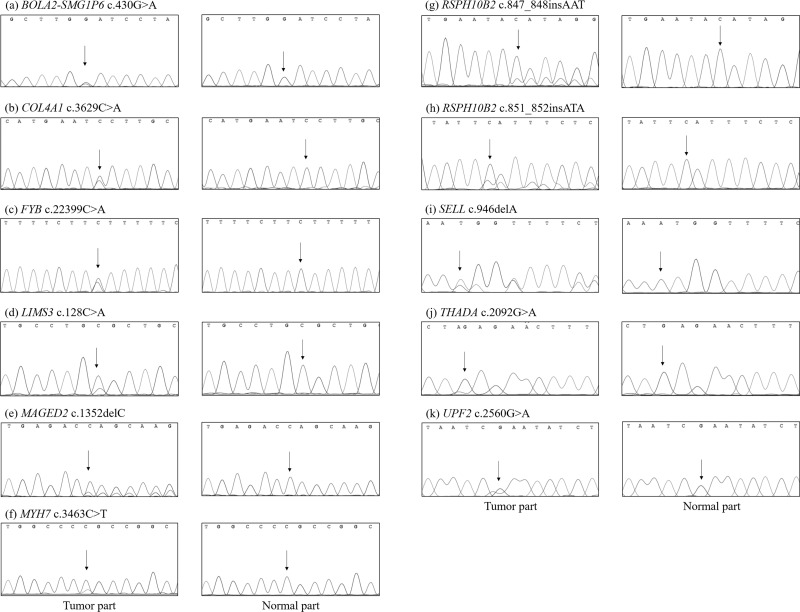
We selected new genetic variants for Sanger sequencing. Novel somatic mutations detected only in the cancer tissues included BOLA2-SMG1P6 p.D144N, COL4A1 p.G1210V, FYB p.E747*, LIMS3 p.R43L, MAGED p.T451fs, MYH7 p.G1155R, RSPH10B2 p.E283delinsE* and p.Y284delins*, SELL p.C316fs, THADA p.Q698*, and UPF2 p.R584* (Fig. 2).
Figure 2.
Sanger sequencing confirmation of noncanonical cancer-related genes identified by WES: (a) BOLA2-SMG1P6, (b) COL4A1, (c) FYB, (d) LIMS3, (e) MAGED, (f) MYH7, (g and h) RSPH10B2, (i) SELL, (j) THADA, and (k) UPF2.
American College of Medical Genetics and Genomics Genes
Of the 24 BC samples, 20.83% (5/24) harbored mutations in American College of Medical Genetics and Genomics genes, including BRCA2, MYH7, MYH11, PCSK9, and TP53. Mutations in TP53 were detected in 12.5% (3/24) of our BC specimens, at p.R213* (rs397516436), p.R248W (rs121912651), and p.R273H (rs28934576). A BRCA2 mutation at p.Q3036E (rs202155613), MYH7 mutation at p.G1155R (a novel mutation), MYH11 mutation at p.R1447Q (rs763467593), and PCSK9 mutation at p.R434W (rs757143429) were detected in 4.17% (1/24) of BC samples.
Altered Pathways
Functional annotation was performed using DAVID. Twelve mutated genes were found in Kyoto Encyclopedia of Genes and Genomes cancer pathways (hsa05200), including AKT1, BRCA2, CDH1, CSF1R, LAMC1, MET, NTRK1, PIK3CA, PIK3CG, RUNX1, SMAD4, and TP53 [false discovery rate (FDR) = 8.6 × 10−4]. Moreover, we identified several cellular pathways that were altered in the BC tissues (Table 2).
Table 2.
Mutated Pathways in Breast Cancer
| Pathways Involved in Carcinogenesis | Mutated Genes |
|---|---|
| hsa04722: Neutrophin signaling pathway | PIK3CG, AKT1, MAP3K1, NTRK1, TP53, PIK3CA, FOXO3 |
| hsa04210: Apoptosis | PIK3CG, AKT1, NTRK1, TP53, PIK3CA, ATM |
| hsa04510: Focal adhesion | PIK3CG, AKT1, MET, ITGA10, PIK3CA, LAMC1, ITGB3 |
| hsa04110: Cell cycle | SMAD4, TP53, ATR, CHEK2, ATM |
| hsa04115: p53 signaling pathway | TP53, ATR, CHEK2, ATM |
| hsa04010: MAPK signaling pathway | AKT1, FGFR4, MAP3K1, NTRK1, TP53, DAXX |
| hsa04062: Chemokine signaling pathway | PIK3CG, AKT1, PTK2B, PIK3CA, FOXO3 |
| hsa04810: Regulation of actin cytoskeleton | PIK3CG, FGFR4, ITGA10, PIK3CA, ITGB3 |
| hsa04150: mTOR signaling pathway | PIK3CG, AKT1, PIK3CA |
| hsa04370: VEGF signaling pathway | PIK3CG, AKT1, PIK3CA |
| hsa04520: Adherens junction | MET, SMAD4, CDH1 |
| hsa04512: ECM–receptor interaction | ITGA10, LAMC1, ITGB3 |
| hsa04144: Endocytosis | FGFR4, NTRK1, MET, CSF1R |
| hsa04012: ErbB signaling pathway | PIK3CG, AKT1, PIK3CA |
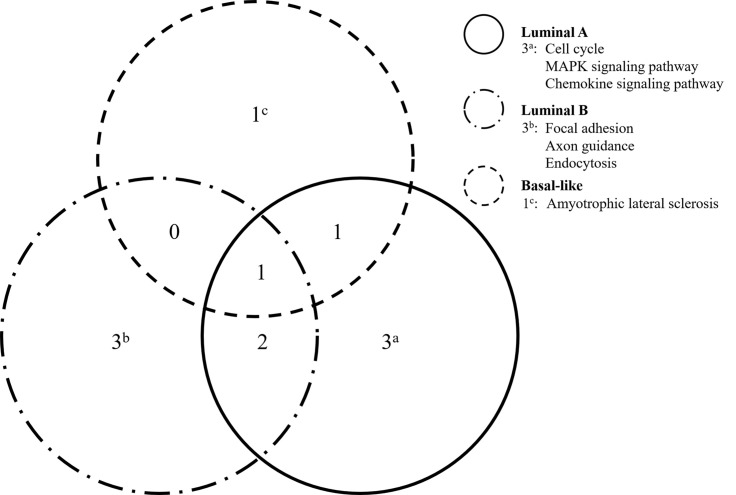
To evaluate the affected pathways according to BC subtype, the patients were classified into four categories: luminal A (n = 9), luminal B (n = 10), HER2 overexpression (n = 1), and basal-like (n = 4) BC. We found three exclusive pathways associated with the genes detected in the luminal A samples: cell cycle (ATM, CHEK2, SMAD4, and TP53), MAPK signaling (AKT1, FGFR4, MAP3K1, and TP53), and chemokine signaling (AKT1, FOXO3, and PIK3CA). Three exclusive pathways were also associated with the luminal B genes: focal adhesion (ITGA10, LAMC1, MET, and PIK3CA), axon guidance (EPHB1, MET, and SRGAP3), and endocytosis (CSF1R, MET, and NTRK1). Basal-like BC was associated with one exclusive pathway: amyotrophic lateral sclerosis (ALS; DAXX and TP53) (Fig. 3).
Figure 3.
Venn diagrams representing the interrelated pathways associated with the identified mutations among luminal A, luminal B, and basal-like BC in Taiwanese patients.
Survival Analysis
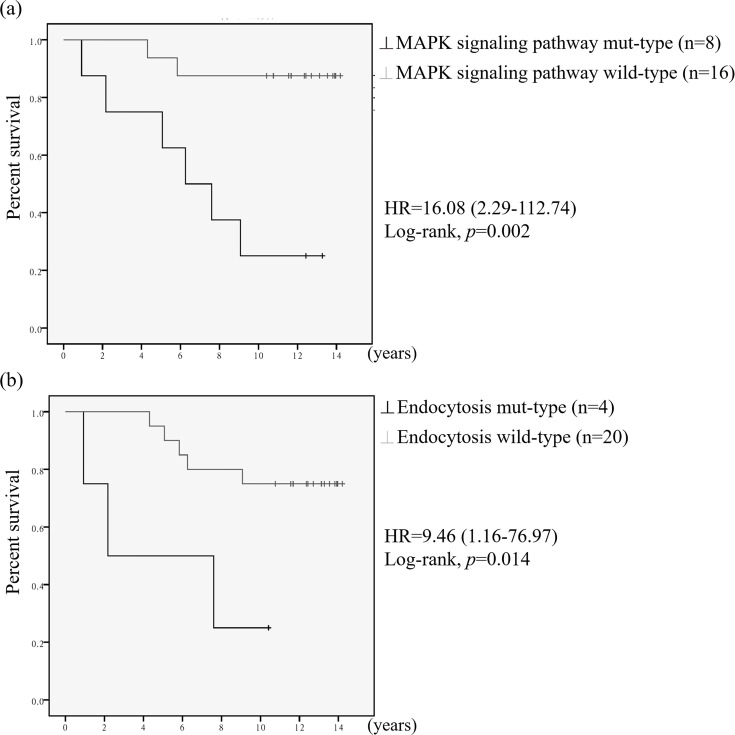
We used Kaplan–Meier curve analysis to assess overall survival. Figure 4 presents the survival curves of patients with mutations in genes involved in the MAPK signaling pathway and endocytosis. The survival curves were significantly different between the two groups of patients. The hazard ratios were 16.08 [95% confidence interval (CI), 2.29–112.74] for the survival of patients with MAPK signaling pathway mutations and 9.46 (95% CI, 1.16–76.97) for that of patients with endocytosis mutations. The average survival was 7.21 years for patients with MAPK signaling pathway mutations versus 13.06 years for patients without those mutations. The average survival was 5.28 years for patients with endocytosis mutations versus 12.18 years for patients without those mutations. Furthermore, we included 2,051 BC samples22 from the cBioPortal database in the analysis, and similar results were obtained (see Supplemental Fig. 1, available at https://github.com/JanGowthChang/Breast-cancer.git). An analysis of 1,918 BC samples in the cBioPortal database from another BC study23 revealed that only the MAPK signaling pathway was significantly correlated with survival (see Supplemental Fig. 2, available at https://github.com/JanGowthChang/Breast-cancer.git). However, two other BC studies (482 and 816 samples, respectively) did not confirm these results24,25. The survival curves for the other validated pathway mutations, presented in supplemental Figure 3 (available at https://github.com/JanGowthChang/Breast-cancer.git), showed no significant difference between the two groups.
Figure 4.
Kaplan–Meier survival curves of patients with mutations in (a) MAPK signaling- and (b) endocytosis-related genes.
DISCUSSION
The present study describes somatic mutations detected in the whole BC exome. We identified several cancer driver and passenger genes among canonical and noncanonical cancer-related genes. Overall, 16.67% of BC cases harbored PIK3CA mutations. Mutations were also found in other canonical cancer-related genes, including TP53 and FKBP9 (12.5% each) and ATM, CHEK2, FOXO3, NTRK1, and NUTM2B (8.33% each). Variants that were not found in the dbSNP, COSMIC, or TCGA database were considered novel. The WES results were further confirmed by Sanger sequencing of DNA from the patients’ tumor and normal tissues. To the best of our knowledge, several sequencing variants have not been reported previously, including those in canonical cancer-related genes (ATR, CSF1R, GATA3, LRP5, MAP3K1, MET, and MTR) and noncanonical cancer-related genes (BOLA2-SMG1P6, COL4A1, FYB, LIMS3, MAGED, MYH7, RSPH10B2, SELL, THADA, and UPF2).
ATR encodes a serine/threonine kinase protein that is involved in sensing DNA damage and activating DNA damage checkpoints, leading to cell cycle arrest26. Somatic mutations in exon 10 of ATR have been identified in endometrioid tumors with DNA mismatch repair defects27. ATR mutations are associated with poor clinical outcomes among patients with endometrioid cancer28. In the present study, we identified a novel mutation, p.V1581fs, in a patient with BC.
The protein encoded by CSF1R is the receptor for colony-stimulating factor 1, a cytokine that controls the production, differentiation, and function of macrophages29. Ligand binding activates CSF1R kinase via oligomerization and transphosphorylation. Mutations in this gene have been associated with metaplastic BC30. In the present study, we identified a novel mutation in this gene, p.R579Q, in a patient with BC.
GATA3 encodes a protein belonging to the GATA family of transcription factors. The protein is an important regulator of T-cell development and plays an important role in endothelial cell biology31. A recent study reported that GATA3 mutations lead to proliferative phenotypes in normal and malignant mammary cells32. In the present study, we identified a novel mutation in GATA3, p.T356delinsTMKS, in a patient with BC.
LRP5 encodes a transmembrane low-density lipoprotein receptor that binds and internalizes ligands via receptor-mediated endocytosis33. LRP5, a coreceptor of Wnt, is located between two other receptors from the Frizzled and Kremen families, which play key roles in the canonical Wnt signaling pathway34. LRP5 plays a central role in skeletal homeostasis, and mutations in LRP5 are associated with many bone density-related diseases, such as osteoporosis–pseudoglioma syndrome, osteoporosis, and high bone mass35,36. In the present study, we identified a novel mutation in LRP5, p.W389*, in a patient with BC.
MAP3K1 encodes a serine/threonine kinase protein belonging to the mitogen-activated protein kinase kinase kinase family and is a member of several signal transduction cascades, including the ERK, JNK, and NF-κB pathways37. MAP3K1 is activated by autophosphorylation and requires magnesium as a cofactor to phosphorylate other proteins. MAP3K1 contains specific domains (PHD, SWIN, and RING motifs) and features (a caspase cleavage site and E3 ligase activity)38. Recent high-throughput genomic studies have revealed oncogenic driver mutations in diverse cancers, including recurrent mutations in MAP3K1 22. In the present study, we identified a novel mutation in MAP3K1, p.T918fs, in a patient with BC.
MET encodes a member of the receptor tyrosine kinase family of proteins, the product of the proto-oncogene MET. MET is activated upon binding to the hepatocyte growth factor ligand, which plays an important role in cell survival, migration, and invasion and embryogenesis39. Mutations in this gene have been found in different solid tumors40. In the present study, we identified a novel mutation in this gene, p.K1161fs, in a patient with BC.
The protein encoded by MTR (5-methyltetrahydrofolate-homocysteine methyltransferase), also known as cobalamin-dependent methionine synthase, catalyzes the methylation of homocysteine to methionine, using 5-methyltetrahydrofolate as a methyl donor and cobalamin (vitamin B12) as a cofactor41. Mutations in MTR have been identified as the underlying cause of methylcobalamin deficiency complementation group G42. Previous studies have investigated the relationship between MTR gene polymorphisms (A2756G, D919G) and the risk of cancer43,44. In the present study, we identified a novel mutation in MTR, p.P1178S, in a patient with BC.
Furthermore, we identified exclusive pathways in three subtypes of BC. Genes mutated in the cell cycle, MAPK signaling, and chemokine signaling pathways were specifically associated with luminal A BC. Liu et al. demonstrated that differential expression of genes in the cell cycle pathway is associated with differential patient outcomes in BC45. In the recently analyzed TCGA PanCancer Atlas collection samples, alterations in the cell cycle pathway were found in the luminal A, luminal B, HER2-enriched, and basal-like BC subtypes at frequencies of 31%, 48%, 40%, and 51%, respectively46. Hembruff et al. reported that deregulation of the chemokine signaling pathway is implicated in cancer progression47. The role of the MAPK signaling pathway in BC has also been explored48.
Gene mutations involved in focal adhesion, axon guidance, and endocytosis processes were specifically associated with luminal B BC. Focal adhesions contain integrins. Felding-Habermann et al. showed that integrin activation regulates metastasis in human BC49. Harburg et al. reported that axon guidance molecules are frequently dysregulated in BC50. Mutations in, and aberrant expression of, endocytosis-regulating genes have been found in multiple human tumors51.
Gene mutations involved in ALS are specifically associated with basal-like BC. The ALS drug riluzole was shown to induce anticancer effects on hepatocellular carcinoma52. Relationships between most ALS genes and various cancers have been identified53.
Compared with the cBioPortal analyses of 2051 BC cases, we found eight signaling pathway mutations that were not correlated with a poor prognosis. Because of our small study cohort, the performed survival analysis with the enormous broadness of the HR confidence is difficult, and the prediction potential clinical associations may not be close to the true condition. Studies with larger BC cohorts involving various international populations are needed to validate the potentially relevant clinical associations observed in the current study.
In agreement with cBioPortal analyses of 2,051 BC cases, we observed that the groups of patients with mutations in the MAPK signaling pathway and endocytosis were correlated with worse prognosis despite our small sample size. These results suggest that these pathways may play an important role in the development of BC.
In summary, we performed WES of BC samples and identified mutations in potential cancer driver and passenger genes. In addition, survival curve analyses showed that the presence of mutations in the MAPK signaling pathway and endocytosis was correlated with a poor prognosis. These results were consistent with cBioPortal analyses of 2,051 BC cases.
ACKNOWLEDGMENTS
This work has been supported in part by China Medical University Hospital grants (DMR107-100 and DMR-108-220).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: A review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–91. [DOI] [PubMed] [Google Scholar]
- 3. Melchor L, Benitez J. The complex genetic landscape of familial breast cancer. Hum Genet. 2013;132(8):845–63. [DOI] [PubMed] [Google Scholar]
- 4. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer 1996;77(11):2318–24. [DOI] [PubMed] [Google Scholar]
- 5. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE, Milne RL, Andrieu N, et al. JAMA 2017;317(23):2402–16. [DOI] [PubMed] [Google Scholar]
- 6. Han MR, Zheng W, Cai Q, Gao YT, Zheng Y, Bolla MK, Michailidou K, Dennis J, Wang Q, Dunning AM, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551(7678):92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michailidou K, Lindstrom S, Dennis J, Beesley J, Hui S, Kar S, Lemacon A, Soucy P, Glubb D, Rostamianfar A, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551(7678):92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–43. [PMC free article] [PubMed] [Google Scholar]
- 9. Goodwin S, McPherson JD, McCombie WR. Coming of age: Ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17(6):333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandler MR, Bilgili EP, Merner ND. A review of whole-exome sequencing efforts toward hereditary breast cancer susceptibility gene discovery. Hum Mutat. 2016;37(9):835–46. [DOI] [PubMed] [Google Scholar]
- 11. Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22(3):568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang K, Li M, Hakonarson H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue):D980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, Kok CY, Jia M, De T, Teague JW, Stratton MR, McDermott U, Campbell PJ. COSMIC: Exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods 2010;7(4):248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–81. [DOI] [PubMed] [Google Scholar]
- 21. Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, Pugh M, Jones L, Russell R, Sammut SJ, Tsui DW, Liu B, Dawson SJ, Abraham J, Northen H, Peden JF, Mukherjee A, Turashvili G, Green AR, McKinney S, Oloumi A, Shah S, Rosenfeld N, Murphy L, Bentley DR, Ellis IO, Purushotham A, Pinder SE, Borresen-Dale AL, Earl HM, Pharoah PD, Ross MT, Aparicio S, Caldas C. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, Penson A, Shen R, Pareja F, Kundra R, Middha S, Cheng ML, Zehir A, Kandoth C, Patel R, Huberman K, Smyth LM, Jhaveri K, Modi S, Traina TA, Dang C, Zhang W, Weigelt B, Li BT, Ladanyi M, Hyman DM, Schultz N, Robson ME, Hudis C, Brogi E, Viale A, Norton L, Dickler MN, Berger MF, Iacobuzio-Donahue CA, Chandarlapaty S, Scaltriti M, Reis-Filho JS, Solit DB, Taylor BS, Baselga J. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 2018;34(3):427–438 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Network TR, Perou CM. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163(2):506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis KA, Mullany S, Thomas B, Chien J, Loewen R, Shridhar V, Cliby WA. Heterozygous ATR mutations in mismatch repair-deficient cancer cells have functional significance. Cancer Res. 2005;65(16):7091–5. [DOI] [PubMed] [Google Scholar]
- 28. Zighelboim I, Schmidt AP, Gao F, Thaker PH, Powell MA, Rader JS, Gibb RK, Mutch DG, Goodfellow PJ. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27(19):3091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496(7446):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edenfield J, Schammel C, Collins J, Schammel D, Edenfield WJ. Metaplastic breast cancer: Molecular typing and identification of potential targeted therapies at a single institution. Clin Breast Cancer 2017;17(1):e1–e10. [DOI] [PubMed] [Google Scholar]
- 31. Ho IC, Vorhees P, Marin N, Oakley BK, Tsai SF, Orkin SH, Leiden JM. Human GATA-3: A lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991;10(5):1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emmanuel N, Lofgren KA, Peterson EA, Meier DR, Jung EH, Kenny PA. Mutant GATA3 actively promotes the growth of normal and malignant mammary cells. Anticancer Res. 2018;38(8):4435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: A union made for bone. J Bone Miner Res. 2004;19(11):1749–57. [DOI] [PubMed] [Google Scholar]
- 34. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med. 2013;19(2):179–92. [DOI] [PubMed] [Google Scholar]
- 35. Biha N, Ghaber SM, Hacen MM, Collet C. Osteoporosis-pseudoglioma in a Mauritanian child due to a novel mutation in LRP5. Case Rep Genet. 2016;2016:9814928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roetzer KM, Uyanik G, Brehm A, Zwerina J, Zandieh S, Czech T, Roschger P, Misof BM, Klaushofer K. Novel familial mutation of LRP5 causing high bone mass: Genetic analysis, clinical presentation, and characterization of bone matrix mineralization. Bone 2018;107:154–60. [DOI] [PubMed] [Google Scholar]
- 37. Pham TT, Angus SP, Johnson GL. MAP3K1: Genomic alterations in cancer and function in promoting cell survival or apoptosis. Genes Cancer 2013;4(11–12):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charlaftis N, Suddason T, Wu X, Anwar S, Karin M, Gallagher E. The MEKK1 PHD ubiquitinates TAB1 to activate MAPKs in response to cytokines. EMBO J. 2014;33(21):2581–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25. [DOI] [PubMed] [Google Scholar]
- 40. Ma PC, Tretiakova MS, MacKinnon AC, Ramnath N, Johnson C, Dietrich S, Seiwert T, Christensen JG, Jagadeeswaran R, Krausz T, Vokes EE, Husain AN, Salgia R. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer 2008;47(12):1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leclerc D, Wilson A, Dumas R, Gafuik C, Song D, Watkins D, Heng HH, Rommens JM, Scherer SW, Rosenblatt DS, Gravel RA. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA 1998;95(6):3059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson A, Leclerc D, Saberi F, Campeau E, Hwang HY, Shane B, Phillips JA 3rd, Rosenblatt DS, Gravel RA. Functionally null mutations in patients with the cblG-variant form of methionine synthase deficiency. Am J Hum Genet. 1998;63(2):409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jokic M, Brcic-Kostic K, Stefulj J, Catela Ivkovic T, Bozo L, Gamulin M, Kapitanovic S. Association of MTHFR, MTR, MTRR, RFC1, and DHFR gene polymorphisms with susceptibility to sporadic colon cancer. DNA Cell Biol. 2011;30(10):771–6. [DOI] [PubMed] [Google Scholar]
- 44. Zhao T, Gu D, Xu Z, Huo X, Shen L, Wang C, Tang Y, Wu P, He J, Gong W, He ML, Chen J. Polymorphism in one-carbon metabolism pathway affects survival of gastric cancer patients: Large and comprehensive study. Oncotarget 2015;6(11):9564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu J, Campen A, Huang S, Peng SB, Ye X, Palakal M, Dunker AK, Xia Y, Li S. Identification of a gene signature in cell cycle pathway for breast cancer prognosis using gene expression profiling data. BMC Med Genomics 2008;1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, et al. Oncogenic signaling pathways in the Cancer Genome Atlas. Cell 2018;173(2):321–37 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hembruff SL, Cheng N. Chemokine signaling in cancer: Implications on the tumor microenvironment and therapeutic targeting. Cancer Ther. 2009;7(A):254–67. [PMC free article] [PubMed] [Google Scholar]
- 48. Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80(2):239–56. [DOI] [PubMed] [Google Scholar]
- 49. Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 2001;98(4):1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harburg GC, Hinck L. Navigating breast cancer: Axon guidance molecules as breast cancer tumor suppressors and oncogenes. J Mammary Gland Biol Neoplasia 2011;16(3):257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harb Perspect Biol. 2013;5(12):a016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seol HS, Lee SE, Song JS, Lee HY, Park S, Kim I, Singh SR, Chang S, Jang SJ. Glutamate release inhibitor, Riluzole, inhibited proliferation of human hepatocellular carcinoma cells by elevated ROS production. Cancer Lett. 2016;382(2):157–65. [DOI] [PubMed] [Google Scholar]
- 53. Taguchi YH, Wang H. Genetic association between amyotrophic lateral sclerosis and cancer. Genes (Basel) 2017;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]