Abstract
Pharmacogenomics has a burgeoning role in cardiovascular medicine, from warfarin dosing to antiplatelet choice, with recent developments in sequencing bringing the promise of personalised medicine ever closer to the bedside. Further scientific evidence, real-world clinical trials, and economic modelling are needed to fully realise this potential. Additionally, tools such as polygenic risk scores, and results from Mendelian randomisation analyses, are only in the early stages of clinical translation and merit further investigation. Genetically targeted rational drug design has a strong evidence base and, due to the nature of genetic data, academia, direct-to-consumer companies, healthcare systems, and industry may meet in an unprecedented manner. Data sharing navigation may prove problematic. The present manuscript addresses these issues and concludes a need for further guidance to be provided to prescribers by professional bodies to aid in the consideration of such complexities and guide translation of scientific knowledge to personalised clinical action, thereby striving to improve patient care. Additionally, technologic infrastructure equipped to handle such large complex data must be adapted to pharmacogenomics and made user friendly for prescribers and patients alike.
Keywords: Pharmacogenomics, Therapeutics, Risk scores, Mendelian randomisation
Introduction
Pharmacogenomics (PGx) has much potential in cardiovascular medicine, with decreases in sequencing costs promising further translation to clinical care. Personalised medicine within the context of PGx advances refers to the increasing ability to stratify patients by genetic information to give each patient drugs, and dosages, to which they will be optimally responsive, at the optimal time, while minimising the risk of adverse drug reactions. It can further inform the optimal starting dose of a drug and guide safe up-titration.
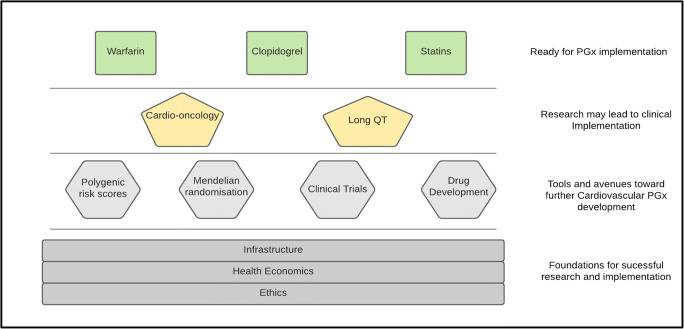
While much work has been undertaken in PGx and there is sufficient evidence to recommend implementation of knowledge to alter clinical practice for prescribing several cardiovascular drugs (most prominently warfarin, clopidogrel, and statins), uptake remains limited to academic arenas, and professional bodies within cardiovascular medicine have not issued a firm position [1]. In addition to those clinical prescribing actions consistently recommended by various PGx guideline groups, there are other areas in which PGx data is earlier in development but shows enormous promise. An example is within cardio-oncology, where genomic variants have been associated with risk of chemotherapy-induced cardiotoxicity [2]. A further example is in drug-induced long QT, a rare but potentially lethal arrhythmic response to a wide array of medication.
However, to deliver such potential advances to the bedside and use contemporary scientific gains to improve patient outcomes, further work is needed. This includes building on the scientific evidence base and advancing PGx use in rational drug design and clinical trials, as well as using exciting newer tools such as polygenic risk scores and Mendelian randomisation (Fig. 1). Furthermore, to aid implementation, further technologic infrastructure is needed. Academia, direct-to-consumer companies, and healthcare systems and industry all have a major role to play if we are to fulfil the long overdue promise of bringing pharmacogenomics into the clinical realm. Ethical issues must also be addressed, and comprehensive economic assessments will inform ethical allocation of resources.
Fig. 1.
Overview of PGx pillars and tools in supporting translation to clinical prescribing
Pharmacogenomics in Clinical Cardiovascular Medicine
Intensive and collaborative research, particularly following completion of the Human Genome Project in 2003, has markedly increased our insight into and understanding of pharmacogenomics. To date, the US Food and Drug Administration (FDA) approved product labels of 298 drugs contain genetic information within them [3]. The majority of these drugs are indicated in oncology (n = 102 drugs) and include both somatic and germline pharmacogenomic biomarkers, followed by psychiatry (n = 35 drugs), infectious diseases (n = 30), neurology (n = 27), anaesthesia (n = 16), and cardiology (n = 15), not including warfarin) [3]. Moreover, the clinical relevance of pharmacogenomics has been assessed for 123 drugs to date by guideline-writing committees, with therapeutic recommendations developed for just over 80 of these drugs and collated together in the PharmGKB repository [4]. These assessments have generally focused on well-researched drug-gene pairs, and often derive therapeutic recommendations to guide clinical prescribing from evaluating the body of evidence, rather than a single pivotal trial.
The majority of these drug-gene clinical assessments have been undertaken by the Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group (DPWG) and the Clinical Pharmacogenetics Implementation Consortium (CPIC), although the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) and other professional societies also contribute [4]. Several drug-gene pairs have overlapping guidance produced by more than one society, although, on the whole, a high rate of concordance exists between DPWG and CPIC published recommendations [5]. Of the assessed drugs, pharmacogenomic therapeutic recommendations are currently available for 12 cardiovascular drugs (Table 1). It is clear that several of these drugs are frequently prescribed in multiple settings including primary care, as well as by cardiology services, and so are relevant to a broad range of physicians.
Table 1.
Cardiovascular drug-gene pairs with actionable therapeutic recommendations in a pharmacogenomics guideline
| Drug | Gene | Guideline | PharmGKB evidence | |||
|---|---|---|---|---|---|---|
| CPIC | DPWG | CPNDS | RNPGx | |||
| Acenocoumarol | VKORC1 | ✓ | ✓ | Level 1A | ||
| CYP2C9 | ✓ | Level 1B | ||||
| Atorvastatin | SLCO1B1 | ✓ | Level 3 | |||
| Clopidogrel | CYP2C19 | ✓ | ✓ | ✓ | Level 1A | |
| Daunorubicin and Doxorubicin | RARG | ✓ | Level 3 | |||
| SLC28A3 | ✓ | Level 3 | ||||
| UGT1A6 | ✓ | Level 3 | ||||
| Flecainide | CYP2D6 | ✓ | Level 2A | |||
| Fluindione | VKORC1 | ✓ | Level 3 | |||
| CYP2C9 | ✓ | ND | ||||
| Metoprolol | CYP2D6 | ✓ | Level 2A | |||
| Phenprocoumon | VKORC1 | ✓ | Level 1A | |||
| Propafenone | CYP2D6 | ✓ | Level 2A | |||
| Simvastatin | SLCO1B1 | ✓ | ✓ | ✓ | Level 1A | |
| Warfarin | VKORC1 | ✓ | ✓ | ✓ | ✓ | Level 1A |
| CYP2C9 | ✓ | ✓ | ✓ | ✓ | Level 1A | |
| CYP4F2 | ✓ | Level 1A | ||||
| rs12777823 | ✓ | Level 1A | ||||
Extended from Dávila-Fajardo et al. [6]
CPIC, the Clinical Implementation Pharmacogenetics Consortium; CPNDS, the Canadian Pharmacogenomics Network for Drug Safety; DPWG, the Royal Dutch Association for the Advancement of Pharmacy-Pharmacogenetics Working Group; ND, not done; RNPGx, the French National Network of Pharmacogenetics
PharmGKB levels of evidence range from 1A (e.g. drug-variant pair is in a CPIC or medical society-endorsed pharmacogenomics guideline) to 4 (evidence based on a case report, non-significant study or in vitro evidence only) [7]
Established Cardiovascular Drug-Gene Associations
The evidence base underpinning drug-gene associations varies but, arguably for cardiology, is highest quality for warfarin, clopidogrel, and simvastatin. Thus, the pharmacogenomics of these drugs is briefly reviewed below; for further information about them, the reader is referred to their CPIC guidelines [8–10].
The main variants associated with warfarin response are rs9923231, a reduction-of-expression variant in VKORC1 (vitamin K epoxide reductase complex subunit 1) that encodes warfarin’s pharmacodynamic target and requires lower warfarin doses; reduction-of-function (ROF) variants in cytochrome P450 2C9 (CYP2C9, e.g. *2, *3, *5, *6, *8, *11) that decrease the metabolism of the more potent S-warfarin enantiomer leading to lower dose requirements; and rs2108622 (*3) in CYP4F2, which decreases vitamin K metabolism resulting in higher levels of reduced (active) vitamin K and so higher warfarin dose requirements [8, 11, 12] Most warfarin pharmacogenomics trials have tested the impact of a genotype-informed warfarin dosing algorithm on time in the therapeutic international normalised ratio range [13]. However, GIFT, the largest warfarin pharmacogenomics randomised controlled trial (RCT, n = 1597 analysed) to date, investigated a clinical composite outcome of INR ≥ 4, major bleeding, death, or venous thromboembolism, and found a significant decrease in the primary endpoint in the genotyped arm relative to the clinically guided arm (10.8% vs 14.7%, p = 0.02) [14].
Real-world implementation in anticoagulation clinics has shown warfarin pharmacogenomics to be feasible and beneficial [15]. Important considerations for warfarin pharmacogenomics include patient ethnicity to avoid misclassification of CYP2C9 metaboliser status, utilisation of pre-emptive or point-of-care genotyping as RCT protocols specified early use of genotyping algorithms (e.g. days 1–5 or 1–11 after starting warfarin), and the wider landscape of oral anticoagulation [13, 14, 16]. Clearly, the uptake of direct acting oral anticoagulants (DOACs) has correspondingly reduced the number of patients starting warfarin [17]. However, genetic analysis of ENGAGE AF-TIMI 48 trial data indicates that patients carrying warfarin- sensitising alleles (in VKORC1 and CYP2C9) have an increased risk of bleeding on warfarin compared to edoxaban, while bleeding risk does not differ between drugs in patients without warfarin-sensitive alleles [18]. This suggests that oral anticoagulation stratification based on VKORC1/CYP2C9 variants may provide an overarching anticoagulation prescribing strategy that could be clinically and cost-effective, but requires prospective testing [19].
Clopidogrel pharmacogenomics focuses on ROF variants in CYP2C19 (e.g. *2, *3) that reduce its biotransformation from prodrug into its active thiol metabolite and leave increased residual platelet reactivity [20]. The clinical indication for antiplatelet therapy appears important for clopidogrel-CYP2C19 with greater utility considered for percutaneous coronary intervention (PCI), particularly after an acute coronary syndrome, given the higher baseline risk of major adverse cardiovascular events (MACE) including stent thrombosis in these settings compared to other lower risk indications [9, 21]. Two recent RCTs, POPular and TAILOR-PCI, have been reported. POPUlar showed CYP2C19-informed antiplatelet stratification (CYP2C19 ROF carriers received ticagrelor or prasugrel, and non-carriers clopidogrel) was non-inferior to standard treatment with ticagrelor/prasugrel for net adverse events (p < 0.001 for non-inferiority) and reduced bleeding (p = 0.04) [22]. However, TAILOR-PCI narrowly missed its primary MACE endpoint comparing CYP2C19-informed antiplatelet stratification to standard care with clopidogrel (4.0% vs 5.9%, p = 0.06), but did observe that genotyping reduced both MACE when multiple events per patient were considered (p = 0.01) and, in post hoc analysis, MACE within the first 3 months (p = 0.001) [23]. Multisite assessment has demonstrated overall feasibility of implementing CYP2C19 and reported higher MACE in CYP2C19 ROF carriers prescribed clopidogrel versus alternative therapy [24, 25].
The rs4149056 (p.V174A) missense variant in the solute carrier organic anion transporter family member 1B1 (SLCO1B1) reduces the intrinsic activity of its encoded hepatic influx transporter, OATP1B1. This variant has been correlated with increased statin exposure, particularly for simvastatin acid, and consistently associated with simvastatin muscle toxicity ranging from mild events to severe myopathy and rhabdomyolysis [26–29]. Although a strong pharmacokinetic association is recognised between atorvastatin and SLCO1B1 rs4149056, its influence on atorvastatin muscle toxicity remains less clear than for simvastatin [30, 31]. In contrast to warfarin and clopidogrel, no prospective statin pharmacogenomics RCT has yet been reported, although the I-PICC RCT is underway [32]. Contra arguments to clinical utility maintain that prescribing statins other than simvastatin, or merely starting with low-dose simvastatin, could obviate any advantage of genetic testing. This approach, however, may underuse simvastatin or result in reluctance to up-titrate simvastatin appropriately. Furthermore, as genetic information is increasingly accessible, this testing would likely be included in panel, rather than stand-alone, pre-emptive testing.
Emerging Cardiovascular Pharmacogenomics
Cardio-oncology is a relatively new but expanding cross-disciplinary subspecialty, reflecting the rapid development of novel therapeutics, the increasing proportion of patients with cancer surviving long term, and the growing recognition of chemotherapeutic-associated cardiotoxicity [33]. Notably, anthracyclines are among the most commonly used chemotherapeutics for paediatric and adult leukaemia, lymphoma, and certain solid organ malignancies (e.g. breast, lung cancer), yet their overall effectiveness is limited by anthracycline-induced cardiotoxicity (ACT). There is high interindividual variability in susceptibility to and severity of ACT, ranging from asymptomatic cardiac function in up to 57% of exposed patients to congestive heart failure in 16–20% [34]. The CPNDS has developed clinical guidance recommending testing rs2229774 (S427L) in retinoic acid receptor gamma (RARG), rs7853758 (L461L) in SLC28A3, and rs17863783 in UGT1A6 in all paediatric cancer patients with an indication for doxorubicin or daunorubicin [34]. The underlying evidence is based on a limited number of observational genetic studies, without functional validation, rather than RCTs, is graded as moderate, and suggested management recommendations for patients at high ACT risk which include increased surveillance, liposomal anthracycline formulations, and alternative chemotherapy where possible [34–37].
There is also growing recognition that rare variants in cardiomyopathy-associated genes, particularly truncating variants in titin, are associated with increased risk of chemotherapeutic-associated cardiotoxicity and especially ACT [38, 39]. Titin is an enormous protein, encoded by ⁓ 363 exons, expressed in cardiac and skeletal muscle [40]. Titin truncating variants (nonsense, frameshift, essential splice site) underlie 25% of familial cases of idiopathic dilated cardiomyopathy, but truncating variants that affect all transcripts are rare in the general population [40, 41]. The majority of dilated cardiomyopathy cases are inherited in an autosomal dominant manner; the exact pathogenesis of titin truncating variants is unknown but a dominant negative mode of action, albeit not through a poisonous-protein mechanism, is thought more likely than haploinsufficiency [42, 43].
Polygenic Risk Scores—Practical Applications
Chronic cardiovascular disease presents a large burden to society [44]. Risk stratification is essential to determine where intervention is advisable, as well as the potential impact of different interventions. Current risk scores, which incorporate clinical and laboratory-based risk factors, can identify individuals at high risk suitable for selective preventative strategies, e.g. prescribing cholesterol-lowering medications for reducing coronary heart disease risk [45]. Such clinical cardiovascular risk calculators fail to identify up to 40% of people who develop cardiovascular disease and utility is limited among young individuals [45, 46].
Cardiovascular disease is polygenic; therefore, a single variant is not informative for assessing disease risk. Instead, genetic risk loading conferred by a combination of variants can predict those at high risk. This genetic risk is most often assessed through polygenic risk scores (PRSs), a weighted sum of a number of risk alleles. PRSs calculating this cumulative genetic burden have recently been shown to correlate with case status in coronary heart disease (CHD) [47].
This year, a number of studies on the role of coronary artery disease (CAD) PRS for risk stratification have been published with conflicting evidence [48–51]. Studies by Marston et al. and Elliott et al. addressed the clinical utility of large genome-wide polygenic risk scores which incorporated more than 6 million single-nucleotide polymorphisms in CAD prediction when compared with clinical risk stratification tools [49, 50]. Both of these studies demonstrated lack of clinical utility when polygenic risk scores were added to pooled cohort equations. These studies join several other published studies that examined polygenic risk scores for CAD across a broad range of population samples [47, 52–54]. Conversely, studies by Marston et al. and Damask et al. which analysed the role of CAD PRS for stratification of patients with pre-existing CAD who were enrolled in the trials of the PCSK9 inhibitors alirocumab and evolucumab found that, among individuals in the placebo arms, a high CAD PRS was strongly associated with cardiovascular events, even after adjusting for baseline traditional risk factors [50, 51]. In the treatment arms, individuals at high genetic risk were found to derive the greatest absolute and relative benefit from PCSK9 inhibitors.
These latter studies provide evidence that a CAD PRS predicts cardiovascular events even in the setting of pre-existing CAD, independent from traditional risk factors, and that clinical application of a CAD PRS in this setting could be used to titrate intensity of low-density lipoprotein cholesterol-lowering therapy. A more recent study by Mars et al., which set out to test the utility of PRSs derived from large-scale genomic information for predicting first disease events in five diseases including CAD, showed that adding PRSs to clinical risk prediction revealed two patterns with implications for clinical utility [55]. Firstly, for early-onset CAD, the CAD PRS identified individuals missed by clinical risk scores, comprising 13% of the early-onset cases. Most cardiovascular risk calculators have been trained with data on middle-aged individuals, and their ability to identify people at risk for early-onset CAD is therefore limited. Improved identification of these high-risk individuals could allow for targeted preventive efforts, e.g. targeting cholesterol-lowering treatments or lifestyle medication may be particularly useful in individuals with a high CAD PRS. Secondly, the CAD PRS improved reclassification of some older individuals into a lower risk category. As age is an important risk driver in most cardiovascular risk calculators and can therefore lead to false positives in older age groups, CAD PRSs may potentially reduce overestimation of risk and subsequent overtreatment, reducing polypharmacy.
Evidence regarding the use of PRSs in other fields of cardiovascular disease, such as Long QT Syndrome (LQTS), is also inconsistent; this condition highlights the tension between predictive value of rare or monogenic variants versus polygenic risk prediction in a phenotype which can be caused by both. Comprehensive genome-wide association analysis has not identified single common variants strongly associated with drug-induced Torsade de Pointes [56]. However, a polygenic risk score of 61 common variants associated with baseline QT interval has been associated with drug-induced QT prolongation and Torsade de Pointes [57]. In one of the largest and most comprehensive such studies of LQTS, Lahrouchi et al. pooled 1656 LQTS cases, finding that aggregate common variants are associated with LQTS and may be a distinct genetic subtype of LQTS [58]. The authors combined 68 primarily common variants previously associated with population QT variation into a PRS and found that a higher risk score was associated with LQTS in both European and Japanese ancestry cohorts. They also showed that genotype-negative individuals (i.e. without an identified rare LQTS variant) have a higher PRS than genotype-positive LQTS cases. This genotype-negative group had a robust phenotype (average QTc approximately 500 ms). These results suggest that a significant proportion of LQTS disease burden is explained by common variation, and that a higher burden of common QT interval-associated variants increases risk of overt LQTS in patients considered genotype-negative. Conversely, a recent study by Turkowski et al. of 423 patients genotyped for 61 variants associated with LQTS found that the variability in QTc is impacted most by the patient’s rare LQTS-causative variant rather than the PRS [59]. The PRS explained < 2% of the QTc in this cohort of patients.
The practical applications of polygenic risk information to stratified screening or for guiding lifestyle and medical interventions in the clinical setting remain to be defined in further studies and must be compared with existing gold standard care. The majority of the cardiovascular risk score literature is limited to European ancestry populations, and few researchers note the limitations that confers in clinical application to a broader and more diverse population (which could then worsen health disparities) [51].
Cost-effectiveness
Cost-effectiveness analysis measures efficacy of different means to achieve a named goal at the same cost. Cost-utility analysis can be understood as a more refined cost-effectiveness analysis, in which the benefit of an intervention is quantified by a multidimensional unit, such as quality adjusted life years (QALYs), which estimates benefit in the form of number of years of perfect quality of health gained from an intervention. As QALYs are multidimensional, all pharmacogenomic (PGx) proposed interventions can be compared to other such interventions as well as differing health interventions to understand the benefit to the populace per economic unit of an available budget.
Methodologic barriers to such studies in PGx include challenges to assessing the cost of the testing itself, as well as cost of integration of the testing within the clinical pathway, assessment of benefits, assessment of utility, and comparison to other possible interventions. In addition to the difficulties of measuring the direct cost/benefit of genomic testing, the lack of uniform practice as to incorporation of these results in the clinical, diagnostic, and therapeutic pathway makes estimates of indirect costs highly variable [60]. Some of these costs could and should be quantified, such as the time in a clinical consultation needed to discuss genomic testing results, but some, such as the burden of variants of unknown significance on clinician resources and accountability would be extremely difficult to quantify.
It also raises the question of costs of upskilling a clinician force who do not normally use genetic data to interpret it appropriately in this limited setting. There would need to be resources in place to support this work as confusion can be anticipated. Where this support would be situated and what the cost of such a service would be is extremely unclear and therefore cannot reasonably be estimated with certainty. There is also downstream cost in needed intervention for any incidentally discovered information. For example, if a potential genetic predisposition to drug-induced long QT syndrome, a condition associated with lethal arrhythmias, is detected, in addition to the prescribing implications, this person may appropriately be referred to an inherited channelopathy specialist and have further investigations which are cost-generating, which would not have been undertaken in absence of this test (and in absence of phenotypic disease expression or family history) [61]. There may be long-term demonstrable economic benefits as a result of these tests if early detection of individuals at risk modifies adverse outcomes, but these would need to be quantified as well (and risk stratification and modification in such cases is highly debatable in the context of asymptomatic screening) [61]. Cost-utility benefit may shift substantially as the cost of genomic testing decreases—thus, analysis should be updated as the cost of genetic testing changes dynamically. Existing cost-effectiveness analyses are sensitive to the cost of genotyping and should thus be adjusted as costs of genetic testing decrease [62]. Furthermore, health economic studies may not be generalisable outside of the geographical context of the study as they are dependent on allele frequency in a given population, which can vary widely between nations [63].
While most of the PGx health economic studies to date have found pharmacogenomics to be cost-effective, with an increasing emphasis on cost-utility analysis, there is uncertainty in these estimates [64–66]. Health outcomes are difficult to measure robustly with a clinical intervention in absence of a randomised controlled (RCT) study. Since very few RCTs have been performed comparing PGx testing guided therapy to gold standard therapeutic intervention without PGx testing, there is uncertainty about clinical benefit, and this cascades down to further extrapolated analysis, including economic analyses [60, 65, 67]. Additionally, it is unclear how patients may react to confrontation with their genetic information and how this may interface with lifestyle choices and medication compliance (adding uncertainty to calculation of benefit) [68].
The role of the cost-utility estimate is to make healthcare spending decisions transparent and objective, quantifying the cost/benefit ratio in a way that can compare various potential different interventions, treating different conditions, in different ways, with the available monetary resources of the health system in question. This addresses a core ethical principle of distributional justice and is important as each spending decision will have an opportunity cost; the cost of not having spent the money elsewhere on a different health intervention. Though some assumptions and uncertainties are unavoidable in health economic estimates, stating these factors and accounting for them in uniform ways across analyses can help to make cost-effectiveness comparisons more robust and will add to transparency in resource allocation decisions, which promotes public trust.
Ethical Issues in Clinical Application
Though PGx holds the key to many proven and possible improvements in clinical care, as detailed above, there remain ethical concerns which must be addressed prior to broad clinical application. These include, principally, confidentiality and data sharing, as well as manifold concerns generated by the use of genetic tests for non-diagnostic purposes. While questions of confidentially and data sharing can arguably be mediated by existing consensus on use of genetic information, pharmacogenomics enters a new dimension as it is a proposed population level screening and would likely be navigated by panels of genes or single-nucleotide polymorphisms leading to a level of genetic identification within a population that has not manifested from other routine uses of genetic testing.
Furthermore, when considering genetic testing in the context of PGx, rather than diagnostic testing, this could be considered a screening test. Comparison with accepted criteria for diverse genetic and non-genetic screening tests is therefore reasonable. The Wilson and Jungner World Health Organization (WHO) screening criteria were published more than 40 years ago but remain the touch stone to mediate the appropriateness of diverse proposed population wide screening measures [69]. It would be difficult to argue that PGx testing currently fulfils these original or modified WHO criteria [70]; specifically encountering problems with pre-symptomatic testing, suitability of various tests available; acceptability to the population; agreed policy on whom to select for this testing, with a not yet clearly defined target population; equity in access; and integration with education, testing, clinical services, and programme management. None of these are out of reach or absolute barriers, but the ethical facets are conjoined with the regulatory and legal infrastructure and must be addressed holistically before PGx unfolds on a population scale.
There are also ethical arguments for implementation of PGx in specific contexts where high quality evidence concludes better outcomes for patients when PGx is used as compared with standard care. This falls under an obligation to minimise harm as medical doctors prescribing therapeutics. This argument hinges on solid evidence of harm in gold standard prescribing care in the absence of PGx use; one such example is evidence for in stent thrombosis on clopidogrel for high-risk ACS patients, where there is an effective alternative therapeutic available and an adverse consequence of not checking for poor metaboliser status is foreseeable.
These are just a few prominent threads of ethical discourse related to PGx and illustrate the need for unified guidance and proactive planning to address ethical barriers to population level PGx implementation. Ideally professional guidelines, as well as national legislation and regulatory bodies, should address all below listed ethical domains prior to expanded PGx use in cardiovascular medicine:
Confidentiality and genetic data in the context of PGx, with specific provision for forensic use and breaches of confidentiality to prevent harm to relatives.
Privacy and data protection, with anticipation of large data repositories and big data sharing. The role of possible collaboration with private industry should be addressed.
Informed consent for PGx panel testing for screening purposes.
Transparency in evolving reclassification of genetic variants with emerging evidence.
Responsibility in the interpretation and actioning of genetic variants identified through PGx testing.
Distributional justice in health economics of PGx testing.
Social justice in PGx testing with limited understanding of variants in persons of diverse and admixed ethnic backgrounds.
Direct-to-Consumer Testing
Direct-to-consumer (DTC) genetic testing emerged in the early 2000s and the industry has rapidly grown, particularly since late 2016 [71–73]. The majority of products target ancestry or paternity testing, although multiple health-related DTC products are available [72]. Concerns over the variable standards of analytical and clinical validity and advertising practices led the FDA to intervene in the USA, stating that health-related DTC tests constitute medical devices, and now, DTC tests of moderate-to-high risk medical purposes require FDA clearance [71, 74, 75]. Subsequently, four 23andMe DTC tests have received FDA marketing authorisation, including a pharmacogenomics DTC panel [75]. Of note though, the DTC pharmacogenomics panel can report general information about whether the consumer has variants that influence drug levels, but cannot relate specific variants to clinical response or treatment recommendation for any specific drug [75]. Furthermore, the FDA issued a safety communication in 2018 specifically regarding DTC pharmacogenomic tests cautioning consumers and healthcare providers that many of the purported drug-gene associations being tested were neither scientifically nor clinically verified and these tests had not been reviewed by the FDA [76]. There is, however, recognition that the growing availability of DTC testing and public involvement will push the need to understand the ethical and social implications of integrating genomics into healthcare practice, as well as putting onus on the healthcare sector to improve workforce genomic literacy [77].
Genomics in Drug Discovery and Development
Genomics provides exciting tools to support drug discovery and development. The clinical goals of pharmacogenomics are to maximise drug efficacy, avoid adverse drug reactions, and target responsive patients. Although the FDA have included details of 298 drug-gene pair associations in drug labelling, only 15% of these associations are based on convincing randomised clinical trial (RCT) data [3, 78]. Genotype-based RCTs have only been initiated over the past 15 years. Using genotype as a biomarker of drug response or toxicity in RCTs enables the use of smaller sample sizes, a decrease in costs, an increase in the likelihood of success, and the minimisation of adverse events.
The results of such genotype-guided RCTs are varying, and highlight the challenges and barriers to delivering pharmacogenetics in a clinical setting [16, 20, 79–83]. Factors beyond the gene-drug relationship will impact widespread test adoption such as stakeholder acceptance of pharmacogenetics, provider and patient education, optimal messaging of pharmacogenetic pharmacotherapy recommendations, and robust information technology infrastructure.
Studies have been carried out that estimate that drug mechanisms with genetic support are twice as likely to succeed, compared to those without it (from phase I to approval) [84, 85]. Therefore, increasing the proportion of discovery and development activities focused on targets with genetic support and allowing genetic data to guide selection of the most appropriate indications should lead to lower rates of failure due to lack of efficacy in clinical development. Genetic resources that integrate all known genetic associations to identify potential causal disease pathways could be an important tool for drug discovery and development. Such a resource could be used to prioritise projects and help reduce attrition rates in clinical trials.
Given the high attrition rates, substantial costs and slow pace of new drug discovery and development, repurposing of ‘old’ drugs to treat both common and rare diseases is increasingly becoming an attractive proposition. This involves the use of de-risked compounds, with potentially lower overall development costs and shorter development timelines. This has recently been used for a number of traditional drugs for the treatment of COVID-19 [86, 87].
Drug repurposing (also called drug repositioning, reprofiling, or re-tasking) is a strategy for identifying new uses for approved or investigational drugs that are outside the scope of the original medical indication [88]. This strategy has improved in the past 20 years based on new discoveries including, more recently, genetic information [89–92]. Thus, where an existing drug targets a gene product or pathway of a disease different from the original indication, fewer clinical trials may be needed to alter the licenced indication, as safety has already been demonstrated. An example of repurposing is sildenafil, initially produced with the expectation of reducing angina, and later found to treat erectile dysfunction and pulmonary hypertension [93, 94]. Evidence exists for repurposing of drugs and candidates for drug development in the context of coronary artery disease, suggesting that in silico analysis using existing databases and genetic findings may be useful to accelerate translation into clinical practice [95, 96]. Clinical trials are now needed to explore the potential value of these agents. Population selection based on genotype could theoretically streamline repurposing.
Mendelian Randomisation
Mendelian randomisation (MR) is a technique which uses genetic proxies for exposures of interest to support causal association with an outcome of interest, under set assumptions [97]. As loci are randomly allocated during miosis events, this can be viewed as a genetic equivalent to a prospective randomised controlled trial, with randomisation at birth [98]. Therefore, MR is a form of experimentation that can add support for a causal relationship to an otherwise observational clinical cohort dataset prone to complex confounding and reverse causality [97].
This is highly relevant to cardiovascular pharmacology and serves as a useful mode of target validation for therapeutic design, as well as drug repurposing [99, 100]. MR can be done using retrospectively collected cohort data to support therapeutic target validation for repurposing prior to clinical trials. One study, for example, used genetic tools to mimic the action of an IL6 inhibitor, such as those used in rheumatoid arthritis (i.e. tocilizumab), to demonstrate decreased odds of coronary artery disease [101]. MR can also provide useful confirmation of a target of interest for drug design or to support a clinical trial. It may also be helpful in predicting negative trial results and adverse effects of drugs, and thereby avoiding taking therapeutics likely to be ineffective or harmful into clinical trials. One group of investigators used a PLA2G7 loss of function variant analogous to the use of the Lp-PLA2 inhibitor darapladib to reach conclusions concordant with negative clinical trials in that there was no effect on major vascular disease. The authors conclude that using such techniques could limit investment in clinical trials that might be predicted to be negative using genetic tools [102]. In another example, MR was used to demonstrate conclusions in keeping with clinical trial findings for ivabridine, using a HCN4 locus variant that mimics the effect of the therapeutic [103]. MR can also discourage further clinical investigation of hypothesised targeted therapy based on observational data that may be biased by confounding. An example is a study that did not find evidence to support a causal association between vitamin D or fatty acid supplementation and altered risk of major depressive disorder (such an association was suggested by observational data and therefore proposed as a potential therapeutic intervention) [104].
MR thus offers added value to existing data and can be used to test the effect of risk variants on an outcome of interest. This could be particularly helpful within the context of PGx, as higher quality evidence could be yielded from observational databases to link PGx variants of interest to adverse outcomes within a drug exposure group and thus predict the effect of PGx-guided changes in therapy to avoid adverse outcomes (via either decreased efficacy or increased toxicity). This may also be a pragmatic way to address demands for increased evidence prior to PGx implementation in the absence of prospective randomised controlled trials for every proposed drug-gene pair (clearly not a viable option due to scale). A Scottish team exemplified this approach by using a genetic variant as a tool to stratify diverse outcomes for stroke patients collecting clopidogrel prescriptions; they demonstrated increased risk in those post-stroke patients taking clopidogrel who had a loss of function variant in the enzyme needed to convert the inactive prodrug into its active metabolite [105]. There are limitations that need to be acknowledged, as with any methodology. Importantly, there is a risk of undetected horizontal pleiotropy if the variant used as a proxy for exposure impacts the outcome through mechanisms other than the modelled exposure [106].
Another potential use of MR in PGx is as a biomarker validation tool within complex systems [100]. This can be a biomarker of interest within the biologic pathway or may indicate genetic metabolism phenotype; MR can support or decry a causal relationship with a cardiovascular outcome of interest, which supports rational targeted therapeutic development and use [100]. An example is a negative MR study that used genetic markers for heightened CRP to conclude that CRP does not have a causal link with coronary disease [107].
MR can also be used as a pharmacovigilance tool [108]. Several studies showed increased risk of diabetes with LDL lipid lowering via two different pharmaceutical agents with differing drug targets and mechanisms of action. In one such study, the investigators used genetic PCSK9 variants as a tool to demonstrate that lower LDL lipids were accompanied by a higher fasting glucose, increased central adiposity, and increased risk of T2DM [109]. Likewise, an additional study used MR with genetic proxies for statin-targeted therapy, HMGCR variants to show that genetically simulated statin therapy, in addition to the desired lowering of LDL cholesterol, increased weight, central adiposity, fasting glucose and insulin levels, and risk of new onset type 2 diabetes [110]. A further study found that genetically simulated exposure to PCSK9 inhibitors increased the risk of Alzheimer’s dementia more than it decreased risk of coronary artery disease [111]. A MR of antihypertensives identified a new association of non-dihydropyridine calcium channel blockers with a small increase in risk of diverticulosis [112]. MR has also been proposed as a pandemic tool of choice to investigate areas of therapeutic debate such as the use of ACEi/ARBS in patients with Covid-19 [113].
MR holds much promise in PGx going forward, in target validation, repurposing, pharmacovigilance, and interfacing with polygenic risk scores. Furthermore, use of MR as a screening tool may increase the efficacy of funds invested in clinical trials. As with other forms of large genetic data resources, academia may collaborate with industry, particularly direct-to-consumer (DTC) testing, in an evolving way in the future (due to the benefits of pooled resources and the scale of DTC testing undertaken). It is important to consider the scientific benefits of increased collaboration as well as the significant potential ethical and regulatory concerns.
Future Directions—Projections
It might be said that the promise of a genomics revolution in healthcare has grown long in the tooth. However, over the last decade, multiple early adopters have implemented pharmacogenomics into clinical practice demonstrating feasibility, patient benefits, and shared lessons learned [24, 25, 114–116]. While these early starters have been mostly specialised academic centres, at least 14 national genomic medicine initiatives are now underway [117]. For example, following on from the 100,000 Genomes Project, the UK has committed to sequencing five million genomes in 5 years, and pharmacogenomics is anticipated to be an integral facet of the NHS Genomic Medicine Service [118, 119].
These initiatives are driving transformative change while addressing implementation barriers and accruing real-world big data for analysis to generate the evidence for further adoption [117]. To illustrate, a recent large-scale pharmacogenomics analysis using UK Biobank (200 drugs; 9 genes; 200,000 participants) has confirmed several drug-gene associations (e.g. warfarin-CYP2C9, simvastatin-SLCO1B1), provided strong evidence for relatively novel associations (e.g. warfarin-rs3814637 in CYP2C19), and discovered new associations (e.g. nicardipine dose and CYP2C19 metaboliser status) [120]. More generally, a transition from current healthcare models to learning healthcare systems/communities that bring healthcare delivery, real-world data collection, evidence generation, implementation, and follow-up outcome monitoring all closer together into an iterative positive cycle will optimise returns on investment in genomic and other novel technologies and so improve healthcare quality, encourage patient engagement, and increase the rate of adoption of validated new findings [121]. This must be coupled with guidance from professional bodies to support prescribers in implementing pharmacogenomic tools that are currently validated for clinical use.
Iterative implementation of cardiovascular pharmacogenomics within such a system will require increased multidisciplinary working, for instance between primary care physicians, clinical pharmacologists, pharmacists, cardiologists, geneticists, bioinformaticians, clinical academics, patient advocacy groups, funding stakeholders, and industry partners (e.g. genomic providers including DTC services and app developers). Coupling of large datasets to artificial intelligence and machine learning approaches will offer further insights, for example, facilitating interpretation of previously uncharacterised combinations of variants. For example, a neural network model has improved CYP2D6 genotype-to-phenotype translation from sequenced data, which may have utility with flecainide and propafenone as well as metoprolol and other beta blockers metabolised by CYP2D6 [122, 123].
Furthermore, while RCTs represent gold standard evidence, there are inherent limitations to pharmacogenomic RCTs including:
the number of drug-gene/variant associations identified in observational data is outstripping the resources and time required to individually test them in RCTs,
differences in variants between ethnicities can limit RCT generalisability,
pharmacogenomic RCTs can require relatively large sample sizes due to only a proportion carrying the variant(s) of interest, and
there remains a lack of consensus on the evidential threshold required for prescription optimisation biomarkers such as pharmacovariants [23, 124].
Thus, real-world big data are expected to play an increasingly prominent role in generating the evidence to inform appropriate utilisation of pharmacogenomics.
Moving forward, polygenic risk scores for cardiovascular diseases combined with clinical risk factors may refine individual risk predictions to facilitate more informed patient-physician interactions regarding the benefits of starting cardiovascular (e.g. primary prevention) drugs for the individual patient. Forthcoming polygenic risk scores are also anticipated to improve adverse drug reaction risk predictions. Advances in prediction of toxicity, such as drug-induced LQTS, may be facilitated by basic science studies using in vitro models as demonstrated by prior work in the context of drug-induced liver injury [125]. Integration of genomic data with other -omics data (e.g. transcriptomics, proteomics, metabolomics) into multi-omics models is improving our understanding of cardiovascular and drug actions; the latter is exemplified by a systems pharmacology approach describing how antiretroviral therapy can alter the activity of an atherosclerotic regulatory gene networks and so may promote coronary artery disease [126, 127]. Importantly, such systems biology approaches, as well as Mendelian randomisation and human gene knockout investigations, are expected to drive development of novel therapeutics in the cardiovascular space, such as novel drugs to stabilise atherosclerotic plaques.
Lastly, pharmacogenomics will also offer a route to understand adverse event signals that emerge from novel therapeutics. Fortunately to date, the anti-PCSK9 siRNA therapeutic, inclisiran, has not shown haematological or immunological adverse events [128]. However, such events and, in particular, thrombocytopaenia, have been reported with a range of antisense oligonucleotide (ASO) therapeutics. It has been observed that phosphorothioate-containing ASOs can bind platelet proteins including platelet factor 4, which is reminiscent of heparin-induced thrombocytopaenia that has been associated with pharmacogenomic signals, suggesting that ASO safety pharmacogenomics studies could be informative [129, 130].
Author contributions
EFM and JCK conceptualised the review. EFM, RMT, and AJ drafted the review. EFM and JCK revised the review. PB and MP provided key input to content and revised the review. All authors have approved the review.
Declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest
MP receives research funding from various organisations including the MRC, NIHR, EU Commission, HDR UK, and Health Education England. He has also received partnership funding for the following: MRC Clinical Pharmacology Training Scheme (co-funded by MRC and Roche, UCB, Eli Lilly, and Novartis); a PhD studentship jointly funded by EPSRC and AstraZeneca; and grant funding from Vistagen Therapeutics. He has also unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb and UCB. He has developed an HLA genotyping panel with MC Diagnostics, but does not benefit financially from this. None of the funding declared above has been used for the current paper. The other authors declare no conflict of interest.
Footnotes
This article belongs to the Topical Collection: Translating genome medicine to treatments
EF Magavern, JC Kaski, P Borry, and M Pirmohamed are joint first and last authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease—implications for personalized medicine. Touyz RM, ed. Pharmacol Rev. 2013;65:987–1009. doi: 10.1124/pr.112.007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminkeng F, Ross CJD, Rassekh SR, Hwang S, Rieder MJ, Bhavsar AP, Smith A, Sanatani S, Gelmon KA, Bernstein D, Hayden MR, Amstutz U, Carleton BC. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016;82:683–695. doi: 10.1111/bcp.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food & Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. 2020.
- 4.PharmGKB. Clinical Guideline Annotations. 2020.
- 5.Bank P. Caudle K, Swen J, Gammal R, Whirl-Carrillo M, Klein T, Relling M, Guchelaar H. Comparison of the guidelines of the clinical pharmacogenetics implementation consortium and the Dutch pharmacogenetics working group. Clin Pharmacol Ther. 2018;103:599–618. doi: 10.1002/cpt.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dávila-Fajardo CL, Díaz-Villamarín X, Antúnez-Rodríguez A, Fernández-Gómez AE, García-Navas P, Martínez-González LJ, et al. Pharmacogenetics in the treatment of cardiovascular diseases and its current progress regarding implementation in the clinical routine. Genes (Basel). MDPI AG. 2019. [DOI] [PMC free article] [PubMed]
- 7.PharmGKB. Clinical annotation levels of evidence. 2020.
- 8.Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, Lee MT, Gage BF, Kimmel SE, Perera MA, Anderson JL, Pirmohamed M, Klein TE, Limdi NA, Cavallari LH, Wadelius M. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther Nature Publishing Group. 2017;102:397–404. doi: 10.1002/cpt.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott SA, Sangkuhl K, Stein CM, Hulot J-S, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, Krauss RM, Roden DM, Feng Q, Cooper-DeHoff RM, Gong L, Klein TE, Wadelius M, Niemi M. The Clinical Pharmacogenomics Implementation Consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean L. Warfarin therapy and VKORC1 and CYP genotype. Med. Genet. Summ. National Center for Biotechnology Information (US); 2012. [PubMed]
- 12.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol Mol Pharmacol. 2009;75:1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirmohamed M, Kamali F, Daly AK, Wadelius M. Oral anticoagulation: a critique of recent advances and controversies. Trends Pharmacol Sci Elsevier Ltd. 2015:153–63. [DOI] [PubMed]
- 14.Gage BF, Bass AR, Lin H, Woller SC, Stevens SM, Al-Hammadi N, Li J, Rodríguez T, Miller JP, McMillin GA, Pendleton RC, Jaffer AK, King CR, BDV W, Porche-Sorbet R, Napoli L, Merritt K, Thompson AM, Hyun G, Anderson JL, Hollomon W, Barrack RL, Nunley RM, Moskowitz G, Dávila-Román V, Eby CS. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT randomized clinical trial. JAMA - J Am Med Assoc American Medical Association. 2017;318:1115–1124. doi: 10.1001/jama.2017.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen AL, Prince C, Fitzgerald G, Hanson A, Downing J, Reynolds J, Zhang JE, Alfirevic A, Pirmohamed M. Implementation of genotype-guided dosing of warfarin with point-of-care genetic testing in three UK clinics: a matched cohort study. BMC Med BioMed Central Ltd. 2019;17:76. doi: 10.1186/s12916-019-1308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, Kesteven P, Christersson C, Wahlström B, Stafberg C, Zhang JE, Leathart JB, Kohnke H, Maitland-van der Zee AH, Williamson PR, Daly AK, Avery P, Kamali F, Wadelius M. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med Massachussetts Medical Society. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 17.Ho KH, Van HM, Leng G. Trends in anticoagulant prescribing: a review of local policies in English primary care. BMC Health Serv Res BioMed Central Ltd. 2020:279. [DOI] [PMC free article] [PubMed]
- 18.Mega JL, Walker JR, Ruff CT, Vandell AG, Nordio F, Deenadayalu N, Murphy SA, Lee J, Mercuri MF, Giugliano RP, Antman EM, Braunwald E, Sabatine MS. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet Lancet Publishing Group. 2015;385:2280–2287. doi: 10.1016/S0140-6736(14)61994-2. [DOI] [PubMed] [Google Scholar]
- 19.Pirmohamed M. Warfarin: the end or the end of one size fits all therapy? J. Pers. Med. MDPI AG; 2018. p. 22. [DOI] [PMC free article] [PubMed]
- 20.Pereira NL, Rihal CS, So DYF, Rosenberg Y, Lennon RJ, Mathew V, Goodman SG, Weinshilboum RM, Wang L, Baudhuin LM, Lerman A, Hasan A, Iturriaga E, Fu YP, Geller N, Bailey K, Farkouh ME. Clopidogrel pharmacogenetics state-of-the-art review and the TAILOR-PCI study. Circ Cardiovasc Interv Lippincott Williams and Wilkins. 2019;12:e007811. doi: 10.1161/CIRCINTERVENTIONS.119.007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JA, Roden DM, Lesko LJ, Ashley E, Klein TE, Shuldiner AR. Clopidogrel: a case for indication-specific pharmacogenetics. Clin. Pharmacol. Ther. NIH Public Access; 2012. p. 774–776. [DOI] [PMC free article] [PubMed]
- 22.Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, t Hof AWJ van, Harst P van der, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, Mosterd A, Herrman J-PR, Dewilde WJM, Janssen PWA, Kelder JC, Postma MJ, Boer A de, Boersma C, Deneer VHM, Berg JM ten. A genotype-guided strategy for Oral P2Y 12 inhibitors in primary PCI. N Engl J Med Massachussetts Medical Society; 2019;381:1621–1631. [DOI] [PubMed]
- 23.Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae J-H, Jeong MH, Chavez I, Gordon P, Abbott JD, Cagin C, Baudhuin L, Fu Y-P, Goodman SG, Hasan A, Iturriaga E, Lerman A, Sidhu M, Tanguay J-F, Wang L, Weinshilboum R, Welsh R, Rosenberg Y, Bailey K, Rihal C. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention. JAMA. 2020;324:761–771. doi: 10.1001/jama.2020.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Empey PE, Stevenson JM, Tuteja S, Weitzel KW, Angiolillo DJ, Beitelshees AL, Coons JC, Duarte JD, Franchi F, Jeng LJB, Johnson JA, Kreutz RP, Limdi NA, Maloney KA, Owusu Obeng A, Peterson JF, Petry N, Pratt VM, Rollini F, Scott SA, Skaar TC, Vesely MR, Stouffer GA, Wilke RA, Cavallari LH, Lee CR. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther Nature Publishing Group. 2018;104:664–674. doi: 10.1002/cpt.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM. Duarte JD, Voora D, Kimmel SE, McDonough CW, Gong Y, Dave CV, Pratt VM, Alestock TD, Anderson RD, Alsip J, Ardati AK, Brott BC, Brown L, Chumnumwat S, Clare-Salzler MJ, Coons JC, Denny JC, Dillon C, Elsey AR, Hamadeh IS, Harada S, Hillegass WB, Hines L, Horenstein RB, Howell LA, Jeng LJB, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv Elsevier Inc. 2018;11:181–191. doi: 10.1016/j.jcin.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nies AT, Niemi M, Burk O, Winter S, Zanger UM, Stieger B, et al. Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. Genome Med Genome Med. 2013;5. [DOI] [PMC free article] [PubMed]
- 27.Elsby R, Hilgendorf C, Fenner K. Understanding the critical disposition pathways of statins to assess drugdrug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther. 2012;92:584–598. doi: 10.1038/clpt.2012.163. [DOI] [PubMed] [Google Scholar]
- 28.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 29.Turner RM, Radman I, Bozina N, Alfirevic A. Pharmacogenetics and statin-related myopathy: what do we know? Pharmacogenomics Future Medicine Ltd. 2020;21:821–825. doi: 10.2217/pgs-2020-0041. [DOI] [PubMed] [Google Scholar]
- 30.Turner RM, Fontana V, Zhang JE, Carr D, Yin P, FitzGerald R, Morris AP, Pirmohamed M. A genome-wide association study of circulating levels of atorvastatin and its major metabolites. Clin Pharmacol Ther Nature Publishing Group. 2020;108:287–297. doi: 10.1002/cpt.1820. [DOI] [PubMed] [Google Scholar]
- 31.Turner RM, Pirmohamed M. Statin-related myotoxicity: a comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J Clin Med. 2019;9:22. doi: 10.3390/jcm9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Integrating pharmacogenetics in clinical care (I-PICC, NCT02871934). 2020.
- 33.Fradley MG. Cardio-oncology fellowship training and education. Curr. Treat. Options Cardiovasc. Med. Springer Healthcare; 2019. p. 1–10. [DOI] [PubMed]
- 34.Aminkeng F, Ross CJD, Rassekh SR, Hwang S, Rieder MJ, Bhavsar AP, et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol Blackwell Publishing Ltd. 2016:683–95. [DOI] [PMC free article] [PubMed]
- 35.Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, Brunham LR, Caron HN, Van Dalen EC, Kremer LC, Van Der Pal HJ, Amstutz U, Rieder MJ, Bernstein D, Carleton BC, Hayden MR, Ross CJD. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet Nature Publishing Group. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visscher H, Ross CJD, Rassekh SR, Sandor GSS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Rogers PC, Rieder MJ, Carleton BC, Hayden MR. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer John Wiley & Sons, Ltd. 2013;60:1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 37.Visscher H, Ross CJD, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, Sandor GS, Caron HN, Van Dalen EC, Kremer LC, Van Der Pal HJ, Brown AMK, Rogers PC, Phillips MS, Rieder MJ, Carleton BC, Hayden MR. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, Ito K, Willcox JA, Arany Z, Li J, Owens AT, Govind R, Nuñez B, Mazaika E, Bayes-Genis A, Walsh R, Finkelman B, Lupon J, Whiffin N, Serrano I, Midwinter W, Wilk A, Bardaji A, Ingold N, Buchan R, Tayal U, et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation Lippincott Williams and Wilkins. 2019;140:31–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linschoten M, Teske AJ, Baas AF, Vink A, Dooijes D, Baars HF, Asselbergs FW. Truncating titin (TTN) variants in chemotherapy-induced cardiomyopathy. J Card Fail Churchill Livingstone Inc. 2017;23:476–479. doi: 10.1016/j.cardfail.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Akinrinade O, Koskenvuo JW, Alastalo TP. Prevalence of titin truncating variants in general population. PLoS One Public Library of Science. 2015;10. [DOI] [PMC free article] [PubMed]
- 41.Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJR, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabish AM, Azzimato V, Alexiadis A, Buyandelger B, Knöll R. Genetic epidemiology of titin-truncating variants in the etiology of dilated cardiomyopathy. Biophys Rev Springer Verlag. 2017:207–23. [DOI] [PMC free article] [PubMed]
- 43.Azad A, Poloni G, Sontayananon N, Jiang H, Gehmlich K. The giant titin: how to evaluate its role in cardiomyopathies. J Muscle Res Cell Motil Springer International Publishing. 2019;40:159–167. doi: 10.1007/s10974-019-09518-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017. Lancet 2017;
- 45.F. Piepoli M. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representati. Int J Behav Med 2017; [DOI] [PubMed]
- 46.Lindbohm JV, Sipilä PN, Mars NJ, Pentti J, Ahmadi-Abhari S, Brunner EJ, Shipley MJ, Singh-Manoux A, Tabak AG, Kivimäki M. 5-year versus risk-category-specific screening intervals for cardiovascular disease prevention: a cohort study. Lancet Public Health. 2019;4:e189–e199. doi: 10.1016/S2468-2667(19)30023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosley JD, Gupta DK, Tan J, Yao J, Wells QS, Shaffer CM, Kundu S, Robinson-Cohen C, Psaty BM, Rich SS, Post WS, Guo X, Rotter JI, Roden DM, Gerszten RE, Wang TJ. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020;323:627. doi: 10.1001/jama.2019.21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC, Elliott P, Tzoulaki I. Predictive accuracy of a polygenic risk score–enhanced prediction model vs a clinical risk score for coronary artery disease. JAMA. 2020;323:636. doi: 10.1001/jama.2019.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marston N, Kamanu F, Nordio F, Gurmu Y, Roselli C, Sever P, Pedersen T, Keech A, Wang H, Lira Pineda A, Giugliano R, Lubitz S, Ellinor P, Sabatine M, Ruff C. Predicting benefit from evolocumab therapy in patients with atherosclerotic disease using a genetic risk score: results from the FOURIER Trial. Circulation (C) 2020 by the American College of Cardiology Foundation and the American Heart Association, Inc.: TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (N.A.M., F.K.K., F.N., Y.G., R.P.G., M.S.S., C.T.R.).; 2020;141:616–623. [DOI] [PMC free article] [PubMed]
- 51.Damask A, Steg P, Schwartz G, Szarek M, Hagstrom E, Badimon L, et al. Patients with high genome-wide polygenic risk scores for coronary artery disease may receive greater clinical benefit from alirocumab treatment in the ODYSSEY OUTCOMES Trial. Circulation (C) 2020 by the American College of Cardiology Foundation and the American Heart Association, Inc.: Regeneron Pharmaceuticals Inc, Tarrytown, NY (A.D., P.B., G.M., R.P., J.D.O., L.A.L., G.D.Y., G.R.A., A.B., C.P.). 2020;141:624–36. [DOI] [PubMed]
- 52.Inouye M, Abraham G, Nelson C, Wood A, Sweeting M, Dudbridge F, et al. Genomic risk prediction of coronary artery disease in nearly 500,000 adults: implications for early screening and primary prevention. bioRxiv. 2018. [DOI] [PMC free article] [PubMed]
- 53.Wünnemann F, Sin Lo K, Langford-Avelar A, Busseuil D, Dubé MP, Tardif JC, et al. Validation of genome-wide polygenic risk scores for coronary artery disease in French Canadians. Circ Genomic Precis Med. 2019. [DOI] [PMC free article] [PubMed]
- 54.Iribarren C, Lu M, Jorgenson E, Martínez M, Lluis-Ganella C, Subirana I, Salas E, Elosua R. Clinical utility of multimarker genetic risk scores for prediction of incident coronary heart disease: a cohort study among over 51 thousand individuals of European ancestry. Circ Cardiovasc Genet. 2016;9:531–540. doi: 10.1161/CIRCGENETICS.116.001522. [DOI] [PubMed] [Google Scholar]
- 55.Mars N, Koskela JT, Ripatti P, Kiiskinen TTJ, Havulinna AS, Lindbohm JV, et al. Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020. [DOI] [PubMed]
- 56.Behr ER, Ritchie MD, Tanaka T, Kääb S, Crawford DC, Nicoletti P, Floratos A, Sinner MF, Kannankeril PJ, Wilde AAM, Bezzina CR, Schulze-Bahr E, Zumhagen S, Guicheney P, Bishopric NH, Marshall V, Shakir S, Dalageorgou C, Bevan S, Jamshidi Y, Bastiaenen R, Myerburg RJ, Schott JJ, Camm AJ, Steinbeck G, Norris K, Altman RB, Tatonetti NP, Jeffery S, Kubo M, Nakamura Y, Shen Y, George AL, Roden DM. Genome wide analysis of drug-induced torsades de pointes: lack of common variants with large effect sizes. PLoS One. 2013;8:e78511. doi: 10.1371/journal.pone.0078511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strauss DG, Vicente J, Johannesen L, Blinova K, Mason JW, Weeke P, et al. Common genetic variant risk score is associated with drug-induced QT prolongation and torsade de pointes risk. Circulation. 2017. [DOI] [PMC free article] [PubMed]
- 58.Lahrouchi N, Tadros R, Crotti L, Mizusawa Y, Postema PG, Beekman L, et al. Transethnic genome-wide association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation. 2020. [DOI] [PMC free article] [PubMed]
- 59.Turkowski KL, Dotzler SM, Tester DJ, Giudicessi JR, Bos JM, Speziale AD, et al. Corrected QT interval-polygenic risk score and its contribution to type 1, type 2, and type 3 long-QT syndrome in probands and genotype-positive family members. Circ Genomic Precis Med. 2020;13. [DOI] [PubMed]
- 60.Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, Wilson R, Bick D, Bottinger EP, Brilliant MH, Eng C, Frazer KA, Korf B, Ledbetter DH, Lupski JR, Marsh C, Mrazek D, Murray MF, O’Donnell PH, Rader DJ, Relling MV, Shuldiner AR, Valle D, Weinshilboum R, Green ED, Ginsburg GS. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niemeijer MN, van den Berg ME, Eijgelsheim M, Rijnbeek PR, Stricker BH. Pharmacogenetics of drug-induced QT interval prolongation: an update. Drug Saf. 2015;38:855–867. doi: 10.1007/s40264-015-0316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meckley LM, Gudgeon JM, Anderson JL, Williams MS, Veenstra DL. A policy model to evaluate the benefits, risks and costs of warfarin pharmacogenomic testing. Pharmacoeconomics. 2010;28:61–74. doi: 10.2165/11318240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y, Krebs K, Milani L, Lauschke VM. Global frequencies of clinically important HLA alleles and their implications for the cost-effectiveness of preemptive pharmacogenetic testing. Clin Pharmacol Ther 2020;cpt.1944. [DOI] [PubMed]
- 64.Berm EJJ, Looff M de, Wilffert B, Boersma C, Annemans L, Vegter S, Boven JFM van, Postma MJ. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second Update of the Literature. Bruns H, ed. PLoS One 2016;11:e0146262 [DOI] [PMC free article] [PubMed]
- 65.Phillips KA, Ann Sakowski J, Trosman J, Douglas MP, Liang S-Y, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genet Med. 2014;16:251–257. doi: 10.1038/gim.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharm J. 2017;17:395–402. doi: 10.1038/tpj.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Swanson KM, Rojas RL, Wang Z, Sauver JLS, Visscher SL, Prokop LJ, Bielinski SJ, Wang L, Weinshilboum R, Borah BJ. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–486. doi: 10.1038/s41436-019-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics. 2013;14:1833–1847. doi: 10.2217/pgs.13.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson J, Jungner G. Principles and practice of screening for disease. Geneva. 1986.
- 70.Andermann, Anne , Blancquaert, Ingeborg, Beauchamp, Sylvie, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. 2008. https://www.who.int/bulletin/volumes/86/4/07-050112/en/#R6 [DOI] [PMC free article] [PubMed]
- 71.Allyse MA, Robinson DH, Ferber MJ, Sharp RR. Direct-to-Consumer Testing 2.0: emerging models of direct-to-consumer genetic testing. Mayo Clin Proc Elsevier Ltd. 2018:113–20. [DOI] [PubMed]
- 72.Phillips AM. ‘Only a click away-DTC genetics for ancestry, health, love. . .and more: a view of the business and regulatory landscape’. Appl Transl Genomics Elsevier B.V.; 2016;8:16–22. [DOI] [PMC free article] [PubMed]
- 73.Regalado Antonio. 2017 was the year consumer DNA testing blew up | MIT Technology Review. 2018.
- 74.Green RC, Farahany NA. Regulation: the FDA is overcautious on consumer genomics. Nature Nature. 2014;505:286–287. doi: 10.1038/505286a. [DOI] [PubMed] [Google Scholar]
- 75.US Food & Drug Administration. Direct-to-consumer tests. 2019.
- 76.US Food & Drug Administration. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA safety communication. 2018.
- 77.Rafi I, Crinson I, Dawes M, Rafi D, Pirmohamed M, Walter FM. The implementation of pharmacogenomics into UK general practice: a qualitative study exploring barriers, challenges and opportunities. J Community Genet Springer. 2020;11:269–277. doi: 10.1007/s12687-020-00468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang B, Canestaro WJ, Choudhry NK. Clinical evidence supporting pharmacogenomic biomarker testing provided in US Food and Drug Administration drug labels. JAMA Intern Med 2014; [DOI] [PubMed]
- 79.Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, Rosenberg YD, Eby CS, Madigan RA, McBane RB, Abdel-Rahman SZ, Stevens SM, Yale S, Mohler ER, Fang MC, Shah V, Horenstein RB, Limdi NA, Muldowney JAS, Gujral J, Delafontaine P, Desnick RJ, Ortel TL, Billett HH, Pendleton RC, Geller NL, Halperin JL, Goldhaber SZ, Caldwell MD, Califf RM, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med Massachussetts Medical Society. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergmeijer TO, Janssen PWA, Schipper JC, Qaderdan K, Ishak M, Ruitenbeek RS, Asselbergs FW, Van’T Hof AWJ, Dewilde WJM, Spanó F, Herrman JPR, Kelder JC, Postma MJ, De Boer A, Deneer VHM, Ten Berg JM. CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients-rationale and design of the Patient Outcome after primary PCI (POPular) genetics study. Am Heart J. 2014;168:16–22.e1. doi: 10.1016/j.ahj.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Peyser B, Perry EP, Singh K, Gill RD, Mehan MR, Haga SB, Musty MD, Milazzo NA, Savard D, Li Y-J, Trujilio G, Voora D. Effects of delivering SLCO1B1 pharmacogenetic information in randomized trial and observational settings. Circ Genomic Precis Med. 2018;11:e002228. doi: 10.1161/CIRCGEN.118.002228. [DOI] [PubMed] [Google Scholar]
- 82.Tardif J-C, Dubé M-P, Pfeffer MA, Waters DD, Koenig W, Maggioni AP, McMurray JJV, Mooser V, White HD, Heinonen T, Black DM, Guertin M-C. Study design of Dal-GenE, a pharmacogenetic trial targeting reduction of cardiovascular events with dalcetrapib. Am Heart J. 2020;222:157–165. doi: 10.1016/j.ahj.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Piccini JP, Abraham WT, Dufton C, Carroll IA, Healey JS, van Veldhuisen DJ, Sauer WH, Anand IS, White M, Wilton SB, Aleong R, Rienstra M, Krueger SK, Ayala-Paredes F, Khaykin Y, Merkely B, Miloradović V, Wranicz JK, Ilkhanoff L, Ziegler PD, Davis G, Emery LL, Marshall D, Kao DP, Bristow MR, Connolly SJ. Bucindolol for the maintenance of sinus rhythm in a genotype-defined HF population. JACC Hear Fail. 2019;7:586–598. doi: 10.1016/j.jchf.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, Sham PC, Li MJ, Wang J, Cardon LR, Whittaker JC, Sanseau P. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–860. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 85.King EA, Wade Davis J, Degner JF. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet 2019; [DOI] [PMC free article] [PubMed]
- 86.Wang X, Guan Y. COVID-19 drug repurposing: a review of computational screening methods, clinical trials, and protein interaction assays. Med Res Rev. 2020. [DOI] [PMC free article] [PubMed]
- 87.Lima WG, Brito JCM, Overhage J, Nizer WS da C. The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): a systematic review. Arch Virol. 2020;165:1729–1737. doi: 10.1007/s00705-020-04693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 89.Xue H, Li J, Xie H, Wang Y. Review of drug repositioning approaches and resources. Int J Biol Sci. 2018;14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2018. [DOI] [PubMed]
- 91.Oprea TI, Mestres J. Drug repurposing: far beyond new targets for old drugs. AAPS J. 2012;14:759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanseau P, Agarwal P, Barnes MR, Pastinen T, Richards JB, Cardon LR, Mooser V. Use of genome-wide association studies for drug repositioning. Nat. Biotechnol. 2012. [DOI] [PubMed]
- 93.Arrowsmith J, Harrison R. Drug repositioning: the business case and current strategies to repurpose shelved candidates and marketed drugs. Bringing New Life to Shelved Assets and Existing Drugs: Drug Repositioning; 2012. [Google Scholar]
- 94.Melnikova I. Rare diseases and orphan drugs. Nat Rev Drug Discov. 2012;11:267–268. doi: 10.1038/nrd3654. [DOI] [PubMed] [Google Scholar]
- 95.Tragante V, Hemerich D, Alshabeeb M, Brænne I, Lempiäinen H, Patel RS, et al. Druggability of coronary artery disease risk loci. Circ Genomic Precis Med. 2018. [DOI] [PMC free article] [PubMed]
- 96.Lempiäinen H, Brænne I, Michoel T, Tragante V, Vilne B, Webb TR, et al. Network analysis of coronary artery disease risk genes elucidates disease mechanisms and druggable targets. Sci Rep. 2018. [DOI] [PMC free article] [PubMed]
- 97.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018:k601. [DOI] [PMC free article] [PubMed]
- 98.Hingorani A, Humphries S. Nature’s randomised trials. Lancet. 2005;366:1906–1908. doi: 10.1016/S0140-6736(05)67767-7. [DOI] [PubMed] [Google Scholar]
- 99.Orho-Melander M. Genetics of coronary heart disease: towards causal mechanisms, novel drug targets and more personalized prevention. J Intern Med. 2015;278:433–446. doi: 10.1111/joim.12407. [DOI] [PubMed] [Google Scholar]
- 100.Denny JC, Van Driest SL, Wei W-Q, Roden DM. The influence of big (clinical) data and genomics on precision medicine and drug development. Clin Pharmacol Ther. 2018;103:409–418. doi: 10.1002/cpt.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. [DOI] [PMC free article] [PubMed]
- 102.Millwood IY, Bennett DA, Walters RG, Clarke R, Waterworth D, Johnson T, et al. Lipoprotein-associated phospholipase A 2 loss-of-function variant and risk of vascular diseases in 90,000 Chinese adults. J Am Coll Cardiol. 2016;67:230–1. [DOI] [PMC free article] [PubMed]
- 103.Legault M-A, Sandoval J, Provost S, Barhdadi A, Lemieux Perreault L-P, Shah S, Lumbers RT, Denus S de, Tyl B, Tardif J-C, Dubé M-P. A genetic model of ivabradine recapitulates results from randomized clinical trials. Mordi I, ed. PLoS One 2020;15:e0236193 [DOI] [PMC free article] [PubMed]
- 104.Milaneschi Y, Peyrot WJ, Nivard MG, Mbarek H, Boomsma DI, W.J.H. Penninx B. A role for vitamin D and omega-3 fatty acids in major depression? An exploration using genomics. Transl Psychiatry. 2019;9:219. doi: 10.1038/s41398-019-0554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tornio A, Flynn R, Morant S, Velten E, Palmer CNA, MacDonald TM, Doney ASF. Investigating real-world clopidogrel pharmacogenetics in stroke using a bioresource linked to electronic medical records. Clin Pharmacol Ther. 2018;103:281–286. doi: 10.1002/cpt.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hemani G, Bowden J, Davey SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27:R195–R208. doi: 10.1093/hmg/ddy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548–8. [DOI] [PMC free article] [PubMed]
- 108.Walker VM, Davey Smith G, Davies NM, Martin RM. Mendelian randomization: a novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int J Epidemiol. 2017;46:2078–2089. doi: 10.1093/ije/dyx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, Hartwig FP, Horta BL, Hyppönen E, Power C, Moldovan M, van Iperen E, Hovingh GK, Demuth I, Norman K, Steinhagen-Thiessen E, Demuth J, Bertram L, Liu T, Coassin S, Willeit J, Kiechl S, Willeit K, Mason D, Wright J, Morris R, Wanamethee G, Whincup P, Ben-Shlomo Y, McLachlan S, Price JF, Kivimaki M, Welch C, Sanchez-Galvez A, Marques-Vidal P, Nicolaides A, Panayiotou AG, Onland-Moret NC, van der Schouw Y, Matullo G, Fiorito G, Guarrera S, Sacerdote C, Wareham NJ, Langenberg C, Scott R, Luan J, Bobak M, Malyutina S, Pająk A, Kubinova R, Tamosiunas A, Pikhart H, Husemoen LL, Grarup N, Pedersen O, Hansen T, Linneberg A, Simonsen KS, Cooper J, Humphries SE, Brilliant M, Kitchner T, Hakonarson H, Carrell DS, McCarty C, Kirchner HL, Larson EB, Crosslin DR, de Andrade M, Roden DM, Denny JC, Carty C, Hancock S, Attia J, Holliday E, O’Donnell M, Yusuf S, Chong M, Pare G, van der Harst P, Said MA, Eppinga RN, Verweij N, Snieder H, LifeLines Cohort study group. Christen T, Mook-Kanamori DO, Gustafsson S, Lind L, Ingelsson E, Pazoki R, Franco O, Hofman A, Uitterlinden A, Dehghan A, Teumer A, Baumeister S, Dörr M, Lerch MM, Völker U, Völzke H, Ward J, Pell JP, Smith DJ, Meade T, Maitland-van der Zee A, Baranova EV, Young R, Ford I, Campbell A, Padmanabhan S, Bots ML, Grobbee DE, Froguel P, Thuillier D, Balkau B, Bonnefond A, Cariou B, Smart M, Bao Y, Kumari M, Mahajan A, Ridker PM, Chasman DI, Reiner AP, Lange LA, Ritchie MD, Asselbergs FW, Casas JP, Keating BJ, Preiss D, Hingorani AD, UCLEB consortium. Sattar N. PCSK9 genetic variants and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2017;5:97–105. doi: 10.1016/S2213-8587(16)30396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JEL, Shah T, Sofat R, Stender S, Johnson PCD, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MCW, Tzoulaki I, Buxbaum SG, A DL van der, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385:351–361. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams DM, Finan C, Schmidt AF, Burgess S, Hingorani AD. Lipid lowering and Alzheimer disease risk: a Mendelian randomization study. Ann Neurol. 2020;87:30–39. doi: 10.1002/ana.25642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gill D, Georgakis MK, Koskeridis F, Jiang L, Feng Q, Wei W-Q, Theodoratou E, Elliott P, Denny JC, Malik R, Evangelou E, Dehghan A, Dichgans M, Tzoulaki I. Use of genetic variants related to antihypertensive drugs to inform on efficacy and side effects. Circulation. 2019;140:270–279. doi: 10.1161/CIRCULATIONAHA.118.038814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teng Y, Xu J, Zhang Y, Liu Z, Zhang S. Mendelian randomization in COVID-19: applications for cardiovascular comorbidities and beyond. EBioMedicine. 2020;57:102847. doi: 10.1016/j.ebiom.2020.102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van der Wouden C, Cambon-Thomsen A, Cecchin E, Cheung K, Dávila-Fajardo C, Deneer V, Dolžan V, Ingelman-Sundberg M, Jönsson S, Karlsson M, Kriek M, Mitropoulou C, Patrinos G, Pirmohamed M, Samwald M, Schaeffeler E, Schwab M, Steinberger D, Stingl J, Sunder-Plassmann G, Toffoli G, Turner R, van Rhenen M, Swen J, Guchelaar H-J. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther. 2017;101:341–358. doi: 10.1002/cpt.602. [DOI] [PubMed] [Google Scholar]
- 115.Van Der WCH, Böhringer S, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VHM, Dolžan V, Ingelman-Sundberg M, Jönsson S, Karlsson MO, Kriek M, Mitropoulou C, Patrinos GP, Pirmohamed M, Rial-Sebbag E, Samwald M, Schwab M, Steinberger D, Stingl J, Sunder-Plassmann G, Toffoli G, Turner RM, Rhenen MH Van. Van ZE, Swen JJ, Guchelaar HJ. Generating evidence for precision medicine: considerations made by the Ubiquitous Pharmacogenomics Consortium when designing and operationalizing the PREPARE study. Pharmacogenet Genomics Lippincott Williams and Wilkins. 2020;30:131–144. doi: 10.1097/FPC.0000000000000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zouk H, Venner E, Lennon NJ, Muzny DM, Abrams D, Adunyah S, Albertson-Junkans L, Ames DC, Appelbaum P, Aronson S, Aufox S, Babb LJ, Balasubramanian A, Bangash H, Basford M, Bastarache L, Baxter S, Behr M, Benoit B, Bhoj E, Bielinski SJ, Bland ST, Blout C, Borthwick K, Bottinger EP, Bowser M, Brand H, Brilliant M, Brodeur W, Caraballo P, Carrell D, Carroll A, Almoguera B, Castillo L, Castro V, Chandanavelli G, Chiang T, Chisholm RL, Christensen KD, Chung W, Chute CG, City B, Cobb BL, Connolly JJ, Crane P, Crew K, Crosslin D, de Andrade M, de la Cruz J, Denson S, Denny J, DeSmet T, Dikilitas O, Friedrich C, Fullerton SM, Funke B, Gabriel S, Gainer V, Gharavi A, Glazer AM, Glessner JT, Goehringer J, Gordon AS, Graham C, Green RC, Gundelach JH, Dayal J, Hain HS, Hakonarson H, Harden MV, Harley J, Harr M, Hartzler A, Hayes MG, Hebbring S, Henrikson N, Hershey A, Hoell C, Holm I, Howell KM, Hripcsak G, Hu J, Jarvik GP, Jayaseelan JC, Jiang Y, Joo YY, Jose S, Josyula NS, Justice AE, Kalla SE, Kalra D, Karlson E, Kelly MA, Keating BJ, Kenny EE, Key D, Kiryluk K, Kitchner T, Klanderman B, Klee E, Kochan DC, Korchina V, Kottyan L, Kovar C, Kudalkar E, Kullo IJ, Lammers P, Larson EB, Lebo MS, Leduc M, Lee MT(M), Leppig KA, Leslie ND, Li R, Liang WH, Lin CF, Linder J, Lindor NM, Lingren T, Linneman JG, Liu C, Liu W, Liu X, Lynch J, Lyon H, Macbeth A, Mahadeshwar H, Mahanta L, Malin B, Manolio T, Marasa M, Marsolo K, Dinsmore MJ, Dodge S, Hynes ED, Dunlea P, Edwards TL, Eng CM, Fasel D, Fedotov A, Feng Q, Fleharty M, Foster A, Freimuth R, McGowan ML, McNally E, Meldrim J, Mentch F, Mosley J, Mukherjee S, Mullen TE, Muniz J, Murdock DR, Murphy S, Murugan M, Myers MF, Namjou B, Ni Y, Obeng AO, Onofrio RC, Taylor CO, Person TN, Peterson JF, Petukhova L, Pisieczko CJ, Pratap S, Prows CA, Puckelwartz MJ, Rahm AK, Raj R, Ralston JD, Ramaprasan A, Ramirez A, Rasmussen L, Rasmussen-Torvik L, Rasouly HM, Raychaudhuri S, Ritchie MD, Rives C, Riza B, Roden D, Rosenthal EA, Santani A, Schaid D, Scherer S, Scott S, Scrol A, Sengupta S, Shang N, Sharma H, Sharp RR, Singh R, Sleiman PMA, Slowik K, Smith JC, Smith ME, Smoller JW, Sohn S, Stanaway IB, Starren J, Stroud M, Su J, Tolwinski K, van Driest SL, Vargas SM, Varugheese M, Veenstra D, Verbitsky M, Vicente G, Wagner M, Walker K, Walunas T, Wang L, Wang Q, Wei WQ, Weiss ST, Wiesner GL, Wells Q, Weng C, White PS, Wiley KL, Jr, Williams JL, Williams MS, Wilson MW, Witkowski L, Woods LA, Woolf B, Wu TJ, Wynn J, Yang Y, Yi V, Zhang G, Zhang L, Rehm HL, Gibbs RA. Harmonizing clinical sequencing and interpretation for the eMERGE III network. Am J Hum Genet Cell Press. 2019;105:588–605. doi: 10.1016/j.ajhg.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, Levy Y, Glazer D, Wilson J, Lawler M, Boughtwood T, Braithwaite J, Goodhand P, Birney E, North KN. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet. 2019;104:13–20. doi: 10.1016/j.ajhg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Department of Health and Social Care. Matt Hancock announces ambition to map 5 million genomes. 2018.
- 119.Robinson J. Everything you need to know about the NHS genomic medicine service. Pharm J Royal Pharmaceutical Society; 2020;
- 120.Mcinnes G, Altman RB. Drug response pharmacogenetics for 200,000 UK Biobank Participants. bioRxiv Cold Spring Harbor Laboratory; 2020;2020.08.09.243311. [PMC free article] [PubMed]
- 121.Mullins CD, Wingate LT, Edwards HA, Tofade T, Wutoh A. Transitioning from learning healthcare systems to learning health care communities. J Comp Eff Res Future Medicine Ltd. 2018;7:603–614. doi: 10.2217/cer-2017-0105. [DOI] [PubMed] [Google Scholar]
- 122.Lee M v d, Allard W, Vossen R, Baak-Pablo R, Menafra R, Deiman B, Deenen M, Neven P, Johansson I, Gastaldello S, Ingelman-Sundberg M, Guchelaar H-J, Swen J, Anvar SY. A unifying model to predict variable drug response for personalised medicine. bioRxiv Cold Spring Harbor Laboratory. 2020;2020:03.02.967554. [Google Scholar]
- 123.Zisaki A, Miskovic L, Hatzimanikatis V. Antihypertensive drugs metabolism: an update to pharmacokinetic profiles and computational approaches. Curr Pharm Des Bentham Science Publishers Ltd. 2014;21:806–822. doi: 10.2174/1381612820666141024151119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turner RM, Pirmohamed M. Cardiovascular pharmacogenomics: expectations and practical benefits. Clin Pharmacol Ther Clin Pharmacol Ther. 2014;95:281–293. doi: 10.1038/clpt.2013.234. [DOI] [PubMed] [Google Scholar]
- 125.Koido M, Kawakami E, Fukumura J, Noguchi Y, Ohori M, Nio Y, Nicoletti P, Aithal GP, Daly AK, Watkins PB, Anayama H, Dragan Y, Shinozawa T, Takebe T. Polygenic architecture informs potential vulnerability to drug-induced liver injury. Nat Med. 2020;26:1541–1548. doi: 10.1038/s41591-020-1023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leon-Mimila P, Wang J, Huertas-Vazquez A. Relevance of multi-omics studies in cardiovascular diseases. Front Cardiovasc Med Frontiers Media SA. 2019:91. [DOI] [PMC free article] [PubMed]
- 127.Frades I, Readhead B, Amadori L, Koplev S, Talukdar HA, Crane HM, Crane PK, Kovacic JC, Dudley JT, Giannarelli C, Björkegren JLM, Peter I. Systems pharmacology identifies an arterial wall regulatory gene network mediating coronary artery disease side effects of antiretroviral therapy. Circ Genomic Precis Med Lippincott Williams and Wilkins. 2019;12:262–272. doi: 10.1161/CIRCGEN.118.002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Landmesser U, Haghikia A, Leiter LA, Wright RS, Kallend D, Wijngaard P, et al. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: a pre-specified analysis from ORION-1. Cardiovasc Res Oxford University Press (OUP). 2020. [DOI] [PubMed]
- 129.Sewing S, Roth AB, Winter M, Dieckmann A, Bertinetti-Lapatki C, Tessier Y, McGinnis C, Huber S, Koller E, Ploix C, Reed JC, Singer T, Rothfuss A. Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. Schulz C, ed. PLoS One Public Library of Science; 2017;12:e0187574 [DOI] [PMC free article] [PubMed]
- 130.Karnes JH, Cronin RM, Rollin J, Teumer A, Pouplard C, Shaffer CM, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost Schattauer GmbH. 2015;113:772–81. [DOI] [PMC free article] [PubMed]