Abstract
Patient: Female, 51-year-old
Final Diagnosis: Drug-induced hypersensitivity syndrome, consistent with DRESS • human herpesvirus 6 reactivation
Symptoms: Liver dysfunction • appearance of a skin rash • eosinophilia • fever
Medication: —
Clinical Procedure: —
Specialty: Allergology • Infectious Diseases
Objective:
Rare disease
Background:
Infection with human herpesvirus 6 (HHV-6) is a recognized risk factor for the development of drug-induced hypersensitivity syndrome (DIHS). DIHS is a systemic autoimmune condition that presents with mucocutaneous lesions of varying severity and comprises 3 subtypes: toxic epidermal necrolysis, Stevens–Johnson syndrome, and drug reaction with eosinophilia and systemic symptoms (DRESS). Here, we describe the case of a 51-year-old woman with a diagnosis of DIHS associated with carbamazepine, reactivation of HHV-6, and acute liver failure, which was consistent with DRESS.
Case Report:
We present the case of a 51-year-old Japanese woman who had been taking carbamazepine for epilepsy for the past 3 weeks. She presented with a fever, liver dysfunction, eosinophilia, and the sudden appearance of a skin rash. Steroid therapy was started for suspected drug-induced liver injury. The skin eruption disappeared, and liver dysfunction showed an improving trend. However, after stopping steroid, the pyrexia and eosinophilia reappeared. Therefore, prednisolone was re-administrated. HHV-6 DNA was detected, so HHV-6 reactivation was confirmed. Carbamazepine was stopped, and the clinical manifestations improved. She was ultimately diagnosed with DIHS, consistent with DRESS, associated with carbamazepine and HHV-6 reactivation, and liver dysfunction was assessed histologically. Therefore, the drug-related hepatotoxicity of carbamazepine played a role in causing liver damage rather than HHV-6 infection at that time.
Conclusions:
We describe a case of DIHS that was also associated with acute liver failure, consistent with DRESS. The case highlights the importance of making the correct diagnosis, as well as the management of mucocutaneous lesions and other systemic conditions (including acute liver failure).
MeSH Keywords: Liver Failure, Acute; Herpesvirus 6, Human; Drug Hypersensitivity Syndrome; Carbamazepine
Background
Drug-induced hypersensitivity syndrome (DIHS) is one of the most serious drug-related reactions, similar to toxic epidermal necrosis and Steven-Johnson syndrome [1]. DIHS is known to have several unique characteristics. Its clinical features include systemic skin disorder, a high pyrexia, liver dysfunction, emerging of atypical lymphocytes, eosinophilia, and lymph-adenopathy. These reactions usually occur 2 to 5 weeks after initiating treatment with the causal agent. A limited number of drugs, including carbamazepine, phenobarbital, allopurinol, and salazosulfapyridine, are implicated in the induction of this syndrome. DIHS is also related to viral reactivation, especially human herpesvirus 6 (HHV-6). Infection with HHV-6 is a recognized risk factor for DIHS development [2]. HHV-6 is a highly seroprevalent virus, and primary infection usually occurs in infancy, followed by a lifelong latent infection in the host. However, in cases of DIHS, HHV-6 reactivation specifically has been shown to occur, typically 2 to 3 weeks after the onset of a rash. In such cases, DIHS shows 2-stage clinical features: an early drug allergic reaction phase and a late HHV-6 reactivation phase. Therefore, both drug allergy and viral infection are considered to contribute to the clinical conditions of DIHS.
We describe a case of DIHS that was also associated with acute liver failure. This scenario was consistent with drug reaction with eosinophilia and systemic symptoms (DRESS) [3]. This situation highlights the importance of making the correct diagnosis, as well as management of mucocutaneous lesions and other systemic conditions (including acute liver failure).
Case Report
A 51-year-old Japanese woman was admitted to our hospital because of an episode of epilepsy in mid-October. She had a history of schizophrenia and was taking multiple medications for it. Three weeks earlier, she was prescribed oral carbamazepine for treatment of epilepsy. As epilepsy was not seen on admission, a further examination was performed. The laboratory data upon hospital admission are shown in Table 1. Her peripheral blood concentrations of carbamazepine and valproic acid were within the normal ranges.
Table 1.
The laboratory data on admission.
| Hematology | Range | ||
| WBC | 13940 | /µL | (3210–9680) |
| RBC | 448 | ×104/µL | (384–492) |
| Hb | 14.0 | g/dL | (11.7–15.1) |
| Plt | 24.3 | ×104/µL | (15.0–36.0) |
| Biochemistry | Range | ||
| TP | 8.5 | g/dL | (6.5–8.2) |
| Alb | 4.9 | g/dL | (4.3–5.4) |
| T-Bil | 0.4 | mg/dL | (0.2–1.2) |
| D-Bil | 0.1 | mg/dL | (0–0.6) |
| AST | 19 | U/L | (10–32) |
| ALT | 22 | U/L | (7–27) |
| LDH | 152 | U/L | (118–257) |
| γ-GTP | 33 | U/L | (5–55) |
| ALP | 247 | U/L | (99–340) |
| ChE | 375 | U/L | (207–452) |
| Na | 136 | mEq/L | (135–148) |
| K | 5.0 | mEq/L | (3.5–5.0) |
| Cl | 102 | mEq/L | (96–111) |
| BUN | 25.2 | mg/dL | (9–20) |
| Cre | 60 | mmol/L | (25–81) |
She developed a fever of ≥38°C around her 5th hospital day and started cefcapene pivoxil hydrochloride hydrate (CFPN-PI). Thereafter, as the patient was found to have abnormally elevated liver enzyme levels [aspartate aminotransferase 276 U/L, alanine aminotransferase (ALT) 498 U/L] with a prolongation of the prothrombin time such as international normalized ratio 1.6, she was referred to our department for further evaluation of liver dysfunction on the 8th hospital day. A physical examination showed no remarkable findings, and no lymph-adenopathy was detected. Abdominal computed tomography showed no findings of cholecystitis, cholangitis, or liver abscess (Figure 1). The Roussel Uclaf Causality Assessment Method (RUCAM) scale was 7 points and 6 points for CFPN-PI and carbamazepine, respectively, so drug-induced liver injury was highly likely. CFPN-PI was withdrawn at that point because of possible involvement of the agent in the deterioration of the liver enzyme levels. A drug-induced lymphocyte stimulation test (DLST) for CFPN-PI and carbamazepine was subsequently performed. DLST was positive for CFPN-PI but negative for carbamazepine. However, despite the cessation of CFPNPI, a skin rash suddenly spread over her entire body as confluent erythematous exanthema on the 11th day. Furthermore, the ALT levels were elevated on day 13. The laboratory data at this point are shown in Table 2. Mild leukocytosis with increased eosinophils and an elevated C-reactive protein level were noted in her peripheral blood. The IgG level decreased to 727 mg/dL. Anti-hepatitis B surface antibody, anti-hepatitis C antibody, anti-nuclear antibodies, and anti-mitochondrial antibodies were negative. No Epstein-Barr virus or cytomegalovirus infection was noted (Table 3).
Figure 1.
(A, B) Computed tomography of the abdomen. Abdominal computed tomography showed no findings of hepatosplenomegaly, cholecystitis, cholangitis, or a liver mass, including abscess.
Table 2.
The laboratory data on day 13 after admission.
| Hematology | ||
| WBC | 12110 | /µL |
| Neutrophils | 66.2 | % |
| Lymphocyte | 8.1 | % |
| Monocyte | 3.2 | % |
| Eosinophils | 20.8 | % |
| Basophils | 0.4 | % |
| LUC | 1.3 | % |
| RBC | 289 | ×104/µL |
| Hb | 9.1 | g/dL |
| Plt | 24.3 | ×104/µL |
| Coagulation | ||
| PT-INR | 1.60 | |
| PT | 47.0 | % |
| APTT | 36.9 | sec |
| AT3 | 81 | % |
| Fibrinogen | 507.8 | mg/dL |
| FDP | 8.6 | µg/dL |
| Biochemistry | ||
| TP | 4.8 | g/dL |
| Alb | 2.1 | g/dL |
| T-Bil | 0.6 | mg/dL |
| D-Bil | 0.4 | mg/dL |
| AST | 143 | U/L |
| ALT | 329 | U/L |
| LDH | 328 | U/L |
| γ-GTP | 990 | U/L |
| ALP | 1244 | U/L |
| ChE | 116 | U/L |
| Na | 131 | mEq/L |
| K | 3.5 | mEq/L |
| Cl | 100 | mEq/L |
| BUN | 24.3 | mg/dL |
| Cre | 2.1 | mg/dL |
| CRP | 12.6 | mg/dL |
| Immunology | ||
| Ferritin | ||
| TSH | 5.27 | µIU/mL |
| FT3 | 1.42 | pg/mL |
| FT4 | 0.80 | ng/dL |
| IgG | 727 | mg/dL |
| IgA | 113 | mg/dL |
| IgM | 126 | mg/dL |
| ANA | <×40 | |
| AMA | <×20 | |
| PR3-ANCA | <×10 | |
| MPO-ANCA | <×10 | |
| Tumor markers | ||
| AFP | 1.2 | ng/mL |
ANA – anti-nuclear antibodies; AMA – anti-mitochondrial antibodies.
Table 3.
Infectious disease markers on day 13 after admission.
| Infection marker | ||
|---|---|---|
| HBs Ag | 0.1 | IU/mL |
| HCV Ab | 0.1 | S/CO |
| HEV IgM | <5 | |
| HEV IgG | <5 | |
| CMV IgM | <×10 | |
| CMV IgG | ×160 | |
| CMV antigen | Negative | |
| EBV-VCA-IgM | <×10 | |
| EBV-VCA-IgG | ×10 | |
| EBV-EBNA | <×10 | |
| HPV B19 IgM | 0.45 | |
| HPV B19 IgG | 1.13 | |
| HSV IgM | <×10 | |
| HSV IgG | ×40 | |
| HHV-6 IgM | ×10 | |
| HHV-6 IgG | ×640 | |
| Tsutsugamushi (Kato, Gilliam) IgM | <×10 | |
| Tsutsugamushi (Kato, Gilliam) IgG | <×10 | |
| Endotoxin | <1.0 | pg/mL |
| β-D-glucan | <6.0 | pg/mL |
HBs Ag – anti-hepatitis B surface antibody; HCV Ab – anti-hepatitis C antibody; CMV – Cytomegalovirus; EBV – Epstein-Barr virus; HPV B19 – human parvovirus B19; HSV – herpes simplex virus; HHV-6 – human herpesvirus 6.
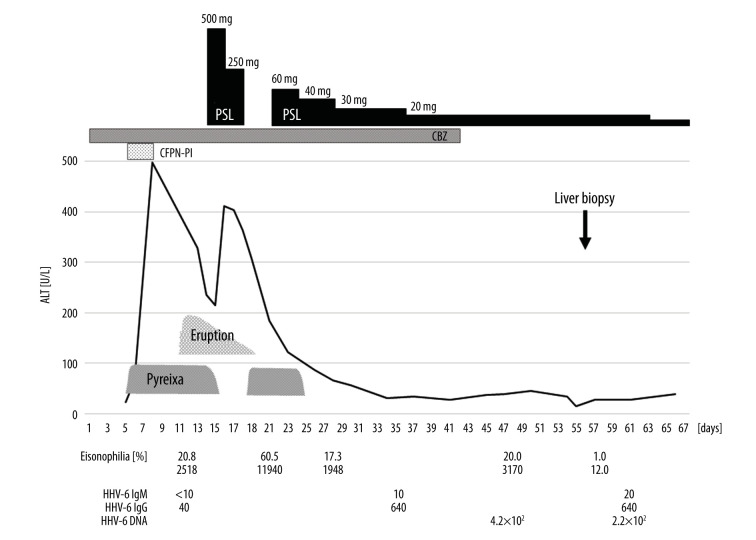
She was placed on steroid pulse treatment using 500 mg/day methylprednisolone injection for 3 consecutive days, followed by 250 mg/day, which was then stopped on day 18. The systemic eruption and high fever disappeared entirely. However, the eosinophil counts, which initially decreased, thereafter increased to 60.5% (11940/µL), and the pyrexia reappeared. Therefore, prednisolone (PSL) was re-administered at the initial daily dose of 60 mg on day 21 and then tapered to 40 mg/day on day 25. As a result, the high fever and eosinophilia gradually disappeared.
Based on the presence of a systemic eruption, high pyrexia, increased number of eosinophils, liver dysfunction, and multiple medications for schizophrenia, we suspected her of having DIHS, presumably caused by carbamazepine. Eosinophilia and systemic liver involvement are consistent with DRESS. Carbamazepine was therefore stopped on day 42. However, the hepatic injury and eosinophilia persisted for a further 2 weeks.
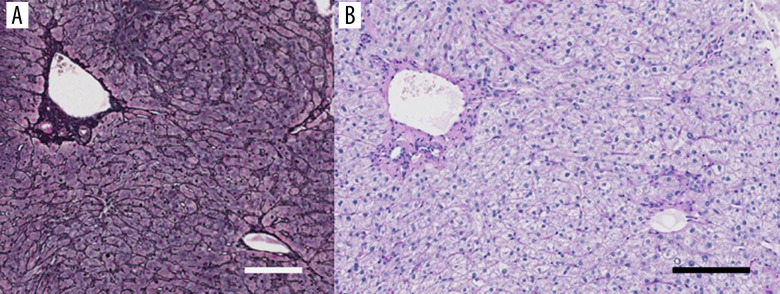
Regarding the HHV-6 virus marker, HHV-6 IgG reached 1: 640 on day 37. Furthermore, HHV-6 DNA (measured by RT-PCR) was detected on day 47. A liver biopsy specimen showed acute-phase liver damage including preservation of the liver architecture without enlargement of the portal tract, and some lymphocytic infiltration into the sinusoids and portal tracts was noted, with no obvious inclusion bodies; these findings suggested HHV-6 infection (Figure 2).
Figure 2.
The histological examination of the liver biopsy specimen. (A) The lobular architecture is well preserved. Grocott’s methenamine sliver impregnation. Bar: 100 µm. (B) Some lymphocytic infiltrations are seen in the sinusoids as well as the portal area, although no fibrous enlargement of the portal tract is seen. No inclusion bodies are found in the hepatocytes. Periodic acid-Schiff-diastase (PAS-D) reaction after diastase digestion. Bar: 100 µm.
PSL was gradually tapered to 10 mg/day, and the patient was ultimately discharged from our hospital on the 67th day (Figure 3).
Figure 3.
Clinical course. Because of fever and elevated liver enzyme levels, cefcapene pivoxil hydrochloride hydrate (CFPN-PI) was immediately withdrawn. However, the eosinophil count increased. In addition, an erythematous rash suddenly developed and promptly spread over the entire body. Steroid pulse therapy was administered for 6 days, which resolved the skin eruption and tended to improve the liver dysfunction. However, after stopping steroid pulse therapy, pyrexia and eosinophilia reappeared. With the re-introduction of prednisolone (PSL) therapy, the clinical manifestations improved. We also confirmed the presence of human herpesvirus 6 (HHV-6). Although carbamazepine was ceased, hepatic injury and eosinophilia persisted for a further 2 weeks. However, these consecutive clinical abnormalities eventually resolved.
Discussion
Initially, drug-induced liver injury was suspected, but DIHS was considered because the RUCAM scale score [4] suggested other symptoms, and the latter did not improve upon discontinuation of antibiotics.
DIHS has 2 defining clinical features: an early drug allergic reaction phase and a late herpes viral (especially HHV-6) reactivation phase [5,6]. DIHS is typically diagnosed by the presence of the following 7 criteria: 1) maculopapular rash developing after starting causative drugs; 2) prolonged clinical symptoms; 3) a fever >38°C; 4) liver abnormalities; 5) leukocytosis, atypical lymphocytosis, and eosinophilia; 6) lymphadenopathy; and 7) human herpesvirus 6 reactivation. The presence of 5 of these criteria is classified as atypical DIHS [3].
Our patient developed skin erythematous eruption after starting carbamazepine, including prolonged clinical symptoms after the discontinuation of carbamazepine, a high fever, acute hepatic injury, and an increased number of eosinophils. Those features met the diagnostic criteria for atypical DIHS, and the type of DIHS was likely to be DRESS [3].
Drugs are commonly associated with DIHS. Symptoms of DIHS occur 2–5 weeks after initiating treatment with the causal agent, such as carbamazepine, phenobarbital, allopurinol, or salazosulfapyridine [5,7–11]. Our patient had received numerous drugs before and after admission to our hospital. Although the DLST results were negative for carbamazepine and positive for CFPN-PI, carbamazepine was suspected as the most likely causal agent, rather than CFPN-PI, because eruption developed despite of cessation of CFPN-PI. Furthermore, a previous report noted that positive DLST reactions were obtained at the recovery stage but not the acute stage in DIHS, regardless of treatment with systemic PSL [12]. The proliferation of regulatory T cells can induce a negative DLST response during the acute phase of DIHS. In addition, the proliferation of regulatory T cells can suppress anti-viral immune responses, resulting in the induction of HHV-6 reactivation [13,14]. Regulatory T cells proliferating during the acute phase of DIHS can also contribute to a decreased B cell population and reduced levels of serum gamma-globulin, particularly IgG [15].
HHV-6 reactivation is a key feature for diagnosing DIHS. Both HHV-6 isolation and increased HHV-6 IgG titers without HHV-6 IgM elevation at 4 to 5 weeks after the onset of rash indicated HHV-6 reactivation in our patient. Regarding acute liver failure, liver abnormalities occur in up to 70% of patients [7], and both drug and HHV-6 are known to cause liver injury. We were able to closely investigate the liver specimen of this case. Although HHV-6 DNA has been detected in peripheral blood, there were no characteristic findings suggesting HHV-6 infection in the liver biopsy specimen. Therefore, we considered that drug-related hepatotoxicity of carbamazepine played an essential etiological role in liver damage rather than viral hepatitis due to HHV-6 infection in the present case of DIHS. However, a previous report found that HHV-6 is not only lymphotropic but can also infect hepatocytes [16], and, generally, it is extremely difficult to detect HHV-6 protein by immunohistochemistry in liver biopsy specimens. Therefore, it is necessary further to investigate manifestations of HHV-6 in liver tissue.
Although we had to consider the early perception of that clinical condition, and then discontinuance of carbamazepine, the primary therapy for DIHS is the administration systemic corticosteroids [7]. However, rapid discontinuance of steroid has been reported to exacerbate DIHS [17]. It is therefore recommended that the steroid dose be reduced gradually. In this case, we consider that the rapid discontinuance of steroid therapy led to an exacerbation of DIHS.
Conclusions
We described a case of DIHS that was also associated with acute liver failure, which was consistent with DRESS. This case study highlights the importance of making the correct diagnosis as well as the management of mucocutaneous lesions and other systemic conditions (including acute liver failure).
Acknowledgments
The authors greatly appreciate the assistance of the many persons who cooperated with this case.
Footnotes
Conflicts of interest
None.
References:
- 1.Hamm RL. Drug-hypersensitivity syndrome: Diagnosis and treatment. J Am Coll Clin Wound Spec. 2012;3(4):77–81. doi: 10.1016/j.jcws.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157(5):934–40. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 3.Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DIHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int. 2019;68(3):301–8. doi: 10.1016/j.alit.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: Comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51(6):2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki Y, Inagi R, Aono T, et al. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol. 1998;134(9):1108–12. doi: 10.1001/archderm.134.9.1108. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K, Yashkawa M, Tohyama M. Human herpesvirus 6 and drug allergy. Curr Opin Allergy Clin Immunol. 2003;3(4):255–60. doi: 10.1097/00130832-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): A reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2005;55(1):1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 8.Aihara Y, Ito SI, Kobayashi Y, et al. Carbamazepine-induced hypersensitivity syndrome associated with transient hypogammaglobulinaemia and reactivation of human herpesvirus 6 infection demonstrated by real-time quantitative polymerase chain reaction. Br J Dermatol. 2003;149(1):165–69. doi: 10.1046/j.1365-2133.2003.05368.x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Fukuda M, Tohyama M, et al. Carbamazepine-induced drug-induced hypersensitivity syndrome in a 14-year-old Japanese boy. Epilepsia. 2008;49(12):2118–21. doi: 10.1111/j.1528-1167.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 10.Tohyama M, Yahata Y, Yasukawa M, et al. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch Dermatol. 1998;134(9):1113–17. doi: 10.1001/archderm.134.9.1113. [DOI] [PubMed] [Google Scholar]
- 11.Miura H, Nakano M, Yoshimura N, et al. Drug-induced hypsersensitivity syndrome associated with reactivation of human herpesvirus 6 presenting with severe hepatic injury. Clin J Gastroenteol. 2008;1(3):127–32. doi: 10.1007/s12328-008-0021-4. [DOI] [PubMed] [Google Scholar]
- 12.Kano Y, Hirahara K, Mitsuyama Y, et al. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy. 2007;62(12):1439–44. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 13.Aota N, Shiohara T. Viral connection between drug rashes and autoimmune diseases: How autoimmune responses are generated after resolution of drug rashes. Autoimmun Rev. 2009;8(6):488–94. doi: 10.1016/j.autrev.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi R, Kano Y, Yamazaki Y, et al. Defective regulatory T cells in patients with severe drug eruptions: Timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. 2009;182(12):8071–79. doi: 10.4049/jimmunol.0804002. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting edge: CD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181(7):4447–51. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki Y, Tajiri H, Tanaka-Taya K, et al. Frequent detection of the human herpesvirus 6-specific genomes in the livers of children with various liver diseases. J Clin Microbiol. 2001;39(6):2173–77. doi: 10.1128/JCM.39.6.2173-2177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe H, Tohyama M, Kamijima M, et al. Occupational trichloroethylene hypersensitivity syndrome with human herpesvirus-6 and cytomegalovirus reactivation. Dermatology. 2010;221(1):17–22. doi: 10.1159/000290775. [DOI] [PubMed] [Google Scholar]