Abstract
Doxorubicin (DOX) is an effective, broad-spectrum antineoplastic agent with serious cardiotoxic side effects, which may lead to the development of heart failure. Current strategies to diagnose, prevent, and treat DOX-induced cardiotoxicity (DIC) are inadequate. Recent evidence has linked the dysregulation and destruction of the vascular endothelium to the development of DIC. Autophagy is a conserved pro-survival mechanism that recycles and removes damaged sub-cellular components. Autophagy-related protein 7 (ATG7) catalyzes autophagosome formation, a critical step in autophagy. In this study, we used endothelial cell-specific Atg7 knockout (EC-Atg7−/−) mice to characterize the role of endothelial cell-specific autophagy in DIC. DOX-treated EC-Atg7−/− mice showed reduced survival and a greater decline in cardiac function compared to wild-type controls. Histological assessments revealed increased cardiac fibrosis in DOX-treated EC-Atg7−/− mice. Furthermore, DOX-treated EC-Atg7−/− mice had elevated serum levels of creatine kinase-myocardial band, a biomarker for cardiac damage. Thus, the lack of EC-specific autophagy exacerbated DIC. Future studies on the relationship between EC-specific autophagy and DIC could establish the importance of endothelium protection in preventing DIC.
Keywords: Autophagy, Cardiomyopathy, Doxorubicin, Endothelium, Heart failure
Highlights
-
•
Recent data suggest that endothelial cells (ECs) may represent a novel target to reduce doxorubicin (DOX)-linked cardiotoxicity.
-
•
We used EC-specific autophagy-related protein 7 knock-out (EC-Atg7−/−) mice to determine how ATG7 loss in ECs affects DIC.
-
•
DOX-treated EC-Atg7−/− mice exhibited reduced survival and cardiac function.
-
•
Cardiac fibrosis and serum creatine kinase-myocardial band levels were increased in DOX-treated EC-Atg7−/− mice.
-
•
Loss of endothelial Atg7 exacerbated DIC phenotypes.
1. Introduction
The anthracycline doxorubicin (DOX) is broadly prescribed to treat solid and hematological cancers, and although effective as a chemotherapeutic agent, its use has been complicated by both acute and chronic cardiotoxic side effects [1]. The cumulative literature suggests that up to 36% of DOX-treated patients develop cardiomyopathy that may progress to heart failure [2]. Indeed, DOX-induced heart failure has been associated with 50% mortality 1 year following diagnosis [2]. The current options for diagnosing, treating, and preventing DOX-induced cardiotoxicity (DIC) are sub-optimal and highlight the urgency to uncover the mechanisms that contribute to the development and progression of DIC [[3], [4], [5]]. Earlier studies on DIC tended to focus on the direct toxic effects of DOX on cardiomyocytes (CMs) [6,7]. Recently, however, there has been a shift in attention toward the vascular endothelium as an indirect target of DOX [1]. Endothelial cells (ECs), support optimal CM function by forming physical barriers that prevent the exposure of toxins to CMs. ECs also secrete paracrine factors such as nitric oxide, endothelin-1, prostacyclin, and neuregulin-1, effectors that contribute to CM survival and function [[8], [9], [10], [11], [12]]. Since DOX is administered intravenously, the ECs that line the vascular lumen are directly exposed to high concentrations of DOX and this may culminate in DOX-induced EC damage thereby preventing endothelium-associated CM protection. Thus, preservation of EC function during DOX therapy may represent a novel therapeutic avenue to help prevent the development of DIC. The toxic effects that DOX exerts on CMs is similar its impact on ECs. The major proposed mechanisms include oxidative stress and mitochondria dysfunction, which ultimately leads to cell dysfunction and death [1].
Autophagy, including macroautophagy, microautophagy, and chaperone-mediated autophagy, is a conserved process of cell preservation whereby damaged cellular components are broken down into their basic constituents via lysosomal degradation [13]. Autophagy is tightly regulated by proteins like B-cell lymphoma 2 because excessive degradation can lead to cell death [14]. Macroautophagy, which accounts for the majority of autophagic events, involves the formation of an autophagosome, a double-layer membrane structure, that engulfs cellular content to be degraded [15]. The autophagosome eventually fuses with the lysosome and the contents are subsequently degraded by acid hydrolases [15]. The formation and maturation of the autophagosome require a plethora of proteins such as autophagy-related gene 7 (ATG7), an essential mediator responsible for catalyzing several steps in the autophagosome maturation process [16]. The ATG7 protein is essential for mitophagy and removal of toxic protein aggregates from oxidative stress, which would be important for cell survival and function after DOX exposure [17,18]. We hypothesized that knocking out the Atg7 gene in ECs would exacerbate DIC. To this end, we report herein the differences in survivability, cardiac function and histology, and levels of creatine kinase-myocardial band (CK-MB) between DOX-treated WT and EC-Atg7−/− mice.
2. Materials and methods
2.1. Animals
All procedures adhered to the guidelines of the Canadian Council on Animal Care and were approved by the Institutional Animal Care Committee (protocol 801). EC-Atg7−/− mice were generated by crossing the double-floxed Atg7 mouse (Atg7flox/flox) with the VE-cadherin Cre transgenic (VE-CadherinCre/) mouse (Jackson Laboratory) [19]. All studies were conducted with male mice only. To determine survivability, 8-10-week-old wild-type (WT) and EC-Atg7−/− mice were administered either DOX (20 mg/kg) or saline (SAL, 0.9% NaCl) intraperitoneally as a single injection, which has been previously characterized [20]. In the cardiac functional assessment studies, WT and EC-Atg7−/− mice underwent baseline echocardiography 2–3 days before treatment with DOX (20 mg/kg) and follow-up echocardiography on Day 3. This timeline was based on survival evaluations. Three days after DOX (20 mg/kg) or SAL (0.9% NaCl) treatment, WT and EC-Atg7−/− mice were euthanized and their heart, lung, and serum collected for morphological and molecular analyses.
2.2. Co-immunofluorescence
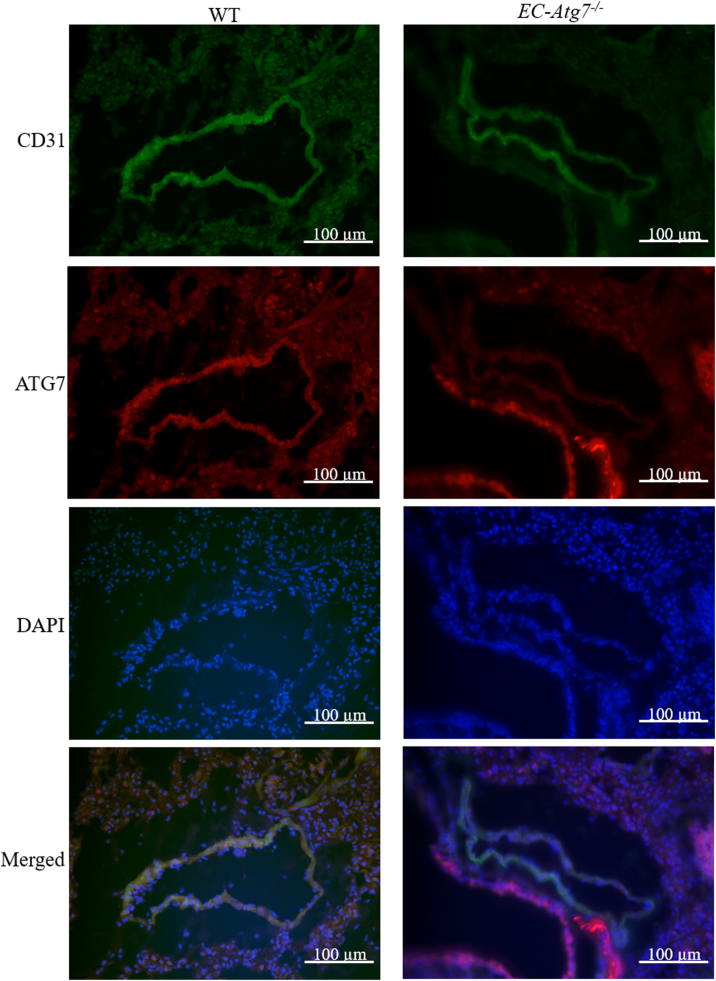
In order to minimize the number of animals used, lung samples were used to confirm the surrogate mouse model. WT and EC-Atg7−/− mice were anesthetized with isoflurane (5%) and euthanized via cervical dislocation. The left lung was isolated and placed in Tissue-Tek® Optimal Cutting Tissue (Sakura) medium-filled cryomolds and 8 μm-thick cryosections were acquired. The sections were incubated with a primary antibody cocktail consisting of anti-rabbit ATG7 (Abcam, Cat# ab133528, 1:500 dilution) and anti-rat cluster of differentiation 31 (CD31) (Abcam, Cat# ab56299, 1:200 dilution). Subsequently, tissue sections were incubated with a secondary antibody cocktail containing goat anti-rabbit Alexa Fluor 555 (Thermo Fisher, Cat#A21428, 1:200 dilution) and goat anti-rat Alexa Fluor 488 (Abcam, Cat#ab150165, 1:200 dilution) before counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). The sections were viewed with an Olympus Upright BX150 widefield microscope at 40× magnification. Images of CD31 expression were captured using the red filter cube with 25 ms exposure times. Images of the ATG7 stained sections were captured using a green filter cube with 90 ms exposure times. Images of the nucleus (DAPI stained) were then captured using a blue filter cube with 4 ms exposure times. All images were taken at a resolution of 4140 × 3096. The three images of each section were superimposed. Since CD31 is only expressed in EC's, the absence of ATG+ in CD31+ cells validated the EC-Atg7−/− mouse model [21].
2.3. Echocardiography assessments
Mice were anesthetized with 2–3% isoflurane via a nose cone. Ultrasound was performed using the Vivo 2100 ultrasound machine and a 40 MHz transducer (VisualSonics). The short-axis motion-mode provided a temporal resolution of the physical and functional aspects of the left ventricle (LV) for each mouse. The transducer was placed transversely along the left chest of each mouse. Images were analyzed using the Vevo LAB 1.7.1 software (VisualSonics). The motion at a segment of the LV wall (anterior epicardium, anterior endocardium, posterior epicardium, posterior endocardium) was used to calculate the functional parameters, LV ejection fraction (EF) and LV fractional shortening (FS).
2.4. Cardiomyocyte vacuolization
Mouse hearts were fixed with 10% neutral buffered formaldehyde for 18–24 h and stored in 70% ethanol. The hearts were processed and embedded in paraffin, and 5 μm thick sections were acquired. To obtain a longitudinal representation of the heart for comparative purposes, three sections were chosen as follows for Hematoxylin and Eosin (H&E) staining and analyses. The first section was the most superficial and was defined as when both cardiac ventricles first became well-defined. The second section was the deepest and was defined as when both cardiac ventricles took up the greatest area. The third section was a section that was located approximately halfway between the first and second. The slides were scanned with the Zeiss Axioscan Scan.Z1 under brightfield settings at 20× magnification. The Zen 2.6 software (Zeiss) was used to view the images. Rectangular boxes were randomly annotated in the LV areas, serving as the areas of interest. A total area of at least 0.7 mm/section (2.4 mm/mouse) was used to quantify the number of vacuoles. Vacuoles were differentiated from tears/rips in the tissue by their circular shape. The average numbers of vacuoles per 1 mm2 of LV tissue were analyzed.
2.5. Cardiac fibrosis
A section with pronounced cardiac ventricles was chosen to be stained with Masson's Trichrome to semi-quantitatively assess collagen deposition. The slides were viewed under the widefield microscope Olympus Upright BX150 at 40× magnification. The areas of interest included the edges of the heart, left ventricle, and the ventricular lumen. Three images captured these aspects: the edge, the left ventricle, and the area around the LV lumen. Two blinded independent assessors rated the images on a scale of 1–10 based on the extent of collagen deposition, with 10 indicating the greatest amount. The average scores between the two assessors were used for the final analysis.
2.6. Serum CK-MB levels
Enzyme-linked immunosorbent assays (ELISAs) were performed using the serum samples of SAL- and DOX-treated WT and EC-Atg7−/− mice to quantify CK-MB levels. Assays were performed in triplicate and according to the manufacturer's protocol (MyBioSource, Cat# MBS705293). Since each sample was assayed in triplicate, one of the readings was eliminated if the coefficient of variation was greater than 10% and if two of the readings were consistently distinct from the third. Statistical analyses were performed using the average of each sample.
2.7. Cardiac apoptosis
Three consecutive sections with pronounced cardiac ventricles were chosen to be stained with cleaved caspase-3 (CC3) to assess the extent of apoptosis. The first section was not incubated with the primary antibody (positive control) and the remaining two were stained with an antibody against CC3. The slides were scanned with the Zeiss Axioscan Scan.Z1 under brightfield settings at 20× magnification. CC3+ cells were only counted if they were also stained with 3,3′-Diaminobenzidine, a nuclear stain.
2.8. Statistical analyses
Survival data were analyzed by the log-rank test on the Kaplan-Meier curves. All other data were analyzed by two-way analysis of variances and the Tukey's test for multiple comparisons. These data are presented as mean ± standard deviation and differences were considered significant if the p value was <0.05.
3. Results
3.1. Knockdown of protein expression in the ECs of EC-Atg7−/− mice
Co-immunofluorescence for CD31 and ATG7 was performed on transverse sections of mouse lungs from WT and EC-Atg7−/− mice. While ATG7 staining was abundant in the CD31+ cells of WT mice, ATG7 staining was not detected in the CD31+ cells of EC-Atg7−/− mice (Fig. 1). This confirmed the EC-specific deletion of Atg7 in EC-Atg7−/− mice. In alignment with our previous study, there was less CD31 staining in the lung sections from EC-Atg7−/− mice [22]. CD31+ non-ECs in WT mice could be stem- or progenitor-cells [23].
Fig. 1.
Loss of ATG7 protein expression in lung ECs of EC-Atg7−/−mice. Representative co-immunofluorescence images of the lung sections (transverse) for WT (left) and EC-Atg7−/− (right) mice. CD31 (green), ATG7 (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) were simultaneously detected (top to bottom). ATG7 was detected in CD31+ cells of WT mice. In contrast, ATG7 was not detected in CD31+ cells from EC-Atg7−/− mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
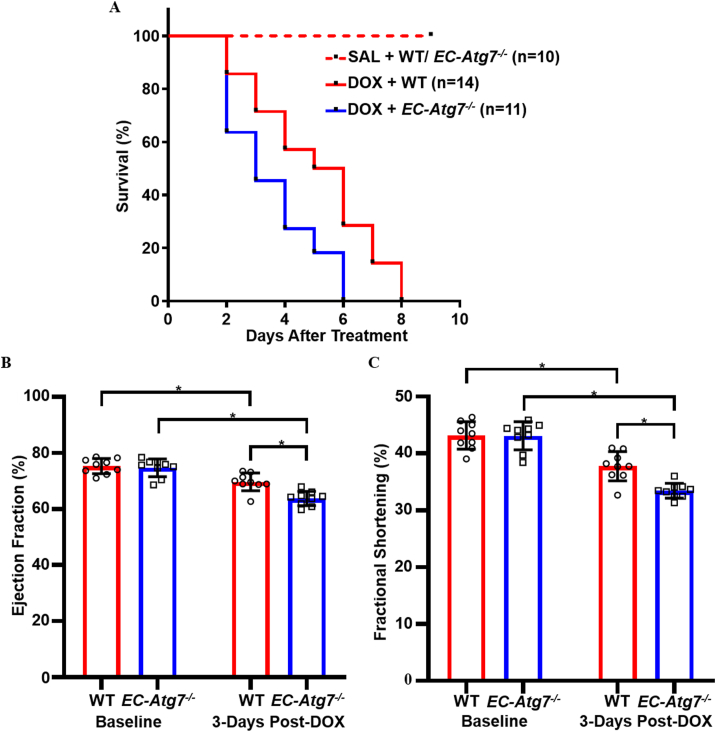
3.2. Reduced survival in DOX-treated EC-Atg7−/− mice
Survival was monitored following initiation of DOX treatment (Fig. 2A). The survivability of DOX-treated EC-Atg7−/− mice (3.5 days ±1.6 days, n = 11) was significantly lower than DOX-treated WT mice (5.1 days ±2.1 days, n = 14) (p < 0.05). A 100% survival at the 4-week time point was noted for the SAL-treated WT (n = 10) and EC-Atg7−/− (n = 10) mice. To the best of our knowledge, this is the first study to show that deficiency in EC-specific autophagy increased DOX-associated mortality.
Fig. 2.
Reduced survival and cardiac function in DOX-treated EC-Atg7−/−mice. (A) Kaplan-Meier survival curves for WT and EC-Atg7−/− mice after DOX (20 mg/kg) treatment. WT mice survived longer than EC-Atg7−/− mice (p < 0.05 using the log-rank test). (B) Left ventricular ejection fraction (LVEF) and (C) Left ventricular fractional shortening (LVFS) in WT (n = 9) and EC-Atg7−/− (n = 9) mice at baseline and 3 days post-DOX treatment. DOX-treated EC-Atg7−/− mice had reduced LVEF and LVFS compared to their WT littermates. Data are presented as mean ± SD. *p < 0.05 using two-way ANOVA and the Tukey test.
3.3. Reduced cardiac function in DOX-treated EC-Atg7−/− mice
Mice underwent ultrasound echocardiography at baseline and Day 3 post-DOX administration to assess changes in cardiac function as defined by LVEF and LVFS. At baseline, there were no differences in LVEF (Fig. 2B) and LVFS (Fig. 2C) measurements between the WT (EF = 75.3% ± 2.7%, FS = 43.2% ± 2.4%, n = 9) and EC-Atg7−/− (EF = 74.7% ± 3.2%, FS = 43.1% ± 2.5%, n = 9) mice. At 3 days post-DOX treatment, LVEF and LVFS were lower in both WT (EF = 69.7% ± 3.2%, p < 0.05; FS = 37.8% ± 2.6%, p < 0.05 compared to baseline) and EC-Atg7−/− mice (EF = 63.8% ± 2.6%, p < 0.05; FS = 33.4% ± 1.3%, p < 0.05 compared to baseline). Notably, the reductions in LVEF and LVFS were less severe in the DOX-treated WT mice (EF = 5.7% ± 4.9%, FS = 5.4% ± 4.1%) when compared to the DOX-treated EC-Atg7−/− mice (EF = 11.0% ± 4.3%, FS = 9.7% ± 2.8%) (p < 0.05). Thus, Atg7 knockout in ECs exacerbated the decline in cardiac function after DOX-treatment, and this may partially account for the increased mortality observed among the DOX-treated EC-Atg7−/− mice.
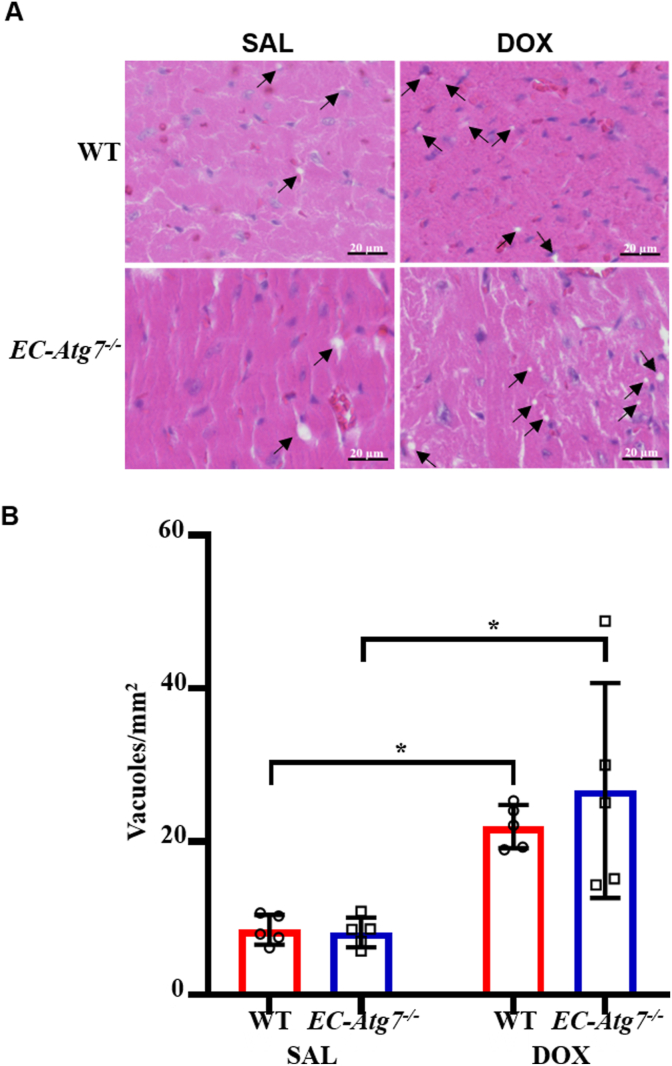
3.4. Increased cardiomyocyte vacuolization after DOX treatment
DOX-mediated CM damage manifests as the loss of myofibers followed by the distension of sacrotubules that lead to vacuole formation [24]. Degenerative LV vacuolization was assessed using H&E-stained paraffinized heart sections from WT and EC-Atg7−/− mice (Fig. 3). Vacuolization was rarely observed in the sections from SAL-treated WT (8.5 ± 1.9 vacuoles/mm2, n = 5) and EC-Atg7−/− (8.1 ± 1.9 vacuoles/mm2, n = 5) mice. Conversely, sections from DOX-treated WT (22.0 ± 2.8 vacuoles/mm2, n = 5) and EC-Atg7−/− (26.7 ± 14.0 vacuoles/mm2, n = 5) mice had significantly greater degenerative vacuolization relative to the sections from the corresponding SAL-treated control groups (p < 0.05), suggesting that intensive CM damage had occurred in the DOX-treated mice. This is consistent with the available literature demonstrating that DOX-treatment induces CM vacuolization [20]. The mean number of vacuoles observed in the LV of DOX-treated WT and EC-Atg7−/− mice were comparable, suggesting that the decline in cardiac function documented in the DOX-treated EC-Atg7−/− mice was not associated with an increase in LV vacuolization.
Fig. 3.
DOX-treated mice demonstrated increased cardiomyocyte vacuolization. (A) Representative hematoxylin and eosin (H&E) images showing the left ventricles (LV) of WT (top) and EC-Atg7−/− (bottom) mice three days after SAL (0.9% NaCl) (left) or DOX (20 mg/kg) (right) treatment. The pink and blue areas represent the cytoplasm and nucleus, respectively. (B) Quantification of vacuole number in the LV of WT and EC-Atg7−/− mice (3 slides/mouse) three days after SAL (n = 5/group) or DOX (n = 5/group) treatment. DOX-treated mice showed increased vacuoles compared to SAL-treated mice. There was no difference in the total number of vacuoles observed between DOX-treated WT and EC-Atg7−/− mice. Data are presented as mean ± SD. *p < 0.05 using two-way ANOVA followed by Tukey's multiple comparison tests. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
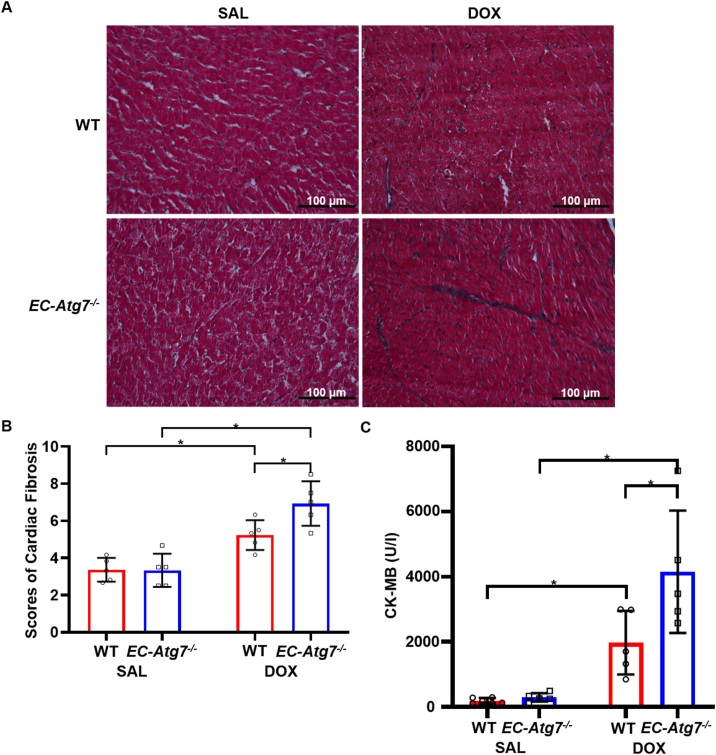
3.5. Increased cardiac fibrosis in DOX-treated EC-Atg7−/− mice
The invasion of cardiac fibroblasts into areas with CM toxicity is a process that normally acts to preserve the structural integrity of the heart [25]. This remodeling process can also promote an unfavorable environment for neighboring CMs through the secretion of paracrine factors and may exacerbate cardiac damage [26]. LV fibrosis was assessed using Masson's Trichrome-stained heart sections to detect collagen deposition secreted from activated cardiac fibroblasts (Fig. 4A and B). Collagen deposition was minimal in the sections from WT (3.4 ± 0.6, n = 5) and EC-Atg7−/− (3.3 ± 0.9, n = 5) mice that had undergone SAL treatment. Collagen deposition was significantly greater in the sections from DOX-treated WT (5.2 ± 0.8, p < 0.05) and EC-Atg7−/− (6.9 ± 1.2, p < 0.05) mice. These findings are consistent with previous rodent studies that reported increased cardiac fibrosis after DOX treatment [20,27]. Furthermore, DOX-treated EC-Atg7−/− mice showed significantly greater collagen deposition compared to DOX-treated WT mice (p < 0.05), suggesting that an increase in fibrosis may have contributed to reduced cardiac function in DOX-treated EC-Atg7−/− mice.
Fig. 4.
DOX-treated EC-Atg7−/−mice demonstrated increased cardiac fibrosis and serum CK-MB. (A) Representative images showing the left ventricle (LV) from WT (top) and EC-Atg7−/− (bottom) mice stained with Masson's trichrome at 3 days after SAL (0.9% NaCl) (left) or DOX (20 mg/kg) (right) treatment. The dark-blue and red areas represent collagen deposition and the cell cytoplasm, respectively. (B) Blinded scoring of cardiac fibrosis though semi-quantification of the extent of collagen deposition in the LV of WT and EC-Atg7−/− mice (1 slide/mouse) three days after SAL (n = 5/group) or DOX (n = 5/group) treatment. DOX-treated mice showed increased cardiac fibrosis compared to SAL-treated animals. In addition, DOX-treated EC-Atg7−/− mice demonstrated increased cardiac fibrosis compared to DOX-treated WT mice. (C) CK-MB levels in the sera of SAL- or DOX-treated WT (n = 5) and EC-Atg7−/− (n = 5) mice were measured by ELISA assays. DOX-treated EC-Atg7−/− mice demonstrated increased serum CK-MB compared to DOX-treated WT mice. Data are mean ± SD. *p < 0.05 using two-way ANOVA and the Tukey test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Increased serum CK-MB in DOX-treated EC-Atg7−/− mice
CK-MB is an enzyme that enters the circulation following CM rupture that is triggered by cardiac damage [28]. ELISA assays were performed on the sera of SAL- and DOX-treated WT and EC-Atg7−/− mice (Fig. 4C). Sera from DOX-treated WT (1971.6 U/I ± 976.3 U/I, n = 5) and EC-Atg7−/− (4150.9 U/I ± 1879.5 U/I, n = 5) mice had higher CK-MB levels compared to those from SAL-treated WT (192.0 U/I ± 82.6 U/I, n = 6) and EC-Atg7−/− (292.6 U/I ± 130.9 U/I, n = 5) mice (p < 0.05). This finding is consistent with those of other groups that observed elevated serum CK-MB levels following DOX treatment in mice [20,29]. Additionally, the serum CK-MB levels of DOX-treated EC-Atg7−/− mice were higher relative to those of DOX-treated WT mice (p < 0.05). This provides additional evidence that DIC was elevated in EC-Atg7−/− mice.
4. Discussion
Autophagy is a tightly regulated cellular process important for tissue homeostasis and function, especially after DOX exposure. The normalization of autophagy, which becomes dysfunction because of DOX, is a cardioprotective mechanism of metformin for individuals receiving DOX [30].
Due to difficulties with culturing ECs from EC-Atg7−/− mice, we were unable to quantify the efficiency of Atg7 deletion in these cells. We observed greater DOX-associated mortality in EC-Atg7−/− mice compared to their WT counterparts. A previous study observed an increase in DOX-associated mortality in mice that were whole-body knock-outs for Uvrag, another gene that is essential for autophagosome formation [20]. Our study demonstrated that the loss of endothelial Atg7 significantly exacerbated DOX-associated mortality in mice. We searched for evidence of increased direct cardiotoxicity and observed a greater decline in LVEF and LVFS in DOX-treated EC-Atg7−/− mice compared to DOX-treated WT controls. To characterize the changes in cardiac function that may occur in parallel with modifications in cardiac morphology, and to explore potential mechanisms that may contribute to changes in cardiac function, we first assessed CM damage/degeneration via quantification of CM vacuolization. Although DOX-treated WT and EC-Atg7−/− mice had increased vacuolization compared to SAL-treated control mice, there was no difference in vacuolization between DOX-treated EC-Atg7−/− and DOX-treated WT mice. It should be noted that vacuoles can only be quantified in cells that are present within the paraffin sections. Significantly damaged CMs with vacuolization in EC-Atg7−/− mice may have undergone cell death by day 3, indicating that CM vacuole quantification may not reflect the full extent of DOX-induced cardiac damage. However, there were no significant CC3 staining, an apoptotic marker, in the cardiac sections of EC-Atg7−/− mice (Supplementary Fig. 1). Exaggerated toxicity of DOX in these mice may lead to rapid necrotic death of CMs [31]. We observed greater LV fibrosis in DOX-treated EC-Atg7−/− mice compared to DOX-WT mice. Cardiac fibrosis has often been associated with systolic and diastolic dysfunction because of its detrimental effects on CMs [32]. Finally, to obtain additional evidence that indicate acute cardiac damage, we assessed serum levels of CK-MB, an enzyme important for energy homeostasis by generating energy buffers in cardiac muscles [28]. EC-Atg7−/− mice demonstrated higher CK-MB levels compared to WT mice after DOX treatment. This is in congruence with our survival, cardiac functional, and cardiac morphological data, which suggests greater DIC in EC-Atg7−/− mice compared to their WT littermates.
There are several limitations to this study. Firstly, non-ATG7-dependent autophagic processes such as microautophagy and chaperone-mediated autophagy account for a small but relevant proportion of autophagic events. Thus, autophagy in the ECs of the EC-Atg7−/− mice we studied were likely reduced, not absent. In addition, microautophagy and chaperone-mediated autophagy, like macroautophagy, has also been observed to remove damaged and oxidized molecules, which can be induced by DOX [33]. Studies examining the extent of autophagic compensation by microautophagy and chaperone-mediated autophagy in the EC-Atg7−/− mouse model are warranted. Secondly, high-dose DOX delivered intraperitoneally can be toxic towards organs such as the liver, kidney, and intestines, with damage at these sites potentially contributing to DOX-associated mortality [34]. In particular, DOX-mediated intestinal release of endotoxin into the circulation and the subsequent immune response can lead to sepsis and rapid death [35]. Knocking out Atg7 in ECs, a major component of the vascular system, may lead to endothelium dysfunction and exacerbate sepsis. Serial echocardiography which provides real-time recordings of cardiac function would afford a more accurate description of the temporal impact of cardiac damage in DOX-associated mortality. Thirdly, our study only explored acute DOX cardiotoxicity. Cardiotoxicity due to chronic DOX exposure continues to be a major clinical challenge because it is difficult to detect in its early stages and can become irreversible, potentially developing into heart failure [5]. The induction of acute cardiotoxicity within 3 days of DOX administration limits the breadth of conclusions applicable to our study. Finally, questions remain regarding the molecular mechanisms by which knocking out Atg7 in ECs seems to accelerate DIC in the EC-Atg7−/− mouse model. Assessing the changes in EC-CM interactions after the deletion of EC-specific Atg7 may help elucidate these mechanisms.
In summary, our study provides evidence that the lack of EC-specific autophagy can heighten DIC. Mechanistic investigations on the relationship between EC-specific autophagy and DIC are warranted to establish the importance of endothelium protection in preventing cardiac damage in patients treated with DOX.
Funding
This study was supported in part by grants from the Canadian Institutes of Health Research to SV. AZL and VZL were the recipients of studentships from the Department of Pharmacology and Toxicology, University of Toronto. BC was the recipient of a St. Michael's Hospital RTC Scholarship. SV holds a Tier 1 Canada Research Chair in Cardiovascular Surgery.
CRediT authorship contribution statement
Albert Z. Luu: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Vincent Z. Luu: Methodology, Validation, Formal analysis, Investigation, Writing - review & editing, Visualization. Biswajit Chowdhury: Methodology, Validation, Formal analysis, Investigation, Visualization. Andrew Kosmopoulos: Investigation, Visualization. Yi Pan: Methodology, Validation, Formal analysis, Investigation. Mohammed Al-Omran: Resources, Writing - review & editing. Adrian Quan: Methodology, Validation, Project administration. Hwee Teoh: Writing - original draft, Writing - review & editing, Visualization. David A. Hess: Conceptualization, Writing - original draft, Writing - review & editing, Supervision. Subodh Verma: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors have no relevant conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.100926.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
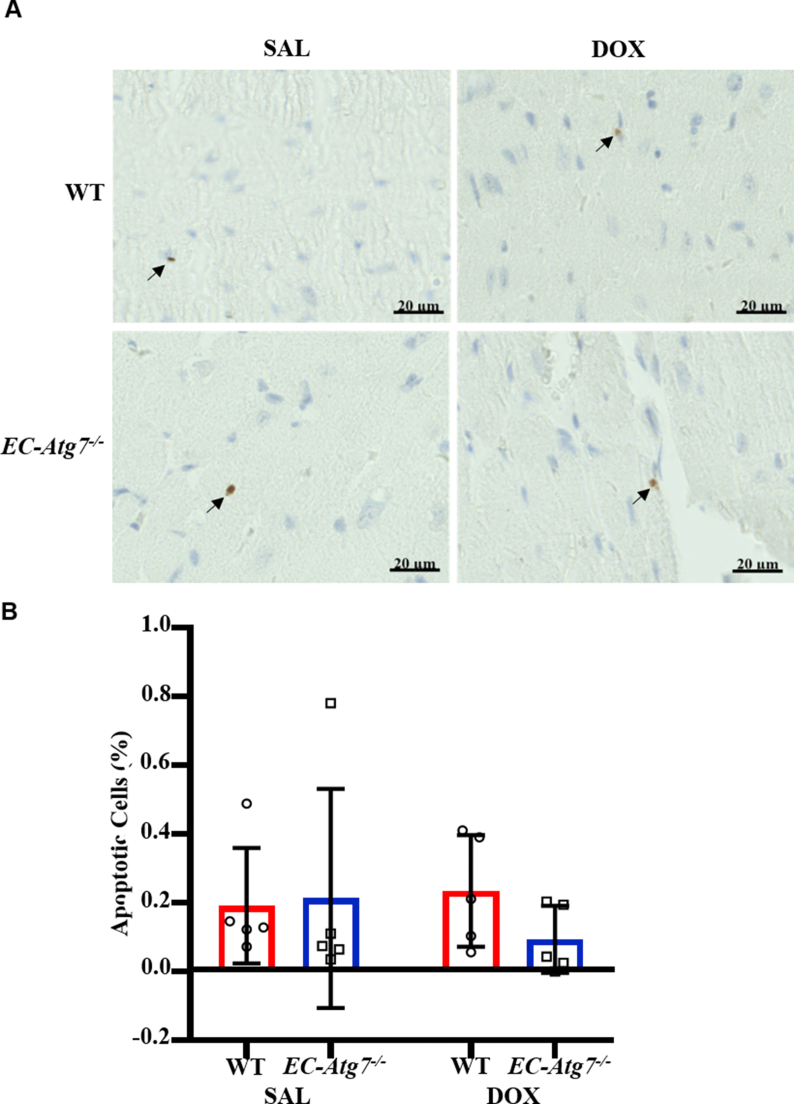
Fig. S1.
Cardiac apoptosis was minimal in all mouse groups.
(A) Representative images showing cleaved caspase-3 staining in the LV of WT (top) and EC-Atg7−/− (bottom) mice three days after SAL (0.9% NaCl) (left) or DOX (20 mg/kg) (right) treatment. Blue staining represents nuclei and brown staining (black arrows) represents cleaved caspase-3 detection. (B) Quantification of apoptotic cells (presence of cleaved caspase-3) in the LV of WT and EC-Atg7−/− mice (2 slides/mouse) three days after SAL (n = 5 mice/group) or DOX (n = 5 mice/group) treatment. There was minimal detection of apoptosis in cardiac tissue of all mouse groups. Data are presented as mean ± SD.
References
- 1.Luu A.Z., Chowdhury B., Al-Omran M., Teoh H., Hess D.A., Verma S. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC. Basic to Transl. Sci. 2018;3:861–870. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Hoff D.D., Layard M.W., Basa P., Davis H.L., Jr., Von Hoff A.L., Rozencweig M., Muggia F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 3.Rahman A.M., Yusuf S.W., Ewer M.S. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int. J. Nanomed. 2007;2:567–583. [PMC free article] [PubMed] [Google Scholar]
- 4.Mitry M.A., Edwards J.G. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Hear. Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee K., Zhang J., Honbo N., Karliner J.S. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childs A.C., Phaneuf S.L., Dirks A.J., Phillips T., Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Canc. Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 7.Solem L.E., Heller L.J., Wallace K.B. Dose-dependent increase in sensitivity to calcium-induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J. Mol. Cell. Cardiol. 1996;28:1023–1032. doi: 10.1006/jmcc.1996.0095. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson E.L., Sidaway J.E., Cross M.J. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol Open. 2016;5:1362–1370. doi: 10.1242/bio.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon M., Shah A.M., Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 11.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim S.L., Lam C.S., Segers V.F., Brutsaert D.L., De Keulenaer G.W. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur. Heart J. 2015;36:2050–2060. doi: 10.1093/eurheartj/ehv132. [DOI] [PubMed] [Google Scholar]
- 13.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pattingre S., Tassa A., Qu X., Garuti R., Liang X.H., Mizushima N., Packer M., Schneider M.D., Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Z., Zhao Y., Li H., Yan M., Zhou L., Chen C., Wang D.W. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging. 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.17 Y., Chiang W.-C., Sumpter R.J., Mishra P., Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168:224–238. doi: 10.1016/j.cell.2016.11.042. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lekli I., Haines D.D., Balla G., Tosaki A. Autophagy: an adaptive physiological countermeasure to cellular senescence and ischaemia/reperfusion-associated cardiac arrhythmias. J. Cell Mol. Med. 2017;21:1058–1072. doi: 10.1111/jcmm.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torisu T., Torisu K., Lee I.H., Liu J., Malide D., Combs C.A., Wu X.S., Rovira I.I., Fergusson M.M., Weigert R., Connelly P.S., Daniels M.P., Komatsu M., Cao L., Finkel T. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med. 2013;19:1281–1287. doi: 10.1038/nm.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An L., Hu X.-W., Zhang S., Hu X., Song Z., Naz A., Zi Z., Wu J., Li C., Zou Y., He L., Zhu H. UVRAG deficiency exacerbates doxorubicin-induced cardiotoxicity. Sci. Rep. 2017;7:43251. doi: 10.1038/srep43251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejana E., Corada M., Lampugnani M.G. Endothelial cell-to-cell junctions. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995;9:910–918. [PubMed] [Google Scholar]
- 22.Singh K.K., Lovren F., Pan Y., Quan A., Ramadan A., Matkar P.N., Ehsan M., Sandhu P., Mantella L.E., Gupta N., Teoh H., Parotto M., Tabuchi A., Kuebler W.M., Al-Omran M., Finkel T., Verma S. The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition. J. Biol. Chem. 2015;290:2547–2559. doi: 10.1074/jbc.M114.604603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y., Sun P., Wang J.-Y., Li Z.-Z., Gao R.-L., Wang X.-Z., Phillips W.D., Liang S.X. Differentiation of CD45-/CD31+ lung side population cells into endothelial and smooth muscle cells in vitro. Int. J. Mol. Med. 2019;43:1128–1138. doi: 10.3892/ijmm.2019.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefrak E.A., Pitha J., Rosenheim S., Gottlieb J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray M.O., Long C.S., Kalinyak J.E., Li H.T., Karliner J.S. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc. Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 27.Pei X.M., Yung B.Y., Yip S.P., Ying M., Benzie I.F., Siu P.M. Desacyl ghrelin prevents doxorubicin-induced myocardial fibrosis and apoptosis via the GHSR-independent pathway. Am. J. Physiol. Endocrinol. Metab. 2014;306:E311–E323. doi: 10.1152/ajpendo.00123.2013. [DOI] [PubMed] [Google Scholar]
- 28.Aydin S., Ugur K., Aydin S., Sahin İ., Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc. Health Risk Manag. 2019;15:1–10. doi: 10.2147/VHRM.S166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S.H., Kim K.-J., Kim J.-H., Kwak J.-H., Song H., Cho J.-Y., Hwang D.Y., Kim K.S., Jung Y.-S. Comparision of doxorubicin-induced cardiotoxicity in the ICR mice of different sources. Lab. Anim. Res. 2017;33:165–170. doi: 10.5625/lar.2017.33.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilinyi R., Czompa A., Czegledi A., Gajtko A., Pituk D., Lekli I., Tosaki A. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018;23 doi: 10.3390/molecules23051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.-W., Shi J., Li Y.-J., Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. 2009;57:435–445. doi: 10.1007/s00005-009-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong P., Christia P., Frangogiannis N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaro I.E., Albornoz A., Molina A., Moreno J., Cordero K., Criollo A., Budini M. Chaperone mediated autophagy in the crosstalk of neurodegenerative diseases and metabolic disorders. Front. Endocrinol. 2018;9:778. doi: 10.3389/fendo.2018.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugazhendhi A., Edison T.N.J.I., Velmurugan B.K., Jacob J.A., Karuppusamy I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26–30. doi: 10.1016/j.lfs.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Xiao H., Siddiqui J., Remick D.G. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]