Highlights
-
•
We investigated two separate supplementary motor area onset focal motor seizures.
-
•
Separate networks to the primary motor area were proved by neurophysiological tools.
-
•
Corticectomy, including these two networks, achieved seizure-free without hemiparesis.
Abbreviations: CCEP, cortico-cortical evoked potential; ECoG, electrocorticogram; EEG, encephaloelectrography; FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; FIQ, full intelligent quotient; HFO, high-frequency oscillation; IVEEG, intracranial video electroencephalography; LC, lateral central; MC, mesial central; MEP, motor evoked potentials; MRI, magnetic resonance imaging; PMA, primary motor area; SISCOM, subtraction ictal single-photon emission computed tomography co-registered to MRI; SMA, supplementary motor area; SPES, single-pulse electrical stimulation
Keywords: Epilepsy surgery, High-frequency oscillation, Cortico-cortical evoked potential, Supplementary motor area, Primary motor area
Abstract
We present a case of drug-resistant focal motor seizures in which separate cortico-cortical epileptic networks within the supplementary motor area (SMA) proper and primary motor area (PMA) were proven by ictal high-frequency oscillation (HFO) and cortico-cortical evoked potential (CCEP). A 12-year-old girl presented with two types seizures: type A, tonic extension and subsequent clonic movements of the right arm; and type B, tonic and clonic movements of the right leg. MRI was normal and karyotype genetic analysis revealed 46,X,t(X;14)(q13;p12). She underwent placement of chronic subdural electrodes over the left hemisphere. We recorded a total of nine seizures during 10 days of epilepsy monitoring. Type A seizures started from the lower part of the left SMA proper and early spread to the hand motor area of the PMA. Type B seizures started from the upper part of the SMA proper and early spread to the leg motor area of the PMA. CCEPs of both SMA proper and PMA activated two identical routes for evoked potentials correlating with separate pathways. Corticectomy of the left SMA proper and PMA achieved seizure-free without hemiparesis. Within a small homunculus of the SMA proper, separate epileptic networks were proven and validated by seizure semiology, ictal HFO, and CCEP.
1. Introduction
The supplementary motor area (SMA) and primary motor area (PMA) form a strong network for the initiation, actual execution, and feedback of movement [1], [2], [3], [4], [5]. SMA is divided into pre-SMA and SMA proper by the vertical anterior-commissural line. Reciprocal bidirectional connectivity has been observed along the rostrocaudal cognitive-motor gradient in the network between the SMA proper and PMA [2], [3], [4], [5]. The somatotopic organization of the SMA proper is rostral to caudal with respect to face, arm, trunk, and leg representations. Sequential seizure propagation according to the somatotopic organization within the SMA proper has been reported [6]. To our knowledge, no studies have investigated whether seizures arise from separate but mutual networks within the SMA proper and PMA.
We report a patient with drug-resistant epilepsy characterized by two types of separate focal motor seizures originating from the SMA proper and spreading to the PMA, with identical mutual cortico-cortical evoked potentials (CCEPs). This is the first report of two identical seizure networks without propagation in the small homunculus of the SMA proper, but a one-to-one connection to the PMA.
2. Patient and methods
2.1. Clinical profile
A 12-year-old left-handed girl presented with clusters of nocturnal focal motor seizures since two years of age. She was born at term without asphyxia and had two healthy younger brothers with normal intelligence.
Daily seizures persisted despite treatment with 15 anti-seizure medications and severely impaired her daily activities. The focal motor seizures were of two types: initially tonic extension and subsequent clonic movements of the right arm (type A) and tonic and clonic movements of the right leg (type B). They occurred 10–30 times/day, primarily during sleep, and lasted for about 30–90 seconds.
The patient manifested muscle weakness in the right arm. The dexterous and alternating movements in the right hand were poor, with stable ambulation. She showed a moderate developmental delay; her full intelligent quotient (FIQ) was 48 at 11 years of age. Karyotype genetic analysis revealed 46,X,t(X;14)(q13;p12). Magnetic resonance imaging (MRI) findings were normal. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) showed hypometabolism of the left SMA proper and PMA (Fig. 1A). Subtraction ictal single-photon emission computed tomography co-registered to MRI (SISCOM) showed an increase in cerebral blood flow in the same areas (Fig. 1B). Scalp video encephaloelectrography (EEG) at 12 years of age captured the two types of seizures; both type A and B seizures started as low-amplitude fast activity from C3 and Cz. A Wada test with propofol showed right hemisphere dominance for language and memory.
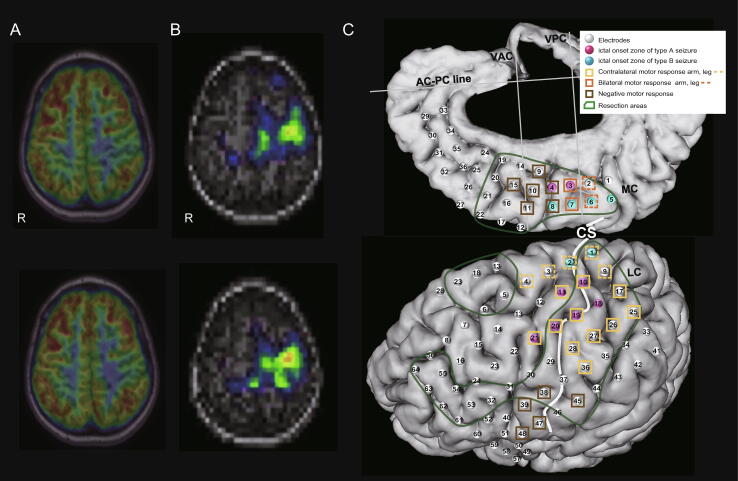
Fig. 1.
FDG-PET, SISCOM, and placement of subdural electrodes superimposed on a 3D brain surface. A: FDG-PET shows hypometabolism in the left SMA proper and PMA. R, right. B: SISCOM shows an increased cerebral blood flow in the two separate areas in the left SMA proper and PMA. R, right. C: A total of 100 subdural grid/strip electrodes (white, red, and blue dots) were implanted to cover the left frontal lobe, PMA, and interhemispheric region, including the SMA proper. The two separate types of seizures were localized - type A (red dots) and B (blue dots). Primary motor functions were widely distributed (light orange squares). Supplemental motor of bilateral motor activation (orange squares) and negative motor areas (brown squares) were localized in the SMA and inferior frontal regions, respectively. Corticectomy was performed over the SMA, part of the PMA, and the frontal lobe, including ictal onset zones and irritative zones with active interictal discharges (green lines). Abbreviations: AC-PC, anterior commissure-posterior commissure; CS, central sulcus; LC, lateral central; MC, mesial central; VAC, vertical line passing through the AC; VPC, vertical line passing through the PC. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Intracranial video EEG
At 12 years of age, the patient underwent intracranial video electroencephalography (IVEEG) using chronic subdural grid/strip electrodes (Unique Medical, Tokyo, Japan) to cover the left frontal lobe, PMA, and interhemispheric region, including the SMA proper (a total of 100 electrodes, Fig. 1C). The electrodes were made of platinum with a recording diameter of 3.0 mm and a center-to-center inter-electrode distance of 10.0 mm. The electrocorticogram (ECoG) was recorded with a bandpass filter of 0.016–600 Hz with a sampling rate of 2000 Hz (EEG 1200, Nihon Kohden, Tokyo, Japan). The reference electrode was placed on the epicranial aponeurosis. The patient was administered carbamazepine, perampanel, and levetiracetam, which were decreased to capture seizures.
2.3. Ictal high-frequency oscillation (HFO)
We evaluated ictal HFO by time-frequency analysis using a short-time Fourier transform (STFT), the details of which can be found in previous studies [7], [8], [9]. We set the onset of the initial conventional ECoG changes as the reference time point (0 s). The spectral power (μV2) was calculated every 10 Hz, and its logarithmic power spectrum (base 10) was computed for the given frequency range and window (25 ms).
2.4. Cortico-cortical evoked potential (CCEP)
In this case, we applied CCEP to investigate the connection between functional and possible epileptic networks. Repetitive single-pulse electrical stimulation (SP-ES) was applied in a bipolar manner to a pair of adjacently placed subdural electrodes by a constant-current stimulator (MEE-1232, Nihon Kohden, Tokyo, Japan). SP-ESs were systematically applied with the following settings: flame-wave pulse, 0.3 ms duration; alternating polarity at 1 Hz; and stimulus intensity, 10 mA. Raw ECoG was recorded with a bandpass filter of 0.08–600 Hz. CCEPs were acquired offline by averaging ECoG signals time-locked to the onset of stimulation.
In this study, SP-ESs were applied to 50 pairs of adjacent electrodes located in the mesial central (middle central electrodes (MC): 16 pairs) and lateral central areas (lateral central electrodes (LC): 34 pairs). The reciprocality of the cortico-cortical connections within the MC and LC was investigated by evaluating whether the area of projection was back to the initial site of stimulation. CCEPs consisted of an early (N1) and a late (N2) negative potential. N1 is an early negative component that occurs 10–50 ms after stimulation. We focused on N1 amplitude [5], [10], [11], [12], [13] and defined the electrode showing the maximum amplitude from baseline (95 ms before the onset of stimulation) as the maximum response.
Ictal HFO and CCEP analyses were performed offline on the data using in-house scripts for MATLAB (Matlab version 8.1.0, The Math Works Inc., Natick, Massachusetts, USA). This study was approved by the Ethics Committee of Osaka City General Hospital (Nos. 1607034 and 1611075). Written informed consent was obtained from all parents.
2.5. Intraoperative cortico-muscular motor evoked potential (MEP)
Monopolar stimulation (train-of-five pulse, duration: 500 ms, interstimulus interval: 4.0 ms, frequency: 250 Hz) was delivered from the electrodes placed at the cortex on the Rolandic areas. MEPs were recorded from muscles in the contralateral upper and lower extremities (abductor pollicis brevis, biceps brachii, brachioradial, anterior tibial) using needle electrodes. The maximum intensity of the stimulation was determined to be 30 mA. When the reaction of the target muscle was negative with the maximum intensity, we regarded the electrode as negative MEP.
3. Results
3.1. Seizures during IVEEG recording and ictal HFO analysis
The patient presented with a total of nine seizures during 10 days of IVEEG recordings with a decrease in carbamazepine and levetiracetam.
Four out of the nine consisted of type A seizures. The low-amplitude fast waves started at the inferior part of the left SMA proper (MC # 2–4, mainly # 3–4) and spread early to the middle part of PMA (LC # 10–11, 18–21). During the type A seizure onset period, interictal spikes and waves were still present at the superior part of the SMA proper (MC # 5–8) and PMA (LC # 1–3) (Fig. 2A). Ictal HFOs at a frequency of 80–120 Hz started at the inferior part of the left SMA proper (MC # 2–4, mainly # 3–4) and spread early to the middle part of PMA (LC # 10–11, 18–21). Subsequently, the HFO increased to higher than 200 Hz (Fig. 2B).
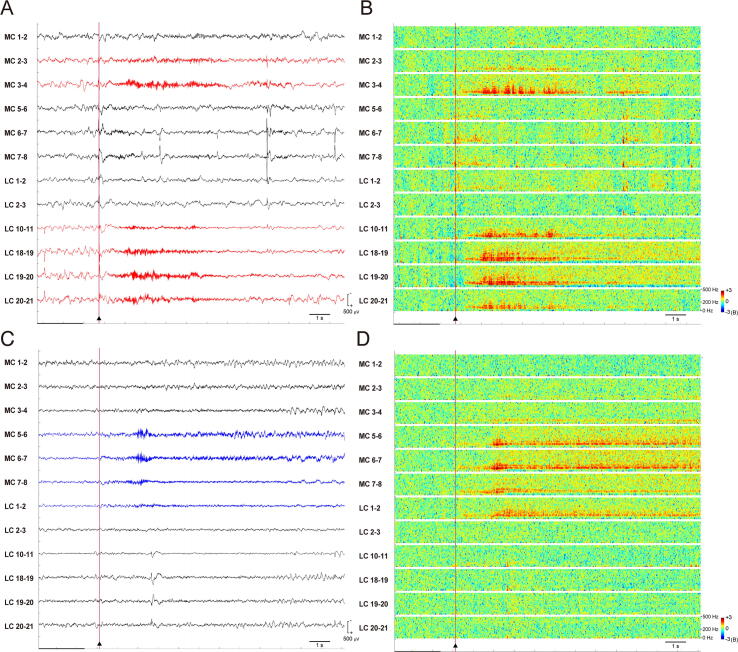
Fig. 2.
Ictal EEG (Bipolar montage; high-frequency filter, 600 Hz; low frequency filter, 1.6 Hz; sampling rate, 2,000 Hz) and time-frequency representation spectrogram of ictal HFOs (X-axis, time; Y axis, 0–500 Hz; color scale [logarithmic], −3 to +3B). Selected channels in the SMA proper and PMA. Type A seizure. A, Type A seizure (red channels) started (cursor) from the lower part of the SMA proper (MC #3, 4) and PMA (LC #10, 11, 18–21) (red dots in Fig. 1C). B, Ictal HFOs of type A seizure were localized identically to the lower part of the SMA proper (MC #3, 4) and PMA (LC #10, 11, 18–21) (red dots in Fig. 1C). Type B seizure. C, Type B seizure (blue channels) started (cursor) from the SMA proper (MC #5–8) and PMA (LC #1, 2) (blue dots in Fig. 1C). D, Ictal HFO was localized identically at the SMA proper (MC #5–8) and PMA (LC #1, 2) (blue dots in Fig. 1C). Abbreviations: LC, lateral central; MC, mesial central. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Three seizures consisted of type B seizures. The low-amplitude fast waves started at the superior part of the left SMA proper (MC # 5–8) and spread early to the superior part of PMA (LC # 1–2). During the type B seizure onset period, interictal spikes and waves were still present in the middle part of PMA (MC # 18–21) (Fig. 2C). Ictal HFOs at a frequency of 80–120 Hz started at the superior part of the left SMA proper (MC # 5–8) and spread early to the superior part of PMA (LC # 1–2). Subsequently, the HFO increased to higher than 200 Hz (Fig. 2D).
The other two seizures simultaneously started from the areas of type A and B seizures on ECoG, while the semiology was almost the same as that of the type A seizure.
3.2. Separate mutual connectivity within the SMA proper and PMA on CCEP
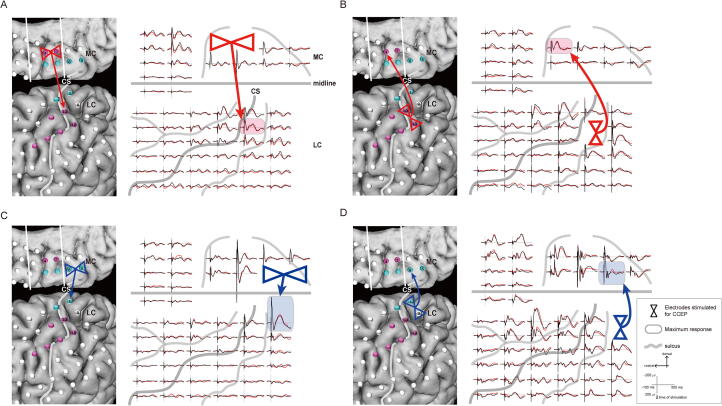
The stimulation of ictal onset electrodes in the SMA proper on CCEP showed the maximum evoked potentials at the early spread to PMA: MC # 3–4 in type A seizure evoked LC #10 (Fig. 3A), and MC # 5–6 in type B seizure evoked LC #1 (Fig. 3C). Stimulation of early spread electrodes in PMA on CCEP showed the maximum evoked potentials at the ictal onset SMA proper electrodes: LC # 10–18 in type A seizure evoked MC #4 (Fig. 3B), and LC # 1–9 in seizure type B seizure evoked MC # 5–6 (Fig. 3D).
Fig. 3.
CCEP of SP-ES at the electrodes in types A and B seizures. A, Left: CCEP response by stimulating MC #3 and 4 (two red triangles) shows a distinct maximum response in LC #10 (red arrow). Right: CCEP waveforms are demonstrated by stimulating MC #3 and 4 (two red triangles). CCEPs show a relatively limited but distinct maximum response in LC #10 (red shaded), except for the adjacent electrodes. B: Left: CCEP response by stimulating LC #10 and 18 (two red triangles) shows a distinct maximum response in MC #4 (red arrow). Right: CCEP waveforms are demonstrated by stimulating LC #10 and 18 (two red triangles). CCEPs showed a distinct maximum response in MC #4 (red shaded). C: Left: CCEP response by stimulating MC #5 and 6 (two blue triangles). CCEPs showed a distinct maximum response in LC #1 (blue arrow). Right: CCEP waveforms are demonstrated by stimulating MC #5 and 6 (two blue triangles). CCEPs show a relatively limited but distinct maximum response in LC #1 (blue shaded). D: Left: CCEP response by stimulating LC #1 and 9 (two blue triangles) shows a distinct maximum response in MC #6 (blue arrow). Right: CCEP waveforms are demonstrated by stimulating LC #1 and 9 (two blue triangles). CCEPs show a distinct maximum response in MC #6 (blue shaded). Abbreviations: CCEP, cortico-cortical evoked potential; CS, central sulcus; LC, lateral central; MC, mesial central; SP-ES, single-pulse electrical stimulation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Resective surgery, pathology, and seizure outcome
Motor mapping showed the arm/leg motor areas at LC electrodes #10, 11, 17, 19–21, 25–28, 36/1–4, and 9 on the PMA. Bilateral motor representatives were confirmed at electrodes MC #2, 3, 6, and 7 on the pre-SMA and SMA proper. Negative motor responses were confirmed at electrodes MC #4, 8, 9–11, and 15 in the frontal lobe (Fig. 1C). Language mapping did not show any language representatives on the left hemisphere grids. The MEP showed that the right lower extremity (LC #2) and right upper extremity (LC #11) were positive with 10 mA stimulation. Other electrodes showed no responses with max 30 mA.
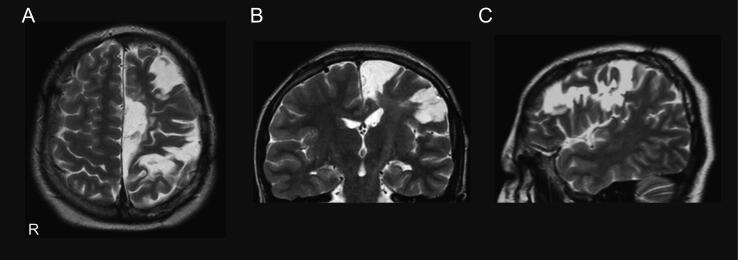
Widespread ictal discharges from SMA to PMA, extensive interictal HFOs, very active spikes over the left frontal region, 10-year-history of drug-resistant frontal lobe epilepsy, cognitive dysfunction, right hand motor weakness, and genetic abnormality indicated developmental and epileptic encephalopathy. Therefore, we performed extensive corticectomy of both mesial and lateral frontal regions, sparing the motor area (Fig. 4). Histopathology showed focal cortical dysplasia (FCD) type I. The patient was seizure-free for two years after surgery, accompanied by a course of carbamazepine, perampanel, and levetiracetam. Currently, she has a mild dexterous movement disorder but is ambulatory. The FIQ score improved to 56 at 14 years of age, two years post-surgery.
Fig. 4.
Postoperative MRI. A, Axial, B, Coronal; C, Sagittal MRI show extensive corticectomy of both mesial and lateral frontal regions, sparing the motor area. R, right.
4. Discussion
4.1. Two separate SMA proper onset focal motor seizures unlike the Jacksonian march
The semiology of the beginning of type A and B seizures in the patient was individual tonic posturing in the contralateral arm and leg: type A, initially tonic extension and subsequently clonic movements of the right arm, and type B, tonic and clonic movements of the right leg.
Cortical stimulation in the human SMA has revealed the presence of somatotopic organization within the SMA [1], [14]. The stimulation can evoke motor symptoms in any part of the body on either side with a tendency toward the contralateral predominance. The head to leg somatotopic localizations are located in the small homunculus lying from rostral to caudal in the SMA [15]. Baumgartner et al. [3] reported the propagation or jumping of both ictal and interictal epileptiform discharges from a part of the SMA to somatotopically corresponding parts of the PMA. The ictal semiology initially consisted of unilateral tonic manifestation and then a clonic seizure involving the same body part.
Our patient demonstrated two identical type A and B seizures arising from a separate location in the SMA proper identified by ictal HFO, corresponding to arm and leg tonic posturing separately. The ictal HFOs in the two SMA areas did not spread to each other during the first 20 seconds after seizure onset. Secondary propagation occurred after the seizure spread to the PMA. Two separate SMA proper onset focal motor seizures did not involve the other ictal onset zones, unlike the Jacksonian march in the PMA.
4.2. No integration within the SMA proper but tightly linked to the PMA
CCEP stimulation of MC # 3–4 of the SMA proper showed the maximum response at LC #10 of the PMA hand motor area, exactly corresponding to the beginning of ictal HFO of the type A seizure. In contrast, CCEP stimulation of LC # 10–18 demonstrated the maximum response at MC #4. CCEP stimulation at electrodes showed the maximum response in the PMA and the “homing” CCEP on exactly the same electrodes as first stimulated in the SMA proper [5]. We confirmed that they were separate, but very close, each located adjacent to the neural network, with reciprocal connections between the SMA proper and PMA.
After resection of the SMA, significant neurological deficits, such as hemiparesis and aphasia, appeared immediately. However, the deficits resolved relatively well soon after surgery. As described in the SMA proper and pre-SMA functions, fine motor and speech function in complex tasks and/or at high speed may be impaired [15], [16], [17], [18].
It is conceivable that the N1 component of the CCEP has been induced by the direct transmission of stimulation and excitation of neurons that project association fibers through the white matter tracts [19], [20], [21]. However, conventional ECoG can detect comprehensive transmission via white matter tracts and subcortical structures. CCEP can be safely performed in children and provide information on the relationship between the functional cortex and epileptogenic zone in patients who require wide corticectomy without MRI lesions [13], [22]. Pathogenesis that involves transmission other than white matter tracts (e.g., subcortical structures, such as the thalamus and brain stem) is also frequently observed in pediatric drug-resistant epilepsy [23], [24], [25]. Therefore, further studies are necessary to differentiate the various networks and assess their pathogenesis.
4.3. Wide resection including the Rolandic areas, SMA, and prefrontal areas
Previous reports demonstrated corticectomy of the Rolandic areas for drug-resistant rolandic epilepsy without MRI lesions [26]. The motor symptoms of the right arm and leg were widely distributed over the pre- to post-central gyri in our patient. In patients with neuronal migration disorders, the motor homunculus of the epileptic brain could be distorted and expanded [27], [28]. For long-lasting drug-resistant focal motor seizures, including those of the SMA and PMA, aggressive surgical resection may be considered as the last resort. Despite extensive left fronto-central cortical resections, our patient recovered from minor finger movement deficits.
5. Conclusion
Ictal HFO and CCEP localized the separate epileptic networks within a small homunculus of the SMA proper and was mutually connected with the PMA in two types of drug-resistant focal motor seizures. CCEP has the potential to identify and increase our understanding of the epileptic network involved in drug-resistant focal epilepsy by outlining functional networks.
6. Ethical publication statement
We confirm that we have read the journal’s position on issues involving ethical publication and affirm that this report is consistent with those guidelines.
7. Author's contributions
All authors contributed substantially to the submitted manuscript. Takeshi Inoue: conception, data collection, manuscript draft, and revision. Takehiro Uda, Ichiro Kuki: data collection, technical advice, manuscript draft, and revision. Naohiro Yamamoto, Shizuka Nagase, Megumi Nukui, Shin Okazaki, Toshiyuki Kawashima, Yoko Nakanishi, Noritsugu Kunihiro, Yasuhiro Matsuzaka: data collection, Hisashi Kawawaki, Hiroshi Otsubo: data analysis, manuscript draft and revision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by MHLW Research program on rare and intractable diseases, Grant number JPMH20FC1039 (to I. Kuki).
Dr. Otsubo was supported by the EpLink - the Epilepsy Research Program of the Ontario Brain Institute (OBI). OBI is an independent non-profit corporation, funded partially by the Ontario government.
References
- 1.Lim S.H., Dinner D.S., Pillay P.K., Lüders H., Morris H.H., Klem G. Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol. 1994;91(3):179–193. doi: 10.1016/0013-4694(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda A., Lüders H.O., Burgess R.C., Shibasaki H. Movement-related potentials recorded from supplementary motor area and primary motor area: role of supplementary motor area in voluntary movements. Brain. 1992;115(4):1017–1043. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner C., Flint R., Tuxhorn I., Van Ness P.C., Kosalko J., Olbrich A. Supplementary motor area seizures: propagation pathways as studied with invasive recordings. Neurology. 1996;46(2):508–511. doi: 10.1212/wnl.46.2.508. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda A., Yazawa S., Kunieda T., Ohara S., Terada K., Mikuni N. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain. 1999;122(5):915–931. doi: 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto R., Nair D.R., LaPresto E., Bingaman W., Shibasaki H., Luders H.O. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain. 2007;130:181–197. doi: 10.1093/brain/awl257. [DOI] [PubMed] [Google Scholar]
- 6.Ohara S., Ikeda A., Kunieda T., Yazawa S., Taki J., Nagamine T. Propagation of tonic posturing in supplementary motor area (SMA) seizures. Epilepsy Res. 2004;62(2–3):179–187. doi: 10.1016/j.eplepsyres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T., Inouchi M., Matsuhashi M., Matsumoto R., Hitomi T., Daifu-Kobayashi M. Interictal slow and high-frequency oscillations: is it an epileptic slow or red slow? J Clin Neurophysiol. 2019;36(2):166–170. doi: 10.1097/WNP.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa K., Matsumoto R., Imamura H., Matsuhashi M., Kikuchi T., Kunieda T. Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin Neurophysiol. 2015;126(1):47–59. doi: 10.1016/j.clinph.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K., Matsumoto R., Matsuhashi M., Usami K., Shimotake A., Kunieda T. High frequency activity overriding cortico-cortical evoked potentials reflects altered excitability in the human epileptic focus. Clin Neurophysiol. 2017;128(9):1673–1681. doi: 10.1016/j.clinph.2017.06.249. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto R., Nair D.R., LaPresto E., Najm I., Bingaman W., Shibasaki H. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(10):2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- 11.Keller C.J., Bickel S., Entz L., Ulbert I., Milham M.P., Kelly C. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Nati Acad Sci USA. 2011;108(25):10308–10313. doi: 10.1073/pnas.1019750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usami K., Matsumoto R., Kobayashi K., Hitomi T., Matsuhashi M., Shimotake A. Phasic REM transiently approaches wakefulness in the human cortex-a single-pulse electrical stimulation study. Sleep. 2017;40(8) doi: 10.1093/sleep/zsx077. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T., Kobayashi K., Matsumoto R., Inouchi M., Togo M., Togawa J. Engagement of cortico-cortical and cortico-subcortical networks in a patient with epileptic spasms: an integrated neurophysiological study. Clin Neurophysiol. 2020;131(9):2255–2264. doi: 10.1016/j.clinph.2020.04.167. [DOI] [PubMed] [Google Scholar]
- 14.Fried I., Katz A., McCarthy G., Sass K.J., Williamson P., Spencer S.S. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11(11):3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landazuri P., Lorella M. Electrical stimulation for mapping of the epileptogenic zone. In: Lhatoo S., Kahane P., Lüders H., editors. Invasive studies of the human epileptic brain principles and practice. 1st ed. Oxford University Press; New York: 2019. pp. 161–172. [Google Scholar]
- 16.Ikeda A., Sato T., Ohara S., Matsuhashi M., Yamamoto J., Takayama M. “Supplementary motor area (SMA) seizure” rather than “SMA epilepsy” in optimal surgical candidates: a document of subdural mapping. J Neurol Sci. 2002;202(1–2):43–52. doi: 10.1016/s0022-510x(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda A. Subdural EEG in frontal lobe epilepsy. In: Lhatoo S., Kahane P., Lüders H., editors. Invasive studies of the human epileptic brain principles and practice. 1st ed. Oxford University Press; New York: 2019. pp. 312–325. [Google Scholar]
- 18.Zentner J., Hufnagel A., Pechstein U., Wolf H.K., Schramm J. Functional results after resective procedures involving the supplementary motor area. J Neurosurg. 1996;85(4):542–549. doi: 10.3171/jns.1996.85.4.0542. [DOI] [PubMed] [Google Scholar]
- 19.Conner C.R., Ellmore T.M., DiSano M.A., Pieters T.A., Potter A.W., Tandon N. Anatomic and electro-physiologic connectivity of the language system: a combined DTI-CCEP study. Comput Biol Med. 2011;41(12):1100–1109. doi: 10.1016/j.compbiomed.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto R., Kunieda T., Nair D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure. 2017;44:27–36. doi: 10.1016/j.seizure.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamao Y., Matsumoto R., Kunieda T., Arakawa Y., Kobayashi K., Usami K. Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp. 2014;35(9):4345–4361. doi: 10.1002/hbm.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T., Kawawaki H., Fukuoka M., Kim K., Nukui M., Kuki I. Intraoperative cortico-cortical evoked potentials show disconnection of the motor cortex from the epileptogenic network during subtotal hemispherotomy. Clin Neurophysiol. 2018;129:455–457. doi: 10.1016/j.clinph.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Steriade M., Timofeev I. Generators of ictal and interictal electroencephalograms associated with infantile spasms: intracellular studies of cortical and thalamic neurons. Int Rev Neurobiol. 2002;49:77–98. doi: 10.1016/s0074-7742(02)49008-3. [DOI] [PubMed] [Google Scholar]
- 24.Aghakhani Y., Bagshaw A.P., Benar C.G., Hawco C., Andermann F., Dubeau F. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- 25.Siniatchkin M., Groppa S., Jerosch B., Muhle H., Kurth C., Shepherd A.J. Spreading photoparoxysmal EEG response is associated with an abnormal cortical excitability pattern. Brain. 2007;130:78–87. doi: 10.1093/brain/awl306. [DOI] [PubMed] [Google Scholar]
- 26.Otsubo H., Chitoku S., Ochi A., Jay V., Rutka J.T., Smith M.L. Malignant rolandic-sylvian epilepsy in children: diagnosis, treatment, and outcomes. Neurology. 2001;57(4):590–596. doi: 10.1212/wnl.57.4.590. [DOI] [PubMed] [Google Scholar]
- 27.Chitoku S., Otsubo H., Harada Y., Jay V., Rutka J.T., Weiss S.K. Extraoperative cortical stimulation of motor function in children. Pediatr Neurol. 2001;24(5):344–350. doi: 10.1016/s0887-8994(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 28.Akai T., Otsubo H., Pang E.W., Rutka J.T., Chitoku S., Weiss S.K. Complex central cortex in pediatric patients with malformations of cortical development. J Child Neurol. 2002;17(5):347–352. doi: 10.1177/088307380201700507. [DOI] [PubMed] [Google Scholar]